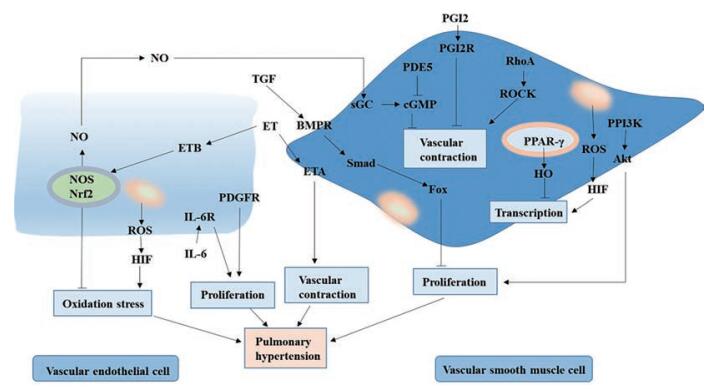
肺动脉高压(pulmonary arterial hypertension, PAH) 被称为“心血管疾病中的癌症”, 病理机制和临床分型复杂, 致病因素多样。早期, PAH主要表现为肺血管内皮功能障碍导致的血管收缩、血栓形成及细胞过度增殖等。血管收缩的发生与内皮素(如一氧化氮、前列腺素) 和血管收缩因子(如内皮素-1、血栓素) 之间的不平衡有关, 肺血管的重构主要与细胞异常增殖有关, 如肺动脉平滑肌细胞(pulmonary arterial smooth muscle cells, PASMCs)、内皮细胞(endothelial cells, ECs) 和成纤维细胞[1]。PASMCs的过度增殖是导致肺血管重构的关键因素, 参与PASMCs增殖的信号通路主要包括PI3K-Akt、RhoA/ROCK、JAK/STAT、T细胞核因子(nuclear factor of activated T-cells, NFAT)、有丝裂原活化的蛋白激酶(mitogen-activated protein kinase, MAPK)、BMP/TGF-β-Smad、Notch、Wnt/β-catenin、活性氧簇(reactive oxygen species, ROS) 和血管活性肠肽(vasoactive intestinal peptide, VIP) 等(图 1)[2]。除血管周围免疫细胞过度积累和血管内浸润程度增加外, PAH患者的血清细胞因子和趋化因子的水平也异常升高, 主要包括CCL2/MCP-1、CCL5/RANTES、CX3CL1/fractalkine、白介素(interleukin, IL)-1α、IL-1β、IL-2、IL-4、IL-5、IL-6、IL-8、IL-10、IL-12、IL-13、干扰素和肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α) 等[2]。
|
Figure 1 Main pathological mechanisms and signaling pathways related to pulmonary arterial hypertension (PAH). ET: Endothelin; ETA: Endothelin A; ETB: Endothelin B; TGF: Transforming growth factor; BMPR: Bone morphogenetic protein receptor; Fox: Forkhead box; PDGFR: Platelet-derived growth factor receptor; IL: Interleukin; ROS: Reactive oxygen species; HIF: Hypoxia-inducible factor; RhoA: Ras homolog gene family member A; ROCK: Rho-associated coiled-coil forming protein kinase; NOS: Nitric oxide synthase; NO: Nitric oxide; GMP: Cyclic guanosine monophosphate; PDE5: Phosphodiesterase-5; PGI2R: Prostaglandin I receptor 2 receptor; sGC: Soluble guanylyl cyclase; Nrf2: Transcription factor NF-E2 related factor 2 |
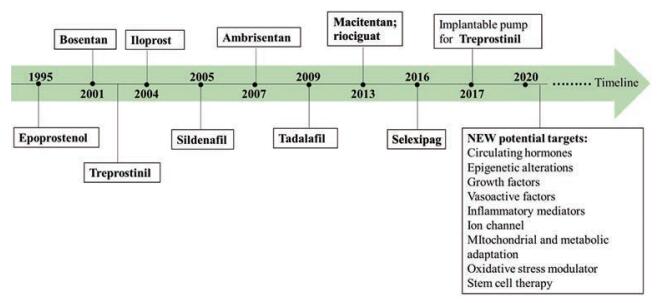
根据药理机制的不同, 抗PAH药物主要包括磷酸二酯酶-5抑制剂、内皮受体拮抗剂、环前列腺素受体激动剂、核因子κB (nuclear factor kappa-B, NF-κB) 抑制剂、受体酪氨酸激酶抑制剂、脂肪酸抗氧化剂、可溶性鸟苷酸环化酶刺激剂、ρ激酶抑制剂、血栓素A2 (thromboxane A 2, TXA2) 受体拮抗剂、内皮型一氧化氮合酶(endothelial nitric oxide synthase, eNOS) 耦合剂、5-羟色胺(5-hydroxytryptamine, 5-HT) 受体拮抗剂、羟甲戊二酰辅酶A (HMG-CoA) 还原酶抑制剂、芳香化酶抑制剂、抗IL-1或IL-6单克隆抗体等[2]。自1995年以来, 一些治疗药物的相继上市(图 2), 提高了患者的存活率, 改善了预后。
|
Figure 2 Approved drugs by Food and Drug Administration to combat PAH |
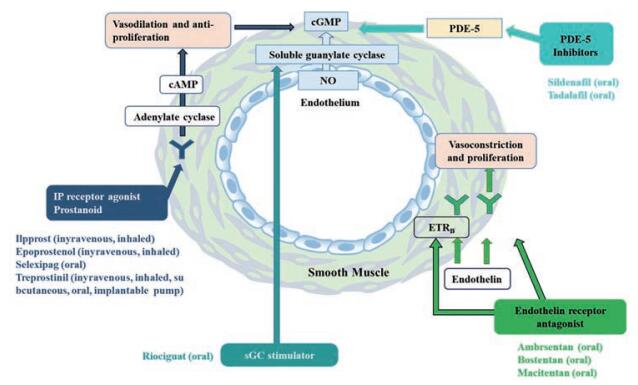
当前针对PAH的临床治疗药物主要包括前列环素类似物和前列环素受体激动剂、内皮素受体拮抗剂、磷酸二酯酶-5抑制剂和可溶性鸟苷酸环化酶抑制剂(图 3)。通过直接调节ECs, 或作用于PASMCs, 这些药物能抑制血管收缩或促进血管舒张, 但只能对血管进行调控, 无法根治PAH; 并且这些药物存在明显不足, 如作用时间短, 用药次数频繁、剂量大等; 且患者容易出现严重心脏毒性、腹泻、头痛等不良反应。另外, PAH的病灶主要位于肺动脉血管, 呈高度弥散性, 药物难以在病灶蓄积。因此, 针对PAH治疗的给药系统的开发显得尤为迫切。本文将对PAH的治疗药物和药物递送策略等进行归纳总结。
|
Figure 3 Singaling pathways and inhibitors/activators to alleviate PAH |
依前列醇、伊洛前列素、曲前列环素(treprostinil, TRE)、贝前列素(beraprost, BPS) 和赛乐西帕(selexipag) 等前列环素途径作用药物为肺血管扩张剂, 具有抗血栓形成、抗增殖和抗炎作用[3, 4]; 常用于治疗特发性PAH和栓塞性PAH。但该类药物存在生物半衰期短和严重不良反应等缺点, 需以递送手段来克服, 从而提高药物疗效。Gupta等[5]利用2-羟丙基-β-环糊精(2-hydroxypropyl-β-cyclodextrin, HP-β-CD) 与前列腺素1 (PGE1) 形成包合复合物, 以双乳液溶剂蒸发法制备负载PGE1-HP-β-CD的可吸入聚乳酸-乙醇酸(polylactic acid-glycolic acid, PLGA) 微粒, 在肺动脉中提供即时和持续的血管舒张作用; 以聚乙烯亚胺(polyethyleneimine, PEI) 修饰PLGA微球, 并采用聚乙烯醇(polyvinyl alcohol, PVA) 作为颗粒的外部涂层, 递送PGE1治疗PAH, 通过吸入给药延长半衰期[6], 药物从聚合物颗粒中连续释放产生持续的肺血管扩张作用, 减少给药频率, 改善平均肺动脉压(mean pulmonary arterial pressure, mPAP)、右心室肥大(right ventricle hypertrophy, RVH) 和肺血管肌肉化和动脉细胞增殖情况[7]。
为解决伊洛前列素包封率低的问题, 并改善其药理作用, Jain等[8]通过薄膜水合法制备了伊洛前列素脂质体, 并采用了阳离子脂质提高药物的包封率, 包封率达60%; 与游离伊洛前列素相比, 负载伊洛前列素的阳离子脂质体增强了肺动脉的血管舒张作用, 并减少了用药剂量。为了延长药物作用时间, 有人制备了TRE缓释制剂(SR): 以曲前列素二乙醇胺和渗透性赋形剂为片芯, 半透膜为包衣材料[9, 10]。口服后, 该制剂能延长药物在II~III级患者的作用时间。将TRE-烷基前药(TPD) 包载于脂质纳米颗粒, 也可以降低给药频率和药物不良反应等[11]。
为有效递送BPS, Akagi等[12]采用乳液溶剂扩散法制备了BPS纳米粒。在野百合碱诱导的PAH模型(MCT-PAH) 中, 气管内滴注后, 与游离BPS相比, 该制剂可显著降低右室压力、RVH以及肺动脉肌化程度, 提高模型大鼠的存活率[12]。
研究发现BPS314d异构体(esuberaprost) 可进一步舒张肺动脉, 但抗增殖作用依赖于NOS途径; 当环腺苷酸(cAMP) 增加时, esuberaprost的效价(EC50 0.4 nmol·L-1) 增强[13]。
1.2 内皮素受体拮抗剂波生坦(bosentan, BOS)、马西替坦、安倍生坦、川芎嗪和西他森坦等药物属于内皮素受体拮抗剂, 通过阻止内皮素-1与内皮素受体结合, 扩张肺血管, 主要用于治疗结缔组织病相关PAH (CTD-PAH)、栓塞性PAH等。
为满足临床需求, 降低药物肝毒性。通过喷雾干燥和喷射研磨, 波生坦水合物(BST) 微粒被制备成了干粉吸入剂(dry powder inhaler, DPI)[14]。由于较小的空气动力学直径和聚集体强度, 使药物有效沉积于肺部, 改善了其对PAH的治疗作用[14]。Hanna等[15]以PLGA和PVA为材料, 利用自发乳化溶剂扩散法制备了控释制剂聚合物胶体(RCRPC), 以促进BOS的递送; 与口服BOS混悬液相比, 气管内给药后, 有效提高了药物的生物利用度(12.71倍), 并持续舒张肺血管。
为优化BOS的给药技术, 开发新的PAH替代疗法, Gimenez等[16]利用ε-聚己内酯(polycaprolactone, PCL) 的特性, 以PCL/丙酮为溶液, 采用电喷涂技术制备了半结晶状的BOS纳米粒; 与游离的BOS相比, 电喷雾纳米粒能延长并控制BOS的释放。基于天然金黄色胡萝卜提取物的固体分散技术[17]或明胶微球[18]也被用于提高安贝生坦的溶解度和生物利用度或肺靶向性。
1.3 NO作用途径——磷酸二酯酶(phosphodiesterase, PDE) 抑制剂PDE抑制剂主要包括西地那非(sildenafil, SIL)、伐地那非(tadalafil, TAD)、米力农和托拉芬特等。通过作用于NO/cGMP途径, PDE5抑制剂实现扩张血管、降低血管收缩的作用[1]; 而PDE3/4抑制剂通过抑制平滑肌细胞的迁移, 逆转肺血管重构[19]。这些抑制剂主要用于治疗成人I级PAH、特发性PAH (IPAH) 和CTD-PAH, 提高运动能力, 延缓临床恶化等[20]。
基于熔融乳化技术制备的SIL固体脂质体(SC-SLN), 通过雾化给药克服了口服给药的缺点, 并且能选择性地靶向肺血管; 相对于侵入性肠胃外产品, 该制剂具有更好的患者依从性[21]。基于类似的方法, 有人制备了SIL纳米脂质体(NLCs), 雾化给药后血管扩张作用明显, 在肺泡巨噬细胞轻度聚集, 相对于SLN, 其安全性更高[22]。
采用水包油包水双乳液溶剂蒸发法, 以PEI为致孔剂制备的多孔SIL-PLGA颗粒, 通过吸入给药后, 延长了药物的释放时间与血管舒张作用, 降低了药物在全身的暴露[23]。另外, 利用振动和喷雾干燥法制备载有SIL的PLGA微粒(MPs), 也能持续释药, 克服多次给药带来的不便, 改善PAH患者生活质量[24]。
研究发现脂质体技术是改善PDE-5抑制剂治疗作用的重要载体[25]。通过水合法制备了葡萄糖醛酸(glucuronic acid, GlcA) 修饰的脂质体(GlcA-Lips), 通过靶向葡萄糖转运体-1靶向递送SIL至PASMCs递送, 抑制了MCT-PAH大鼠的血管重塑; 尾静脉给药后, caspase-3、eNOS和环鸟苷酸(cyclic guanosine monophosphate, cGMP) 的表达增强, 磷酸化ERK1/2和己糖激酶-2的表达降低, 肺动脉压力、内膜增厚及RVH明显降低。相较于无表面修饰的脂质体, GlcA-Lips的作用效果更好, 安全性更高[25]。以1, 2-二肉豆蔻酰基-sn-甘油-3-磷酸胆碱为脂材制备的脂质体也能潜在促进PDE-5抑制剂的递送[26]。
另外, 研究人员开发了多种基于TAD给药的制剂与给药系统。包括片剂[27]、可吸入型PLGA纳米粒[28]、纳米DPI[29]、可吸入纳米乳剂[30]等。通过乳液溶剂蒸发法制备的TAD可吸入PLGA纳米粒, 能持续释放24 h, 且雾化性能良好, 吸入后主要沉积在肺深处[28]。喷雾干燥技术制备的TAD纳米DPI; 给药后, 药物能有效蓄积于病灶, 提高了药物的生物利用度和治疗功效, 减少了系统不良反应的发生[29]。TAD的可吸入纳米乳剂, 不仅提高了药物溶解度, 也提升了cGMP的水平, 且无鼻腔毒性[30]。
1.4 NO作用途径——鸟苷酸环化酶吸入一氧化氮(nitric oxide, NO) 可改善原发性和继发性PAH患者的氧合作用并降低肺动脉压力(pulmonary artery pressure, PAP), 用于新生儿或成人急性PAH的治疗[31]。可溶性鸟苷酸环化酶(soluble guanylyl cyclase, sGC) 是心肺系统中的一种酶, 也是NO的受体; 当NO与sGC结合时, 该酶催化信号分子cGMP的合成, 从而调节血管张力、增殖、纤维化和炎症过程。上市药物利奥西瓜(riociguat) 利用该机制治疗WHO FC II或III中PAH患者以及在WHO FC II或III中CTEPH或持续/复发性PAH患者, 但尚无儿童应用研究。
NONOate 2-(二甲胺基) 乙基酪氨酸/NO气雾剂可改善氧合作用, 且能选择性舒张肺血管, 无明显的全身毒性。而吸入硝酸甘油能特异地降低PAP, 抑制高肺血流量引起的肺血管重塑[32]。为了促进二亚乙基三胺NONOate (DETA NONOate, DN) 递送, 有人以溶剂蒸发和水合法制备DN脂质体, 并与多肽CARSKNKDC (CAR) 偶联得到一种长效脂质体; 气管内给药后, 该脂质体能聚集于肺部, 作用于PASMCs, 舒张肺血管, 降低PAP而不改变全身性动脉压力(mean systemic arterial pressure, mSAP)。另外, 基于脂质/蛋白质/糖基构成的干粉微粒也被用于递送sGC激动剂(BAY 41-2272、BAY 41-8543和BAY 58-2667)[33]: 在急性PAH的清醒羔羊模型中, 吸入给药后能产生剂量依赖性的肺血管扩张作用, 有效提升了cGMP的水平, 并增强了动脉氧合作用而不降低mSAP。
1.5 钙通道阻滞剂(CCB)在去极化过程中, 硝苯地平、地尓硫卓和氨氯地平等钙通道阻滞剂通过抑制钙离子流入心脏和血管平滑肌, 扩张肺血管, 可用于治疗低氧和慢性栓塞型PAH。
舌下给药硝苯地平片剂可以降低肺和全身血管阻力, 增加心输出量, 改善血液氧合作用。为降低给药频率, 增强患者依从性, Saigal等[34]以PVA为释放调节剂, 通过喷雾干燥法制备了硝苯地平微球, 控制药物释放。口服氨氯地平可在PAH患者中引起急性肺血管扩张[35]。
1.6 酪氨酸酶抑制剂(tyrosine kinase inhibitor, TKI)通过作用于血小板源性生长因子(platelet derived growth factor receptor, PDGF) 受体、血管内皮生长因子受体、c-kit和Raf等, TKI能抑制酪氨酸激酶活性, 抑制细胞增殖, 改善PAH/IPAH症状[36-38]。这类主要药物包含伊马替尼、尼罗替尼和索拉菲尼。为提高药物疗效, 利用穿膜肽CAR能促进药物向靶细胞/组织转运的特点, 有人制备了CAR-伊马替尼混合物, 静脉注射或舌下给药后, 能有效增强伊马替尼的肺血管扩张作用, 但无全身性血管扩张的不良反应; 舌下给药与静脉注射疗效一致, 说明靶向作用于肺部可有效提高疾病的疗效[39]。另外, 为降低给药剂量及给药次数, Akagi等[40]用乳化溶剂扩散法以PLGA为囊材制备伊马替尼纳米混悬液(Ima-NPs), 单次气管给药1 mg·kg-1后, 能产生持续的抗增殖作用, 抑制小动脉重构, 有效地抑制了MCT-PAH的发展。
1.7 线粒体调节剂线粒体调节剂通过促进葡萄糖氧化, 抑制丙酮酸脱氢酶激酶, 增强氧化磷酸化作用, 促进H2O2和细胞色素C的释放, 诱导线粒体依赖性细胞的凋亡, 减少细胞增殖, 改善肺血管重构从而治疗PAH[41-43]。线粒体调节剂主要包括二氯乙酸盐(dichloroacetate, DCA)、曲美他嗪和雷诺嗪。口服DCA可以预防和逆转MCT-PAH和低氧型PAH, 降低死亡率[41]。2019年, 曲美他嗪胶囊已完成II期临床试验, 结果表明, 通过阻断Randle循环间接影响葡萄糖氧化, 该制剂能改善右心室功能[44]。
1.8 抗炎和免疫调节剂PAH患者炎症反应严重, 体内炎症免疫反应调节紊乱。抗炎和免疫调节剂通过抑制促炎因子的激活, 调节代谢功能; 降低肺中Plrp5/6、细胞周期蛋白D1和WISP 1等表达可防止肺血管重塑, 增加肺泡形成, 减少出血并发症, 治疗重症PAH[45, 46]。主要治疗药物包含甲基巴多索隆、他克莫司(tacrolimus, TAC)、利妥昔单抗、Msed蛋白和ICG001 (表 1)[40, 47-49]。
| Table 1 Anti-inflammatory and immunomodulatory agents |
药物递送技术能潜在地改善这些药物的治疗作用。如利用PEG-PLGA制备纳米粒, 递送NF-κB信号通路阻滞剂; 对MCT-PAH模型气管内给药后, 可促进内源型NO的生成, 有效抑制肺血管重塑, 提高生存率[50]。
TAC在PAH治疗的应用也时有报道。TAC的上市剂型主要有胶囊剂、干混悬剂、缓释固体制剂等。针对TAC治疗存在的问题如生物利用度低和不良反应明显等, 研究人员设计了一些递药系统, 对其进行优化改善。如采用喷雾干燥法制备了TAC的聚合物纳米粒, 不仅提高了TAC的溶解度, 且可控制药物释放; 以DPI的形式进行肺部给药后, 药物能明显沉积于肺部[51]。采用二棕榈酰磷脂酰胆碱和二棕榈酰磷脂酰甘油为赋形剂, 通过喷雾干燥法制备的TAC-磷脂气溶胶微粒, 也能促进药物的肺部递送[52]。
1.9 Rho激酶抑制剂Rho激酶抑制剂能有效抑制5-HT诱导的PASMCs增殖和细胞周期, 抑制肺血管重构; 也能减轻肺小动脉ECs的损伤, 抑制胶原纤维的表达[53, 54]。Rho激酶抑制剂主要用于治疗慢性HPAH和MCT-PAH, 这类药物主要包括法舒地尔(fasudil, FSD) 和氮杂吲哚-1 (azaindole-1) 等。
为了促进FSD的肺部递送, 研究人员设计了多种前体药物和递药系统。如偶联CAR提高了FSD的肺部靶向性, 并延长了血管舒张时间[55]。共递送CAR和FSD能有效提升大鼠右心室收缩压(right ventricular systolicpressure, RVSP) 的短期降压作用, 并且舌下给药和静脉注射具有相同疗效[39]。另外, 前药法舒地尔盐-二氯乙酸盐(FDCA) 可增强ROCK II的活性; 在PDGF-BB和低氧诱导的PASMCs和ECs中, 抑制了TNF-α和IL-6的表达; 口服FDCA能舒张肺血管, 抑制血管重构[56]。将大鼠血液中的红细胞进行低渗裂解, 通过聚碳酸酯膜挤出制备的纳米红血球, 作为可吸入性载体递送FSD, 抑制Rho激酶活性, 延长药物的半衰期[57]。将FSD包载于DSPE-PEG 5000胶束中, 表面用CAR肽修饰以靶向肺组织, 吸入给药后, 延长了循环时间和肺部保留时间, 降低了mPAP且不影响mSAP[58]。通过水化挤出法, 采用硫酸铵诱导的跨膜电化学梯度包载FSD制备脂质体, 雾化给药后能舒张肺血管和下调生长因子、细胞增殖标志物和基质蛋白等, 上调凋亡标志物的表达, 缓解PAH的症状, 为非侵入式、控释和肺部靶向治疗提供了选择[59]。注射给药后, CAR修饰FSD脂质体, 半衰期延长了34倍, 可长期降低低氧诱导的PAH (SU-PAH) 或MCT-PAH的mPAP。吸入给予FSD磁性脂质体后, 磁场作用能促进药物在肺血管系统中的积聚, 半衰期和生物利用度分别延长了27倍和14倍, 增强了PASMCs的摄取, 并抑制PASMCs的增殖[60]。
其他非经典的Rho激酶抑制剂如氟西汀, 也能潜在治疗PAH。通过抑制RhoA-ROCK和Akt信号通路, 氟西汀能抑制MCT-PAH大鼠肺动脉的重构[61]。灌胃后, 氟西汀可抑制PASMCs增殖, 诱导PASMCs凋亡并上调Kv1.5通道, 降低Bcl-2和Bcl-xL蛋白的表达, 增加caspase 3蛋白和mRNA的表达, 降低PAP和RVH, 并抑制肺动脉内侧壁厚度的增加等[62]。RP5063是一种新型化学实体, 是5-HT调节剂, 研究表明, 灌胃给药后RP5063可改善肺血流动力学, 减少RVH, 恢复动脉氧合作用, 延缓肺血管的重塑[63, 64]。
1.10 羟甲戊二酰辅酶A (HMG-CoA) 拮抗剂HMG-CoA拮抗剂可靶向肺小动脉和CD11b炎症细胞, 具有抗炎和抗增殖作用, 抑制肺血管重塑, 可用于治疗MCT-PAH[65, 66]。治疗药物主要有匹伐他汀(pitavastatin)、西立伐他汀等。纳米递药系统的设计能潜在地改善这类药物的治疗作用。如气管内滴注匹伐他汀纳米粒PLGA-NPs后, 14天内粒子能有效聚集在肺巨噬细胞和肺小动脉[65]。以基于PLGA-PVA制备的匹伐他汀纳米粒, 低剂量静脉给药后能延缓PAH的发展, 提高存活率[66]。吸入给药西立伐他汀脂质体能减少PA的增生和肺小动脉的阻塞, 降低mPAP并改善右心室功能等[67]。
1.11 5-脂氧合酶(5-LO) 抑制剂RF22-c是一种强效的5-LO抑制剂。以羟丙基甲基纤维素、大豆卵磷脂和Softisan 100为材料, 通过溶剂扩散技术制备RF22-c-脂质纳米粒(SLNs), 提高了药物RF22-c的缓释性能与肺靶向性, 使RF22-c以高浓度聚集在肺动脉病变处, 提高了RF22-c的生物利用度[68]; 腹腔注射SLNs可降低MCT-PAH大鼠的mPAP和毛细血管前阻力, 抑制血管重塑, 降低RVH[68]。
1.12 过氧化物酶体增殖物激活受体-γ (PPAR-γ)激动剂PPAR-γ与PAH的发病机制有关, PPAR-γ激动剂罗格列酮(rosiglitazone, ROSI) 通过抑制5-HT2BR的表达、减轻动脉粥样硬化病变并阻止受损动脉壁的重塑, 达到抑制血管收缩扩张肺动脉的目的[69, 70]。Rashid等[71]以双乳剂-溶剂蒸发法制备的ROSI-PLGA微粒, 对SU-PAH大鼠气管内给药后, 增加了eNOS、PPAR-γ和NOX-4的表达, 同时能促进肺动脉血管的舒张, 降低mPAP, 减少PASMCs的增殖。
1.13 mTOR抑制剂mTOR的异常激活与PAH的肺血管重塑有关, mTOR抑制剂雷帕霉素(rapamycin, RAP) 可通过下调Akt/mTOR信号通路抑制PASMCs增殖, 从而抑制肺血管重构。为了改善RAP的治疗作用, 研究者制备了基于PEG-PCL的RAP NPs, 静脉给药后该纳米粒能蓄积于病变的脉管系统, 抑制PASMCs的增殖, 降低全身性不良反应[72]。
1.14 联合递送药物联合治疗在临床上已被证明比单一疗法更有效, 也被纳入了世界卫生组织心功能分级的推荐方法中。目前临床上是不同药物的简单加和, 并无两种药物的联合递送方案, 因此联合递送药物的临床前研究成为炙手可热的研究方向之一。脂质体是最为常见的共递送载体。如FSD/超氧化物歧化酶(superoxide dismutase, SOD) 脂质体, 静脉注射和雾化肺部给药后, SOD和FSD的生物半衰期延长了3倍[73]; 偶联CAR多肽后, 能进一步提高肺靶向性[74]; 与普通药物或混合药物相比, 包含两种药物的CAR修饰脂质体可降低胶原蛋白沉积、动脉的肌肉化, 增加肺中SOD含量, 降低pSTAT-3和p-MYPT1的表达, 减少药物总摄入量、全身暴露量, 并降低给药频率[74]。为了同时作用于Rho激酶和NO信号通路, 协同治疗PAH, FSD和DN被包封于CAR修饰的脂质体中, 雾化给药后, 该脂质体能选择性降低MCT-PAH/SU-PAH大鼠的mPAP, 降低肌肉化程度、内侧动脉壁厚度和胶原蛋白沉积程度, 提升cGMP的水平等[75]。另外, 基于PLGA的载体也被用于共载SC和ROSI, 联合治疗PAH[76]。对SU-PAH大鼠吸入给药后, 颗粒可选择性地降低mPAP、增加心输出量和降低总肺阻力, 改善心功能; 同时通过减少RVH补偿心室功能不全, 减少胶原蛋白沉积和肺动脉肌肉化减慢疾病进展[76]。
1.15 其他递送其他的治疗药物如紫杉醇(paclitaxel, PTX)、肝素、穿心莲内酯等也能潜在地治疗PAH。有学者报道表明, 由于壳的高度水合和尺寸较小, PTX-PEG 5000-DSPE胶束能逃脱肺泡巨噬细胞系统的清除, 延长了药物载体的肺停留时间: 雾化给药后在肺中高度蓄积, 并保护药物免受其他生物屏障的影响, 对肺的靶向效率为6.57, 比静脉途径高了132倍[77]。Octaarginine (R8) 修饰脂质体也能潜在提高PTX的疗效。给药后, 体系能抑制PASMCs的增殖, 逆转表型转化, 抑制细胞迁移, 并降低MCT-PAH大鼠的mPAP、RV/ (LV+S) 和远端小肺动脉壁厚度等[78]。除此之外, PEG-PLGA微粒、葡聚糖载体等被用于促进低分子肝素[79]、穿心莲内酯[80]的肺部递送。
2 基因治疗基因递送在基因相关疾病的治疗中发挥着重要作用, 并可诱导机体对经由呼吸道进入的病原体产生免疫力。有关于PAH治疗的microRNA研究主要包括miR-17、miR-12、miR-92a、miR-17×92、miR-145、miR-181a-5p、miR-324-5p、miR-204a、miR-205和miR-495等[81], 通过作用于PASMCs或ECs, 从而改善血管重塑、血管生成、右心室压力和右心衰等。由于存在酶降解、快速清除和不稳定等问题, 研究者们常以脂质体、病毒等载体促进这些药物的递送。研究表明, PEI修饰的PLGA纳米粒能作为非病毒基因载体递送DNA[82]。DOTAP-脂质体阳离子能提高siRNA在肺组织中的蓄积, 并减少在其他器官中的蓄积和药物浓度[83]。antimiRNA-145具有良好抗重塑作用与心脏修复作用。基于脂多胺结构的阳离子脂质体能有效且低毒地向肺部递送antimiRNA-145, 降低了内源性miRNA-145的水平, 抑制了肺小动脉重构, 改善了小动脉的闭塞性, 促进了右心室功能的恢复[84]。
3 细胞疗法近年来, 干细胞技术取得了快速的发展, 如通过用骨髓衍生的内皮祖细胞(endothelial progenitor cells, EPCs) 治疗, 可再生肺血管内皮, EPC的移植可显著改善mPAP、心输出量, 降低肺血管阻力与小肺动脉内侧厚度, 促进新生血管生成等。如将骨髓来源的ELPCs植入MCT-PAH模型, 可恢复肺微血管的结构和功能[85]。EPC疗法也能与药物如西地那非发生协同作用[86]。
利用肺源性间充质干细胞(mesenchymal stem cells, MSCs) 具备的分化及分泌特性, 静脉注射从MCT-PAH大鼠中分离的MSCs, 可减弱肺小动脉狭窄, 减少肺泡间隔增厚和RVSP等[87]; 与未接受细胞治疗的模型大鼠相比, MSCs疗法降低了RVH, 改善了右心室功能[87]。静脉注射过表达eNOS的MSCs不仅促进内皮细胞的再生, 从而增加肺小动脉的数量, 而且可以增强内皮细胞中NO的分泌, 减少肺动脉的收缩作用, 降低PAP, 并改善PAH相关的右心室损伤, 延长生存时间[88]。除此之外, 气管内注射MSC可以改善大鼠的内皮依赖性反应并缓解MCT-PAH的症状[89]。
通过抑制PASMCs增殖, 调节AMPK/BMP/Smad信号通路, 脂联素基因修饰的脂肪干细胞可有效延缓PAH的进程[90]。同时, 将过表达血管内皮生长因子(VEGF)-A的同基因PASMCs注射到肺微血管系统, 可有效抑制MCT-PAH的发生和发展, 抑制血管重塑和RVH等[91]。
4 结论和展望随着研究深入, 除了增强血管扩张、减少血管收缩外, 针对抑制细胞增殖、改善血管重构、减少炎性浸润、利用再生内皮细胞增加肺动脉血管的数量等治疗策略在临床前研究不断取得进展。与之相关的药物递送方法不断更新, 从而提高了药物稳定性, 延长生物半衰期, 提高了生物利用度, 减少了不良反应。目前PAH治疗以口服、注射和吸入给药途径为主(图 4)。尽管如此, 传统的给药技术难以对PAH实现有效治疗。特别是, PAH的发病机制与免疫失调相关紧密, 生物药物是调节免疫的有效方法之一, 但往往生物利用度低, 无法实现有效治疗[92]。因此针对大分子药物递送技术的开发显得极为迫切。应加快新型递药系统或技术如脂质体、药物晶体[93, 94]、凝胶和长效微球等在PAH的应用[95]。通过肺部给药或半侵入给药方法如肌肉注射等, 从而实现肺部的高浓度药物治疗或长效治疗, 增强临床治疗的安全性和有效性, 并提高患者的依从性[96]。目前针对于PAH治疗的新型制剂多为气雾剂、脂质体和胶束等递药系统(图 4), 但其临床转化率低, 主要原因包括靶向性差、难以规模化生产等。而配体的修饰能从一定程度上提高药物的靶向率, 增加细胞的摄取量, 降低药物的清除率。

|
Figure 4 Drug delivery systems and administration routes to treat PAH |
除了经典的治疗药物, 新的靶向药物和疗法等在近10年取得了一定的进步, 提高了模型动物的存活率, 如PTX、低分子肝素、穿心莲内酯、基因治疗与细胞疗法等。尽管如此, 将动物实验应用于临床仍然受到极大限制。PAH的作用靶点大多作用于细胞自噬通路, 以促进细胞死亡, 主要包括PI3K/AKT/mTOR、MAPK和NF-κB等。但基于细胞GPCR、离子通道、代谢、表观遗传学、生长因子受体、转录因子和炎症等靶标的治疗方法, 研究甚少(图 2)。
作者贡献: 何伟负责论文选题、设计及论文修改; 蒋小宏负责论文撰写与修改; 邢续扬、王孝春和尹莉芳负责更改论文中的常见错误与语句矛盾等。
利益冲突: 本文所有作者均声明不存在利益冲突。
| [1] |
Avitabile CM, Vorhies EE, Ivy DD. Drug treatment of pulmonary hypertension in children[J]. Paediatr Drugs, 2020, 22: 123-147. DOI:10.1007/s40272-019-00374-2 |
| [2] |
Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension[J]. Circ Res, 2014, 115: 165-175. DOI:10.1161/CIRCRESAHA.113.301141 |
| [3] |
Ivy DD, Doran A, Claussen L, et al. Weaning and discontinuation of epoprostenol in children with idiopathic pulmonary arterial hypertension receiving concomitant bosentan[J]. Am J Cardiol, 2004, 93: 943-946. DOI:10.1016/j.amjcard.2003.12.031 |
| [4] |
Ruan KH. Advance in understanding the biosynthesis of prostacyclin and thromboxane A2 in the endoplasmic reticulum membrane via the cyclooxygenase pathway[J]. Mini Rev Med Chem, 2004, 4: 639-647. DOI:10.2174/1389557043403710 |
| [5] |
Gupta V, Davis M, Hope-Weeks LJ, et al. PLGA microparticles encapsulating prostaglandin E 1-hydroxypropyl-beta-cyclodextrin (PGE1-HPbetaCD) complex for the treatment of pulmonary arterial hypertension (PAH)[J]. Pharm Res, 2011, 28: 1733-1749. DOI:10.1007/s11095-011-0409-6 |
| [6] |
Gupta V, Ahsan F. Influence of PEI as a core modifying agent on PLGA microspheres of PGE(1), a pulmonary selective vasodilator[J]. Int J Pharm, 2011, 413: 51-62. DOI:10.1016/j.ijpharm.2011.04.017 |
| [7] |
Gupta V, Gupta N, Shaik IH, et al. Inhaled PLGA particles of prostaglandin E(1) ameliorate symptoms and progression of pulmonary hypertension at a reduced dosing frequency[J]. Mol Pharm, 2013, 10: 1655-1667. DOI:10.1021/mp300426u |
| [8] |
Jain PP, Leber R, Nagaraj C, et al. Liposomal nanoparticles encapsulating iloprost exhibit enhanced vasodilation in pulmonary arteries[J]. Int J Nanomedicine, 2014, 9: 3249-3261. |
| [9] |
Zare P, Heller D. Treprostinil[M]. Treasure Island (FL): StatPearls Publishing, 2020.
|
| [10] |
Skoro-Sajer N, Lang I. Extended-release oral treprostinil for the treatment of pulmonary arterial hypertension[J]. Expert Rev Cardiovasc Ther, 2014, 12: 1391-1399. DOI:10.1586/14779072.2014.975209 |
| [11] |
Leifer FG, Konicek DM, Chen KJ, et al. Inhaled treprostinil-prodrug lipid nanoparticle formulations provide long-acting pulmonary vasodilation[J]. Drug Res, 2018, 68: 605-614. DOI:10.1055/s-0044-100374 |
| [12] |
Akagi S, Nakamura K, Matsubara H, et al. Intratracheal administration of prostacyclin analogue-incorporated nanoparticles ameliorates the development of monocrotaline and sugen-hypoxia-induced pulmonary arterial hypertension[J]. J Cardiovasc Pharmacol, 2016, 67: 290-298. DOI:10.1097/FJC.0000000000000352 |
| [13] |
Shen L, Patel JA, Norel X, et al. Pharmacology of the single isomer, esuberaprost (beraprost-314d) on pulmonary vascular tone, IP receptors and human smooth muscle proliferation in pulmonary hypertension[J]. Biochem Pharmacol, 2019, 166: 242-252. DOI:10.1016/j.bcp.2019.05.026 |
| [14] |
Lee HJ, Kang JH, Lee HG, et al. Preparation and physicochemical characterization of spray-dried and jet-milled microparticles containing bosentan hydrate for dry powder inhalation aerosols[J]. Drug Des Devel Ther, 2016, 10: 4017-4030. DOI:10.2147/DDDT.S120356 |
| [15] |
Hanna LA, Basalious EB, On EL. Respirable controlled release polymeric colloid (RCRPC) of bosentan for the management of pulmonary hypertension: in vitro aerosolization, histological examination and in vivo pulmonary absorption[J]. Drug Deliv, 2016, 24: 188-198. |
| [16] |
Gimenez VM, Sperandeo N, Faudone S, et al. Preparation and characterization of bosentan monohydrate/epsilon-polycaprolactone nanoparticles obtained by electrospraying[J]. Biotechnol Prog, 2019, 35: e2748. DOI:10.1002/btpr.2748 |
| [17] |
Deshmane S, Deshmane S, Shelke S, et al. Enhancement of solubility and bioavailability of ambrisentan by solid dispersion using Daucus carota as a drug carrier: formulation, characterization, in vitro, and in vivo study[J]. Drug Dev Ind Pharm, 2018, 44: 1001-1011. DOI:10.1080/03639045.2018.1428339 |
| [18] |
Zeng FB, Lu B, Yang H, et al. Studies on gelatin microsphere loaded ligustrazine hydrochloride for lung targeting[J]. Acta Pharm Sin (药学学报), 1996, 31: 132-137. |
| [19] |
Pullamsetti S, Krick S, Yilmaz H, et al. Inhaled tolafentrine reverses pulmonary vascular remodeling via inhibition of smooth muscle cell migration[J]. Respir Res, 2005, 6: 128. DOI:10.1186/1465-9921-6-128 |
| [20] |
Bhogal S, Khraisha O, Al Madani M, et al. Sildenafil for pulmonary arterial hypertension[J]. Am J Ther, 2019, 26: e520-e526. DOI:10.1097/MJT.0000000000000766 |
| [21] |
Makled S, Nafee N, Boraie N. Nebulized solid lipid nanoparticles for the potential treatment of pulmonary hypertension via targeted delivery of phosphodiesterase-5-inhibitor[J]. Int J Pharm, 2017, 517: 312-321. DOI:10.1016/j.ijpharm.2016.12.026 |
| [22] |
Nafee N, Makled S, Boraie N. Nanostructured lipid carriers versus solid lipid nanoparticles for the potential treatment of pulmonary hypertension via nebulization[J]. Eur J Pharm Sci, 2018, 125: 151-162. DOI:10.1016/j.ejps.2018.10.003 |
| [23] |
Rashid J, Patel B, Nozik-Grayck E, et al. Inhaled sildenafil as an alternative to oral sildenafil in the treatment of pulmonary arterial hypertension (PAH)[J]. J Control Release, 2017, 250: 96-106. DOI:10.1016/j.jconrel.2017.02.003 |
| [24] |
Beck-Broichsitter M, Bohr A, Aragao-Santiago L, et al. Formulation and process considerations for the design of sildenafil-loaded polymeric microparticles by vibrational spray-drying[J]. Pharm Dev Technol, 2017, 22: 691-698. DOI:10.3109/10837450.2015.1098661 |
| [25] |
Li B, He W, Ye L, et al. Targeted delivery of sildenafil for inhibiting pulmonary vascular remodeling[J]. Hypertension, 2019, 73: 703-711. DOI:10.1161/HYPERTENSIONAHA.118.11932 |
| [26] |
Bowles EA, Feys D, Ercal N, et al. Liposomal-delivery of phosphodiesterase 5 inhibitors augments UT-15C-stimulated ATP release from human erythrocytes[J]. Biochem Biophys Rep, 2017, 12: 114-119. |
| [27] |
Lu M, Xing H, Yang T, et al. Dissolution enhancement of tadalafil by liquisolid technique[J]. Pharm Dev Technol, 2017, 22: 77-89. DOI:10.1080/10837450.2016.1189563 |
| [28] |
Varshosaz J, Taymouri S, Hamishehkar H, et al. Development of dry powder inhaler containing tadalafil-loaded PLGA nanoparticles[J]. Res Pharm Sci, 2017, 12: 222-232. DOI:10.4103/1735-5362.207203 |
| [29] |
Teymouri Rad R, Dadashzadeh S, Vatanara A, et al. Tadalafil nanocomposites as a dry powder formulation for inhalation, a new strategy for pulmonary arterial hypertension treatment[J]. Eur J Pharm Sci, 2019, 133: 275-286. DOI:10.1016/j.ejps.2019.04.001 |
| [30] |
Elbardisy B, Galal S, Abdelmonsif DA, et al. Intranasal tadalafil nanoemulsions: formulation, characterization and pharmacodynamic evaluation[J]. Pharm Dev Technol, 2019, 24: 1083-1094. DOI:10.1080/10837450.2019.1631846 |
| [31] |
Jacobs BR, Brilli RJ, Ballard ET, et al. Aerosolized soluble nitric oxide donor improves oxygenation and pulmonary hypertension in acute lung injury[J]. Am J Respir Crit Care Med, 1998, 158: 1536-1542. DOI:10.1164/ajrccm.158.5.9802114 |
| [32] |
Puikuan K, Chunyu Z, Jin F, et al. Inhalation of nebulized nitroglycerin, a nitric oxide donor, for the treatment of pulmonary hypertension induced by high pulmonary blood flow[J]. Heart Vessels, 2006, 21: 169-179. DOI:10.1007/s00380-005-0876-y |
| [33] |
Evgenov OV, Kohane DS, Bloch KD, et al. Inhaled agonists of soluble guanylate cyclase induce selective pulmonary vasodilation[J]. Am J Respir Crit Care Med, 2007, 176: 1138-1145. DOI:10.1164/rccm.200707-1121OC |
| [34] |
Saigal A, Ng WK, Tan RB, et al. Development of controlled release inhalable polymeric microspheres for treatment of pulmonary hypertension[J]. Int J Pharm, 2013, 450: 114-122. DOI:10.1016/j.ijpharm.2013.04.011 |
| [35] |
Woodmansey PA, O'Toole L, Channer KS, et al. Acute pulmonary vasodilatory properties of amlodipine in humans with pulmonary hypertension[J]. Heart, 1996, 75: 171-173. DOI:10.1136/hrt.75.2.171 |
| [36] |
Minami M, Arita T, Iwasaki H, et al. Comparative analysis of pulmonary hypertension in patients treated with imatinib, nilotinib and dasatinib[J]. Br J Haematol, 2017, 177: 578-587. DOI:10.1111/bjh.14608 |
| [37] |
Patterson KC, Weissmann A, Ahmadi T, et al. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension[J]. Ann Intern Med, 2006, 145: 152-153. DOI:10.7326/0003-4819-145-2-200607180-00019 |
| [38] |
Kimura G, Kataoka M, Inami T, et al. Sorafenib as a potential strategy for refractory pulmonary arterial hypertension[J]. Pulm Pharmacol Ther, 2017, 44: 46-49. DOI:10.1016/j.pupt.2017.03.009 |
| [39] |
Toba M, Alzoubi A, O'Neill K, et al. A novel vascular homing peptide strategy to selectively enhance pulmonary drug efficacy in pulmonary arterial hypertension[J]. Am J Pathol, 2014, 184: 369-375. DOI:10.1016/j.ajpath.2013.10.008 |
| [40] |
Akagi S, Nakamura K, Miura D, et al. Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension[J]. Int Heart J, 2015, 56: 354-359. DOI:10.1536/ihj.14-338 |
| [41] |
McMurtry MS, Bonnet S, Wu X, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis[J]. Circ Res, 2004, 95: 830-840. DOI:10.1161/01.RES.0000145360.16770.9f |
| [42] |
Hawwa N, Menon V. Ranolazine: clinical applications and therapeutic basis[J]. Am J Cardiovasc Drugs, 2013, 13: 5-16. DOI:10.1007/s40256-012-0003-2 |
| [43] |
Sutendra G, Bonnet S, Rochefort G, et al. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension[J]. Sci Transl Med, 2010, 2: 44ra58. |
| [44] |
Evgenov OV, Kohane DS, Bloch KD, et al. Inhaled agonists of soluble guanylate cyclase induce selective pulmonary vasodilation[J]. Am J Resp Crit Care, 2007, 176: 1138-1145. DOI:10.1164/rccm.200707-1121OC |
| [45] |
Spiekerkoetter E, Sung YK, Sudheendra D, et al. Low-dose FK506(tacrolimus) in end-stage pulmonary arterial hypertension[J]. Am J Respir Crit Care Med, 2015, 192: 254-257. DOI:10.1164/rccm.201411-2061LE |
| [46] |
Sommer N, Droege F, Gamen KE, et al. Treatment with low-dose tacrolimus inhibits bleeding complications in a patient with hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension[J]. Pulm Circ, 2019, 9: 2045894018805406. |
| [47] |
Akagi S, Nakamura K, Miura D, et al. Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension[J]. Int Heart J, 2015, 56: 354-359. DOI:10.1536/ihj.14-338 |
| [48] |
Alapati D, Rong M, Chen S, et al. Inhibition of LRP5/6-mediated Wnt/beta-catenin signaling by Mesd attenuates hyperoxia-induced pulmonary hypertension in neonatal rats[J]. Pediatr Res, 2013, 73: 719-725. DOI:10.1038/pr.2013.42 |
| [49] |
Alapati D, Rong M, Chen S, et al. Inhibition of beta-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia[J]. Am J Respir Cell Mol Biol, 2014, 51: 104-113. DOI:10.1165/rcmb.2013-0346OC |
| [50] |
Kimura S, Egashira K, Chen L, et al. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension[J]. Hypertension, 2009, 53: 877-883. DOI:10.1161/HYPERTENSIONAHA.108.121418 |
| [51] |
Wang Z, Cuddigan JL, Gupta SK, et al. Nanocomposite microparticles (nCmP) for the delivery of tacrolimus in the treatment of pulmonary arterial hypertension[J]. Int J Pharm, 2016, 512: 305-313. DOI:10.1016/j.ijpharm.2016.08.047 |
| [52] |
Brousseau S, Wang Z, Gupta SK, et al. Development of aerosol phospholipid microparticles for the treatment of pulmonary hypertension[J]. AAPS PharmSciTech, 2017, 18: 3247-3257. DOI:10.1208/s12249-017-0821-2 |
| [53] |
Sun XZ, Tian XY, Wang DW, et al. Effects of fasudil on hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats[J]. Eur Rev Med Pharmacol Sci, 2014, 18: 959-964. |
| [54] |
Chen XY, Dun JN, Miao QF, et al. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses 5-hydroxytryptamine-induced pulmonary artery smooth muscle cell proliferation via JNK and ERK1/2 pathway[J]. Pharmacology, 2009, 83: 67-79. DOI:10.1159/000178814 |
| [55] |
Gupta N, Patel B, Nahar K, et al. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats[J]. Eur J Pharm Biopharm, 2014, 88: 1046-1055. DOI:10.1016/j.ejpb.2014.10.012 |
| [56] |
Qi L, Lv T, Cheng Y, et al. Fasudil dichloroacetate (FDCA), an orally available agent with potent therapeutic efficiency on monocrotaline-induced pulmonary arterial hypertension rats[J]. Bioorg Med Chem Lett, 2019, 29: 1812-1818. DOI:10.1016/j.bmcl.2019.05.006 |
| [57] |
Gupta N, Patel B, Ahsan F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: preparation and characterization[J]. Pharm Res, 2014, 31: 1553-1565. DOI:10.1007/s11095-013-1261-7 |
| [58] |
Gupta N, Ibrahim HM, Ahsan F. Peptide-micelle hybrids containing fasudil for targeted delivery to the pulmonary arteries and arterioles to treat pulmonary arterial hypertension[J]. J Pharm Sci, 2014, 103: 3743-3753. DOI:10.1002/jps.24193 |
| [59] |
Gupta V, Gupta N, Shaik IH, et al. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension[J]. J Control Release, 2013, 167: 189-199. DOI:10.1016/j.jconrel.2013.01.011 |
| [60] |
Nahar K, Absar S, Patel B, et al. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature[J]. Int J Pharm, 2014, 464: 185-195. DOI:10.1016/j.ijpharm.2014.01.007 |
| [61] |
Wang HM, Wang Y, Liu M, et al. Fluoxetine inhibits monocrotaline-induced pulmonary arterial remodeling involved in inhibition of RhoA-Rho kinase and Akt signalling pathways in rats[J]. Can J Physiol Pharmacol, 2012, 90: 1506-1515. DOI:10.1139/y2012-108 |
| [62] |
Zhai FG, Zhang XH, Wang HL. Fluoxetine protects against monocrotaline-induced pulmonary arterial hypertension: potential roles of induction of apoptosis and upregulation of Kv1.5 channels in rats[J]. Clin Exp Pharmacol Physiol, 2009, 36: 850-856. DOI:10.1111/j.1440-1681.2009.05168.x |
| [63] |
Bhat L, Hawkinson J, Cantillon M, et al. RP5063, a novel, multimodal, serotonin receptor modulator, prevents monocrotaline-induced pulmonary arterial hypertension in rats[J]. Eur J Pharmacol, 2017, 810: 92-99. DOI:10.1016/j.ejphar.2017.05.048 |
| [64] |
Bhat L, Hawkinson J, Cantillon M, et al. RP5063, a novel, multimodal, serotonin receptor modulator, prevents Sugen 5416-hypoxia-induced pulmonary arterial hypertension in rats[J]. Eur J Pharmacol, 2017, 810: 83-91. DOI:10.1016/j.ejphar.2017.05.052 |
| [65] |
Chen L, Nakano K, Kimura S, et al. Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension[J]. Hypertension, 2011, 57: 343-350. DOI:10.1161/HYPERTENSIONAHA.110.157032 |
| [66] |
Ichimura K, Matoba T, Koga JI, et al. Nanoparticle-mediated targeting of pitavastatin to small pulmonary arteries and leukocytes by intravenous administration attenuates the progression of monocrotaline-induced established pulmonary arterial hypertension in rats[J]. Int Heart J, 2018, 59: 1432-1444. DOI:10.1536/ihj.17-683 |
| [67] |
Lee Y, Pai SB, Bellamkonda RV, et al. Cerivastatin nanoliposome as a potential disease modifying approach for the treatment of pulmonary arterial hypertension[J]. J Pharmacol Exp Ther, 2018, 366: 66-74. DOI:10.1124/jpet.118.247643 |
| [68] |
Liparulo A, Esposito R, Santonocito D, et al. Formulation and characterization of solid lipid nanoparticles loading RF22-c, a potent and selective 5-LO inhibitor, in a monocrotaline-induced model of pulmonary hypertension[J]. Front Pharmacol, 2020, 11: 83. DOI:10.3389/fphar.2020.00083 |
| [69] |
Crossno JT Jr, Garat CV, Reusch JE, et al. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling[J]. Am J Physiol Lung Cell Mol Physiol, 2007, 292: L885-L897. DOI:10.1152/ajplung.00258.2006 |
| [70] |
Liu Y, Tian XY, Mao G, et al. Peroxisome proliferator-activated receptor-gamma ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor[J]. Hypertension, 2012, 60: 1471-1478. DOI:10.1161/HYPERTENSIONAHA.112.198887 |
| [71] |
Rashid J, Alobaida A, Al-Hilal TA, et al. Repurposing rosiglitazone, a PPAR-gamma agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH[J]. J Control Release, 2018, 280: 113-123. DOI:10.1016/j.jconrel.2018.04.049 |
| [72] |
Segura-Ibarra V, Amione-Guerra J, Cruz-Solbes AS, et al. Rapamycin nanoparticles localize in diseased lung vasculature and prevent pulmonary arterial hypertension[J]. Int J Pharm, 2017, 524: 257-267. DOI:10.1016/j.ijpharm.2017.03.069 |
| [73] |
Gupta N, Al-Saikhan FI, Patel B, et al. Fasudil and SOD packaged in peptide-studded-liposomes: properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs[J]. Int J Pharm, 2015, 488: 33-43. DOI:10.1016/j.ijpharm.2015.04.031 |
| [74] |
Gupta N, Rashid J, Nozik-Grayck E, et al. Cocktail of superoxide dismutase and fasudil encapsulated in targeted liposomes slows PAH progression at a reduced dosing frequency[J]. Mol Pharm, 2017, 14: 830-841. DOI:10.1021/acs.molpharmaceut.6b01061 |
| [75] |
Rashid J, Nahar K, Raut S, et al. Fasudil and DETA NONOate, loaded in a peptide-modified liposomal carrier, slow PAH progression upon pulmonary delivery[J]. Mol Pharm, 2018, 15: 1755-1765. DOI:10.1021/acs.molpharmaceut.7b01003 |
| [76] |
Rashid J, Nozik-Grayck E, McMurtry IF, et al. Inhaled combination of sildenafil and rosiglitazone improves pulmonary hemodynamics, cardiac function, and arterial remodeling[J]. Am J Physiol Lung Cell Mol Physiol, 2019, 316: L119-L130. DOI:10.1152/ajplung.00381.2018 |
| [77] |
Gill KK, Nazzal S, Kaddoumi A. Paclitaxel loaded PEG(5000)-DSPE micelles as pulmonary delivery platform: formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation[J]. Eur J Pharm Biopharm, 2011, 79: 276-284. DOI:10.1016/j.ejpb.2011.04.017 |
| [78] |
Yin Y, Wu X, Yang Z, et al. The potential efficacy of R8-modified paclitaxel-loaded liposomes on pulmonary arterial hypertension[J]. Pharm Res, 2013, 30: 2050-2062. DOI:10.1007/s11095-013-1058-8 |
| [79] |
Patel B, Gupta V, Ahsan F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low mole-cular weight heparin[J]. J Control Release, 2012, 162: 310-320. DOI:10.1016/j.jconrel.2012.07.003 |
| [80] |
Mali AJ, Bothiraja C, Purohit RN, et al. In vitro and in vivo performance of novel spray dried andrographolide loaded scleroglucan based formulation for dry powder inhaler[J]. Curr Drug Deliv, 2017, 14: 968-980. |
| [81] |
Carregal-Romero S, Fadon L, Berra E, et al. MicroRNA nanotherapeutics for lung targeting. Insights into pulmonary hypertension[J]. Int J Mol Sci, 2020, 21: 3253. DOI:10.3390/ijms21093253 |
| [82] |
Bivas-Benita M, Romeijn S, Junginger HE, et al. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium[J]. Eur J Pharm Biopharm, 2004, 58: 1-6. DOI:10.1016/j.ejpb.2004.03.008 |
| [83] |
Garbuzenko OB, Saad M, Betigeri S, et al. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug[J]. Pharm Res, 2009, 26: 382-394. DOI:10.1007/s11095-008-9755-4 |
| [84] |
McLendon JM, Joshi SR, Sparks J, et al. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension[J]. J Control Release, 2015, 210: 67-75. DOI:10.1016/j.jconrel.2015.05.261 |
| [85] |
Takahashi M, Nakamura T, Toba T, et al. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs[J]. Tissue Eng, 2004, 10: 771-779. DOI:10.1089/1076327041348563 |
| [86] |
Zhao YD, Courtman DW, Deng Y, et al. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease[J]. Circ Res, 2005, 96: 442-450. DOI:10.1161/01.RES.0000157672.70560.7b |
| [87] |
Umar S, de Visser YP, Steendijk P, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension[J]. Am J Physiol Heart Circ Physiol, 2009, 297: H1606-H1616. DOI:10.1152/ajpheart.00590.2009 |
| [88] |
Kanki-Horimoto S, Horimoto H, Mieno S, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension[J]. Circulation, 2006, 114: I181-I185. |
| [89] |
Baber SR, Deng W, Master RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction[J]. Am J Physiol Heart Circ Physiol, 2007, 292: H1120-H1128. DOI:10.1152/ajpheart.00173.2006 |
| [90] |
Luo L, Zheng W, Lian G, et al. Combination treatment of adipose-derived stem cells and adiponectin attenuates pulmonary arterial hypertension in rats by inhibiting pulmonary arterial smooth muscle cell proliferation and regulating the AMPK/BMP/Smad pathway[J]. Int J Mol Med, 2018, 41: 51-60. |
| [91] |
Campbell AI, Zhao Y, Sandhu R, et al. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension[J]. Circulation, 2001, 104: 2242-2248. DOI:10.1161/hc4201.097838 |
| [92] |
Xiao Q, Li X, Li Y, et al. Biological drug and drug delivery-mediated immunotherapy[J]. Acta Pharm Sin B, 2020. DOI:10.1016/j.apsb.2020.12.018 |
| [93] |
Teng C, Lin C, Huang F, et al. Intracellular codelivery of anti-inflammatory drug and anti-miR 155 to treat inflammatory disease[J]. Acta Pharm Sin B, 2020, 10: 1521-1533. DOI:10.1016/j.apsb.2020.06.005 |
| [94] |
Du X, Hou Y, Huang J, et al. Cytosolic delivery of the immunological adjuvant poly I: C and cytotoxic drug crystals via a carrier-free strategy significantly amplifies immune response[J]. Acta Pharm Sin B, 2021. DOI:10.1016/j.apsb.2021.03.014 |
| [95] |
He W, Xing X, Wang X, et al. Nanocarrier-mediated cytosolic delivery of biopharmaceuticals[J]. Adv Funct Mater, 2020, 30: 1910566. DOI:10.1002/adfm.201910566 |
| [96] |
He W, Kapate N, Shields IVCW, et al. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases[J]. Adv Drug Deliv Rev, 2020, 165-166: 15-40. DOI:10.1016/j.addr.2019.12.001 |
 2021, Vol. 56
2021, Vol. 56


