2. 军事科学院军事医学研究院辐射医学研究所, 北京 100850
2. Beijing Institute of Radiation Medicine, Beijing 100850, China
水凝胶(hydrogels) 系指凝胶基质与药物制成混悬或乳状液型的水性黏稠半固体制剂。在水介质中, 凝胶通过物理交联(如缠结、微晶)或化学交联维持其稳定性[1, 2]。由于凝胶含水量丰富, 具有一定的膨胀力和柔韧性, 因此在药物递送、组织工程、生物传感器等领域应用广泛[3, 4]。由于其组成、化学结构、生物降解性及其他理化性质(如光学性能、机械性质、酸碱度等) 差异很大[5, 6], 需根据药物性质、给药途径和剂量等因素具体选择[7-9]。
环境敏感型水凝胶(environmentally sensitive hydrogels) 会根据体内环境的某些影响因素(如温度、pH、离子、溶剂、光等) 不同而发生相转化: 通常呈低黏性液体, 给药后聚合物分散状态或构象基于外部环境改变在用药部位发生相转变, 成为半固体凝胶, 在刺激去除后能可逆返回到初始状态[10]。环境敏感型水凝胶的发展依赖于环境敏感型聚合物即“智能”聚合物的发展。这些聚合物一般以单分子态存在, 在生理环境中依赖于各种刺激发生聚集、交联, 进而形成黏弹性凝胶。环境敏感型水凝胶具有许多优点, 如制备简单、易于给药、患者依从性好、局部停留时间长和刺激性等[11, 12]。其临床给药途径多样, 可经皮肤、眼、口腔、鼻腔、阴道和直肠等途径实现局部或全身给药, 用于治疗湿疹[13]、低压低氧脑损伤[14]、牙周炎[15]、创面愈合[16]和创伤后应激障碍(post-traumatic stress disorder, PTSD) 等多种疾病, 所递送的药物种类也覆盖了小分子、生物大分子和siRNA等, 具有广阔临床应用前景。本综述将从分类、常用环境敏感型聚合物和给药途径角度概括环境敏感型水凝胶最新研究进展。
1 环境敏感型水凝胶分类根据响应因素不同, 环境敏感型水凝胶可分为温度敏感型、pH敏感型、离子敏感型、光敏感型、酶敏感型和磁敏感型等(图 1)。随着新型高分子聚合物的发展, 双重、三重或更多重环境敏感型水凝胶也屡见不鲜。
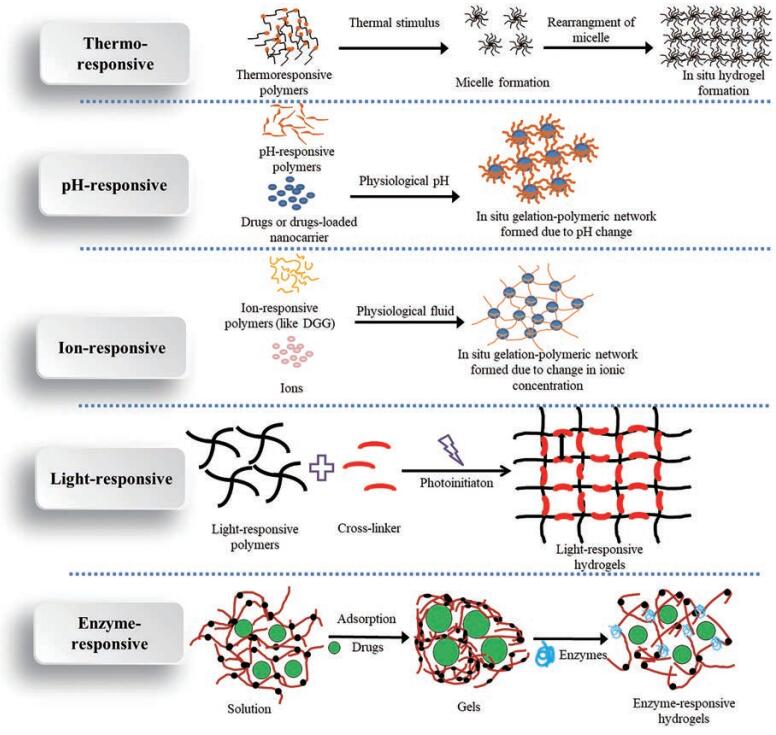
|
Figure 1 The mechanism of environmentally sensitive hydrogels. DGG: Deacetylated gellan gum |
温敏型水凝胶系指其网络结构受温度变化影响而产生体积变化进而发生溶胶-凝胶转变[17]。此类型凝胶的重要特征是在临界溶液温度(lower critical solution temperature, LCST) 以下是溶液, 在LCST以上形成凝胶[18, 19]。常用温度敏感型聚合物包括聚(N-异丙基丙烯酰胺) (poly N-isopropyl acrylamide, pNIPAAm) 和泊洛沙姆(poloxamer), 均可在一定温度环境下形成凝胶[20], 已成功用于中药单体, 如粉防己碱[21]、葛根素[22]和小分子化合物, 如布洛芬[23]等。
温度敏感型水凝胶作为研究最多的敏感型凝胶可通过多种途径给药(如鼻腔、眼表、注射等)。姜黄素可通过调节神经内分泌免疫因子达到抗抑郁作用。载姜黄素壳聚糖温度敏感型水凝胶的黏度在29~30 ℃内迅速增加, 具有良好的黏膜黏附性能。该凝胶在体外释药10 h时累积释放率达到55%, 可增加海马和纹状体中去甲肾上腺素、多巴胺、5-羟色胺及其代谢物的浓度。因此, 鼻用温度敏感型水凝胶可成为治疗神经疾病的有效给药方式[24]。莫西沙星常用来治疗牙周炎, 采用泊洛沙姆和壳聚糖作为基质制备的莫西沙星温度敏感型水凝胶在室温下为溶液态更容易注射。凝胶进入口腔后环境温度迅速升高至37 ℃, 溶液态转变为凝胶而长期滞留在牙周袋中, 可持续释放药物 > 8 h[25]。以pNIPAAm和透明质酸为基质制备了载KCl温度敏感型水凝胶: 药物含量为91%~96%, pH值6.0~7.5, 胶凝温度为33 ℃[26]。该凝胶具有缓释效果, 能有效抑制白色念珠菌生长。
温度敏感型水凝胶能迅速将药物局限在局部注射部位, 避免药物流失从而提高肿瘤局部治疗效率, 减少了对正常细胞、组织的不良反应[27]。钆中子捕获疗法(gadolinium neutron capture therapy, Gd-NCT) 是肿瘤治疗新方法, 其关键在于Gd-NCT能被递送到肿瘤内并保持足够高浓度。采用脂质体包裹钆后再分散到温度敏感型水凝胶中, 能明显延长Gd在瘤内滞留时间[28]。7-乙基-10-羟基喜树碱(7-ethyl-10-hydroxycamptothecin, SN-38) 内酯形式(SN-38 A) 具有活性。SN-38脂质体温度敏感型水凝胶在25 ℃呈溶液状态, 在37 ℃形成凝胶; 可增加内酯稳定性, 延长药物释放, 提高局部药物浓度, 降低全身毒性[29]。
温度敏感型水凝胶也常用于脑部疾病治疗药物靶向递送。以泊洛沙姆188和407为基质制备粉防己碱鼻用温度敏感型水凝胶(tetrandrine temperature-sensitive gel, TTG) 可在鼻腔内停留4 h, 能明显改善PTSD模型小鼠海马、前额叶皮层及杏仁核部位的病理变化[30]。有研究比较了葛根素、葛胺酮(G20)、知母皂苷BⅡ和大麻二酚(cannabidiol, CBD) 4种药物鼻用温敏水凝胶对小鼠缺氧性脑损伤的预防作用。结果表明G20和CBD水凝胶可显著延长常压密闭缺氧小鼠标准缺氧耐受时间、外周血中红细胞计数和血红蛋白含量; 减少小鼠脑组织中炎性细胞数量, 改善核固缩深染现象。其良好的抗脑缺氧效果可能与提高机体抗氧化能力、清除自由基和神经细胞保护作用有关[31]。
1.2 pH敏感型水凝胶pH敏感型水凝胶体系中含有大量可解离基团, 其胶凝行为是电荷间相互排斥作用导致分子链伸展与相互缠结造成的。以卡波姆(carbopol) 为例, 结构中含大量羧基(约56%~68%), 当pH < 4时, 羧基几乎不解离, 聚合物在水中分散并溶胀, 但不溶解, 黏性很低。无机碱或有机碱可使羧基解离, 负电荷间排斥作用导致分子链膨胀、伸展, 低浓度卡波姆溶液形成澄明溶液, 在浓度较大时, 分子链相互缠结而形成具有一定强度和弹性的半透明状凝胶。pH值为6~12时, 卡波姆凝胶黏度最大[32]。
以乙二醇二甲基丙烯酸酯为交联剂, 采用共引发剂过亚硫酸铵和硫酸氢钠引发水性介质中自由基聚合, 制备以泊洛沙姆407和聚丙烯酸为原料的pH敏感型水凝胶, 可在pH 6.8时形成凝胶, 其热力学性质稳定, 药物释放遵循一级动力学、Higuchi方程, 生物相容性好[33]。载伊曲康唑pH敏感型水凝胶体外角膜渗透性优于伊曲康唑滴眼剂和伊曲康唑混悬剂, 可用于治疗真菌性角膜炎以克服常规剂型的局限性[34]。由聚(甲基丙烯酸-g-乙二醇) 和丙烯酰基改性带胆固醇的支链淀粉纳米凝胶是一种新型pH敏感型水凝胶; 与普通凝胶相比, 该纳米凝胶载药量和释放率明显增加[35]。You等[36]制备的载氢化可的松琥珀酸钠的魔芋胶-黄原胶-甘油-海藻酸钠凝胶可防止药物在肠道较高的pH值下快速释放。该凝胶在pH 7.4时缓释效果显著(4 h, 70.20 %), 可使药物特异性到达结肠治疗溃疡性结肠炎。羧甲基壳聚糖-聚乙烯醇/银纳米复合pH敏感型水凝胶在pH 2.1 (模拟胃液) 和pH 7.4 (模拟肠液) 条件下溶胀性能优于羧甲基壳聚糖-聚乙烯醇凝胶, 具有优异抗菌性能。该凝胶中的药物释放随银粒子含量增加而增加[37]。
鼻腔给予磷酸川芎嗪pH敏感型原位凝胶后, 在大鼠脑中分布速度较快, 鼻腔给药后5 min即可在脑部纹状体区测出, 表明给药后能很快分布到脑部, 约28 min左右达到峰值。且在模型组中药物含量为正常组的1.22倍, 说明病理状况下药物在脑部消除减慢, 生物利用度增加, 可能对急性脑缺血模型大鼠具有保护作用[38]。经穿透肽CRGDK修饰具有肿瘤靶向性的pH敏感多柔比星前体药物纳米粒(prodrug nanoparticles, PDNPs) 注射后, 首先通过CRGDK介导主动靶向至肿瘤血管和肿瘤细胞上过表达的神经纤毛蛋白-1受体, 然后通过热诱导自聚集进行溶胶-凝胶转变, 形成可生物降解凝胶(PDNPs-gel), 将大量PDNPs锚定在肿瘤部位。从PDNPs-gel中释放的PDNPs可有效渗透到肿瘤组织内, 在肿瘤细胞内酸性环境触发下PDNPs最终释放出药物发挥作用[39]。pH敏感型水凝胶还可与其他剂型复合使用。将万古霉素制备成脂质体, 再整合到pH敏感型水凝胶体系中, 该体系在滴眼后即刻形成凝胶, 可延长眼部停留时间并维持药物释放, 抗菌作用明显、刺激性低[40]。
除小分子药物外, pH敏感型水凝胶也可用于递送大分子药物(如多肽、蛋白质)。采用索拉胶与2-丙烯酰胺基-2-甲基-1-丙烷磺酸发生接枝共聚反应制备pH敏感凝胶可实现胰岛素递送, 使胰岛素释放速率可根据环境pH值变化而调节。该凝胶在胃内(pH 1.2) 释药量相对较低(24 h内释放率约为26.1%), 而在肠道条件下(pH 7.4) 则明显增加(6 h内释放率超过50%)[41]。载葡萄糖氧化酶、过氧化氢酶和胰岛素的pH敏感型水凝胶当遇到低血糖症时, 酶可以将葡萄糖转化为葡萄糖酸, 降低了局部pH值, 相邻碱性氨基酸侧链相互排斥导致胰岛素释放[42]。
1.3 离子敏感型水凝胶离子敏感型水凝胶主要是由于某些高分子聚合物对外界离子强度响应从而发生结构或构象的可逆变化, 完成由溶液向凝胶的转化。成人鼻腔中每天鼻液量为1.5~2 mL, 并富含阳离子(Na+、K+、Ca2+); 泪液中也有丰富的Na+、K+和Ca2+, 因此离子敏感型水凝胶适用于鼻用、眼用制剂。
去乙酰结冷胶(deacetylated gellan gum, DGG) 是最常见的离子敏感型聚合物。丹皮酚是一种潜在的中枢神经保护剂, 因水溶性差、体内代谢快而限制了其临床应用。采用DGG制备载丹皮酚固体脂质体纳米粒的鼻用离子敏感型水凝胶细胞毒性较低, 可在脑区靶向分布, 尤其是嗅球、小脑和纹状体[43]。多奈哌齐纳米脂质体离子敏感型水凝胶可用于治疗阿尔茨海默病。与市售制剂相比, 其在大鼠脑中药物分布较高, 在血浆中的药物浓度较低, 大鼠认知功能明显改善[44]。通过反相蒸发法和酸碱度梯度法制备了马来酸噻吗洛尔脂质体DGG离子敏感型水凝胶, 与滴眼剂相比表观渗透系数增加了1.93倍, 在角膜表面滞留时间更长, 能迅速降低眼压, 明显延长有效作用时间[45]。以结冷胶为基质、碳酸钙为交联剂、Ca2+为离子源制备了盐酸伊托必利漂浮型口服凝胶, 其Tmax延长, 说明吸收时间延长; 0.12 h凝胶制剂血浆药物浓度比普通制剂增高近90%[46]。为了缩短胶凝时间和提高凝胶机械强度, 可考虑离子敏感型水凝胶与其他机制联合应用, 如pH敏感和温度敏感等。
1.4 光敏感型水凝胶光敏感型水凝胶是一类可响应光信号而发生物理或化学性质变化的智能型凝胶。在光照下, 光敏基团经过光电离过程所产生的离子扰乱了凝胶介质的渗透平衡; 水分子和离子被驱入或驱出凝胶网络以补偿渗透性不平衡, 最终导致宏观表现上凝胶溶胀或收缩[47]。组成光敏水凝胶的聚合物通常由骨架和光敏部分组成, 光敏部分捕获光信号, 通过光反应(异构化、裂解或二聚化) 将其转化为化学信号影响结构改变。
光敏型水凝胶具有精度高、产热低的特点[48]。偶氮苯是最常用的光开关分子之一。偶氮苯类交联剂经紫外线照射会去交联而引发液-固相转变[49]。通过四氟偶氮苯连接两个脲基-嘧啶酮成功构建了新型可见光敏感型超分子聚合物, 在可见光照射下, 两种异构体之间可实现可逆异构化(图 2A)[50]。环己烷1,3,5-三羧酰胺核心提供面对面的氢键键合和平面构象, 从而诱导超分子聚合物自组装。环己烷1,3,5-三羧酰胺核心被三个芳基偶氮唑臂取代后能形成三脚结构凝胶, 通过动态共价化学键实现凝胶组成与降解(图 2B)[51]。卤素键具有定向性高、强度可调、疏水性好和原子尺寸大等特点, 是构建超分子的重要驱动力。以偶氮吡啶作为卤素键受体, 1,2-二(2,3,5,6-四氟-4-碘苯基) 二氮烯作为卤素键供体与可见光敏感部分连接构建了光敏感型水凝胶, 该凝胶在绿光照射下可实现从凝胶到溶液的转变, 而蓝光照射下发生相反过程(图 2C)[52]。
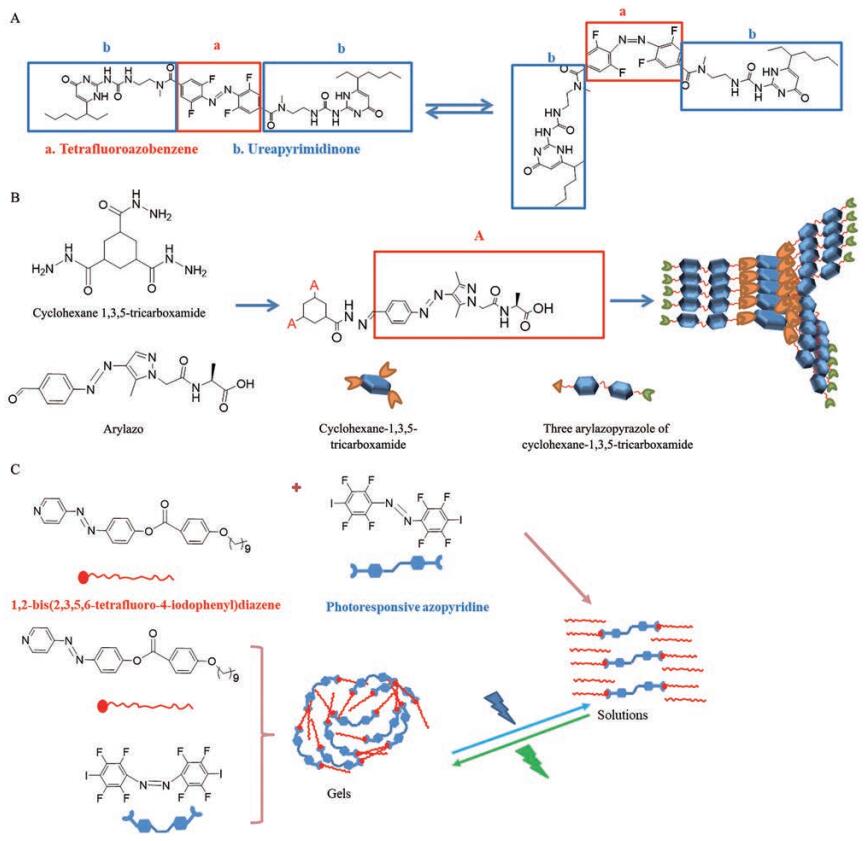
|
Figure 2 Reversible photoisomerization of tetrafluoroazobenzene (A); tripod structure of cyclohexane 1,3,5-tricarboxamide (B); supramolecular composed of halogen bonds (C) |
新技术的出现进一步促进了光敏感型水凝胶的发展。低分子量自组装肽可通过改变其结构网络响应外部环境的变化来实现药物递送。用6-硝基过氧化丙烯酰羰基-二苯丙氨酸自组装形成载异硫氰酸胰岛素荧光素(insulin-FITC) 的纳米纤维凝胶, 该凝胶在紫外线照射下能降解产生二苯丙氨酸, 进一步促进凝胶降解和insulin-FITC释放[53]。另一种新型自组装肽凝胶上的羧基能与钙离子通过配位作用形成凝胶载药, 在365 nm紫外光照射下, 凝胶降解连续释放药物, 为药物有效包裹、时空可控释放提供了新思路[54]。
1.5 酶敏感型水凝胶将可以与某种酶发生特定反应的多肽分子通过物理或化学方式与凝胶基质连接, 可在酶作用下降解, 从而释放出所包裹或连接的药物。改变处方和交联参数可调节凝胶降解速度及降解时间[55]。由多肽CRDTEGE-ARGSVIDRC修饰的硫醇聚乙二醇凝胶对聚集蛋白聚糖酶敏感, 可被软骨细胞降解, 并产生透明样工程软骨用于软骨再生[56]。利用酶促交联将3-(4-羟基苯基)-丙酸-乙二醇壳聚糖制备成可注射、可生物降解的凝胶, 可被溶菌酶降解释放所载蛋白质, 改变乙酰化程度可调节释放速率[57]。结冷胶分别采用二乙烯基砜、巯基细胞黏附肽进行功能化修饰后, 通过对金属蛋白酶-1敏感的二硫代肽交联剂, 与谷丙转氨酶或肽功能化谷丙转氨酶连接形成凝胶。该凝胶可被金属蛋白酶1生物降解并使内皮细胞黏附和增殖[58]。
现有酶敏感水凝胶只能在特定酶存在的条件下释放药物, 并不能根据病理生理过程中酶变化实现时空可控性释放。因此, 研究持续响应性智能化酶敏感释药凝胶的前景广阔。
1.6 磁敏感型水凝胶磁敏感型水凝胶一般载顺磁性氧化铁纳米粒(superparamagnetic iron oxide nanoparticles, SPIONs), 其一个重要的生物学机制是在外磁场作用下可被远程加热[59]。因此可利用热疗与放疗、化疗结合提高肿瘤治疗效果。基于SPIONs的凝胶生物相容性较好, 可选择性杀死M059K胶质母细胞瘤细胞[60]。通过聚醚砜、pNIPAAm纳米粒凝胶和氧化铁磁性纳米粒协同作用可在80 ℃高温下控制纳米多孔屏障层的溶胀和去溶胀, 以实现药物可控释放, 且这种多孔屏障层结构可通过非溶剂诱导相分离工艺条件来控制, 有望用作生物大分子递送[61]。
1.7 氧化还原敏感型水凝胶氧化还原敏感型水凝胶作为水性基质允许水溶性分子扩散, 其结构中结合的氧化还原对通过电子传导而发生快速还原和氧化, 因此能基于外界氧化还原环境改变而响应性释药[62]。由水溶性氧化还原聚合物交联形成的网络在水中膨胀而不溶解[63]。采用β-环糊精、十二烷基改性聚丙烯酸和氧化还原敏感型客体二茂铁羧酸(ferrocenecarboxylic acid, FCA) 构建了氧化还原敏感型水凝胶, 还原态FCA呈凝胶状, 而在氧化状态下则表现为溶液[64]。将转铁蛋白通过二硫键锚定在中空介孔二氧化硅纳米粒表面而制备的多功能纳米载体可在谷胱甘肽存在下降解[65], 尤其适用于肿瘤靶向药物递送和释放。
1.8 多重敏感型水凝胶出于增加凝胶机械强度、加快胶凝速度等不同目的, 双重或三重等多重复合敏感型水凝胶应运而生。
1.8.1 pH/温度敏感型基于聚乙二醇-聚氨基碳酸酯共聚物制备了新型pH和温度双重敏感水凝胶用来递送人类生长激素, 该凝胶在pH 6.0、23 ℃下以溶液形式存在, 该凝胶在生理条件(pH 7.4、37 ℃) 下形成可生物降解的注射用凝胶。在大鼠背部注射后可形成均一多孔结构, 且在注射部位和周围组织没有炎症[66]。温度敏感的pNIPAAm与pH敏感的共聚单体衣康酸通过自由基聚合可制备成载对乙酰氨基酚的温度/pH双重敏感水凝胶。加入衣康酸降低了凝胶最低胶凝化温度, 提高了凝胶力学性能; 当pH为6.8时最大释药量为74%[67]。采用泊洛沙姆407和去乙酰结冷胶制备的温度/离子双重敏感载酮咯酸氨丁三醇鼻用凝胶具有生物黏附性, 可持续释放药物, 鼻黏膜刺激性小, 具有明显镇痛作用[68]。
1.8.2 温度/离子敏感型采用泊洛沙姆407和去乙酰结冷胶制备的苯甲酸利扎曲普坦鼻用温度/离子敏感型原位凝胶的胶凝温度为31.5 ℃, 较鼻腔正常温度的下限稍低, 可保证在正常鼻腔内完全胶凝。同时鼻腔内离子强度可满足去乙酰结冷胶的离子敏感性, 使胶凝时间缩短(约25 s), 可在鼻腔内迅速胶凝。体外释药表明, 5 min内释放低于20%, 突释作用较小, 同时具有明显缓释效果[69]。同样采用泊洛沙姆407和去乙酰结冷胶制备知母皂苷BII温度/离子双重敏感水凝胶经鼻腔给药后具有脑靶向性, 能明显改善脑部注射脂多糖导致的空间记忆力和自发活动下降, 可用于预防阿尔茨海默病[70]。
2 环境敏感型水凝胶常用聚合物环境敏感型水凝胶主要由天然或合成高分子聚合物组成, 常用天然高分子材料包括壳聚糖和海藻酸盐, 半合成高分子材料包括纤维素衍生物等, 合成高分子材料包括泊洛沙姆和pNIPAAm等。从响应因素分类具体可分为温度敏感型、pH敏感型和离子敏感型等(图 3)。常见构成水凝胶聚合物本身不具有敏感型, 需与敏感基团连接后才可作为环境敏感型水凝胶基质, 如壳聚糖和海藻酸等。
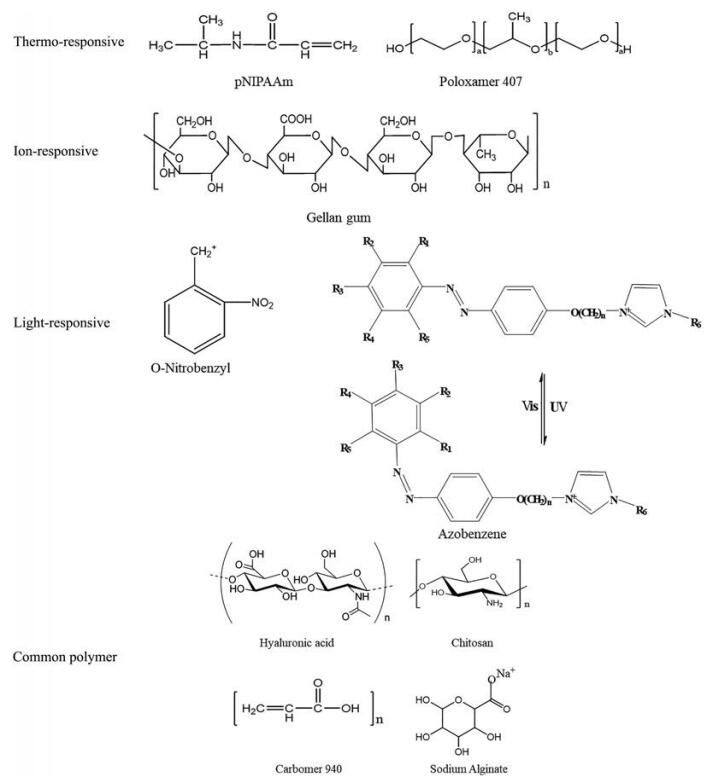
|
Figure 3 The chemical structure of environmentally sensitive polymers and common hydrogel polymers |
pNIPAAm是常见的合成温敏性聚合物, 由N-取代丙烯酰胺和甲基丙烯酰胺单体合成, 其胶凝温度约为32 ℃, 低于此温度时以无序线团状存在。pNIPAAm与其他聚合物结合可调节LCST以满足不同相转变需求。
pNIPAAm通过半互穿聚合物网络策略制备了木质素温敏水凝胶, 所添加的木质素有利于改善凝胶疏水性以获得更好的机械强度, 在LCST附近表现出疏水性、亲水性之间的良好转换[71]。采用硫酸催化纯碱木质素合成了酚醛木质素, 再使酚醛木质素与NIPAAm通过热聚合获得了温敏水凝胶。与纯碱木质素凝胶相比, 由于接枝在pNIPAAm疏水侧链的反应位点增加和空间变小, 所得凝胶交联度较高和LCST较低[72]。由pNIPAAm和海藻酸盐制成的温敏水凝胶在室温下溶解于水或磷酸盐缓冲溶液中, 当温度升高到胶凝温度以上时形成凝胶。载多柔比星后可实现持续释放, 细胞毒作用明显[73]。N,N'-亚甲基双丙烯酰胺(N,N'-methylenebisacrylamide, MBA) 与pNIPAAm交联形成pNIPAAm-MBA凝胶, 其溶胀能力随MBA浓度增加而显著下降, 这是由于共聚过程中交联效率较高所致。冷冻-扫描电镜发现pNIPAAm-MBA孔径较小、孔密度较高。差示扫描量热分析证实了pNIPAAm-MBA凝胶的温度敏感性[74]。pNIPAAm凝胶最大缺点是不可生物降解。将被乙烯基修饰的聚γ-谷氨酸与pNIPAAm通过交联聚合可形成具有可降解性的水凝胶, 该凝胶同时具备pH敏感性和温敏性[75]。
2.2 温度敏感型聚合物——泊洛沙姆泊洛沙姆也是常用温度敏感型聚合物, 是由聚氧乙烯(polyoxyethylene, PEO) 和聚氧丙烯(polyoxypropylene, PPO) 组成的PEO-PPO-PEO型三嵌段共聚物。作为温敏水凝胶基质使用时, 可选择不同型号泊洛沙姆, PEO与PPO比例不同则胶凝温度不同(表 1)。当聚合物浓度达到临界胶束浓度(critical micelle concentration) 时, 泊洛沙姆水溶液在其胶凝化温度(gelation temperature) 附近可呈现从溶胶到凝胶(胶凝温度) 以及从凝胶到溶胶(胶熔温度) 的相变过程。常用泊洛沙姆型号包括泊洛沙姆407和泊洛沙姆188等。
| Table 1 Commonly used poloxamer types. HLB: Hydrophile-lipophile balance |
泊洛沙姆407凝胶可实现药物长达72 h恒定释放[76]。由泊洛沙姆407和188制备得到的马来酸替莫洛尔眼用温敏水凝胶, 在生理温度下与人工泪液接触即可发生相转变形成半固体凝胶, 延长眼表滞留时间, 提高眼部生物利用度, 有效降低眼压[77]。以戊二醛作为交联剂连接羧甲基壳聚糖和泊洛沙姆, 可形成三维交联凝胶, 其胶凝温度为32~33 ℃。负载奈非那克的凝胶在35 ℃、pH 7.4时释放速率最大, 且对人角膜上皮细胞无明显毒性, 可作为眼科药物递送[78]。1% (w/w) 壳聚糖冰醋酸溶液和泊洛沙姆水溶液等量混合后, 可形成稳定、均匀的温度敏感型水凝胶。该凝胶在4~30 ℃之间可注射, 在生理条件下可生物降解[79]。基于泊洛沙姆的温敏水凝胶常用于经皮给药中[80]。同样, 由泊洛沙姆407和海藻酸盐制成的温度敏感型水凝胶被用于经皮递送帕金森病治疗药物司立吉林, 可实现持续、可控释放[81]。由泊洛沙姆407和羧甲基纤维素钠制备的温敏水凝胶可装载中药有效成分, 用于皮肤局部疾病治疗[82, 83]。
2.3 离子敏感型聚合物——去乙酰结冷胶DGG是目前最常用的离子敏感型聚合物, 是酰基全部或部分被去除的一种线性阴离子杂多糖, 因空间阻碍作用明显减弱, 形成凝胶能力增强, 能与多价阳离子形成三维凝胶结构, 大量吸收水或体液, 并在聚合物长链间隙中充分包载药物, 已成功应用于药物递送[84]。
将白藜芦醇纳米悬浮液分散在DGG溶液中制备成离子敏感水凝胶, 0.6% (w/v) DGG凝胶就具有良好的胶凝能力和离子敏感性。通过鼻腔给药, 绕过了血脑屏障, 药物脑中的生物利用度增加了2.88倍, 脑靶向效率提高了458.2%[85]。使用天然多糖和DGG制备的酮替芬离子敏感型水凝胶与普通滴剂相比, 在180天储存期内, 制剂黏度几乎无变化, 且该凝胶可持续释放药物从而有效延长药物作用时间[86]。由于鼻液、泪液含有丰富离子(如K+、Na+、Cl-), 因此, 离子敏感型可单纯或与其他机制联合应用于鼻腔、眼内给药。
2.4 光敏感型聚合物中常见光敏基团光敏感型聚合物由骨架和光敏基团组成。根据光敏基团光化学反应机制不同, 光敏感基质可分为3类: 光异构型、光裂解型和光聚合型。常见的光异构基团包括偶氮苯、螺吡喃和二芳基乙烯等; 光裂解型基团包括邻硝基苄基和香豆素等; 光聚合基团包括肉桂酸酯和蒽等。
由于易合成和可逆异构化, 偶氮苯及其衍生物是使用最广泛的光敏基团。偶氮苯及其衍生物存在顺式和反式两种异构体。紫外光下会发生反式结构向顺式结构转变的过程, 而在可见光下又恢复到反式结构。两种结构的可逆性转变会引起分子极性、分子尺寸和空间位阻等变化, 进而导致凝胶体系形状、体积、溶胀性质及功能改变, 最终引起溶液-凝胶转变。4,4-二烯丙氧基-偶氮苯和八氢硅氧烷之间通过氢化硅烷化反应得到的偶氮苯桥连立方硅倍半氧烷网络(图 4A) 在干燥环境中保持较高的热稳定性, 但非极性溶剂中具有可逆光异构化行为[87]。通过化学桥接偶氮苯和带有1,4-苯二甲酰胺连接基的联苯树枝状分子合成了一种不对称凝胶(图 4B): 在紫外光下发生异构化, 形成牢固的氢键[88]。β-环糊精二聚体和四邻甲氧基取代的偶氮苯二聚体之间通过相互作用制备了全可见光敏感性超分子凝胶(图 4C), 相对于传统的紫外线光敏感型分子, 可用于生物医学和智能化给药等领域[89]。
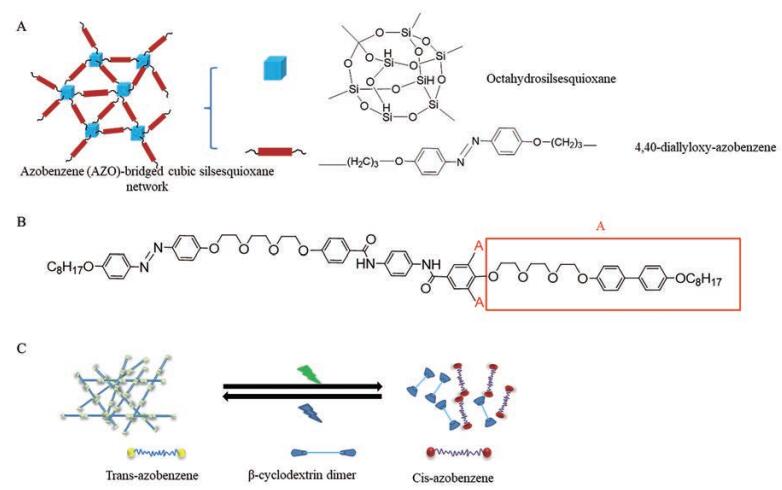
|
Figure 4 Reversible photoisomerization of tetrafluoroazobenzene (A); cyclohexane 1,3,5-tricarboxamide tripod structure (B); supramolecular composed of halogen bonds (C) |
光裂解型聚合物的分子主链或侧基中一般存在光裂解型基团, 在特定光照条件下发生断链, 导致凝胶网络的溶胀度和极性等理化性质发生改变。其中, 邻硝基苄基及其衍生物是运用最多的一类光裂解型基团。邻硝基苄基的光致裂解反应往往是不可逆的, 利用这种裂解反应, 可不同程度破坏聚合物网络, 进而达到有效调控凝胶释药的目的。花青素和多肽共聚物形成的凝胶对光和温度均敏感, 其溶液-凝胶相转变可被螺吡喃结构的反向光致变色作用所控制(图 5A)[90]。基于四臂星形聚合物聚乙二醇-b-聚(γ-O-硝基苯基-L-谷氨酸) 的疏水相互作用而设计的光降解注射自愈性凝胶具有良好的可注射性和自愈能力。在紫外光照射下, 对硝基苄基酯基被裂解, 由疏水结构域转化为亲水结构域, 有效释放了所载疏水性药物(图 5B)[91]。
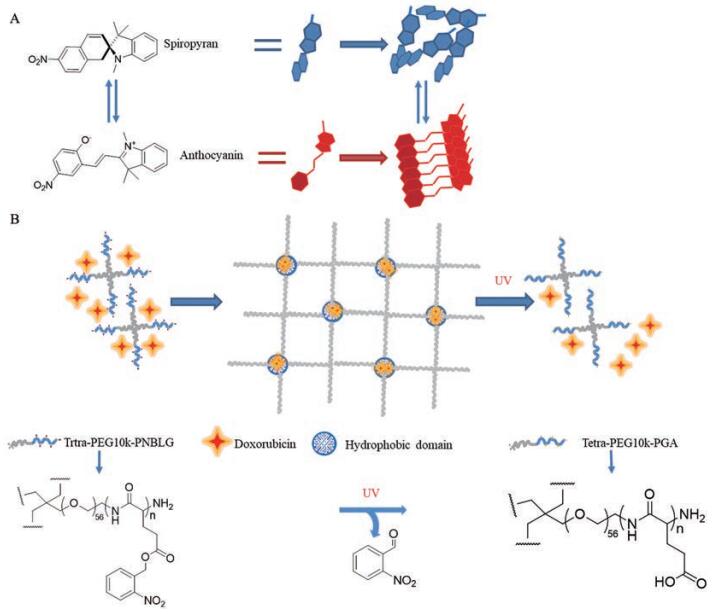
|
Figure 5 Reverse photochromism structure of spiropyran (A); the structure of photodegradable injection self-healing gel (B). PEG: Polyethylene glycol; PNBLG: p-Nitrophenoxycarbonyl-γ-O-nitrobenzyl-L-glutamate; PGA: 3-Phosphoglycerate |
壳聚糖作为一种天然阳离子多糖, 经修饰或与其他辅料联用能同时表现出温度敏感性和pH敏感性[92], 适用于肿瘤治疗和皮肤疾病治疗。当然, 由于壳聚糖伯胺基团在水中能形成阳离子凝胶网络, 通过在酸性条件下(pH < pKa) 膨胀和在碱性条件下(pH > pKa) 收缩可表现出一定对pH的响应行为[93]。由聚乙二醇接枝壳聚糖制成的可注射温度/pH敏感型水凝胶在低温下以溶液状态注射, 在体温下可形成凝胶, 并能实现药物持续释放[94]。温敏性可注射N-己酰二醇壳聚糖凝胶表现出明显溶液-凝胶转变, 这可能是基于己酰基之间的疏水相互作用[95]。用硫脲修饰壳聚糖, 再接枝一定比例聚L-丙交酯、聚N-异丙基丙烯酰胺、聚丙烯酰胺可形成温敏性胶束[96]。
由脱乙酰壳多糖、丙烯酸、(2-二甲基氨基) 甲基丙烯酸乙酯通过原位自由基聚合形成的pH敏感型水凝胶机械性能提高, 可用于递送牛血清白蛋白和5-氟尿嘧啶。该凝胶对肝癌细胞具有一定细胞毒性[97]。将羧甲基壳聚糖与甘油磷酸二钠盐水合物交联形成温敏型水凝胶, 胶凝时间明显缩短, 生物活性和化学稳定性提高。该凝胶未来可用于骨种植体以实现长期固定及维持表面性质的稳定性[98]。采用壳聚糖、羟丙基甲基纤维素和甘油联合制备了新型温敏水凝胶, 其中羟丙基甲基纤维素通过疏水相互作用促进凝胶化; 高浓度甘油破坏聚合物水鞘, 促进疏水区域形成, 降低相变温度。这种新型温敏水凝胶在15 min内可迅速形成凝胶, 细胞毒性较低, 可生物降解, 在生物医学领域应用广泛[99]。
2.5.2 透明质酸(hyaluronic acid, HA)HA是一种由D-葡萄糖醛酸和N-乙酰葡萄糖胺组成的天然酸性黏多糖, 具有优越的亲水性和可降解性。HA凝胶对透明质酸酶和自由基敏感, 通过对HA链上羟基、羧基和乙酰氨基位点进行化学修饰[100]可改变HA凝胶物理和化学性质, 可作为酶敏感型、温度敏感型和光敏感型水凝胶基质。酪胺化HA凝胶具有优良的抗酶解性能, 通过控制HA含量可控制不同降解时间, 最长可在体内维持1个月[101]。酪胺化HA与关节软骨通过光交联反应连结, 可修复关节软骨缺陷[102]。将pNIPAAm接枝到HA上可得到温度敏感型水凝胶, 1 min内即可完成溶液-凝胶态转变[103]。因此, HA凝胶在组织修复和药物递送领域研究较多。
2.5.3 卡波姆(Carbopol)卡波姆是由丙烯酸与烷基蔗糖交联而成的一种高分子聚合物。卡波姆在很低浓度下即能形成具有生物黏附性的高黏度凝胶, 其常用浓度为0.1%~3%, 在pH 6~12时黏度最大, 当pH < 3或pH > 12时黏度下降。与其他辅料联用或经修饰后具有环境敏感性。由卡波姆和羟丙基甲基纤维素组成的伊曲康唑pH敏感水凝胶具有较高黏度和较强黏膜黏附力, 可在口腔内停留6 h以保持有效药物作用时间且药物释放可达80%[104]。由白芨多糖、生物活性天然高分子羧甲基壳聚糖与卡波姆940物理共混后制备的pH敏感型水凝胶3 h释放率超过90%。该凝胶可促进胶原纤维和新生血管形成, 用于促进创面愈合[105]。
2.5.4 海藻酸钠(sodium alginic acid)海藻酸钠是β-D-甘露糖醛酸和α-L-古洛糖醛酸按照(1→4) 糖苷键连接而成的线型聚合物, 具有良好的生物相容性、可生物降解性和pH敏感性。采用自由基聚合技术以乙二醇二甲基丙烯酸酯作为交联剂、海藻酸钠为基质制备了pH敏感型水凝胶, 其溶胀率随丙烯酸和交联剂浓度增加而降低, 而随海藻酸钠浓度增加而增加[106]。通过半互穿聚合物网络和微波辐射, 在聚乙烯吡咯烷酮存在下, 得到海藻酸钠-聚(丙烯酸-共-丙烯酰胺) 新型凝胶表现出良好的pH敏感性, 可用于药物递送[107]。
3 环境敏感型水凝胶不同给药途径环境敏感型水凝胶作为一种智能化给药系统, 能基于体内生理或病理条件变化而发生响应, 因此给药途径多样, 根据用药部位可分为经皮、眼用、口服(包括口腔用)、鼻用、阴道用和直肠用凝胶等。
3.1 眼部给药眼部给药可用于治疗青光眼、损伤修复、老年性黄斑病变和糖尿病视网膜病变等。但目前常用的滴眼液给药后受到泪液冲刷等因素影响流失严重, 导致局部作用时间较短。而眼用环境敏感型水凝胶一般以亲水性聚合物为载体, 给药后基于温度、离子等外界因素改变从溶液转变成半固体凝胶态, 基于生物黏附性在眼表停留可延长药物作用时间, 减少用药次数, 且生物相容性较好[108] (图 6)。有研究比较了4种常用眼用制剂的流变学性质, 包括眼用凝胶、溶液型滴眼液、混悬型滴眼液和眼膏剂。结果表明, 在线性黏弹区内, 眼用凝胶剂弹性模量大于黏性模量, 具有一定屈服应力和触变性, 为剪切变稀型非牛顿流体, 其优点为在低剪切时呈高黏度, 有利于制剂在贮藏时的稳定性和均匀性; 在高剪切(即在使用时), 黏度快速降低有利于凝胶剂在眼部涂布, 提高使用舒适性。屈服应力是指流体发生流动时所需的最小剪切应力值。眼用凝胶剂均具有一定大小的屈服应力值, 可保证凝胶剂在挤出涂布时不会像滴眼液一样极易流出眼外而降低生物利用度[109]。
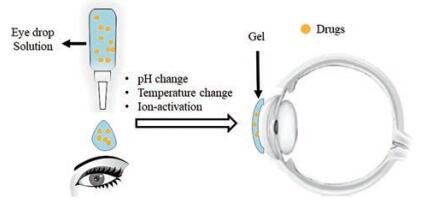
|
Figure 6 The advantages of ophthalmic gels compared with solution |
以泊洛沙姆188、407和羧甲基纤维素为基质制备的伏康唑温敏型眼用凝胶具有持续释药特性, 且给药后在角膜、结膜或虹膜中未观察到明显刺激性[110]。将泊洛沙姆407、188溶解在pH7.4磷酸盐缓冲液中制备得到替莫洛尔马来酸酯温度敏感型水凝胶, 可增强房水中药物吸收并提高眼部生物利用度, 能维持持续降低眼压1周, 对青光眼治疗效果优于普通滴眼液[77]。基于pNIPAAm和透明质酸制备的温敏型酮康唑眼用凝胶在体试验中没有观察到明显眼部刺激、发红或其他刺激性, 具有抗白色念珠菌的作用[26]。对147例眼周注射透明质酸凝胶的患者随访发现, 水肿(11%)、蓝灰色素异常(31.3%) 和轮廓不规则(30.5%) 等并发症中90%较轻微, 相对较安全[111]。
以泊洛沙姆407、188为凝胶基质, 透明质酸钠和羧甲基纤维素钠为生物黏附性材料, 制备了用于治疗初发型干眼病的左卡尼汀温度敏感型水凝胶。滴眼给药后, 该凝胶在家兔眼表滞留时间约为25 min, 为溶液剂5倍[112]。珍珠明目滴眼液具有清肝、明目和止痛等功效, 可用于早期老年性白内障、慢性结膜炎和视疲劳等。以泊洛沙姆407、188为凝胶基质, 卡波姆934为生物黏附性材料, 制成眼用温敏型凝胶后能显著延长药物在眼部滞留时间, 是市售滴眼液7倍, 角膜表观透过系数为5.58 cm·s-1[113]。
3.2 口腔黏膜给药环境敏感型水凝胶适用于口服给药, 尤其是口腔黏膜局部给药, 具有局部停留时间延长、起效快和使用方便的优点, 适用于口腔溃疡、口腔黏膜肉芽肿性疾病、牙龈炎和牙周炎等。
口腔黏膜炎是接受化放疗的肿瘤患者经常遇到的不良反应。促红细胞生成素具有抗炎、抗氧化和促伤口愈合的效果, 采用三甲基壳聚糖将其制备成温敏水凝胶后, 可用于口腔黏膜炎的预防和治疗[114]。牙周炎是由营养不良引起的细菌性口腔炎症。环境敏感型水凝胶给药后能强烈地黏附在牙齿表面, 并提供足够的机械强度, 以确保在牙周袋中长时间释药[115]。以泊洛沙姆407、结冷胶和卡波姆934为凝胶基质制备了盐酸莫西沙星凝胶, 其凝胶温度为36 ℃, 胶凝时间为102 s。该凝胶可明显改善牙周炎症状[116]。
辛伐他汀温度敏感型水凝胶药物含量为89%~92%, pH值为6.5~7.0, 胶凝化温度为37 ℃。体外释放较慢, 口服后能有效促进牙槽骨缺损的骨再生修复[117]。以姜黄素为主药, 羟丙基-β-环糊精(hydroxypropyl-β-cyclodextrin) 为吸收促进剂, 泊洛沙姆为凝胶基质, 制备一种新型姜黄素水凝胶。牙周袋内注射后, 牙周组织炎症程度及牙周破坏程度比模型组和空白凝胶组减轻[118]。10名健康成人使用可注射透明质酸凝胶进行牙齿间乳头缺失后的美学重建。局麻后, 将0.2 mL透明质酸凝胶直接注射到牙齿间乳头底部, 21天后再次注射。每月随访反映, 6个月后疗效显著, 患者顺应性良好, 2/3患者会选择再次接受手术[119]。
3.3 阴道给药阴道局部给药是治疗女性疾病时首选方式, 如真菌性/细菌性阴道炎、宫颈糜烂等。常规溶液剂阴道给药后易流失, 需反复多次给药; 而具有生物黏附作用的凝胶可避免上述问题。
阴道给药通常以温敏水凝胶为主。以乙二醇甲壳素和孕酮制备的温敏水凝胶, 在阴道环境中迅速形成稳定凝胶, 无明显刺激性[120]。载蟾蜍毒和雄黄的泊洛沙姆188、407温敏感型水凝胶可实现药物持续释放, 无明显局部刺激感[121]。用泊洛沙姆188和407制备的伏立康唑-羟丙基-β-环糊精包合物温度敏感型水凝胶显示出良好的黏膜黏附强度, 可实现持续8 h以上持续释放, 阴道局部吸收率更高[122]。以泊洛沙姆407、海藻酸钠制备了能抑制奈瑟氏球菌的卷曲乳杆菌(Lactobacillus crispatus strain) 温度敏感型水凝胶, 其胶凝温度和流变特性适于阴道给药, 其中卷曲乳杆菌可在凝胶中存活6个月[123]。
以羟丙基甲基纤维素作为生物黏合剂、结冷胶作为离子敏感聚合物、NaCl作为等渗调节剂, 制备了载克林霉素离子敏感型水凝胶, 具有高澄清度、无刺激性、载药量高、滞留时间长和生物利用度高的优点[124]。阴道给药姜黄素水凝胶能明显抑制白色念珠菌生长、阴道黏膜水肿及炎性增生, 且降低炎性因子TNF-α及IL-1β表达, 可用于治疗霉菌性阴道炎[125]。
3.4 注射给药局部注射水凝胶主要用于瘤内/瘤旁、牙周袋内和关节腔内注射, 能缓慢释放药物在给药局部维持较长时间高浓度[126]。室温下将壳聚糖溶液注入肿瘤内, 在β-甘油磷酸酯作用下可自动凝胶化, 用于在体内实现安全、高效的结肠癌热疗和化疗[27]。顺铂作为有效化疗药物之一, 其临床应用受到严重肾毒性的限制。聚(N-苯甘氨酸) 与α-环糊精超分子自组装可制备一种可注射顺铂凝胶[127], 其具有近红外敏感性和温度敏感性, 而且可通过溶液-凝胶转变触发顺铂按需释放, 从而增强靶向抗肿瘤活性, 降低非靶点毒性。
环境敏感型水凝胶也适用于关节腔内注射。将透明质酸和氟离子在水溶液中简单混合得到一种关节腔内注射吡罗昔康温敏感型水凝胶, 可持续释药, 生物利用度较高, 可用于治疗关节炎[128]。由聚己内酰胺-聚醋酸乙烯酯-聚乙二醇制备的他克莫司自组装温敏纳米凝胶经关节腔局部注射后, 在局部注射部位滞留时间较长, 疗效呈剂量依赖性, 可用于治疗类风湿关节炎[129]。利用壳聚糖-甘油-饱和硼砂溶液构建用于关节腔注射的马钱子碱缓释壳聚糖温敏水凝胶在常温下为溶液态, 37 ℃时转变为凝胶态, 生物相容性好, 可用于治疗骨关节炎[130]。
3.5 鼻腔给药鼻腔内表面积大, 毛细血管丰富, 也特别适用于环境敏感型水凝胶给药, 尤其鼻腔独有的三叉神经、嗅神经通路为脑靶向递送提供了一种新策略, 可用于神经退行性疾病[131]、创伤后应激障碍[132]、脑肿瘤、缺氧性脑损伤[14, 133]、微波辐射脑损伤[21]、神经修复、焦虑[134]/抑郁[135]等精神疾病的治疗(图 7)。
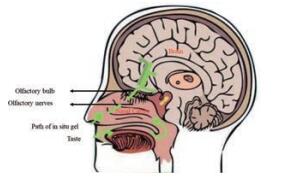
|
Figure 7 The illustration schemed of nose-brain pathway |
将奥啡肽脂质体制剂加至以泊洛沙姆407、卡波姆934为基质的温敏感型水凝胶中, 可持续释放药物 > 5 h, 且该凝胶可提高肽在鼻黏膜的渗透系数和渗透速率[136]。以壳聚糖为基质制备的布洛芬温度敏感型水凝胶在30~35 ℃能快速凝胶化, 提高了布洛芬在人鼻上皮细胞中的转运, 有利于鼻-脑途径给药[137]。以泊洛沙姆407、188和壳聚糖作为基质的石杉甲温度/pH双重敏感水凝胶可在29~34 ℃、pH 6.5条件下迅速凝胶化。药代动力学结果表明, 其经鼻腔给药可达到与静脉给药相似的脑分布特性[138]。将白藜芦醇纳米悬浮液分布在离子敏感型聚合物DGG溶液中制得凝胶经鼻腔给药后脑靶向效率可达458.2%、直接转运百分比为78.18%, 脑中生物利用度增加2.88倍[85]。采用温度敏感型聚合物泊洛沙姆407制备的粉防己碱温度敏感型水凝胶可明显提高微波脑损伤导致的空间记忆力和自发活动下降。与口服给药相比, 其达峰时间缩短(4.8 h vs 8.4 h), 脑靶向指数是口服给药的2.26倍[21]。
3.6 经皮给药由于环境敏感型水凝胶具有成胶迅速、载药量高、释放可控和刺激性小等优点, 在经皮给药制剂中应用很广泛, 可用于痤疮[139]、湿疹[13]和促创面愈合等。以泊洛沙姆407为基质制备了两性霉素B温敏型水凝胶用于皮肤和阴道念珠菌病的治疗; 该凝胶在32 ℃时发生胶凝, 90天内稳定, 对念珠菌抑制效果明显, 未引起明显皮肤刺激性[140]。载骨髓间充质干细胞的温敏水凝胶在用药局部可快速实现溶液-凝胶转变, 显著促进伤口闭合、上皮细胞增殖和上皮再形成, 并减少伤口及周围组织的炎症反应[141]。以油酸和丙二醇为渗透促进剂制备了氯诺昔康凝胶的透皮贴剂。该贴片在4 ℃条件下可稳定6.5个月, 体内镇痛和抗炎活性较好, 可作为口服药物的替代给药[142]。
3.7 直肠给药与固体栓剂相比, 凝胶经直肠给药更为简便, 患者顺应性好。载甲氨蝶呤凝胶与口服给药相比, Tmax、Cmax降低, 适于治疗儿童呕吐[143]。用泊洛沙姆407、羟丙基甲基纤维素和海藻酸钠制备的布洛芬温敏水凝胶与固体栓剂相比, 药物累积释放量显著增加[144]。载磺胺嘧啶pH敏感型水凝胶可用于结肠靶向递送, 能在结肠pH 7.4环境下选择性释放药物[145]。
4 商品化水凝胶及环境敏感型水凝胶现状环境敏感型水凝胶是基于水凝胶基础发展而来, 应该说目前正处于研究阶段。水凝胶基于多年的发展, 产品集中于口服、阴道、经皮和眼部给药。口服给药主要用于口腔护理和预防心绞痛, 包括基质型和储库型两种类型: 基质型通过机械混合使药物完全分散在聚合物中; 储库型则是以聚合物为壳、药物作为内核。药物释放速率由水凝胶性质、壳厚度、药物理化性质决定[146, 147]。阴道用上市凝胶产品以控制阴道气味为主, 如月经期间气味、尿失禁或由细菌失衡引起的气味等。对经皮给药而言, 水凝胶通过保湿作用进一步促进药物通过皮肤水合作用高效渗透吸收, 适合局部和全身应用[148]。眼部凝胶给药可在眼表面停留相当长的时间, 用来保持眼部湿润, 如用来治疗慢性干眼症的Hylo®Gel。下面列举了市场上应用于药物递送的一些水凝胶产品(表 2)。
| Table 2 Hydrogel-based commercial products used in drug delivery system |
水凝胶除用于不同给药途径药物递送外, 也常用于组织工程, 这是因为水凝胶与许多组织和细胞外基质结构类似[149]。由于水凝胶舒适、稳定、生物惰性且不影响视觉效果, 因此可用作隐形眼镜, 还可作为填充剂, 注射后修复面部软组织缺损, 最常用基质为可生物降解的牛胶原蛋白和透明质酸(表 3)。
| Table 3 Hydrogel-based commercial products used in tissue engineering applications, wound dressing, contact lens, hygiene applications and fillers |
对环境敏感型水凝胶而言, 目前市场上应用较多的是温敏水凝胶, 该机制明确, 安全性高(表 4)。
| Table 4 Environmentally sensitive hydrogel-based products on the market |
环境敏感型水凝胶能基于不同生理环境特点在用药局部形成黏附性较好的半固体, 局部滞留时间长有利于持续释药, 制备工艺相对简单、易于实现工业化, 还能与脂质体、微乳、纳米粒形成复合制剂改善药物性质实现多样化应用。但应清楚地看到, 环境敏感型水凝胶广泛应用还面临很多瓶颈, 主要问题如下: ①辅料种类相对较少, 对其流变学、机械性能、响应速率、体内行为和安全性等还缺乏深入、系统的研究。必要时还需进一步修饰以达到不同应用目的。在全面阐明相关辅料理化性质基础上, 实现环境敏感型聚合物的合规化对相关产品研发至关重要; ②本身缓控释作用不明显, 尤其小分子药物突释现象较明显, 需与其他剂型结合实现缓控释, 如包合物、脂质体、微粒和纳米粒等; ③目前环境敏感型水凝胶多以经皮、眼表等浅表性给药方式为主, 其口服、注射给药途径的应用尚需依赖于安全性的全面确证; ④缺乏相关质量标准的建立, 如环境敏感型水凝胶黏度范围、流变学曲线具体何种标准?敏感型聚合物分子量及其分布范围与流变学、黏度、机械性能之间联系如何?目前均无确切数据, 严重制约了环境敏感型水凝胶发展; ⑤环境敏感型水凝胶可能更适用于化学药物递送。中药复方由于成分复杂, 可能会影响胶凝机制。生物大分子与水凝胶长时间接触, 可能影响其稳定性。
未来能实现时空可控、按需给药的环境敏感型水凝胶迎合了精准化、个体化和智能化医疗的发展趋势, 给药途径广泛, 具有广阔应用前景。
作者贡献: 张圆圆负责文献查阅、文章撰写; 杜丽娜、金义光负责总结分析。
利益冲突: 本文不存在任何与本稿件相关的利益冲突。
| [1] |
Buwalda SJ, Boere KW, Dijkstra PJ, et al. Hydrogels in a historical perspective: from simple networks to smart materials[J]. J Control Release, 2014, 190: 254-273. DOI:10.1016/j.jconrel.2014.03.052 |
| [2] |
Dragan ES. Design and applications of interpenetrating polymer network hydrogels. A review[J]. Chem Eng J, 2014, 243: 572-590. DOI:10.1016/j.cej.2014.01.065 |
| [3] |
Sharpe LA, Daily AM, Horava SD, et al. Therapeutic applications of hydrogels in oral drug delivery[J]. Expert Opin Drug Deliv, 2014, 11: 901-915. DOI:10.1517/17425247.2014.902047 |
| [4] |
Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery[J]. Molecules, 2019, 24: 603. DOI:10.3390/molecules24030603 |
| [5] |
Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: their characteristics and the mechanisms behind them[J]. Gels, 2017, 3: 6. DOI:10.3390/gels3010006 |
| [6] |
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery[J]. Nat Rev Mater, 2016, 1: 16071. DOI:10.1038/natrevmats.2016.71 |
| [7] |
Hanafy NA, Leporatti S, El-Kemary MA. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system[J]. Appl Sci, 2019, 9: 825. DOI:10.3390/app9050825 |
| [8] |
Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products[J]. Eur Polym J, 2015, 65: 252-267. DOI:10.1016/j.eurpolymj.2014.11.024 |
| [9] |
Ahmed TA, El-Say KM. Transdermal film-loaded finasteride microplates to enhance drug skin permeation: two-step optimization study[J]. Eur J Pharm Sci, 2016, 88: 246-256. DOI:10.1016/j.ejps.2016.03.015 |
| [10] |
Jeong B, Kibbey MR, Birnbaum JC, et al. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA[J]. Macromolecules, 2000, 33: 8317-8322. DOI:10.1021/ma000638v |
| [11] |
Kasiński A, Zielińska-Pisklak M, Oledzka E, et al. Smart hydrogels-synthetic stimuli-responsive antitumor drug release systems[J]. Int J Nanomedicine, 2020, 15: 4541-4572. DOI:10.2147/IJN.S248987 |
| [12] |
Singh B, Khurana RK, Garg B, et al. Stimuli-responsive systems with diverse drug delivery and biomedical applications: recent updates and mechanistic pathways[J]. Crit Rev Ther Drug Carrier Syst, 2017, 34: 209-255. DOI:10.1615/CritRevTherDrugCarrierSyst.2017017284 |
| [13] |
Zhu SQ, Pang LL, Ma JQ, et al. The treatment of chronic eczema in mice by matrine hydrogels[J]. J Int Pharm Res (国际药学研究杂志), 2020, 47: 731-737. |
| [14] |
Ou G, Ma JQ, Zhu L, et al. Preparation of adenosine triphosphate liposome hydrogel and research of its anti-hypoxia effect[J]. Acta Pharm Sin (药学学报), 2020, 55: 1288-1295. |
| [15] |
Ruan H, Yu Y, Liu Y, et al. Preparation and characteristics of thermoresponsive gel of minocycline hydrochloride and evaluation of its effect on experimental periodontitis models[J]. Drug Deliv, 2016, 23: 525-531. DOI:10.3109/10717544.2014.929195 |
| [16] |
Lee Y, Bae JW, Lee JW, et al. Enzyme-catalyzed in situ forming gelatin hydrogels as bioactive wound dressings: effects of fibroblast delivery on wound healing efficacy[J]. J Mater Chem B, 2014, 2: 7712-7718. DOI:10.1039/C4TB01111B |
| [17] |
Klouda L, Perkins KR, Watson BM, et al. Thermoresponsive, in situ cross-linkable hydrogels based on N-isopropylacrylamide: fabrication, characterization and mesenchymal stem cell encapsulation[J]. Acta Biomater, 2011, 7: 1460-1467. DOI:10.1016/j.actbio.2010.12.027 |
| [18] |
Koetting MC, Peters JT, Steichen SD, et al. Stimulus-responsive hydrogels: theory, modern advances, and applications[J]. Mater Sci Eng R Rep, 2015, 93: 1-49. DOI:10.1016/j.mser.2015.04.001 |
| [19] |
Kuckling D. Stimuli-responsive gels[J]. Gels, 2018, 4: 60. DOI:10.3390/gels4030060 |
| [20] |
Tang S, Floy M, Bhandari R, et al. Synthesis and characterization of thermoresponsive hydrogels based on N-isopropylacrylamide crosslinked with 4, 4'-dihydroxybiphenyl diacrylate[J]. ACS Omega, 2017, 2: 8723-8729. DOI:10.1021/acsomega.7b01247 |
| [21] |
Zhang LH, Pang LL, Zhu SQ, et al. Intranasal tetrandrine temperature-sensitive in situ hydrogels for the treatment of microwave-induced brain injury[J]. Int J Pharm, 2020, 583: 119384. DOI:10.1016/j.ijpharm.2020.119384 |
| [22] |
Ma JQ, Pang LL, Zhu SQ, et al. Comparative study of oral and intranasal puerarin for prevention of brain injury induced by acute high-altitude hypoxia[J]. Int J Pharm (国际药学研究杂志), 2020, 591: 120002. DOI:10.1016/j.ijpharm.2020.120002 |
| [23] |
Gholizadeh H, Cheng S, Pozzoli M, et al. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders[J]. Expert Opin Drug Deliv, 2019, 16: 453-466. DOI:10.1080/17425247.2019.1597051 |
| [24] |
Qi XJ, Liu XY, Tang LM, et al. Anti-depressant effect of curcumin-loaded guanidine-chitosan thermo-sensitive hydrogel by nasal delivery[J]. Pharm Dev Technol, 2020, 25: 316-325. DOI:10.1080/10837450.2019.1686524 |
| [25] |
Sheshala R, Quah SY, Tan GC, et al. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels[J]. Drug Deliv Transl Res, 2019, 9: 434-443. DOI:10.1007/s13346-018-0488-6 |
| [26] |
Zhu M, Wang J, Li N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery[J]. Artif Cells Nanomed Biotechnol, 2018, 46: 1282-1287. DOI:10.1080/21691401.2017.1368024 |
| [27] |
Zheng Y, Wang W, Zhao J, et al. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer[J]. Carbohydr Polym, 2019, 222: 115039. DOI:10.1016/j.carbpol.2019.115039 |
| [28] |
Le UM, Shaker DS, Sloat BR, et al. A thermo-sensitive polymeric gel containing a gadolinium (Gd) compound encapsulated into liposomes significantly extended the retention of the Gd in tumors[J]. Drug Devel Indus Pharm, 2008, 34: 413-418. DOI:10.1080/03639040701662495 |
| [29] |
Bai X, Bao Z, Bi S, et al. Chitosan-based thermo/pH double sensitive hydrogel for controlled drug delivery[J]. Macromol Biosci, 2018, 18: 5. |
| [30] |
Pang LL, Gao Y, Zhang LH, et al. Intranasal tetrandrine temperature-sensitive gel for treatment of post-traumatic stress disorder[J]. Acta Pharm Sin (药学学报), 2019, 54: 1680-1687. |
| [31] |
Ma JQ, Pang LL, Zhu SQ, et al. Effect of four drug-loaded hydrogels on prevention of hypoxic brain damage[J]. J Int Pharm Res (国际药学研究杂志), 2019, 46: 516-521. |
| [32] |
Zhu HY, Yang FX. Advances of pH-sensitive in situ gels research[J]. Qilu Pharm Aff (齐鲁药事), 2006, 25: 486-488. |
| [33] |
Nasir N, Ahmad M, Minhas MU, et al. pH-responsive smart gels of block copolymer pluronic F127-co-poly(acrylic acid) for controlled delivery of ivabradine hydrochloride: its toxicological evaluation[J]. J Polym Res, 2019, 26: 212. DOI:10.1007/s10965-019-1872-8 |
| [34] |
Jaiswal M, Kumar M, Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis[J]. Colloids Surf B Biointerfaces, 2015, 130: 23-30. DOI:10.1016/j.colsurfb.2015.03.059 |
| [35] |
Cinay GE, Erkoc P, Alipour M, et al. Nanogel-integrated pH-responsive composite hydrogels for controlled drug delivery[J]. ACS Biomater Sci Eng, 2017, 3: 370-380. DOI:10.1021/acsbiomaterials.6b00670 |
| [36] |
You YC, Dong LY, Dong K, et al. In vitro and in vivo application of pH-sensitive colon-targeting polysaccharide hydrogel used for ulcerative colitis therapy[J]. Carbohydr Polym, 2015, 130: 243-253. DOI:10.1016/j.carbpol.2015.03.075 |
| [37] |
Gholamali I, Asnaashariisfahani M, Alipour E. Silver nanoparticles incorporated in pH-sensitive nanocomposite hydrogels based on carboxymethyl chitosan-poly (vinyl alcohol) for use in a drug delivery system[J]. Regen Eng Transl Med, 2019, 6: 138-153. |
| [38] |
Liu HW, Yan YL, Zhou LL. Comparison of the brain pharmacokinetics of nasal tetramethylpyrazine phosphate pH-sensitive in situ gel in normal rats and model rats[J]. Acta Pharma Sin (药学学报), 2012, 47: 677-679. |
| [39] |
Liu H, Shi X, Wu D, et al. Injectable, biodegradable, thermosensitive nanoparticles-aggregated hydrogel with tumor-specific targeting, penetration, and release for efficient postsurgical prevention of tumor recurrence[J]. ACS Appl Mater Interfaces, 2019, 11: 19700-19711. DOI:10.1021/acsami.9b01987 |
| [40] |
Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation[J]. J Pharm Pharmacol, 2019, 71: 1209-1221. DOI:10.1111/jphp.13106 |
| [41] |
Qi X, Wei W, Li J, et al. Salecan-based pH-sensitive hydrogels for insulin delivery[J]. Mol Pharm, 2017, 14: 431-440. DOI:10.1021/acs.molpharmaceut.6b00875 |
| [42] |
Li X, Fu M, Wu J, et al. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery[J]. Acta Biomater, 2017, 51: 294-303. DOI:10.1016/j.actbio.2017.01.016 |
| [43] |
Sun Y, Li L, Xie H, et al. Primary studies on construction and evaluation of ion-sensitive in situ gel loaded with paeonol-solid lipid nanoparticles for intranasal drug delivery[J]. Int J Nanomedicine, 2020, 15: 3137-3160. DOI:10.2147/IJN.S247935 |
| [44] |
Butani S. Fabrication of an ion-sensitive in situ gel loaded with nanostructured lipid carrier for nose to brain delivery of donepezil[J]. Asian J Pharm, 2018, 12: 2838. |
| [45] |
Yu S, Wang QM, Wang X, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate[J]. Int J Pharm, 2015, 480: 128-136. DOI:10.1016/j.ijpharm.2015.01.032 |
| [46] |
Rao MRP, Shelar SU. Controlled release ion sensitive floating oral in situ gel of a prokinetic drug using gellan gum[J]. Indian J Pharm Edu Res, 2015, 49: 158-167. DOI:10.5530/ijper.49.2.13 |
| [47] |
Dehghany M, Zhang H, Naghdabadi R, et al. A thermodynamically-consistent large deformation theory coupling photochemical reaction and electrochemistry for light-responsive gels[J]. J Mech Physics Solids, 2018, 116: 239-266. DOI:10.1016/j.jmps.2018.03.018 |
| [48] |
Unger K, Salzmann P, Masciullo C, et al. Novel light-responsive biocompatible hydrogels produced by initiated chemical vapor deposition[J]. ACS Appl Mater Interfaces, 2017, 9: 17408-17416. DOI:10.1021/acsami.7b01527 |
| [49] |
Ayer MA, Schrettl S, Balog S, et al. Light-responsive azo-containing organogels[J]. Soft Matter, 2017, 13: 4017-4023. DOI:10.1039/C7SM00601B |
| [50] |
Zhan TG, Lin MD, Wei J, et al. Visible-light responsive hydrogen-bonded supramolecular polymers based on ortho-tetrafluorinated azobenzene[J]. Polym Chem, 2017, 8: 7384-7389. DOI:10.1039/C7PY01612C |
| [51] |
Chu CW, Stricker L, Kirse TM, et al. Light-responsive arylazopyrazole gelators: from organic to aqueous media and from supramolecular to dynamic covalent chemistry[J]. Chemistry, 2019, 25: 6131-6140. DOI:10.1002/chem.201806042 |
| [52] |
Tong X, Qiu Y, Zhao X, et al. Visible light-triggered gel-to-sol transition in halogen-bond-based supramolecules[J]. Soft Matter, 2019, 15: 6411-6417. DOI:10.1039/C9SM01310E |
| [53] |
Roth-Konforti ME, Comune M, Halperin-Sternfeld M, et al. UV light-responsive peptide-based supramolecular hydrogel for controlled drug delivery[J]. Macromol Rapid Commun, 2018, 39: 1800588. DOI:10.1002/marc.201800588 |
| [54] |
Zhang YL, Chang R, Duan HZ, et al. Metal ion and light sequentially induced sol-gel-sol transition of a responsive peptide-hydrogel[J]. Soft Matter, 2020, 16: 7652-7658. DOI:10.1039/D0SM00442A |
| [55] |
Skaalure SC, Akalp U, Vernerey FJ, et al. Tuning reaction and diffusion mediated degradation of enzyme-sensitive hydrogels[J]. Adv Healthc Mater, 2016, 5: 432-438. DOI:10.1002/adhm.201500728 |
| [56] |
Skaalure SC, Chu S, Bryant SJ. An enzyme-sensitive PEG hydrogel based on aggrecan catabolism for cartilage tissue engineering[J]. Adv Healthc Mater, 2015, 4: 420-431. DOI:10.1002/adhm.201400277 |
| [57] |
Gohil SV, Padmanabhan A, Kan HM, et al. Degradation-dependent protein release from enzyme sensitive injectable glycol chitosan hydrogel[J]. Tissue Eng Part A, 2020. DOI:10.1089/ten.TEA.2020.0124 |
| [58] |
da Silva LP, Jha AK, Correlo VM, et al. Gellan gum hydrogels with enzyme-sensitive biodegradation and endothelial cell biorecognition sites[J]. Adv Healthc Mater, 2018, 7: 1700686. DOI:10.1002/adhm.201700686 |
| [59] |
Milcovich G, Lettieri S, Antunes FE, et al. Recent advances in smart biotechnology: hydrogels and nanocarriers for tailored bioactive molecules depot[J]. Adv Colloid Interface Sci, 2017, 249: 163-180. DOI:10.1016/j.cis.2017.05.009 |
| [60] |
Meenach SA, Hilt JZ, Anderson KW. Poly(ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy[J]. Acta Biomater, 2010, 6: 1039-1046. DOI:10.1016/j.actbio.2009.10.017 |
| [61] |
Lin X, Nguyen Quoc B, Ulbricht M. Magnetoresponsive poly(ether sulfone)-based iron oxide cum hydrogel mixed matrix composite membranes for switchable molecular sieving[J]. ACS Appl Mater Interfaces, 2016, 8: 29001-29014. DOI:10.1021/acsami.6b09369 |
| [62] |
He J, Zhang A, Zhang Y, et al. Novel redox hydrogel by in situ gelation of chitosan as a result of template oxidative polymerization of hydroquinone[J]. Macromolecules, 2011, 44: 2245-2252. DOI:10.1021/ma1029532 |
| [63] |
Heller A. Electron-conducting redox hydrogels: design, characteristics and synthesis[J]. Curr Opin Chem Biol, 2006, 10: 664-672. DOI:10.1016/j.cbpa.2006.09.018 |
| [64] |
Tomatsu I, Hashidzume A, Harada A. Redox-responsive hydrogel system using the molecular recognition of β-cyclodextrin[J]. Macromol Rapid Commun, 2006, 27: 238-241. DOI:10.1002/marc.200500793 |
| [65] |
Tian Y, Guo R, Jiao Y, et al. Redox stimuli-responsive hollow mesoporous silica nanocarriers for targeted drug delivery in cancer therapy[J]. Nanoscale Horiz, 2016, 1: 480-487. DOI:10.1039/C6NH00139D |
| [66] |
Phan VH, Thambi T, Duong HT, et al. Poly(amino carbonate urethane)-based biodegradable, temperature and pH-sensitive injectable hydrogels for sustained human growth hormone delivery[J]. Sci Rep, 2016, 6: 1-12. DOI:10.1038/s41598-016-0001-8 |
| [67] |
Fallon M, Halligan S, Pezzoli R, et al. Synthesis and characterisation of novel temperature and pH sensitive physically cross-linked poly (N-vinylcaprolactam-co-itaconic acid) hydrogels for drug delivery[J]. Gels, 2019, 5: 41. DOI:10.3390/gels5030041 |
| [68] |
Li X, Du L, Chen X, et al. Nasal delivery of analgesic ketorolac tromethamine thermo- and ion-sensitive in situ hydrogels[J]. Int J Pharm, 2015, 489: 252-260. DOI:10.1016/j.ijpharm.2015.05.009 |
| [69] |
Wang J, He W. Preparationand in-vitro properties of thermo-ion sensitive in-situ gel of rizatriptan benzoate for in-tranasal administration[J]. China Pharmacist (中国药师), 2019, 22: 1143-1145. |
| [70] |
Chen W, Li R, Zhu S, et al. Nasal timosaponin BII dually sensitive in situ hydrogels for the prevention of Alzheimer's disease induced by lipopolysaccharides[J]. Int J Pharm, 2020, 578: 119115. DOI:10.1016/j.ijpharm.2020.119115 |
| [71] |
Xia J, Liu Z, Chen Y, et al. Fabrication of thermo-sensitive lignocellulose hydrogels with switchable hydrophilicity and hydrophobicity through an sipn strategy[J]. RSC Adv, 2019, 9: 29600-29608. DOI:10.1039/C9RA05575D |
| [72] |
Jiang P, Sheng X, Yu S, et al. Preparation and characterization of thermo-sensitive gel with phenolated alkali lignin[J]. Sci Rep, 2018, 8: 1-10. |
| [73] |
Liu M, Song X, Wen Y, et al. Injectable thermoresponsive hydrogel formed by alginate-g-poly(N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system[J]. ACS Appl Mater Interfaces, 2017, 9: 35673-35682. DOI:10.1021/acsami.7b12849 |
| [74] |
Jovancic P, Vilchez A, Molina R. Synthesis of thermo-sensitive hydrogels from free radical copolymerization of NIPAAm with MBA initiated by atmospheric plasma treatment[J]. Plasma Proc Polym, 2016, 13: 752-760. DOI:10.1002/ppap.201500194 |
| [75] |
Yang N, Wang Y, Zhang QS, et al. γ-Polyglutamic acid mediated crosslinking PNIPAAm-based thermo/pH-responsive hydrogels for controlled drug release[J]. Polym Degrad Stabil, 2017, 144: 53-61. DOI:10.1016/j.polymdegradstab.2017.07.028 |
| [76] |
Giuliano E, Paolino D, Cristiano MC, et al. Rutin-loaded poloxamer 407-based hydrogels for in situ administration: stability profiles and rheological properties[J]. Nanomaterials, 2020, 10: 1069. DOI:10.3390/nano10061069 |
| [77] |
Zeng Y, Chen J, Li Y, et al. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: in vitro characterization, in vivo pharmacokinetics and pharmacodynamics study[J]. Life Sci, 2018, 212: 80-86. DOI:10.1016/j.lfs.2018.09.050 |
| [78] |
Yu S, Zhang X, Tan G, et al. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery[J]. Carbohydr Polym, 2017, 155: 208-217. DOI:10.1016/j.carbpol.2016.08.073 |
| [79] |
Dahake PT, Baliga SM, Punse T, et al. Formulation and physical characterization of bio-degradable chitosan-poloxamer gel base for local drug delivery[J]. J Drug Deliv Ther, 2020, 10: 59-66. |
| [80] |
Chatterjee S, Hui PC, Kan CW. Thermoresponsive hydrogels and their biomedical applications: special insight into their applications in textile based transdermal therapy[J]. Polymers, 2018, 10: 480. DOI:10.3390/polym10050480 |
| [81] |
Chen CC, Fang CL, Al-Suwayeh SA, et al. Transdermal delivery of selegiline from alginate-pluronic composite thermogels[J]. Int J Pharm, 2011, 415: 119-128. DOI:10.1016/j.ijpharm.2011.05.060 |
| [82] |
Wang W, Wat E, Hui PC, et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment[J]. Sci Rep, 2016, 6: 24112. DOI:10.1038/srep24112 |
| [83] |
Wang WY, Hui PCL, Wat E, et al. Enhanced transdermal permeability via constructing the porous structure of poloxamer-based hydrogel[J]. Polymers, 2016, 8: 406. DOI:10.3390/polym8110406 |
| [84] |
Das M, Giri TK. Hydrogels based on gellan gum in cell delivery and drug delivery[J]. J Drug Deliv Sci Technol, 2020, 56: 101586. DOI:10.1016/j.jddst.2020.101586 |
| [85] |
Hao J, Zhao J, Zhang S, et al. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery[J]. Colloids Surf B Biointerfaces, 2016, 147: 376-386. DOI:10.1016/j.colsurfb.2016.08.011 |
| [86] |
Zhu L, Ao J, Li P. A novel in situ gel base of deacetylase gellan gum for sustained ophthalmic drug delivery of ketotifen: in vitro and in vivo evaluation[J]. Drug Des Devel Ther, 2015, 9: 3943-3949. |
| [87] |
Guo S, Okubo T, Kuroda K, et al. A photoresponsive azobenzene-bridged cubic silsesquioxane network[J]. J Sol Gel Sci Technol, 2016, 79: 262-269. DOI:10.1007/s10971-016-4074-4 |
| [88] |
Choi YJ, Kim JT, Yoon WJ, et al. Azobenzene molecular machine: light-induced wringing gel fabricated from asymmetric macrogelator[J]. ACS Macro Lett, 2018, 7: 576-581. DOI:10.1021/acsmacrolett.8b00167 |
| [89] |
Yan H, Qiu Y, Wang J, et al. Wholly visible-light-responsive host-guest supramolecular gels based on methoxy azobenzene and β-cyclodextrin dimers[J]. Langmuir, 2020, 36: 7408-7417. DOI:10.1021/acs.langmuir.0c00964 |
| [90] |
Wang W, Hu J, Zheng M, et al. Multi-responsive supramolecular hydrogels based on merocyanine-peptide conjugates[J]. Org Biomol Chem, 2015, 13: 11492-11498. DOI:10.1039/C5OB01912E |
| [91] |
Zhao D, Tang Q, Zhou Q, et al. A photo-degradable injectable self-healing hydrogel based on star poly(ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier[J]. Soft Matter, 2018, 14: 7420-7428. DOI:10.1039/C8SM01575A |
| [92] |
Fathi M, Sahandi Zangabad P, Majidi S, et al. Stimuli-responsive chitosan-based nanocarriers for cancer therapy[J]. Bioimpacts, 2017, 7: 269-277. DOI:10.15171/bi.2017.32 |
| [93] |
Wang B, Wu X, Li J, et al. Thermosensitive behavior and antibacterial activity of cotton fabric modified with a chitosan-poly(N-isopropylacrylamide) interpenetrating polymer network hydrogel[J]. Polymers, 2016, 8: 110. DOI:10.3390/polym8040110 |
| [94] |
Bhattarai N, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermoreversible hydrogel[J]. Macromol Biosci, 2005, 5: 107-111. DOI:10.1002/mabi.200400140 |
| [95] |
Li Z, Shim H, Cho MO, et al. Thermo-sensitive injectable glycol chitosan-based hydrogel for treatment of degenerative disc disease[J]. Carbohydr Polym, 2018, 184: 342-353. DOI:10.1016/j.carbpol.2018.01.006 |
| [96] |
Pourjavadi A, Bagherifard M, Doroudian M. Synthesis of micelles based on chitosan functionalized with gold nanorods as a light sensitive drug delivery vehicle[J]. Int J Biol Macromol, 2020, 149: 809-818. DOI:10.1016/j.ijbiomac.2020.01.162 |
| [97] |
Che Y, Li D, Liu Y, et al. Physically cross-linked pH-responsive chitosan-based hydrogels with enhanced mechanical performance for controlled drug delivery[J]. RSC Adv, 2016, 6: 106035-106045. DOI:10.1039/C6RA16746B |
| [98] |
Zhang N, He J, Wu F. Tuning the gelation behavior and cellular response of thermo-sensitive chitosan hydrogels[J]. Mater Lett, 2020, 260: 126903. DOI:10.1016/j.matlet.2019.126903 |
| [99] |
Wang T, Chen L, Shen T, et al. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol[J]. Int J Biol Macromol, 2016, 93: 775-782. DOI:10.1016/j.ijbiomac.2016.09.038 |
| [100] |
Walimbe T, Panitch A, Sivasankar PM. A review of hyaluronic acid and hyaluronic acid-based hydrogels for vocal fold tissue engineering[J]. J Voice, 2017, 31: 416-423. DOI:10.1016/j.jvoice.2016.11.014 |
| [101] |
Raia NR, Partlow BP, McGill M, et al. Enzymatically crosslinked silk-hyaluronic acid hydrogels[J]. Biomaterials, 2017, 131: 58-67. DOI:10.1016/j.biomaterials.2017.03.046 |
| [102] |
Donnelly PE, Chen T, Finch A, et al. Photocrosslinked tyramine-substituted hyaluronate hydrogels with tunable mechanical properties improve immediate tissue-hydrogel interfacial strength in articular cartilage[J]. J Biomater Sci Polym Ed, 2017, 28: 582-600. DOI:10.1080/09205063.2017.1289035 |
| [103] |
Tan H, Ramirez CM, Miljkovic N, et al. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering[J]. Biomaterials, 2009, 30: 6844-6853. DOI:10.1016/j.biomaterials.2009.08.058 |
| [104] |
Nief RA, Tamer MA, Abd Alhammid SN. Mucoadhesive oral in situ gel of itraconazole using pH-sensitive polymers: preparation, and in vitro characterization, release and rheology study[J]. Drug Invent Today, 2019, 11: 1451-1455. |
| [105] |
Huang Y, Shi F, Wang L, et al. Preparation and evaluation of bletilla striata polysaccharide/carboxymethyl chitosan/carbomer 940 hydrogel for wound healing[J]. Int J Biol Macromol, 2019, 132: 729-737. DOI:10.1016/j.ijbiomac.2019.03.157 |
| [106] |
Jalil A, Khan S, Naeem F, et al. The structural, morphological and thermal properties of grafted pH-sensitive interpenetrating highly porous polymeric composites of sodium alginate/acrylic acid copolymers for controlled delivery of diclofenac potassium[J]. Desig Mono Polym, 2017, 20: 308-324. DOI:10.1080/15685551.2016.1259834 |
| [107] |
Tally M, Atassi Y. Optimized synthesis and swelling properties of a pH-sensitive semi-IPN superabsorbent polymer based on sodium alginate-g-poly(acrylic acid-co-acrylamide) and polyvinylpyrrolidone and obtained via microwave irradiation[J]. J Polym Res, 2015, 22: 181. DOI:10.1007/s10965-015-0822-3 |
| [108] |
Chowhan A, Giri TK. Polysaccharide as renewable responsive biopolymer for in situ gel in the delivery of drug through ocular route[J]. Int J Biol Macromol, 2020, 150: 559-572. DOI:10.1016/j.ijbiomac.2020.02.097 |
| [109] |
Wu XL, Ma JF, Fan XY, et al. Research in rheological properties of four types of ophthalmic preparations[J]. Acta Pharma Sin (药学学报), 2017, 52: 146-152. |
| [110] |
Okur NU, Yozgatli V, Okur ME, et al. Improving therapeutic efficacy of voriconazole against fungal keratitis: thermo-sensitive in situ gels as ophthalmic drug carriers[J]. J Drug Deliv Sci Technol, 2019, 49: 323-333. DOI:10.1016/j.jddst.2018.12.005 |
| [111] |
Mustak H, Fiaschetti D, Goldberg RA. Filling the periorbital hollows with hyaluronic acid gel: long-term review of outcomes and complications[J]. J Cosmet Dermatol, 2018, 17: 611-616. DOI:10.1111/jocd.12452 |
| [112] |
Huang PQ, Gao LL, Yu YC, et al. Preparation and quality evaluation of levocarnitine thermosensitive in situ gel[J]. Acta Pharm Sin (药学学报), 2019, 54: 1115-1122. |
| [113] |
Song LN, Li HR, Wang HY, et al. Preparation and properties of thermosensitive in situ gel for ophthalmic formulation containing pearl hydrolyzate[J]. Acta Pharm Sin (药学学报), 2016, 51: 1622-1628. |
| [114] |
Rezazadeh M, Jafari N, Akbari V, et al. A mucoadhesive thermosensitive hydrogel containing erythropoietin as a potential treatment in oral mucositis: in vitro and in vivo studies[J]. Drug Deliv Transl Res, 2018, 8: 1226-1237. DOI:10.1007/s13346-018-0566-9 |
| [115] |
Yadav R, Kanwar IL, Haider T, et al. In situ gel drug delivery system for periodontitis: an insight review[J]. Fut J Pharm Sci, 2020, 6: 1-13. DOI:10.1186/s43094-019-0015-8 |
| [116] |
Swain GP, Patel S, Gandhi J, et al. Development of moxifloxacin hydrochloride loaded in-situ gel for the treatment of periodontitis: in-vitro drug release study and antibacterial activity[J]. J Oral Biol Craniofac Res, 2019, 9: 190-200. |
| [117] |
Ruan H, Yu Y, Guo X, et al. The possibility of healing alveolar bone defects with simvastatin thermosensitive gel: in vitro/in vivo evaluation[J]. Drug Des Devel Ther, 2018, 12: 1997-2003. DOI:10.2147/DDDT.S163986 |
| [118] |
Hao WY, Zhang LH, Zheng ZJ, et al. Therapeutic effect of curcumin hydrogel on rats with periodontitis[J]. Chin J Mod Appl Pharm (中国现代应用药学), 2019, 36: 1773-1778. |
| [119] |
Awartani FA, Tatakis DN. Interdental papilla loss: treatment by hyaluronic acid gel injection: a case series[J]. Clin Oral Investig, 2016, 20: 1775-1780. DOI:10.1007/s00784-015-1677-z |
| [120] |
Almomen A, Cho S, Yang CH, et al. Thermosensitive progesterone hydrogel: a safe and effective new formulation for vaginal application[J]. Pharm Res, 2015, 32: 2266-2279. DOI:10.1007/s11095-014-1616-8 |
| [121] |
Zhang S, Zhang Y, Wang Z, et al. Temperature-sensitive gel-loaded composite nanomedicines for the treatment of cervical cancer by vaginal delivery[J]. Int J Pharm, 2020, 586: 119616. DOI:10.1016/j.ijpharm.2020.119616 |
| [122] |
Deshkar SS, Palve VK. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery[J]. J Drug Deliv Sci Technol, 2019, 49: 277-285. DOI:10.1016/j.jddst.2018.11.023 |
| [123] |
N'Guessan Gnaman KC, Bouttier S, Yeo A, et al. Characterization and in vitro evaluation of a vaginal gel containing Lactobacillus crispatus for the prevention of gonorrhea[J]. Int J Pharm, 2020, 588: 119733. DOI:10.1016/j.ijpharm.2020.119733 |
| [124] |
Patel P. Formulation and evaluation of clindamycin hcl in situ gel for vaginal application[J]. Int J Pharm Investig, 2015, 5: 50-56. DOI:10.4103/2230-973X.147233 |
| [125] |
Chen X, Chen WY, Ma PP, et al. Curcumin temperature-sensitivein situhydrogels for treatment of vaginal candidiasis[J]. J Int Pharm Res (国际药学研究杂志), 2017, 44: 947-952. |
| [126] |
Askari E, Seyfoori A, Amereh M, et al. Stimuli-responsive hydrogels for local post-surgical drug delivery[J]. Gels, 2020, 6: 14. DOI:10.3390/gels6020014 |
| [127] |
Ruan C, Liu C, Hu H, et al. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy[J]. Chem Sci, 2019, 10: 4699-4706. DOI:10.1039/C9SC00375D |
| [128] |
Jung YS, Park W, Park H, et al. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and pluronic F-127 for sustained NSAID delivery[J]. Carbohydr Polym, 2017, 156: 403-408. DOI:10.1016/j.carbpol.2016.08.068 |
| [129] |
Wu H, Wang K, Wang H, et al. Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy[J]. Colloids Surf B Biointerfaces, 2017, 149: 97-104. DOI:10.1016/j.colsurfb.2016.10.013 |
| [130] |
Chen ZP, Liu W, Chen HX, et al. Brucine chitosan thermosensitive hydrogel for intra-articular injection[J]. Acta Pharm Sin (药学学报), 2012, 47: 652-656. |
| [131] |
Du LN, Jin YG. Brain-targeted nasaldrug delivery systems for the treatment of neurodegenerative diseases[J]. J Int Pharm Res (国际药学研究杂志), 2016, 43: 104-109. |
| [132] |
Pang LL, Gao Y, Zhang LH, et al. Intranasal tetrandrine temperature-sensitive gel for treatment of post-traumatic stress disorder[J]. Acta Pharm Sin (药学学报), 2019, 54: 1680-1687. |
| [133] |
Ma JQ, Pang LL, Zhu SQ, et al. Effect of four drug-loaded hydrogels on prevention of hypoxic brain damage[J]. J Int Pharm Res (国际药学研究杂志), 2019, 46: 516-521. |
| [134] |
Beirami E, Oryan S, Seyedhosseini Tamijani SM, et al. Intranasal insulin treatment alleviates methamphetamine induced anxiety-like behavior and neuroinflammation[J]. Neurosci Lett, 2017, 660: 122-129. DOI:10.1016/j.neulet.2017.09.026 |
| [135] |
Naresh WR, Dilip DV, Sunil KP. Xyloglucan based nasal in situ gel formulation of mirtazapine for treatment of depression[J]. Indian J Pharm Edu Res, 2020, 54: 210-219. DOI:10.5530/ijper.54.2s.77 |
| [136] |
Mura P, Mennini N, Nativi C, et al. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin[J]. Eur J Pharm Biopharm, 2018, 122: 54-61. DOI:10.1016/j.ejpb.2017.10.008 |
| [137] |
Gholizadeh H, Cheng S, Pozzoli M, et al. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders[J]. Expert Opin Drug Deliv, 2019, 16: 453-466. DOI:10.1080/17425247.2019.1597051 |
| [138] |
Chen Y, Cheng G, Hu R, et al. A nasal temperature and pH dual-responsive in situ gel delivery system based on microemulsion of huperzine a: formulation, evaluation, and in vivo pharmacokinetic study[J]. AAPS PharmSciTech, 2019, 20: 301. DOI:10.1208/s12249-019-1513-x |
| [139] |
Qiu HY, Bi HY, Chen X, et al. Curcumin hydrogel combined with photodynamics to treat acne[J]. J Int Pharm Res (国际药学研究杂志), 2017, 44: 33-39. |
| [140] |
Sosa L, Calpena AC, Silva-Abreu M, et al. Thermoreversible gel-loaded amphotericin B for the treatment of dermal and vaginal candidiasis[J]. Pharmaceutics, 2019, 11: 312. DOI:10.3390/pharmaceutics11070312 |
| [141] |
Lei Z, Singh G, Min Z, et al. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for the management of severe skin wound healing[J]. Mater Sci Eng C Mater Biol Appl, 2018, 90: 159-167. DOI:10.1016/j.msec.2018.04.045 |
| [142] |
Hashmat D, Shoaib MH, Ali FR, et al. Lornoxicam controlled release transdermal gel patch: design, characterization and optimization using co-solvents as penetration enhancers[J]. PLoS One, 2020, 15: e0228908. DOI:10.1371/journal.pone.0228908 |
| [143] |
AMAE Razek, Hasan AA, Sabry SA, et al. Metoclopramide hydrochloride thermally sensitive rectal in situ gelling system, a novel out-patient treatment for vomiting in pediatric age[J]. J Drug Deliv Sci Technol, 2019, 50: 9-17. DOI:10.1016/j.jddst.2019.01.001 |
| [144] |
Liu Y, Wang X, Liu Y, et al. Thermosensitive in situ gel based on solid dispersion for rectal delivery of ibuprofen[J]. AAPS PharmSciTech, 2018, 19: 338-347. DOI:10.1208/s12249-017-0839-5 |
| [145] |
Abbasi M, Sohail M, Minhas MU, et al. Novel biodegradable pH-sensitive hydrogels: an efficient controlled release system to manage ulcerative colitis[J]. Int J Biol Macromol, 2019, 136: 83-96. DOI:10.1016/j.ijbiomac.2019.06.046 |
| [146] |
Yang WW, Pierstorff E. Reservoir-based polymer drug delivery systems[J]. J Lab Autom, 2012, 17: 50-58. DOI:10.1177/2211068211428189 |
| [147] |
Caccavo D, Lamberti G, Cafaro MM, et al. Mathematical modelling of the drug release from an ensemble of coated pellets[J]. Br J Pharmacol, 2017, 174: 1797-1809. DOI:10.1111/bph.13776 |
| [148] |
Kong BJ, Kim A, Park SN. Properties and in vitro drug release of hyaluronic acid-hydroxyethyl cellulose hydrogels for transdermal delivery of isoliquiritigenin[J]. Carbohydr Polym, 2016, 147: 473-481. DOI:10.1016/j.carbpol.2016.04.021 |
| [149] |
Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications[J]. Biomaterials, 2003, 24: 4337-4351. DOI:10.1016/S0142-9612(03)00340-5 |
 2021, Vol. 56
2021, Vol. 56


