随着人们生活方式的改变和人口老龄化加速, 痛风/高尿酸血症的发病率持续上升, 已经成为全球共同的健康问题[1]。高尿酸血症是指血清尿酸盐的浓度超过了尿酸盐溶解度的极限(6.8 mg·dL-1), 是痛风的主要致病因素。高尿酸血症主要由外源性嘌呤过量摄入、内源性过度产生和排泄不足引起。黄嘌呤氧化酶(xanthine oxidase, XOD) 催化次黄嘌呤和黄嘌呤的氧化羟基化反应, 生成尿酸(uric acid, UA), 并在黄素中心还原氧, 生成活性氧。XOD活性过高会导致产生尿酸增多, 引发高尿酸血症。同时, 过氧化氢和超氧阴离子增多, 增加了氧化应激的可能性。因此, XOD是治疗组织氧化损伤、痛风和其他与高尿酸血症相关的疾病的有效靶点。目前, 临床使用的XOD抑制剂种类较少、存在或多或少的药物不良反应, 寻找新的高效低毒的XOD抑制剂具有十分重要的意义。相比化学合成类药物, 天然产物更为安全, 且药理作用不局限于单纯降血尿酸作用, 为高尿酸血症治疗药物研发提供了更多选择。
1 XODXOD由一个同源二聚体组成, 每个亚基有3个结构域: 一个钼辅因子(Mo)、两个铁硫中心(2Fe-2S) 和一个黄素腺嘌呤二核苷酸(flavin adenine dinucleotide, FAD)。其中, 氧化反应发生在Mo中心附近[2] (图 1A)。
|
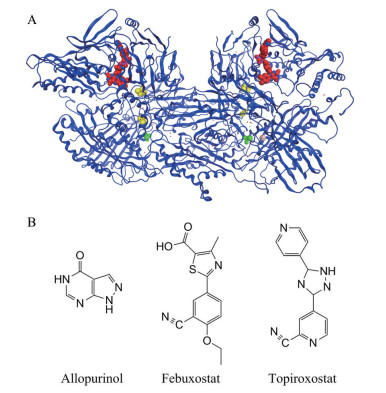
Figure 1 The structure of xanthine oxidase (XOD) and its inhibitors. A: Schematic diagram of XOD structure and its cofactor distribution (Bos taurus XOD (PDB ID: 3B9J)) structure superposition diagram[2]. Green: Molybdenum cofactor; Yellow: Iron-sulfur center; Red: Flavin adenine dinucleotide; B: XOD inhibitors in clinic |
在生物体内XOD发挥着重要的生理和病理作用。一方面, 其对杂环化合物的氧化降解实现了药物和有害中间代谢产物解毒的重要作用[3]。另一方面, 催化次黄嘌呤和黄嘌呤的氧化羟基化反应, 生成尿酸, 并在黄素中心还原氧, 生成超氧阴离子自由基或过氧化氢等活性氧, 它们的升高会造成氧化应激损伤和缺血再灌注损伤, 引起代谢综合征和心血管疾病等[4, 5]。
2 临床黄嘌呤氧化酶抑制剂临床治疗高尿酸血症和痛风的首选药物是XOD抑制剂[6], 如别嘌呤醇(allopurinol)、非布司他(febuxostat) 和托吡司他(topiroxostat) 等(图 1B)。XOD抑制剂与Mo中心的催化活性位点结合, 防止底物次黄嘌呤和黄嘌呤发生羟化反应, XOD抑制剂不仅减少尿酸生成, 还能减少氧化应激, 从而缓解高尿酸血症及其相关疾病的病程[7]。
别嘌呤醇是XOD竞争性抑制剂, 在体内迅速氧化成其活性代谢产物氧嘌呤醇, 该活性代谢物以时间依赖性方式通过共价键与XOD活性中心钼结合而抑制XOD[4, 8, 9], 据报道其半数抑制浓度(IC50) 值为0.2~50 μmol·L-1 [9]。除降低尿酸盐以外, 别嘌呤醇可通过抗氧化、抗神经炎症以及激活腺苷受体的作用来治疗神经性疼痛[10], 还可以抑制过量葡萄糖诱导的滋养层细胞白细胞介素-1β (interleukin-1β, IL-1β) 和活性氧的产生, 对于预防患有糖尿病的孕妇的胎盘功能障碍和不良妊娠结局(如先兆子痫) 有积极作用[11]。别嘌呤醇和其他嘌呤类似物的严重不良反应是别嘌呤醇超敏综合征(allopurinol hypersensitivity syndrome, AHS)[12], 常出现发热、皮疹和嗜酸性粒细胞过敏等。
非布司他是XOD非竞争性抑制剂, 其与XOD活性中心钼存在多重弱的相互作用, IC50值为7 nmol·L-1, 比别嘌呤醇的降尿酸活性更有效、更持久[13, 14]。非布司他可通过抑制炎症及其导致的纤维化变化, 对免疫球蛋白A肾病具有保护作用[15], 还可通过降低内质网应激、减少氧化应激, 从而改善高尿酸血症患者的左心室舒张等病症[16]。但是, 也有研究证明其全因死亡率和心血管死亡率均高于别嘌呤醇治疗者[17]。
托吡司他是XOD竞争性抑制剂, 其与XOD活性中心钼形成共价键, 从而抑制XOD活性[18], IC50值为5.3 nmol·L-1。托吡司他在降低高尿酸血症患者血清尿酸(serum uric acid, sUA) 的同时, 还可降低血清总胆固醇和低密度脂蛋白胆固醇浓度, 改善血管内皮功能, 预防动脉粥样硬化等疾病的发生[19, 20]。此外, 托吡司他还可减少合并高尿酸血症的3期慢性肾病患者的尿白蛋白排泄, 表现出一定的肾脏保护作用[21]。然而, 在使用托吡司他的患者中肝功能异常的发生率很高[21], 限制了其使用。
3 具有黄嘌呤氧化酶抑制作用的天然产物药用植物中具有大量能够有效抑制XOD活性的成分, 这些成分主要包括黄酮类化合物、多酚类化合物、生物碱类化合物、三萜类化合物、苷类化合物和有机酸类化合物等[22]。对近年来发现的具有XOD抑制作用的药用植物提取物或单体化合物简述如下。
3.1 药用植物提取物目前研究表明, 药用植物化学成分非常复杂, 不仅具有降血糖、抗炎、抗氧化和抗肿瘤等药理作用, 还具有一定的XOD抑制作用, 但发挥XOD抑制作用的活性成分/分子结构还有待进一步研究。若能探明具体起效物质, 得到相应的化学结构并将其研发成药, 对合并炎症和代谢综合征等多种疾病的患者非常有益, 不仅可以减少用药种类、改善依从性, 还能减少药物之间的相互作用等不良反应。
甜叶菊(Stevia rebaudiana) 是菊科甜叶菊属植物, 别称“蜜叶”。甜菊糖苷等提取物有抗高血糖、高血压、炎症和肿瘤, 预防急性慢性肝损伤、利尿和免疫调节等作用[23-26]。Mehmood等[27]制备了富含类黄酮和绿原酸的甜叶菊残渣提取物(stevia residue extract, STVRE), 体外实验结果表明, STVRE抑制XOD的IC50值为8.78 ± 0.89 μg·mL-1。在体内实验中, STVRE通过抑制XOD活性、改善氧化应激和炎症反应, 对果糖联合氧嗪酸钾引起的高尿酸血症模型小鼠起到有益的作用。
高原荨麻(Urtica hyperborea) 是荨麻科荨麻属植物, 生物活性成分主要包括黄酮类、生物碱、木脂素、香豆素、萜类、甾体、有机酸和挥发油等, 临床用于治疗消化不良、高血压和免疫调节等[28, 29]。Han等[30]发现, 高原荨麻醇提取物在体外和体内实验中均表现出降低尿酸的作用, 其主要作用机制为抑制XOD和腺苷脱氨酶(adenosine deaminase, ADA) 的活性, 调节尿酸盐转运蛋白1 (urate transporter 1, URAT1) 和有机阴离子转运蛋白1 (organic anion transporter 1, OAT1) 的表达。但是, 其有效成分还有待进一步研究。
铁皮石斛(Dendrobium catenatum) 是兰科石斛属植物, 其主要活性成分为多糖、生物碱、黄酮类、酚类及木质素类等, 具有抗氧化、增强免疫力、治疗糖尿病和血管疾病的药理作用[31-34]。Lou等[35]研究发现, 铁皮石斛叶的大孔树脂提取物可通过抑制XOD/ADA系统有效治疗高尿酸血症, 但其有效成分仍然未知。
桑叶是桑科桑属植物桑(Morus alba) 的干燥叶, 其含有的主要活性成分是黄酮类、生物碱、植物甾醇、γ-氨基丁酸和桑叶多糖等, 具有降血压、降血糖血脂、抗炎和抗肿瘤等作用[36]。Wan等[37]发现, 桑叶的乙醇提取物能以剂量依赖性的方式竞争性抑制XOD活性, IC50值为1 104.76 ± 7.1 μg·mL-1。该作用仍需通过体内实验进行验证, 具体起效成分有待进一步研究。
土茯苓(Smilax glabra) 为百合科菝葜属植物光叶菝葜的干燥根茎, 其主要成分为皂苷、鞣质、树脂、生物碱、微量挥发油、甾醇, 以及落新妇苷及黄酮类, 具有利尿和抗炎解毒的作用[38, 39]。Ding等[40]研究显示, 土茯苓水提物可通过多靶点、多途径协同有效降低高尿酸血症模型小鼠的血尿酸水平, 起到肾脏保护作用。其机制可能是通过有效抑制肝脏XOD活性以减少尿酸生成, 下调肾脏URAT1及葡萄糖转运体9 (glucose transporter 9, GLUT9) mRNA的表达以促进肾脏尿酸排泄, 降低IL-1β和肿瘤坏死因子α (tumor necrosis factor-α, TNF-α) 等炎症因子的表达, 以缓解炎症造成的肾脏损害。
杜仲叶为杜仲科杜仲属植物杜仲(Eucommia ulmoides) 的叶, 含有与树皮中相似的活性成分, 包括黄酮类、环烯醚萜类、苯丙素类、木脂素类、多糖类以及杜仲橡胶等。主要药理作用为降血压、降血脂、降血糖和抗炎抗病毒等[41]。Fu等[42]研究发现, 杜仲叶的乙酸乙酯提取物能抑制XOD活性, IC50值为2.47 mg·mL-1, 具体活性成分未明。
藜蒿(Artemisia selengensis) 为菊科蒿属植物, 其主要生物活性成分包括藜蒿叶多糖、黄酮类和酚酸类等, 具有抗炎、抗氧化、抗癌和降血压等作用[43]。研究[44]发现藜蒿提取物中富含的黄酮类、绿原酸类、双咖啡酰奎宁酸类物质对XOD有抑制作用, 但作用机制还有待进一步研究。
栀子为茜草科栀子属栀子(Gardenia jasminoides) 的果实, 其生物活性成分包括环烯醚萜类、二萜类、三萜类、黄酮类和三萜皂苷类等, 具有抗炎、抗肿瘤、抗血栓和保护肝脏等作用[45]。Zhu等[46]研究发现, 栀子中的栀子苷和西红花苷-I终浓度为100 μmol·L-1时对XOD的抑制率分别为44.5%和44.6%, 但对XOD的mRNA表达没有显著影响, 其抑制XOD的作用机制有待进一步研究。
海带(Laminaria japonica) 又名昆布, 是海带科多年生食用藻类。其主要活性成分是海带多糖, 具有抗病毒、降血糖、抗炎、抗肿瘤、抗氧化、抗菌和改善肠道微生态等作用[47, 48]。Yan等[49]研究发现, 海带多糖在体外对XOD具有明显的抑制作用, IC50值为0.88 mg·mL-1。体内实验显示, 其可明显降低氧嗪酸钾所致的高尿酸血症模型小鼠的sUA水平, 尤其对模型小鼠肝脏中XOD抑制作用显著。因此, 有望开发成为新的抗高尿酸血症药物。
辣木(Moringa oleifera) 为辣木科辣木属热带落叶乔木。其主要活性成分为黄酮类、生物碱类、糖苷类、有机酸和有机酸酯等, 具有降血糖、降血脂、抗氧化、消炎和抑制肿瘤等作用[50]。Zhong等[51]研究发现, 辣木叶的乙醇提取物对XOD具有竞争性抑制作用, IC50值为10.55 mg·mL-1。Liang等[52]研究表明, 口服辣木叶70%乙醇提取物(1、2和4 g·kg-1) 对氧嗪酸钾造成的高尿酸血症小鼠肝脏XOD有一定抑制作用, 但其体外抑制XOD活性与体内降尿酸作用并不平行, 推测可能存在其他机制。
金线莲(Anoectochilus roxburghii) 是兰科开唇兰属的一种多年生草本植物。其主要活性成分有金线莲苷、多糖和黄酮等, 具有降血糖、保肝降脂、清除自由基、抗炎和抗肿瘤等药理作用[53]。Xu等[54]实验表明, 1 000 μg·mL-1金线莲水提物对XOD抑制率可达96.7%, IC50值为97.09 μg·mL-1, 能显著降低由氧嗪酸钾造成的高尿酸血症小鼠的血尿酸水平, 并有效改善其肾脏功能、加速尿酸的排泄。
3.2 从植物中提取的单体化合物药用植物中大量蕴含的黄酮类、多酚类、生物碱类、三萜类、苷类、多糖类和有机酸类化合物等均能较好地抑制XOD活性, 且大多具有可参考的化学结构骨架, 体外分子对接实验结果初步提示了其作用特点, 这些天然产物来源的活性物质可作为研发新型XOD抑制剂的先导化合物。
3.2.1 芦荟中芦荟大黄素芦荟(Aloe vera var. chinensis) 是芦荟科芦荟属植物, 用于预防或治疗皮肤病、抗病毒、代谢性疾病、心血管疾病和癌症等, 其主要生物活性物质包括芦荟素、多糖、芦荟大黄素和芦荟精油等[55]。
芦荟大黄素是芦荟植物中的主要蒽醌类物质, 具有一定的XOD抑制活性[56]。Shi等[57]在此基础上设计、合成和评估了一系列芦荟大黄素衍生物, 用作XOD抑制剂。其中, 有4种化合物1~4表现出一定的XOD抑制活性, IC50值分别为2.79、3.87、8.43和18.67 μmol·L-1。对接结构分析表明, 化合物1的游离4, 5-羟基和侧链的甲酰基对XOD活性的抑制起重要作用(图 2)。

|
Figure 2 Aloe emodin derivatives with XOD inhibitory activity |
细叶买麻藤(Gnetum parvifolium) 是买麻藤科买麻藤属植物, 富含生物活性化合物, 如黄酮类和芪类化合物, 主要具有降血压、抗氧化、抗癌和抗菌作用[58-60]。
Tang等[61]从细叶买麻藤中提取出4种对XOD具有抑制活性的物质, 分别为白皮杉醇(piceatannol)、土大黄苷(rhaponiticin)、白藜芦醇(resveratrol) 和异丹叶大黄素(isorhapontigenin), 均显示出良好的自由基清除能力和一定的XOD抑制作用, 其抑制XOD的IC50值分别为6.44、5.997、3.88和46.75 μmol·L-1。动力学参数表明, 白皮杉醇对XOD的抑制方式为竞争型, 而土大黄苷、白藜芦醇和异丹叶大黄素的抑制方式为非竞争型。该研究为开发新结构类型的XOD抑制剂提供了实验依据(图 3)。Feng等[62]研究还表明, 白藜芦醇能够抑制单钠尿酸盐诱导RAW264.7巨噬细胞产生的炎症反应, 并通过Nrf2/HO-1信号通路提高巨噬细胞抗氧化能力。

|
Figure 3 Compounds with XOD inhibitory activity in Gnetum parvifolium |
尖蕾狗牙花(Tabernaemontana bufalina) 是夹竹桃科狗牙花属植物, 其含有的主要活性成分是生物碱, 具有抗肿瘤、镇痛以及对阿片类药物的身体依赖性和精神依赖性的防治作用等[63, 64]。
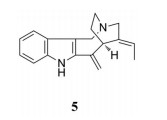
Shi等[65]对尖蕾狗牙花的生物碱成分进行系统检查, 鉴定出78种生物碱, 其中, 化合物5表现出强的XOD抑制作用, IC50值为0.65 μmol·L-1。对接结构分析表明, 该化合物与酶的活性位点残基之间形成了许多强疏水键, 封闭了该酶的钼中心。这一结果为进一步开发新的XOD抑制剂提供了可参考的化学结构信息(图 4)。
|
Figure 4 Structure of compound with XOD inhibitory activity in Tabernaemontana bufalina |
丹参(Salvia miltiorrhiza) 为唇形科鼠尾草属植物丹参的干燥根和根茎, 其主要生物活性成分有丹参酮ⅡA和隐丹参酮、丹酚酸A和丹酚酸B、紫草酸等, 具有抗心血管疾病、抗肿瘤和抗代谢紊乱等作用[66]。
Liu等[67]研究表明, 丹参中的酚酸类物质紫草酸为XOD竞争性抑制剂, 其IC50值为9.66 μmol·L-1。其通过清除超氧化物自由基从而达到抗炎和降尿酸的作用。Fu等[68]研究发现, 丹酚酸A竞争性抑制XOD, 而丹酚酸C为XOD混合型抑制剂, 其IC50值分别为73.17和9.07 μmol·L-1 (图 5)。

|
Figure 5 Compounds with XOD inhibitory activity in Salvia miltiorrhiza |
毛冬青(Ilex pubescens) 为冬青科冬青属植物毛冬青的根, 其主要生物活性成分有三萜类、酚、酸类、苯丙素类、环烯醚萜类和黄酮类等, 具有抗凝、降压、保护心脏和脑组织、抗炎及免疫等作用[69]。
Zhou等[70]研究发现, 毛冬青的70%乙醇水溶液提取物具有显著的XOD抑制活性, 进一步分离出了4种皂苷, 其中化合物6的XOD抑制活性稍强, IC50值为18.39 μmol·L-1 (图 6)。

|
Figure 6 Structure of compound with XOD inhibitory activity in Ilex pubescens |
香椿叶为楝科香椿属植物香椿(Toona sinensis) 的叶, 其主要生物活性物质包括黄酮类的没食子酸、芦丁、槲皮素等和多糖类, 具有抗氧化、抗肿瘤和降血脂等作用[71]。
Yuk等[72]研究发现, 香椿叶的乙醇提取物中五烷基戊糖对XOD有较强抑制作用, 其IC50值为2.79 μmol·L-1, 为非竞争性抑制剂(图 7)。

|
Figure 7 Compounds with XOD inhibitory activity in Toona sinensis leaves |
葛根为豆科葛属植物葛(Pueraria lobata) 的干燥根, 其主要有效成分葛根素具有血管舒张、心脏保护、抗肿瘤、抗炎和抗氧化等作用[73]。
Shen[74]的研究显示, 葛根素属于XOD的竞争性抑制剂。Shi等[75]研究发现, 适宜剂量的葛根素可以通过抑制XOD活性抑制尿酸生成, 并增加尿酸在尿液中的溶解度, 保持一定的排泄量, 从而抑制机体的sUA水平升高(图 8)。

|
Figure 8 Puerarin with XOD inhibitory activity in Pueraria lobata |
姜黄素为多酚类化合物, 存在于植物郁金、姜黄、莪术和菖蒲等的根茎中, 具有抗炎、抗氧化、调脂、抗病毒、抗感染、抗肿瘤、抗凝、抗肝纤维化和抗动脉粥样硬化等作用[76]。
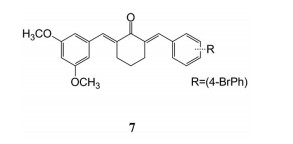
Ao等[77]设计、合成和评估了一系列姜黄素衍生物作为XOD抑制剂。化合物7在体外可强效抑制XOD活性, IC50值为5.7 ± 0.8 nmol·L-1, 同时降低了氧嗪酸钾诱导的高尿酸血症小鼠的sUA水平。其可能的作用机制是通过调节XOD活性和URAT1表达来改善尿酸的过量产生和排泄不足(图 9)。
|
Figure 9 Curcumin derivative monomers with XOD inhibitory effect |
高良姜(Alpinia officinarum) 为姜科山姜属高良姜的干燥根, 目前从高良姜中分离出的药效成分主要为挥发油、黄酮类及二芳基庚烷, 其中黄酮类的高良姜素具有抗氧化、抗肿瘤和抗炎等作用[78]。
研究发现[79, 80], 高良姜素可通过抑制XOD活性减少尿酸生成, 分别降低次黄嘌呤联合氧嗪酸钾、酵母膏联合氧嗪酸钾造成的高尿酸血症小鼠的血尿酸浓度(图 10)。

|
Figure 10 Galangin with XOD inhibitory activity in galangal |
研究报道, 植物中富含的山柰酚[81]、槲皮素[82]、木犀草素[83, 84]、芹菜素、木犀草苷、陈皮苷、金丝桃苷[85]、肉桂醛[86, 87]、毛蕊花糖苷[88]和新木脂素[89]等均具有XOD抑制作用。
山柰酚为黄酮醇类化合物, 存在于多种植物中, 如山柰的根茎、茶叶、椰菜和巫榛子等。其具有抗肿瘤、抗炎、抗氧化、抗菌和抗病毒等作用。Wang等[81]研究发现, 山柰酚可竞争性抑制XOD, IC50值为2.18 ± 0.02 μmol·L-1, 进一步的研究仍在进行中(图 11)。

|
Figure 11 Kaempferol with XOD inhibitory activity |
木犀草素为黄酮类化合物, 存在于多种植物中, 如全叶青兰、辣椒、野菊花、金银花和紫苏等。其具有抗炎、抗氧化和抗肿瘤等作用[83]。Yan[84]的实验结果表明, 木犀草素还是一种XOD竞争性抑制剂, 其IC50值为4.79 ± 0.02 μmol·L-1 (图 12)。

|
Figure 12 Luteolin with XOD inhibitory activity |
槲皮素为黄酮醇类化合物, 广泛存在于植物的花、叶和果实中, 已知多种中草药中含有槲皮素, 如槐米、菊花、车前子、桑寄生、仙鹤草、山楂、银杏叶、刺五加、鱼腥草和番石榴叶等。槲皮素具有抗氧化及清除自由基的作用, 还具有抗炎、抗病毒、抗肿瘤、降糖降脂及免疫调节等作用[90-93]。槲皮素及其体内代谢产物还对XOD具有抑制作用[82], 可有效抑制高尿酸血症模型小鼠肝组织的XOD活力, 导致酶底物的结合亲和力降低, 显著降低高尿酸血症小鼠sUA水平(图 13)。

|
Figure 13 Quercetin with XOD inhibitory activity |
高尿酸血症是痛风发病的基础, 与代谢综合征、肾病和心血管疾病等慢性疾病密切相关且常常伴行。高尿酸血症治疗可能是临床上治疗与代谢综合征相关疾病的新选择。而XOD抑制剂又是高尿酸血症治疗的首选药物。近年来研究发现, XOD抑制剂在降低血清尿酸的同时, 又能减少高尿酸诱导的炎症和组织氧化损伤, 具有很好的应用前景, 值得深入研究。特别是在药学、体内药效、安全性等成药性研究和临床研究方面, 还有待进一步阐明。
天然产物中蕴含着大量具有XOD抑制活性的组分和多种结构的单体化合物。许多活性物质还具有抗炎、抗菌、抗氧化、保护肝脏和肾脏等其他药理作用, 在降低血尿酸的同时, 还可能进一步阻止/延缓代谢综合征等疾病的发生发展, 功效更加全面。发挥天然产物的多靶点、网络药理学作用, 改善药物代谢性质、提高药效和安全性, 是研发新型治疗高尿酸血症及其合并代谢综合征药物的良好途径, 相信在未来能得到充分应用。
作者贡献: 姜楠是文章框架的构思者并负责内容的撰写和文献整理; 叶菲指导论文写作; 张晓琳和田金英对论文进行了修改和检查。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Yu Y, Zhou Q, Yang N, et al. An analysis of current situation of global clinical practice guidelines on gout[J]. Drug Eval (药品评价), 2018, 15: 9-15. |
| [2] |
Yamaguchi Y, Matsumura T, Ichida K, et al. Human xanthine oxidase changes its substrate specificity to aldehyde oxidase type upon mutation of amino acid residues in the active site: roles of active site residues in binding and activation of purine substrate[J]. J Biochem, 2007, 141: 513-524. DOI:10.1093/jb/mvm053 |
| [3] |
Harrison R. Structure and function of xanthine oxidoreductase: where are we now?[J]. Free Radical Biol Med, 2002, 33: 774-797. DOI:10.1016/S0891-5849(02)00956-5 |
| [4] |
Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors[J]. Curr Med Chem, 2002, 9: 195-217. DOI:10.2174/0929867023371229 |
| [5] |
Stockert AL, Shinde SS, Anderson RF, et al. The reaction mechanism of xanthine oxidase: evidence for two-electron chemistry rather than sequential one-electron steps[J]. J Am Chem Soc, 2002, 124: 14554-14555. DOI:10.1021/ja027388d |
| [6] |
Terkeltaub RA. Clinical practice. Gout[J]. N Engl J Med, 2003, 349: 1647-1655. DOI:10.1056/NEJMcp030733 |
| [7] |
Nishikawa T, Nagata N, Shimakami T, et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice[J]. Sci Rep, 2020, 10: 815. DOI:10.1038/s41598-020-57784-3 |
| [8] |
Krakoff IH. Use of allopurinol in preventing hyperuricemia in leukemia and lymphoma[J]. Cancer, 1966, 19: 1489-1496. DOI:10.1002/1097-0142(196611)19:11<1489::AID-CNCR2820191105>3.0.CO;2-F |
| [9] |
Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol[J]. Pharmacol Rev, 2006, 58: 87-114. DOI:10.1124/pr.58.1.6 |
| [10] |
Abad ASS, Falanji F, Ghanbarabadi M, et al. Assessment of anti-nociceptive effect of allopurinol in a neuropathic pain model[J]. Brain Res, 2019, 1720: 146238. DOI:10.1016/j.brainres.2019.04.033 |
| [11] |
Negi M, Mulla MJ, Han CS, et al. Allopurinol inhibits excess glucose-induced trophoblast IL-1β and ROS production[J]. Reproduction, 2020, 159: 73-80. DOI:10.1530/REP-19-0422 |
| [12] |
Lu JM, Yao Q, Chen C. 3, 4-Dihydroxy-5-nitrobenzaldehyde (DHNB) is a potent inhibitor of xanthine oxidase: a potential therapeutic agent for treatment of hyperuricemia and gout[J]. Biochem Pharmacol, 2013, 86: 1328-1337. DOI:10.1016/j.bcp.2013.08.011 |
| [13] |
Zhou H, Li X, Li Y, et al. Synthesis and bioevaluation of 1-phenylimidazole-4-carboxylic acid derivatives as novel xanthine oxidoreductase inhibitors[J]. Eur J Med Chem, 2020, 186: 111883. DOI:10.1016/j.ejmech.2019.111883 |
| [14] |
Takano Y, Hase-Aoki K, Horiuchi H, et al. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase[J]. Life Sci, 2005, 76: 1835-1847. DOI:10.1016/j.lfs.2004.10.031 |
| [15] |
Inoue MK, Yamamotoya T, Nakatsu Y, et al. The xanthine oxidase inhibitor febuxostat suppresses the progression of IgA nephropathy, possibly via its anti-inflammatory and anti-fibrotic effects in the gddY mouse model[J]. Int J Mol Sci, 2018, 19: 3967. DOI:10.3390/ijms19123967 |
| [16] |
Kim H, Baek CH, Chang JW, et al. Febuxostat, a novel inhibitor of xanthine oxidase, reduces ER stress through upregulation of SIRT1-AMPK-HO-1/thioredoxin expression[J]. Clin Exp Nephrol, 2020, 24: 205-215. DOI:10.1007/s10157-019-01804-8 |
| [17] |
White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout[J]. N Engl J Med, 2018, 378: 1200-1210. DOI:10.1056/NEJMoa1710895 |
| [18] |
Matsumoto K, Okamoto K, Ashizawa N, et al. FYX-051:a novel and potent hybrid-type inhibitor of xanthine oxidoreductase[J]. J Pharmacol Exp Ther, 2011, 336: 95-103. DOI:10.1124/jpet.110.174540 |
| [19] |
Tohyo S. Topiroxostat influences circulating lipid concentrations in hyperuricemic patients[J]. Int J Clin Pharmacol Ther, 2019, 57: 567-570. DOI:10.5414/CP203478 |
| [20] |
Higa S, Shima D, Tomitani N, et al. The effects of topiroxostat on vascular function in patients with hyperuricemia[J]. J Clin Hypertens, 2019, 21: 1713-1720. DOI:10.1111/jch.13707 |
| [21] |
Hisayuki K, Hidekatsu Y, Mariko H. Renoprotective effect of xanthine oxidase inhibitor, topiroxostat[J]. J Clin Med Res, 2019, 11: 614-616. DOI:10.14740/jocmr3913 |
| [22] |
Mehmood A, Ishaq M, Zhao L, et al. Natural compounds with xanthine oxidase inhibitory activity: a review[J]. Chem Biol Drug Des, 2019, 93: 387-418. DOI:10.1111/cbdd.13437 |
| [23] |
Lemus-Mondaca R, Vega-Galvez A, Zura-Bravo L, et al. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects[J]. Food Chem, 2012, 132: 1121-1132. DOI:10.1016/j.foodchem.2011.11.140 |
| [24] |
Cosola C, Sabatino A, di Bari I, et al. Nutrients, nutraceuticals, and xenobiotics affecting renal health[J]. Nutrients, 2018, 10: 808. DOI:10.3390/nu10070808 |
| [25] |
Chatsudthipong V, Muanprasat C. Stevioside and related compounds: therapeutic benefits beyond sweetness[J]. Pharmacol Ther, 2009, 121: 41-54. DOI:10.1016/j.pharmthera.2008.09.007 |
| [26] |
Ramos-Tovar E, Hernandez-Aquino E, Casas-Grajales S, et al. Stevia prevents acute and chronic liver injury induced by carbon tetrachloride by blocking oxidative stress through Nrf2 upregulation[J]. Oxid Med Cell Longev, 2018, 2018: 3823426. |
| [27] |
Mehmood A, Zhao L, Wang C, et al. Stevia residue extract alone and combination with allopurinol attenuate hyperuricemia in fructose-PO-induced hyperuricemic mice[J]. J Food Biochem, 2020, 44: e13087. |
| [28] |
Akbay P, Basaran AA, Undeger U, et al. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L[J]. Phytother Res, 2003, 17: 34-37. DOI:10.1002/ptr.1068 |
| [29] |
Su RN. Research on the Material Basis and Mechanism for Hypouricemia and Anti-BPH of Tibetan Medicine of Urtica hyperborea Jacq. ex Wedd. (藏药高原荨麻降尿酸、抗良性前列腺增生物质基础及作用机制研究)[D]. Nanchang: Jiangxi University of Traditional Chinese Medicine, 2019.
|
| [30] |
Han S, Wei R, Han D, et al. Hypouricemic effects of extracts from Urtica hyperborea Jacq. ex Wedd. in hyperuricemia mice through XOD, URAT1, and OAT1[J]. Biomed Res Int, 2020, 2020: 2968135. |
| [31] |
Huang K, Li Y, Tao S, et al. Purification, characterization and biological activity of polysaccharides from Dendrobium officinale[J]. Molecules, 2016, 21: 701. DOI:10.3390/molecules21060701 |
| [32] |
Luo QL, Tang ZH, Zhang XF, et al. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale[J]. Int J Biol Macromol, 2016, 89: 219-227. DOI:10.1016/j.ijbiomac.2016.04.067 |
| [33] |
Ruijun W, Shi W, Yijun X, et al. Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia[J]. Int J Biol Macromol, 2015, 72: 771-775. DOI:10.1016/j.ijbiomac.2014.09.026 |
| [34] |
Sun L, Wang L, Zhou Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum[J]. Carbohydr Polym, 2012, 87: 1206-1210. DOI:10.1016/j.carbpol.2011.08.097 |
| [35] |
Lou XJ, Wang YZ, Lei SS, et al. Beneficial effects of macroporous resin extract of Dendrobium candidum leaves in rats with hyperuricemia induced by a high-purine diet[J]. Evid Based Complement Alternat Med, 2020, 2020: 3086106. |
| [36] |
Lai LL, Peng XF, Leng EN, et al. Advances in study on pharmacological effects of Mulberry leaves[J]. Anhui Med Pharm J (安徽医药), 2016, 20: 2210-2214. |
| [37] |
Wan L, Chen G, Jian S, et al. Antioxidant and xanthine oxidase inhibitory properties and LC-MS/MS identification of compoundsof ethanolic extract from mulberry leaves[J]. Acta Sci Pol Technol Aliment, 2018, 17: 313-319. |
| [38] |
Liu JC, Wang T. Research progress of rhizome Dioscorea and rhizoma Smilacis Glabrae on treatment of hyperuricemia[J]. J Liaoning Univ Tradit Chin Med (辽宁中医药大学学报), 2018, 20: 79-81. |
| [39] |
Zhang XX, Sun WF, Hou Y, et al. Clinical observation on Fufang Tufuling Keli treating 40 cases of hyperuricemia: a randomize-controlled study[J]. J Tradit Chin Med (中医杂志), 2016, 57: 41-45. |
| [40] |
Ding R, Hong Q, Geng XD, et al. Mechanism of rhizoma Smilacis Glabrae in the treatment of hyperuricemia mouse model[J]. Chin J Integr Tradit West Nephrol (中国中西医结合肾病杂志), 2019, 20: 97-100. |
| [41] |
Liu C, Guo FF, Ping XJ, et al. Research advances in chemical constituents and pharmacological activities of different parts of Eucommia ulmoides[J]. China J Chin Mater Med (中国中药杂志), 2020, 45: 497-512. |
| [42] |
Fu G, Tong H, Zeng H, et al. Antioxidant and xanthine oxidase inhibitory activity of Eucommia ulmoides oliver leaf extracts[J]. Pak J Pharm Sci, 2018, 31: 1333-1339. |
| [43] |
Xie X, Tu ZC, Wang H, et al. Antioxidant and enzyme inhibitory activities of extracts from various parts of wild and cultivated Artemisia selengensis Turcz[J]. J Chin Inst Food Sci Tech (中国食品学报), 2020, 20: 58-65. |
| [44] |
Cao WW, Wu T, Fang YJ, et al. Identification of 1, 4-dicaffeoylquinic acid from Artemisia selengensis leaves and structure activity relationship of dicaffeoylquinic acid compounds inhibiting xanthine oxidase and inhibiting sodium urate induced IL-1β in vitro[J]. J Chin Inst Food Sci Tech (中国食品学报), 2020, 20: 1-8. |
| [45] |
Zou Y, Zhou M. Research progress on chemical constituents and pharmacological effects of Gardenia jasminoides Ellis[J]. Jiangxi Chem Ind (江西化工), 2019, 18: 47-48. |
| [46] |
Zhu JX, Li XW, Zeng JX, et al. Effects of active components from Gardenia jasminoides Ellis on XOD activity and mRNA expression in A549 cells[J]. Tradit Chin Med J (中医药通报), 2015, 14: 65-68. |
| [47] |
Malyarenko OS, Zdobnova EV, Silchenko AS, et al. Radiosensitizing effect of the fucoidan from brown alga Fucus evanescens and its derivative in human cancer cells[J]. Carbohydr Polym, 2019, 205: 465-471. DOI:10.1016/j.carbpol.2018.10.083 |
| [48] |
Du B, Feng JX, Jin WG. Research progress on structural elucidation and biological activities of Laminaria japonica polysaccharide[J]. Chin J Mar Drug (中国海洋药物), 2020, 39: 50-59. |
| [49] |
Yan CW, Li J, Jiang L, et al. Inhibitory effects on xathine oxidase activity and regulation on hyperuricemia in mice of Laminaria japonica polysaccharides[J]. Per Ocean Univ China (Nat Sci) (中国海洋大学学报(自然科学版)), 2015, 45: 50-55. |
| [50] |
Ji EH, Xu J, Wei JY. Active constituents and pharmacological effect of Moringa oleifera leaves research[J]. Chin J Exp Tradit Med Form (中国实验方剂学), 2021, 27: 212-223. |
| [51] |
Zhong YY, Zhou JM, Ye MF. Inhibition of Moringa leaf extract on xanthine oxidase activity[J]. Food Ind (食品工业), 2020, 41: 55-58. |
| [52] |
Liang WJ, He JS, Tian Y. Study on extract of Moringa oleifera leaves reducing the level of uric acid in hyperuricemia mice and its mechanism[J]. J Anhui Agric Sci (安徽农业科学), 2017, 45: 108-109, 112. |
| [53] |
Chen YQ, Lin YH, Zou YH. Research progress on identification of crude drugs, active ingredients and pharmacological effects of Anoectochilus[J]. Chin Tradit Pat Med (中成药), 2020, 42: 2141-2144. |
| [54] |
Xu GH, Zhao SF, Luo YQ. Studies of Anoectochilus roxburghii extracts inhibiting xanthine oxidase activity in vitro and reducing the level of uric acid in hyperuricemia mice[J]. Strait Pharm J (海峡药学), 2017, 29: 12-14. |
| [55] |
Akaberi M, Sobhani Z, Javadi B, et al. Therapeutic effects of Aloe spp. in traditional and modern medicine: a review[J]. Biomed Pharmacother, 2016, 84: 759-772. DOI:10.1016/j.biopha.2016.09.096 |
| [56] |
Park MY, Kwon HJ, Sung MK. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages[J]. Biosci Biotechnol Biochem, 2009, 73: 828-832. DOI:10.1271/bbb.80714 |
| [57] |
Shi DH, Huang W, Li C, et al. Design, synthesis and molecular modeling of aloe-emodin derivatives as potent xanthine oxidase inhibitors[J]. Eur J Med Chem, 2014, 75: 289-296. DOI:10.1016/j.ejmech.2014.01.058 |
| [58] |
Fang Y, Cao Z, Hou Q, et al. Cyclin D1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells[J]. Mol Cancer Ther, 2013, 12: 1492-1503. DOI:10.1158/1535-7163.MCT-12-0922 |
| [59] |
Fang Y, Yu Y, Hou Q, et al. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP[J]. J Biol Chem, 2012, 287: 35234-35243. DOI:10.1074/jbc.M112.389494 |
| [60] |
Kongkachuichai R, Charoensiri R, Yakoh K, et al. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand[J]. Food Chem, 2015, 173: 838-846. DOI:10.1016/j.foodchem.2014.10.123 |
| [61] |
Tang X, Tang P, Ma L, et al. Screening and evaluation of xanthine oxidase inhibitors from Gnetum parvifolium in China[J]. Molecules, 2019, 24: 2671. DOI:10.3390/molecules24142671 |
| [62] |
Feng J, Huang X, Li HY, et al. Mechanism of resveratrol inhibiting monosodium urate induced oxidative damage of RAW264.7 macrophages[J]. Acta Pharm Sin (药学学报), 2020, 55: 2368-2374. |
| [63] |
Chen YM, Zhou LH, Zhang XD, et al. Antinociceptive effects of total Ervatamia yunnansis alkaloid in mice[J]. J Pharm Pract (药学实践杂志), 2006, 24: 203-205. |
| [64] |
Xuan WD, Chen HS, Yuan ZX, et al. Antiaddictive indole alkaloids in Ervatamia yunnanensis and their bioactivity[J]. Acad J Second Milit Med Univ (第二军医大学学报), 2006, 27: 92-96. |
| [65] |
Shi BB, Chen J, Bao MF, et al. Alkaloids isolated from Tabernaemontana bufalina display xanthine oxidase inhibitory activity[J]. Phytochemistry, 2019, 166: 112060. DOI:10.1016/j.phytochem.2019.112060 |
| [66] |
Jiang X, Shi L. Research progress on active constituents and pharmacological effects of Salvia miltiorrhiza[J]. J Pharm Res (药学研究), 2017, 36: 166-169. |
| [67] |
Liu X, Chen R, Shang Y, et al. Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats[J]. Chem Biol Interact, 2008, 176: 137-142. DOI:10.1016/j.cbi.2008.07.003 |
| [68] |
Fu Y, Mo HY, Gao W, et al. Affinity selection-based two-dimensional chromatography coupled with high-performance liquid chromatography-mass spectrometry for discovering xanthine oxidase inhibitors from Radix Salviae Miltiorrhizae[J]. Anal Bioanal Chem, 2014, 406: 4987-4995. DOI:10.1007/s00216-014-7902-9 |
| [69] |
Mei L, Niu RJ, Jiang L, et al. Advances in the study on the chemical constituents and pharmacological activities of Ilex pubescens Hook. et Arn[J]. Biol Chem Engin (生物化工), 2018, 4: 129-131. |
| [70] |
Zhou ZL, Feng ZC, Fu CY, et al. A new triterpene saponin from the roots of Ilex pubescens[J]. Nat Prod Res, 2013, 27: 1343-1347. DOI:10.1080/14786419.2012.738208 |
| [71] |
Gu QY. Isolation and Analysis of Chemical Constituents in Chinese Toon (中药香椿叶化学成分的分离与分析)[D]. Zhenjiang: Jiangsu University, 2016.
|
| [72] |
Yuk HJ, Lee YS, Ryu HW, et al. Effects of Toona sinensis leaf extract and its chemical constituents on xanthine oxidase activity and serum uric acid levels in potassium oxonate-induced hyperuricemic rats[J]. Molecules, 2018, 23: 3254. DOI:10.3390/molecules23123254 |
| [73] |
Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects[J]. Phytother Res, 2014, 28: 961-975. DOI:10.1002/ptr.5083 |
| [74] |
Shen QR. The Screening and Study on the Inhibition Kinetics of Xanthine Oxidase Inhibitors from Chinese Herbal Medicine (中药黄嘌呤氧化酶抑制剂的筛选及抑制动力学研究)[D]. Nanchang: Nanchang University, 2015.
|
| [75] |
Shi S, Zhang RT, Shang XY, et al. Effect of Pueraria lobata extract on serum uric acid in hyperuricemia rats[J]. Food Sci Technol (食品科技), 2014, 39: 216-220. |
| [76] |
Sun LL, Li Q, Tian ZH, et al. Research progress on chemical constituents and pharmacological effects of Curcuma longa[J]. J Shandong Univ Tradit Chin Med (山东中医药大学学报), 2019, 43: 207-212. |
| [77] |
Ao GZ, Zhou MZ, Li YY, et al. Discovery of novel curcumin derivatives targeting xanthine oxidase and urate transporter 1 as anti-hyperuricemic agents[J]. Bioorg Med Chem, 2017, 25: 166-174. DOI:10.1016/j.bmc.2016.10.022 |
| [78] |
Xiong YG, Shen Y, Zhang H. Advances in pharmacological activity of Alpinia officinaruim Hance[J]. Cent South Pharm (中南药学), 2017, 15: 1418-1421. |
| [79] |
Pu ZQ, Wang QL, Xu XM. Separation, purification of galangin and its effect on reducing uric acid[J]. J Jiangsu Univ (Med) (江苏大学学报(医学版)), 2017, 27: 338-343. |
| [80] |
Lu H, Chen XW, Yao H. Pharmacological effect and cellular mechanism of galangin on uric acid nephropathy[J]. Cent South Pharm (中南药学), 2020, 18: 1098-1102. |
| [81] |
Wang Y, Zhang G, Pan J, et al. Novel insights into the inhibitory mechanism of kaempferol on xanthine oxidase[J]. J Agric Food Chem, 2015, 63: 526-534. DOI:10.1021/jf505584m |
| [82] |
An R. Studies on Extraction and Effects on Hyperuricemia of Quercetin from Bud of Sophora japonica L. (槐米中槲皮素的提取及其对高尿酸血症的影响)[D]. Tianjin: Tianjin University of Science and Technology, 2010.
|
| [83] |
Baek KS, Yi YS, Son YJ, et al. Comparison of anticancer activities of Korean red ginseng-derived fractions[J]. J Ginseng Res, 2017, 41: 386-391. DOI:10.1016/j.jgr.2016.11.001 |
| [84] |
Yan JK. Study on the Inhibition Mechanism of Luteolin on Xanthine Oxidase and α-Glucosidase (木犀草素对黄嘌呤氧化酶、α-葡萄糖苷酶抑制机理的探讨)[D]. Nanchang: Nanchang University, 2014.
|
| [85] |
Ma WT. Screening Inhibition of Xanthine Oxidase, Lipoxygenase, Cyclooxygenase and Antitumor Activity In Vitro (黄嘌呤氧化酶、脂氧化酶和环氧化酶抑制剂的筛选及其体外抗肿瘤活性研究)[D]. Wuhan: Hubei University of Traditional Chinese Medicine, 2016.
|
| [86] |
Ye YZ, Hu JL, Gong MJ, et al. The treatment and mechanism study on hyperuricemia of couplet medicines polydatin and cinnamaldehyde[J]. J Chin Med Mater (中药材), 2020, 43: 423-428. |
| [87] |
Huang CY, Yeh TF, Hsu FL, et al. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin[J]. Molecules, 2018, 23: 1107. DOI:10.3390/molecules23051107 |
| [88] |
Wan Y, Zou B, Zeng H, et al. Inhibitory effect of verbascoside on xanthine oxidase activity[J]. Int J Biol Macromol, 2016, 93: 609-614. DOI:10.1016/j.ijbiomac.2016.09.022 |
| [89] |
Tsai SF, Lee SS. Neolignans as xanthine oxidase inhibitors from Hyptis rhomboides[J]. Phytochemistry, 2014, 101: 121-127. DOI:10.1016/j.phytochem.2014.01.016 |
| [90] |
Granato M, Rizzello C, Gilardini Montani MS, et al. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways[J]. J Nutr Biochem, 2017, 41: 124-136. DOI:10.1016/j.jnutbio.2016.12.011 |
| [91] |
Rojas A, Del Campo JA, Clement S, et al. Effect of quercetin on hepatitis C virus life cycle: from viral to host targets[J]. Sci Rep, 2016, 6: 31777. DOI:10.1038/srep31777 |
| [92] |
Lee JY, Park W. Anti-inflammatory effect of Wogonin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid[J]. Molecules, 2015, 20: 6888-6900. DOI:10.3390/molecules20046888 |
| [93] |
Klimaszewska-Wisniewska A, Halas-Wisniewska M, Izdebska M, et al. Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton[J]. Acta Histochem, 2017, 119: 99-112. DOI:10.1016/j.acthis.2016.11.003 |
 2021, Vol. 56
2021, Vol. 56


