经皮药物递送(transdermal drug delivery, TDD) 是通过皮肤向全身递送药物, 具有避免肝脏首过效应和胃肠代谢、提高生物利用度、减少药物总剂量、有助于避免情绪创伤和针刺伤害从而提高依从性等优点[1-3]。但由于皮肤的角质层屏障, 使得TDD系统只适用于少数药物的递送。
微针(microneedle, MN) 首先报道于1976年, 可有效克服传统经皮系统缺点[4]。微针的针长150~1 500 μm, 基座宽度50~250 μm, 尖端直径1~25 μm, 每个贴片阵列为几十到几百根针。微针通过刺穿角质层产生孔道(微孔道, microchannels), 不损伤真皮中的神经末梢和血管, 不会导致出血和疼痛[5]。通过微针递药起效快, 可自行给药, 涉及领域包括生物大分子(活性肽、蛋白质和疫苗) 和小分子药物的经皮递送、美容行业和临床诊断等[6]。
微针的市场需求广泛, 但目前尚缺少评价微针产品质量的统一标准体系, 这已成为其大规模产业化应用的障碍。微针对皮肤孔道的穿刺效果及闭合时间的影响[7, 8], 关乎安全性和药物渗透性, 是微针临床应用前必须明确的重要问题之一。本文仅以这两方面内容, 系统综述目前已有的相关文献, 对其影响因素及评价方法进行了比较, 为微针产品质量的标准化和安全应用提供参考。
1 微针穿刺后孔道的形成与闭合 1.1 孔道的形成皮肤一般分为表皮、真皮以及皮下组织共三层。表皮的厚度为75~150 μm, 最外层是角质层, 由15~20层死亡的扁平角质细胞组成, 厚度为10~20 μm, 称为皮肤“最坚硬的外壳”, 是药物经皮吸收的最主要屏障[9, 10]; 表皮下是1~4 mm的真皮层, 由弹性纤维、网状纤维、胶原纤维和细胞外基质组成; 皮下组织主要是由疏松的结缔组织和脂肪小叶组成, 起缓冲作用, 厚度因人而异[11]。微针可以刺破皮肤, 暂时打破角质层屏障, 创建微孔道, 延伸至真皮层(图 1)。药物通过微针所创建的孔道被组织吸收, 通常有3种方式: ①微针刺入皮肤后, 移除微针, 再施加药液进行给药; ②将药材和基质材料混合, 涂覆在微针的表面或制备成可溶性微针, 微针刺入皮肤时, 药物随基质的溶解而释放; ③药液通过空心微针注射到孔道中, 被相应组织吸收[10]。

|
Figure 1 Schematic figure of applying microneedles to the skin |
微针刺破皮肤后, 皮肤组织的高弹性回缩特性和组织修复过程使微孔道逐渐变小, 直至闭合。这种破坏是可逆、轻微的, 不同于胶带去除角质层会造成不可逆的破坏[12]。孔道的闭合时间取决于皮肤屏障被破坏的程度, 药物和微生物可通过孔道进行传递。因而, 孔道的闭合是确定药物递送有效性和安全性的重要因素。
2 评价孔道形成与闭合的方法目前用于评价孔道形成与闭合的方法主要有: 染色法、组织切片法、液体绷带、扫描电子显微镜(scanning electron microscopy, SEM)、共聚焦激光扫描显微镜(confocal laser scanning microscopy, CLSM)、计算机断层扫描、超声扫描、光学相干断层扫描(optical coherence tomography, OCT)、经表皮水分丢失(transepidermal water loss, TEWL) 和电阻法(表 1)[13-33]。
| Table 1 Methods to evaluate the formation and closure of skin microchannel |
每种评价方法因其特有的性质而适用不同的范围, 染色法、SEM和液体绷带法通常用来观察形成的孔道表面大小; 组织学切片、超声扫描、计算机断层扫描、CLSM和OCT可用来观察孔道的深度; TEWL法和电阻法常用于观察孔道的闭合。
每一种方法的准确性和可靠性都会受到不同因素的影响, 因而选择不同的测量方法, 测量结果会有差异。通过TEWL法观察微针刺穿皮肤后皮肤屏障功能的恢复情况, 发现370和770 μm长的微针穿刺后皮肤在约4~5 h后恢复其屏障功能, 但钙黄绿素成像的结果有所不同: 370 μm长微针, 孔道闭合时间为12 h, 而770 μm长微针, 孔道闭合时间为18 h[23]。不过也有研究显示两种测量方法所得的皮肤闭合时间一致, TEWL法和染色法显示180和280 μm两种长度的微针刺入志愿者皮肤后的闭合时间都为24 h[34]。
对于同种测量方法, 操作步骤不同也会导致结果的差异。使用CLSM观察300 μm长的微针刺入皮肤的深度。染色后穿刺, 测量深度为130 μm, 穿刺后染色, 测量深度为170 μm[35]。因此, 研究者要根据实际情况, 选择合适的测量方法。
3 影响孔道形成与闭合的因素微针对皮肤孔道形成与闭合的影响因素是复杂的, 主要有以下几方面(图 2)。
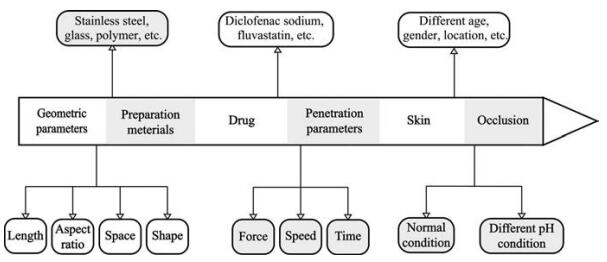
|
Figure 2 Factors affecting the formation and closure of skin microchannels after microneedles application |
现有微针的长度在150~1 500 μm, 长度不同对皮肤孔道产生直接影响。微针长度显著影响形成孔道的大小。由于皮肤弹性和微针机械强度等影响, 使得微针不能完全刺入皮肤中, 形成孔道的深度是微针自身深度的1/4~3/4[35-37]。Donnelly等[38]使用给药器将长度为280、350、600和900 μm的微针刺入新生猪皮中, 形成孔道的深度分别为257、293、470和789 μm, 形成孔道的深度随长度增加而显著增加。该研究者发现在手动给药时, 刺入深度增加的比例小于微针长度增加的比例[39]。为了克服不完全刺入的问题, 研究者开发了箭头状微针, 将长600 μm的金字塔状可溶性微针叠加在长为300、600和900 μm金属轴上, 刺入深度分别为300、600和900 μm, 可大幅度提高微针的刺入深度[40]。
3.1.1.2 微针的长宽比微针的长宽比即锐度不同对孔道大小的影响有所差别。目前, 微针的长宽比在2~4之间居多[41-43]。Carcamo-Martinez等[44]制备了4种不同长宽比(2.5~4.5) 的微针, 刺入深度随长宽比的减小而减小, 当长宽比为2.5, 刺入深度显著小于针长的80%, 表明微针的长宽比低于一定值, 才会对刺入深度产生显著影响。
3.1.1.3 微针的密度微针的密度过高, 会导致“钉子床”效应(nail bed), 即微针贴片上的微针数量多、密度高, 在给定的作用力下无法有效突破角质层屏障, 导致刺入失败[45-47]。微针间距低于150 μm, 会显著降低刺入率。因而, 多数微针间距高于150 μm[42, 48]。随着微针的间距增加, 形成的孔道大小增加、刺入率增加。将间距为50和300 μm的微针刺入石蜡膜中, 测得刺入深度分别为280和320 μm[49]。Donnelly等[38]支持无“钉子床”效应, 发现间距为30~600 μm、长为600 μm微针形成的孔道深度都为475 μm。
3.1.1.4 微针的形状发展至今, 微针形状不断变化: 圆锥形[50]、棱锥形[18]、铅笔形[17]、箭头形[40]和十字形[51]等(图 3)。研究者发现基底为三角形、正方形和六边形的铅笔状微针, 刺入深度为340、343和197 μm[21]。片状、八棱锥和30G注射针形微针刺入皮肤的深度为285、225和225 μm[52]。微针尖端的形状也对刺入深度产生显著影响, 尖端空心的微针在皮肤上形成很浅的环印, 尖端实心的微针穿刺深度可达200 μm[53]。微针形状带有棱角、尖锐, 刺入皮肤更深。

|
Figure 3 Schematic figure of microneedles shapes. A: Cone; B: Pyramid; C: Pencil (pyramid); D: Pencil (cone); E: Arrow; F: Cross |
金属、玻璃、陶瓷及各种高分子聚合物材料都可用于微针的制备, 材料不同会影响微针的机械强度, 从而影响皮肤孔道的形成与闭合。微针材料越硬, 机械强度越佳, 形成孔道越大。长为750 μm的不锈钢微针, 刺入深度为300 μm[36], 而长为1 080 μm的玻璃微针, 刺入深度为100~300 μm[54]。可溶性微针的基质材料和所载药物种类、占比等对孔道形成有不同的影响。Dillon等[55]制备加载两种不同药物的微针, 五肽胃泌素微针的刺入深度达405 μm, 而辛卡利特微针的刺入深度达284 μm。类似的结果还有两种不同分子质量的聚乙二醇(PEG) 和Gantrez® S-97共混制备成微针, 含PEG 10 000微针的刺入深度达554 μm, 含PEG 200微针的刺入深度达392 μm[56]。浓度为0%、25%、50%和75%的γ-聚谷氨酸(γ-PGA)水凝胶微针暴露于相对湿度55%环境3 h后, 刺入深度为415、418、647和650 μm, 只有50%和75%的γ-PGA水凝胶微针可完全刺入(图 4)[57]。造成上述结果的原因与材料的韧脆性和吸水性等性质密不可分。
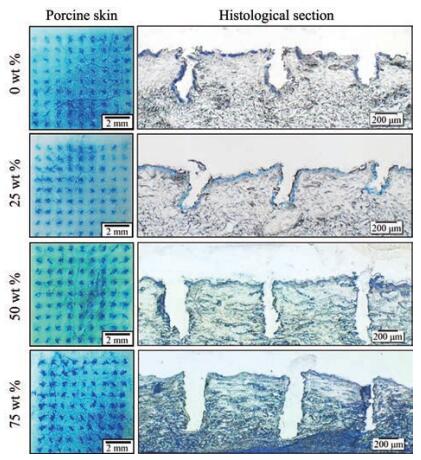
|
Figure 4 In vitro skin insertion capability of microneedles (MNs) containing 0, 25, 50, and 75 wt% hydrogel after exposure to a relative humidity environment of 55% for 3 h. The left column shows the bright-field micrographs of porcine cadaver skin after MNs insertion and staining with blue tissue marking dye. The right column shows the corresponding histological section of MNs puncture sites. (Adapted from Ref. 57 with permission. Copyright © 2015 Acta Materialia Inc) |
在一定范围内, 形成孔道的大小随刺入力的增大而增大。Donnelly等[38]使用4.4、7.0、11.0和16.4 N的刺入力将长600 μm的微针刺入皮肤中, 刺入深度分别为330、400、470和520 μm, 彼此之间的差异具有显著性。类似的结果还有染色法显示微针形成孔道的直径大小取决于刺入力[36]。刺入力超过20 N时, 力值对刺入深度没有影响, 20、30和50 N的刺入力形成孔道的深度为140~180 μm, 没有显著差异[58]。
3.1.3.2 刺入速度刺入力和速度是两个不可分离的因素, 很多研究者会使用微针专用给药器将微针有效地刺入皮肤[17, 52]。刺入速度对形成孔道大小的影响与刺入力的影响相似, Lhernould等[59]控制微针的刺入速度为0.01、0.1、1和10 m·s-1, 刺入深度为200、400、500和500 μm。因此, 微针进行给药时, 控制刺入速度≥ 1 m·s-1。
3.1.3.3 刺入时间刺入时间保持在30 s以上, 微针可有效刺入皮肤中。Larraneta等[49]保持刺入时间为1和30 s, 刺入深度为270和330 μm。也有其他研究者持相同结果, 刺入时间为1、10、30和60 s, 形成孔道的深度分别为250、250、381和318 μm[59]。
3.1.4 受试者的皮肤差异性别、年龄和部位等不同会导致皮肤的差异性, 对孔道的产生有不同的影响。将微针刺入不同年龄的志愿者皮肤上, 不论微针数量、长度如何变化, 青年组形成的孔道大于老年组的孔道[60]。另有研究者发现性别对孔道的形成没有显著影响[61]。
3.2 影响孔道闭合的影响因素 3.2.1 微针的几何参数 3.2.1.1 微针的长度长度对孔道闭合时间的影响没有显著差异。Gomaa等[62]将长度为400、600和1 000 μm的微针刺入离体皮肤, TEWL法显示初始值有所差别, 但闭合时间都在25 h内。但也有研究者持相反实验结果, 染色法显示长度为250、500和1 000 μm的微针形成孔道的闭合时间分别为2、3和8 h, TEWL法显示250和500 μm长的微针形成孔道在3 h内闭合, 而1 000 μm长的微针形成孔道并未在3 h内闭合[63]。
3.2.1.2 微针的密度微针间距越大, 形成孔道越深, 但孔道数量增加, 皮肤损伤程度增大, 对孔道闭合时间的影响是复杂的。Gupta等[64]在相同面积的不锈钢板上制备10和50根两种数量的微针阵列, 刺入志愿者皮肤中, 两种间距微针形成孔道的闭合时间为2 h, 在闭塞条件下, 微针数量增大5倍, 孔道闭合时间增加10倍。Li等[65]将间距为400、600和800 μm的微针刺入皮肤中, TEWL和染色法显示孔道闭合时间分别为24、24和48 h内(图 5)。

|
Figure 5 Effect of space of microneedles on the formation and closure of microchannels. A: TEWL; B: Methylene blue staining. n = 6, x± s. ***P < 0.001 vs space 600 μm (0 h). (Adapted from Ref. 65 with permission. Copyright © 2021 Acta Pharmaceutica Sinica) |
形状不同对皮肤孔道闭合的影响较小, 将圆锥气泡微针和圆锥实心微针刺入小鼠体内, TEWL法显示孔道的闭合时间为9~12 h, 没有差异性[66]。具有同样结果的还有十字形微针和棱锥形微针, 形成孔道的闭合时间为4 h[25, 51]。
3.2.2 微针的制备材料金属及可溶性等多种材料制备成的微针, 形成孔道的闭合时间为24 h内[12, 23]; 而溶胀型材料制备成微针, 形成孔道的闭合速度较慢, 在皮肤上施用水凝胶颗粒聚-N-异丙基丙烯酰胺和聚乳酸-羟基乙酸共聚物制备的微针, 3天后孔道完全闭合[67]。
3.2.3 药物非甾体抗炎药通过抑制环氧合酶发挥作用, 环氧合酶是炎症反应不可缺少的物质, 而孔道闭合与炎症有关。因而, 部分药物可影响孔道的闭合。用微针刺破皮肤后, 涂覆双氯芬酸钠凝胶和安慰剂凝胶, 电阻法结果显示安慰剂组孔道的闭合时间为0.43~1.67天, 双氯芬酸钠凝胶组孔道的闭合时间长达7天[2]。Banks等[68]每日将3%双氯芬酸钠的凝胶涂覆在微针处理后的皮肤上, 孔道的开放时间长达7天。结果显示, 非特异性环加氧酶抑制剂双氯芬酸钠可有效延长微孔道的寿命。上述研究中双氯芬酸钠是每日使用。Ghosh等[69]将双氯芬酸钠和纳曲酮以酯键相连形成共价结合药物, 7天内两次涂覆在孔道上, 结果显示孔道在7天内未闭合, 单纯施用纳曲酮的孔道闭合时间为4天。氟伐他汀通过抑制胆固醇的合成, 也可增加微孔道的开放时间长达7天[70]。
3.2.4 受试者的皮肤差异Kelchen等[60]研究年龄对孔道闭合的影响, 青年组的皮肤孔道闭合时间在24 h内, 而老年组的孔道闭合时间大于24 h。这与老年人皮肤结构变化、弹性降低而导致皮肤屏障功能损伤增大有关。
3.2.5 闭塞条件微针刺破皮肤, 皮肤屏障遭到破坏, TEWL值增加, 开始修复。在闭塞条件下, 皮肤修复趋于缓慢, 皮肤表面的pH值升高, 也阻碍了屏障的恢复。
3.2.5.1 正常条件下的闭塞将微针刺入大鼠腹部皮肤, TEWL法结果显示, 非闭塞环境下, 孔道在4 h内完全闭合, 在聚乙烯医用胶带或不同pH缓冲溶液覆盖条件下, 孔道的闭合时间延长至120 h内, 亚甲蓝染色进一步证实了该实验结果[24]。当使用半透膜来进行闭塞, 屏障功能恢复正常[24]。
闭塞条件会加大微针参数对孔道闭合时间的差异性, 在未闭塞条件下, 不同长度、宽度的微针形成的孔道都在2 h内闭合; 在闭塞条件下, 当微针长度由500 μm增加至750 μm, 闭合时间由22 h延长至30 h[64]。移除闭塞后, 孔道会在1~2 h内快速闭合[24, 64]。
3.2.5.2 不同pH值条件下的闭塞研究显示pH值对伤口的恢复有显著影响[71, 72], 但pH值对微针形成的孔道闭合没有显著影响。将微针刺入皮肤, 使用不同pH值溶液进行闭塞, 孔道的闭合时间保持一致[24, 73]。损伤较小时, pH值的变化不足以影响皮肤屏障功能的恢复。
4 结语本文对微针造成皮肤孔道的形成与闭合所使用的评价方法和影响因素进行了全面的回顾和分析。每种评价方法都有其优缺点, 研究者应根据自己的需要, 选择合适的方法。单一的评价方法不能全面地评价孔道的形成与闭合, 需要综合运用多种现代先进技术, 探索建立一种更加全面科学的评价方法用于微针穿刺的评价。
皮肤孔道形成与闭合的影响因素也多种多样, 由微针的几何参数和微针的制备材料可确定制备出满足要求的微针; 由刺入参数和受试者皮肤差异可选用不同的微针给药方式、辅助给药器及给药部位; 由处理部位的闭塞条件和药物可确定微针给药的周期, 进而设计缓控释微针给药方式。尽管本文总结了影响孔道形成与闭合的因素及规律, 考虑到研究者们彼此之间使用的微针参数和实验条件等各不相同, 可能会导致结果差异, 仍有待于进一步系统性探索研究影响皮肤孔道形成与闭合的关键因素及规律, 为微针阵列产品及其评价的标准化和规范化提供基础数据。
关于孔道的形成与闭合也存在一些亟待解决的问题。孔道在开放期间, 细菌等微生物可通过孔道进入到身体中, 引发感染。因此, 微针制剂的灭菌或无菌保障、抗菌及保证细菌不会进入孔道是未来微针产业应用所面临的重要问题。相信随着科学技术的发展和研究的不断深入, 微针在质量可控性、有效性和安全性等方面的诸多问题都将得到有效解决和足够的保障。
作者贡献: 李蓉蓉是本文的主要撰写者; 王缘协助查询相关文献和图片的整理; 刘哲、修雪亮、刘勇和王延妮对本文提出许多修改意见; 马凤森提出本文的思路并参与文章撰写及修改。
利益冲突: 本文的研究内容无任何利益冲突。
| [1] |
Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery[J]. Nat Rev Drug Discov, 2004, 3: 115-124. DOI:10.1038/nrd1304 |
| [2] |
Brogden NK, Milewski M, Ghosh P, et al. Diclofenac delays micropore closure following microneedle treatment in human subjects[J]. J Control Release, 2012, 163: 220-229. DOI:10.1016/j.jconrel.2012.08.015 |
| [3] |
Xie Y, Xu B, Gao Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array[J]. Nanomedicine, 2005, 1: 184-190. DOI:10.1016/j.nano.2005.03.001 |
| [4] |
Duarah S, Sharma M, Wen J. Recent advances in microneedle-based drug delivery: special emphasis on its use in paediatric population[J]. Eur J Pharm Biopharm, 2019, 136: 48-69. DOI:10.1016/j.ejpb.2019.01.005 |
| [5] |
Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety[J]. Drug Deliv, 2010, 17: 187-207. DOI:10.3109/10717541003667798 |
| [6] |
Dou JJ, Yan JH, Xu K, et al. Transdermal delivery of Gentiana macrophylla complex components system under micro-needle conditions[J]. Acta Pharm Sin (药学学报), 2011, 46: 1137-1143. |
| [7] |
Zhang ZB, Fang DM. Research progress of microneedle technology in transdermal drug delivery system[J]. Tianjin Pharm (天津药学), 2018, 30: 40-43. |
| [8] |
Shen RX, Zhu ZZ, Tong XL, et al. Preparation and evaluation of soluble microneedle patch loaded with salmon calcitonin[J]. Chin J Pharms (中国医药工业杂志), 2018, 49: 1264-1271. |
| [9] |
Ma CM, Cai JL. The research advancement and the application on importing drugs through the skin microneedle in plastic surgery[J]. Chin J Aesthetic Med (中国美容医学杂志), 2012, 21: 860-863. |
| [10] |
Hu DL. The Experimental Research of Insertion of Microneedle and Microneedle Array into Silicon Membrane (微针及其阵列刺入硅胶实验研究)[D]. Dalian: Dalian University of Technology, 2016.
|
| [11] |
Hou JJ. The Theoretical and Experimental Study of Microneedle Array Inserting into Skin (微针阵列刺入皮肤的理论与实验研究)[D]. Dalian: Dalian University of Technology, 2013.
|
| [12] |
Liu S, Jin MN, Quan YS, et al. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin[J]. Eur J Pharm Biopharm, 2014, 86: 267-276. DOI:10.1016/j.ejpb.2013.10.001 |
| [13] |
Zhan HH, Huang YC, Ma FS, et al. Quality evaluation of lidocaine hydrochloride rapid onset local anesthesia preparation based on microneedles technology[J]. Acta Pharm Sin (药学学报), 2018, 53: 1371-1376. |
| [14] |
Arya JM, Dewitt K, Scott-Garrard M, et al. Rabies vaccination in dogs using a dissolving microneedle patch[J]. J Control Release, 2016, 239: 19-26. DOI:10.1016/j.jconrel.2016.08.012 |
| [15] |
Aung NN, Ngawhirunpat T, Rojanarata T, et al. Fabrication, characterization and comparison of alpha-arbutin loaded dissolving and hydrogel forming microneedles[J]. Int J Pharm, 2020, 586: 119508. DOI:10.1016/j.ijpharm.2020.119508 |
| [16] |
Park JH, Choi SO, Seo S, et al. A microneedle roller for transdermal drug delivery[J]. Eur J Pharm Biopharm, 2010, 76: 282-289. DOI:10.1016/j.ejpb.2010.07.001 |
| [17] |
Chen MC, Huang SF, Lai KY, et al. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination[J]. Biomaterials, 2013, 34: 3077-3086. DOI:10.1016/j.biomaterials.2012.12.041 |
| [18] |
Fonseca DFS, Costa PC, Almeida IF, et al. Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment[J]. Carbohydr Polym, 2020, 241: 116314. DOI:10.1016/j.carbpol.2020.116314 |
| [19] |
Liu D, Yu B, Jiang G, et al. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats[J]. Mater Sci Eng C Mater Biol Appl, 2018, 90: 180-188. DOI:10.1016/j.msec.2018.04.055 |
| [20] |
Nguyen HX, Banga AK. Delivery of methotrexate and characterization of skin treated by fabricated PLGA microneedles and fractional ablative laser[J]. Pharm Res, 2018, 35: 68-88. DOI:10.1007/s11095-018-2369-6 |
| [21] |
Loizidou EZ, Inoue NT, Ashton-Barnett J, et al. Evaluation of geometrical effects of microneedles on skin penetration by CT scan and finite element analysis[J]. Eur J Pharm Biopharm, 2016, 107: 1-6. DOI:10.1016/j.ejpb.2016.06.023 |
| [22] |
Economidou SN, Pere CPP, Reid A, et al. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery[J]. Mater Sci Eng C Mater Biol Appl, 2019, 102: 743-755. DOI:10.1016/j.msec.2019.04.063 |
| [23] |
Kalluri H, Kolli CS, Banga AK. Characterization of microchannels created by metal microneedles: formation and closure[J]. AAPS J, 2011, 13: 473-481. DOI:10.1208/s12248-011-9288-3 |
| [24] |
Kalluri H, Banga AK. Formation and closure of microchannels in skin following microporation[J]. Pharm Res, 2011, 28: 82-94. DOI:10.1007/s11095-010-0122-x |
| [25] |
Mao J, Wang H, Xie Y, et al. Transdermal delivery of rapamycin with poor water-solubility by dissolving polymeric microneedles for anti-angiogenesis[J]. J Mater Chem B, 2020, 8: 928-934. DOI:10.1039/C9TB00912D |
| [26] |
Liu S, Zhang S, Duan Y, et al. Transcutaneous immunization of recombinant staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge[J]. Vaccine, 2019, 37: 3810-3819. DOI:10.1016/j.vaccine.2019.05.055 |
| [27] |
Coulman SA, Birchall JC, Alex A, et al. In vivo, in situ imaging of microneedle insertion into the skin of human volunteers using optical coherence tomography[J]. Pharm Res, 2011, 28: 66-81. DOI:10.1007/s11095-010-0167-x |
| [28] |
Fercher AF. Optical coherence tomography-development, principles, applications[J]. Z Med Phys, 2010, 20: 251-276. DOI:10.1016/j.zemedi.2009.11.002 |
| [29] |
Verbaan FJ, Bal SM, van den Berg DJ, et al. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin[J]. J Control Release, 2007, 117: 238-245. DOI:10.1016/j.jconrel.2006.11.009 |
| [30] |
Yan G, Warner KS, Zhang J, et al. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery[J]. Int J Pharm, 2010, 391: 7-12. DOI:10.1016/j.ijpharm.2010.02.007 |
| [31] |
Elkeeb R, Hui X, Chan H, et al. Correlation of transepidermal water loss with skin barrier properties in vitro: comparison of three evaporimeters[J]. Skin Res Technol, 2010, 16: 9-15. DOI:10.1111/j.1600-0846.2009.00406.x |
| [32] |
Elmahjoubi E, Frum Y, Eccleston GM, et al. Transepidermal water loss for probing full-thickness skin barrier function: correlation with tritiated water flux, sensitivity to punctures and diverse surfactant exposures[J]. Toxicol In Vitro, 2009, 23: 1429-1435. DOI:10.1016/j.tiv.2009.06.030 |
| [33] |
Prausnitz MR. The effects of electric current applied to skin: a review for transdermal drug delivery[J]. Adv Drug Deliv Rev, 1996, 18: 395-425. DOI:10.1016/0169-409X(95)00081-H |
| [34] |
Haq MI, Smith E, John DN, et al. Clinical administration of microneedles: skin puncture, pain and sensation[J]. Biomed Microdevices, 2009, 11: 35-47. DOI:10.1007/s10544-008-9208-1 |
| [35] |
Bal S, Kruithof AC, Liebl H, et al. In vivo visualization of microneedle conduits in human skin using laser scanning microscopy[J]. Laser Phys Lett, 2010, 7: 242-246. DOI:10.1002/lapl.200910134 |
| [36] |
Pearton M, Saller V, Coulman SA, et al. Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression[J]. J Control Release, 2012, 160: 561-569. DOI:10.1016/j.jconrel.2012.04.005 |
| [37] |
Moussi K, Bukhamsin A, Hidalgo T, et al. Biocompatible 3D printed microneedles for transdermal, intradermal, and percutaneous applications[J]. Adv Engineer Mater, 2019, 22: 1-10. |
| [38] |
Donnelly RF, Garland MJ, Morrow DI, et al. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution[J]. J Control Release, 2010, 147: 333-341. DOI:10.1016/j.jconrel.2010.08.008 |
| [39] |
Donnelly RF, Moffatt K, Alkilani AZ, et al. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet[J]. Pharm Res, 2014, 31: 1989-1999. DOI:10.1007/s11095-014-1301-y |
| [40] |
Chu LY, Prausnitz MR. Separable arrowhead microneedles[J]. J Control Release, 2011, 149: 242-249. DOI:10.1016/j.jconrel.2010.10.033 |
| [41] |
Hu L, Liao Z, Hu Q, et al. Novel Bletilla striata polysaccharide microneedles: fabrication, characterization, and in vitro transcutaneous drug delivery[J]. Int J Biol Macromol, 2018, 117: 928-936. DOI:10.1016/j.ijbiomac.2018.05.097 |
| [42] |
Xenikakis I, Tzimtzimis M, Tsongas K, et al. Fabrication and finite element analysis of stereolithographic 3D printed microneedles for transdermal delivery of model dyes across human skin in vitro[J]. Eur J Pharm Sci, 2019, 137: 104976. DOI:10.1016/j.ejps.2019.104976 |
| [43] |
Zhang Y, Jiang G, Yu W, et al. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats[J]. Mat Sci Eng C, 2018, 85: 18-26. DOI:10.1016/j.msec.2017.12.006 |
| [44] |
Carcamo-Martinez A, Anjani QK, Permana AD, et al. Coated polymeric needles for rapid and deep intradermal delivery[J]. Int J Pharm, 2020, 2: 100048. |
| [45] |
Andersen TE, Andersen AJ, Petersen RS, et al. Drug loaded biodegradable polymer microneedles fabricated by hot embossing[J]. Microelectron Eng, 2018, 195: 57-61. DOI:10.1016/j.mee.2018.03.024 |
| [46] |
Chen Y, Chen BZ, Wang QL, et al. Fabrication of coated polymer microneedles for transdermal drug delivery[J]. J Control Release, 2017, 265: 14-21. DOI:10.1016/j.jconrel.2017.03.383 |
| [47] |
Yung KL, Xu Y, Kang C, et al. Sharp tipped plastic hollow microneedle array by microinjection moulding[J]. J Micromech Microeng, 2012, 22: 15-16. |
| [48] |
Hsueh KJ, Chen MC, Cheng LT, et al. Transcutaneous immunization of Streptococcus suis bacterin using dissolving microneedles[J]. Comp Immunol Microbiol Infect Dis, 2017, 50: 78-87. DOI:10.1016/j.cimid.2016.12.001 |
| [49] |
Larraneta E, Moore J, Vicente-Perez EM, et al. A proposed model membrane and test method for microneedle insertion studies[J]. Int J Pharm, 2014, 472: 65-73. DOI:10.1016/j.ijpharm.2014.05.042 |
| [50] |
Vora LK, Courtenay AJ, Tekko IA, et al. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules[J]. Int J Biol Macromol, 2020, 146: 290-298. DOI:10.1016/j.ijbiomac.2019.12.184 |
| [51] |
Uddin MJ, Scoutaris N, Economidou SN, et al. 3D printed microneedles for anticancer therapy of skin tumours[J]. Mat Sci Eng C Mater Biol Appl, 2020, 107: 110248. DOI:10.1016/j.msec.2019.110248 |
| [52] |
Maaden VK, Varypataki EM, Yu H, et al. Parameter optimization toward optimal microneedle-based dermal vaccination[J]. Eur J Pharm Sci, 2014, 64: 18-25. DOI:10.1016/j.ejps.2014.08.004 |
| [53] |
Ye R, Yang J, Li Y, et al. Fabrication of tip-hollow and tip-dissolvable microneedle arrays for transdermal drug delivery[J]. ACS Biomater Sci Eng, 2020, 6: 2487-2494. DOI:10.1021/acsbiomaterials.0c00120 |
| [54] |
Martanto W, Moore JS, Couse T, et al. Mechanism of fluid infusion during microneedle insertion and retraction[J]. J Control Release, 2006, 112: 357-361. DOI:10.1016/j.jconrel.2006.02.017 |
| [55] |
Dillon C, Hughes H, O'Reilly NJ, et al. Dissolving microneedle based transdermal delivery of therapeutic peptide analogues[J]. Int J Pharm, 2019, 565: 9-19. DOI:10.1016/j.ijpharm.2019.04.075 |
| [56] |
Khan S, Minhas MU, Tekko IA, et al. Evaluation of microneedles-assisted in situ depot forming poloxamer gels for sustained transdermal drug delivery[J]. Drug Deliv Transl Res, 2019, 9: 764-782. DOI:10.1007/s13346-019-00617-2 |
| [57] |
Chen MC, Ling MH, Kusuma SJ. Poly-gamma-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin[J]. Acta Biomater, 2015, 24: 106-116. DOI:10.1016/j.actbio.2015.06.021 |
| [58] |
Vicente-Perez EM, Quinn HL, McAlister E, et al. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo[J]. Pharm Res, 2016, 33: 3072-3080. DOI:10.1007/s11095-016-2032-z |
| [59] |
Lhernould MS, Deleers M, Delchambre A. Hollow polymer microneedles array resistance and insertion tests[J]. Int J Pharm, 2015, 480: 152-157. DOI:10.1016/j.ijpharm.2015.01.019 |
| [60] |
Kelchen MN, Siefers KJ, Converse CC, et al. Micropore closure kinetics are delayed following microneedle insertion in elderly subjects[J]. J Control Release, 2016, 225: 294-300. DOI:10.1016/j.jconrel.2016.01.051 |
| [61] |
Vicente-Perez EM, Larraneta E, McCrudden MTC, et al. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo[J]. Eur J Pharm Biopharm, 2017, 117: 400-407. DOI:10.1016/j.ejpb.2017.04.029 |
| [62] |
Gomaa YA, Morrow DI, Garland MJ, et al. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: assessments by transepidermal water loss[J]. Toxicol In Vitro, 2010, 24: 1971-1978. DOI:10.1016/j.tiv.2010.08.012 |
| [63] |
Martanto W, Davis SP, Holiday NR, et al. Transdermal delivery of insulin using microneedles in vivo[J]. Pharm Res, 2004, 21: 947-952. DOI:10.1023/B:PHAM.0000029282.44140.2e |
| [64] |
Gupta J, Gill HS, Andrews SN, et al. Kinetics of skin resealing after insertion of microneedles in human subjects[J]. J Control Release, 2011, 154: 148-155. DOI:10.1016/j.jconrel.2011.05.021 |
| [65] |
Li RR, Wang Y, Liu Y, et al. Effects of metal or dissolving microneedles and its parameters of operation on the formation and closure of skin microchannels[J]. Acta Pharm Sin (药学学报), 2021, 56: 1163-1169. |
| [66] |
Kim S, Yang H, Eum J, et al. Implantable powder-carrying microneedles for transdermal delivery of high-dose insulin with enhanced activity[J]. Biomaterials, 2020, 232: 119733. DOI:10.1016/j.biomaterials.2019.119733 |
| [67] |
Kim M, Jung B, Park JH. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin[J]. Biomaterials, 2012, 33: 668-678. DOI:10.1016/j.biomaterials.2011.09.074 |
| [68] |
Banks SL, Paudel KS, Brogden NK, et al. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin[J]. Pharm Res, 2011, 28: 1211-1219. DOI:10.1007/s11095-011-0372-2 |
| [69] |
Ghosh P, Pinninti RR, Hammell DC, et al. Development of a codrug approach for sustained drug delivery across microneedle-treated skin[J]. J Pharm Sci, 2013, 102: 1458-1467. DOI:10.1002/jps.23469 |
| [70] |
Ghosh P, Brogden NK, Stinchcomb AL. Fluvastatin as a micropore lifetime enhancer for sustained delivery across microneedle-treated skin[J]. J Pharm Sci, 2014, 103: 652-660. DOI:10.1002/jps.23844 |
| [71] |
Elias PM, Tsai J, Menon GK, et al. The potential of metabolic interventions to enhance transdermal drug delivery[J]. J Investig Dermatol Symp Proc, 2002, 7: 79-85. DOI:10.1046/j.1523-1747.2002.19632.x |
| [72] |
Schneider LA, Korber A, Grabbe S, et al. Influence of pH on wound-healing: a new perspective for wound-therapy?[J]. Arch Dermatol Res, 2007, 298: 413-420. DOI:10.1007/s00403-006-0713-x |
| [73] |
Ghosh P, Brogden NK, Stinchcomb AL. Effect of formulation pH on transport of naltrexone species and pore closure in microneedle-enhanced transdermal drug delivery[J]. Mol Pharm, 2013, 10: 2331-2339. DOI:10.1021/mp3007083 |
 2021, Vol. 56
2021, Vol. 56


