2. 浙江大学药学院, 药理毒理研究所, 浙江 杭州 310058
2. Institute of Pharmacology and Toxicology, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China
在我国, 肺癌的发病率与死亡率仍高居首位, 每年约有78万人罹患肺癌, 其5年生存率低于20%, 且85%肺癌患者为非小细胞肺癌(non-small cell lung cancer, NSCLC)[1]。近年来, 对晚期NSCLC的治疗已经由传统化疗转变为由遗传突变和分子水平指导为基础的疾病精细分类疗法[2], 特别是表皮生长因子受体(epidermal growth factor receptor, EGFR) 突变、无融合淋巴瘤激酶(anaplastic lymphoma kinase, ALK) 易位、c-ROS肉瘤致癌因子-受体酪氨酸激酶(ROS proto-oncogene 1, receptor tyrosine kinase, ROS1) 重排以及B-raf原癌基因丝氨酸/苏氨酸激酶(B-Raf proto oncogene serine/threonine protein kinase, BRAF) 突变, 这些患者在使用了精准的靶向治疗后其无进展生存期(progression-free survival, PFS) 有了显著改善[3-7]。其中EGFR抑制剂治疗EGFR突变的腺癌已成为NSCLC靶向治疗的先驱[8, 9]。开发新一代抑制剂来应对EGFR获得性耐药也成为该领域的研究热点。
EGFR基因位于7p12号染色体上, 由28个外显子和27个内含子组成[10, 11]。EGFR是ERBB家族受体酪氨酸激酶之一, ERBB家族由4个成员组成: EGFR (也称为ERBB1/HER1)、ERBB2/HER2/NEU、ERBB3/HER3和ERBB4/HER4。对于野生型EGFR, 需要特定的配体与EGFR的胞外域结合, 导致同二聚体和异二聚体的形成[12, 13]。聚合物刺激受体固有的酪氨酸激酶活性, 并触发特定酪氨酸残基的自磷酸化, 通过信号转导子启动多个下游通路, 如Ras/Raf/丝裂原活化蛋白激酶通路(Ras/Raf/mitogen-activated protein kinase pathway, Ras/MAPK)、磷脂酰肌醇3激酶/AKT通路(phosphatidylinositol 3-kinase/AKT pathway, PI3K/AKT) 和信号转导和转录激活因子通路(signal transducers and activators of transcription pathway, STAT), 从而参与调节增殖和凋亡[14-16]。EGFR突变使蛋白质结构的平衡在没有配体刺激下从非活性状态转变为活性状态, 从而导致EGFR和其他HER家族蛋白的持续磷酸化[17]。
在所有NSCLC的EGFR激酶结构域突变中, EGFR外显子21中的亮氨酸到精氨酸(L858R) 的单点突变和外显子19中至少3个氨基酸残基的可变缺失通常被称为经典EGFR激活突变, 且这些突变对EGFR抑制剂敏感[18-21]。但是, 并非所有激活的EGFR突变对EGFR抑制剂都敏感。EGFR外显子20插入(ex20ins) 在所有NSCLC病例中占比较低, 占所有已证明EGFR突变的癌症的10%~12%[22-24]。这些突变是继常见的致敏EGFR突变(即外显子19缺失和外显子21 L858R突变) 之后的第三大常见的EGFR突变亚型。外显子20中的框内碱基对插入导致EGFR的组成性激活, 但是与经典的激活性EGFR突变不同, EGFR外显子20的插入导致该类患者对当前临床上可用的EGFR抑制剂产生新抗性[24, 25]。近年来, 临床前工作一直在寻找EGFR外显子20插入突变的NSCLC患者中EGFR抑制剂耐药的原因, 也针对这些突变开发了相应的药物。本文将对外显子20插入对EGFR结构和EGFR抑制剂敏感性影响的研究现状进行综述以及对目前临床治疗策略进行梳理, 希望为临床治疗提供新的思路。
1 外显子20插入对EGFR结构和EGFR抑制剂敏感性影响的研究现状EGFR的激活状态取决于C-螺旋(外显子20插入的C末端) 的状态, C-螺旋由外向内旋转从而允许与稳定二聚化EGFR的活性位点进行特异性相互作用[26, 27]。EGFR中外显子19的缺失通过从环上去除残基而限制了C螺旋的旋转, 从而使C螺旋不再是向外的非活性构象, 而是转变为向内的活性构象[28]。正是通过这种机制, 外显子19缺失突变将使EGFR由非活跃态转向活跃状态, 从而促进组成型受体活化。外显子20突变中的大多数位于残基M766之后, 处于激酶N瓣内C螺旋末端附近, 并有一小部分映射到C螺旋中间(影响氨基酸E762至Y764) (图 1)[29-31]。总之, EGFR外显子20插入突变表征为在EGFR蛋白的氨基酸762和774之间聚集的3至21 bp的框内插入或重复[17, 32]。然而, 与外显子19缺失不同, 外显子20插入通常直接按顺序插入环中, 从另一个方向将C螺旋转变为主动构象[33]。
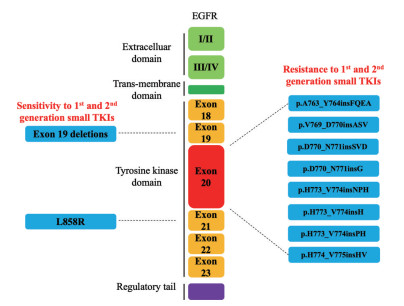
|
Figure 1 Epidermal growth factor receptor (EGFR) receptor structure, EGFR exon 20 insertion is a common type. The data can be accessed on COSMICv92 (https://cancer.sanger.ac.uk/cosmic), and the mutation types are those with a sample size greater than 10 after screening by non-small cell lung cancer (NSCLC), adenocarcinomas, and EGFR exon 20 insertion |
EGFR外显子20插入突变是异质的, 插入位置可能会影响药物和ATP结合的动力学, 最终决定对EGFR抑制剂的耐药性或敏感性。在众多的插入类型中, 仅有A763_Y764insFQEA的突变, 展现出对吉非替尼和厄洛替尼的高度敏感[32]。三名A763_Y764insFQEA插入的患者在接受厄洛替尼治疗后显示出肿瘤消退或保持稳定。A763_Y764insFQEA插入的3D建模表明, 在C螺旋自身内部残基764之前发生的外显子20插入可能具有与L858R或外显子19更为相似的激活机制和结构[28, 34]。D770_N771insSVD和p.V769_D770insASV等其他外显子20插入则与常见的L858R或外显子19的激活机制和结构不同(部分突变结构如表 1所示), 这也使其具有对酪氨酸激酶抑制剂(tyrosine kinase inhibitors, TKI) 的耐药性, 而如何克服这个难题是现在面对的问题。
| Table 1 Part of the EGFR exon 20 insertion was identified by Maria E Arcila and Khedoudja Nafa[24]. Note: Due to the very low mutation peaks, sanger sequencing cannot be used to characterize a 6 bp insertion |
一项分子结构的研究发现, 某些EGFR外显子20中以及相应的ERBB2/HER2外显子20插入引起结构变化, 从而限制了ATP结合袋的大小[35]。因此, 如图 2所示, 较大的药物很难与预期的靶标结合。所以, 在外显子20突变的EGFR肿瘤中, ATP结合袋的结构较小, TKI无法再与靶标结合[7, 36], 使EGFR得以保持活性, 致癌信号持续存在。使用单克隆抗体(monoclonal antibody, mAb) 或更小的新一代TKI可能会避免外显子20插入改变所产生的耐药性[37]。其次, T790M突变使L858R突变型EGFR的ATP结合亲和力几乎恢复到野生型受体水平, 这消除了可逆性ATP竞争性抑制剂吉非替尼和厄洛替尼对突变型的选择性, 使治疗窗口变窄[38-40], 而第三、四代TKI的出现或许能够解决这些问题[41, 42]。
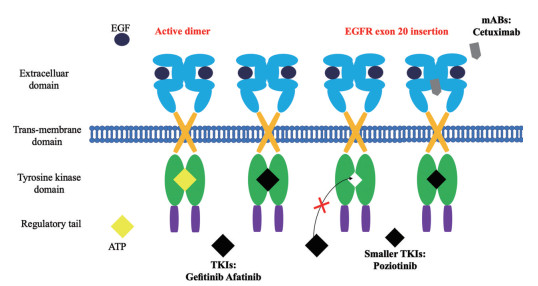
|
Figure 2 Unlike EGFR tumors with non-exon 20 mutations, in EGFR tumors with exon 20 mutations, the structure of the ATP binding pocket is small, and tyrosine kinase inhibitors (TKI) can no longer bind. Receptors remain active so that oncogenic signals persist |
先前的研究也表明, 不同的EGFR突变可驱动不同的下游信号传导蛋白[43, 44]。那么不同类型的EGFR外显子20插入突变激活的下游信号通路极有可能不相同, 而这些通路与更常见的L858R或外显子19缺失EGFR突变激活的下游信号通路的重叠程度同样需要更多的研究[45]。不同EGFR外显子20插入所特有的下游途径激活的未来表征可能会产生选择性靶向这些途径的突变特异性疗法。
2 外显子20插入的NSCLC治疗策略研究 2.1 EGFR抑制剂 2.1.1 第一代EGFR抑制剂Gefitinib和erlotinib是可逆的ATP竞争性抑制剂, 其可逆地与EGFR的ATP结合袋结合。Ⅲ期随机试验已经证明, 在具有EGFR突变的肺癌患者中, 这些TKI在PFS方面优于常规化疗, 并成为EGFR突变NSCLC治疗的金标准, 可实现高达72%的反应率(response rate, RR) 和近10个月的PFS[46, 47]。相比之下[47-49], 除了A763_Y764insFQEA突变外, 对临床数据的回顾性分析显示, 第一代EGFR抑制剂在绝大多数EGFR外显子20插入突变型NSCLC患者中效果不佳[18, 50]。
2.1.2 第二代EGFR抑制剂Neratinib、dacomitinib和afatinib都在特定的半胱氨酸残基(C797) 上与EGFR形成不可逆的共价相互作用[20, 51, 52]。尽管有令人鼓舞的临床前数据, 但由于对野生型EGFR的抑制作用, 第二代TKI与第一代TKI相比有较严重的不良事件, 使该药物的临床可用浓度尚未达到T790M肿瘤的治疗范围[32, 53]。
当前, 仅有有限的临床证据支持在外显子20插入突变体NSCLC中使用第二代EGFR抑制剂[54]。结合Ⅱ Lux-Lung 2期、Ⅲ Lux-Lung 3和6期的结果进行分析, 虽然阿法替尼在罕见的EGFR点突变(G719X、S786I和L861Q) 中具有临床活性, 但是afatinib治疗的EGFR外显子20插入的患者RR为8.7%, PFS为2.7个月, 效果不佳[55-57]。但是临床前实验中, 在770位引入甘氨酸的插入物展现出对dacomitinib的独特敏感性, 为具有D770delinsGY突变的患者提供了潜在的治疗途径[58]。而且在2011年的Ⅰ期临床试验中, 在6名具有不同EGFR外显子20插入突变的患者中, 只有携带D770delinsGY突变的患者对dacomitinib表现出部分反应[59, 60]。然而, 常见的外显子20插入片段在770位缺少甘氨酸, 因此, 大多数外显子20插入患者不太可能受益于dacomitinib的治疗。
2.1.3 第三代EGFR抑制剂Osimertinib和rociletinib是第三代EGFR抑制剂, 与EGFR的C797半胱氨酸残基共价结合并保持了对双突变L858R/T790M或第19外显子del/T790M EGFR的选择性, 以及在表达常见EGFR外显子20插入片段的异种移植物中的体内作用[61-63]。一例报告携带EGFR V769_D770InsASV变体的患者, 在osimertinib治疗后出现了肿瘤缩小[64]。但是, 仍然需要更多的临床证据支持在外显子20插入突变体NSCLC中使用第三代EGFR抑制剂[65]。
Ikemuraa和Yasuda等[66-68]利用基于分子动力学模拟的模型计算EGFR外显子20插入突变对EGFR-TKI的敏感性的影响。该模型计算了外显子20插入突变体(包括单例) 对第三代EGFR-TKI奥西替尼的敏感性(图 3)。可以从中看出, 仅有A763_Y764insFQEA外显子20插入的突变具有对EGFR-TKI的高敏感性。此外, Zhao等[69]通过分子动力学模拟发现, HER2 ex20ins通过改变HER2激酶的构象结构, 并限制活性状态的激酶构象, 致使配体依赖性激酶的活化。
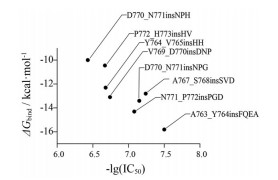
|
Figure 3 Plot of ΔG bind values against negative lg transformed the half maximal inhibitory concentration (IC50) values. Each EGFR exon 20 insertion mutation is indicated by a dot |
通过使用Ba/F3细胞对TKI的体外敏感性数据(表 2)[24, 25, 70]可以看出: EGFR外显子20插入让外显子20突变的EGFR肿瘤恢复到野生型的水平, 这与常见的致敏EGFR突变(即外显子19缺失和外显子21 L858R突变) 不同[71]。而且可以看出不同的突变对于不同TKI的敏感性不同, 通过精确检测目标突变, 为每种突变选择最合适的TKI, 同时继续开展体外研究, 收集罕见突变的临床数据, 可能可以提供新的临床治疗决策。
| Table 2 Summary of the in vitro sensitivities of Ba/F3 cells expressing EGFR mutation to various TKI IC50 values (nmol·L-1) of < 10 is shown with *. When the exact value was not described in the literature, the approximate number was estimated from each figure. Wild type and typical exon 19 mutation are listed for comparison |
对EGFR外显子20插入突变体具有选择性的化合物的开发对于限制患者由于野生型EGFR受抑制产生的毒性至关重要。最近开发的几种新抑制剂化合物, 被证明可直接靶向EGFR外显子20插入(如TAS6417共价修饰突变EGFR的ATP结合位点797位半胱氨酸残基)。尽管这些进展仍处于临床前阶段, 但已发表的数据表明, 这些化合物在携带EGFR外显子20插入突变的NSCLC患者中可能具有重要的临床活性[72]。如TAS6417[73]、化合物1A[61, 74]和TAK-788[75, 76]等都显示出对肿瘤的抑制作用。但是在临床试验前, 这些药物的不良药代动力学特性(包括其口服生物利用度低、半衰期短和清除率高) 尚待解决。
TAS6417, 也称为CLN-081, 是一种新型小分子, 它通过共价修饰突变EGFR的ATP结合位点797位半胱氨酸残基来抑制EGFR, 该突变在外显子20中具有框内插入突变[73]。在具有EGFR外显子20突变或外显子19突变的肺癌患者的基因工程细胞和细胞系以及具有KRAS突变的细胞中建立的体外基因工程研究中, TAS6417展现出较好的效果, 与大多数批准/开发中的EGFR抑制剂相比, 具有更广泛的活性谱和更宽的治疗范围[77]。可惜的是, 虽然用TAS6417进行的治疗在具有EGFR外显子20突变的小鼠中抑制了PI3K/AKT途径和RAS/MAPK途径, 但对那些具有EGFR外显子20突变的小鼠没有抗肿瘤作用, TAS6417在NSCLC患者中的临床评估尚未开始, 因此, 在EGFR外显子20插入阳性患者中, 抑制剂的突变体选择性是否足以达到良好的治疗窗且毒性低尚待确定。
2.2 其他靶向抑制剂 2.2.1 PoziotinibPoziotinib (HM781-36B) 是EGFR和HER2外显子20插入的共价不可逆抑制剂[78]。Poziotinib与afatinib相似, 是一种柔软的喹唑啉衍生物, 但它具有较小的尺寸和更大的卤化度, 并且具有更高的柔软性。3D建模预测, 这些结构优势使poziotinib能够避开外显子20插入所产生的空间变化, 并在药物结合袋中更紧密地结合[35], 可能可以避免由于ATP结合袋的结构较小TKI无法结合的问题。
使用携带外显子20插入的工程Ba/F3细胞的体外分析表明, poziotinib有效地抑制了具有EGFR或HER2外显子20突变的细胞的生长[79]。在该细胞系中, 对poziotinib的生长增殖抑制作用比其他TKI更有效。与afatinib相比, poziotinib可显著减少肿瘤体积, 并在12周时出现持久无进展迹象[35, 80]。但是, 由于poziotinib在野生型EGFR中也发挥了体外活性, 因此治疗范围可能很窄。
2.2.2 LuminespibLuminespib (AUY922) 是一种基于异噁唑基间苯二酚的热休克蛋白90 (heat shock protein 90, HSP90) 抑制剂, 与第一代格尔德霉素HSP90抑制剂不同。第二代抑制剂效力增强, 拥有更少的不良反应和更优的药代动力学。HSP90是一种分子伴侣, 可维持细胞蛋白质稳定性并协助折叠[81, 82]。重要的是, 在NSCLC中, HSP90介导了许多重要的致癌驱动蛋白的调控, 包括EGFR和ALK, 而且HSP90抑制剂也显示出对癌细胞更强的抗肿瘤特性和选择性[83], 尽管迄今为止尚未批准HSP90抑制剂, 但在许多治疗选择受到限制的癌症类型中, 正在积极探索其潜力[84]。最近, 临床前数据表明, EGFR外显子20插入突变激酶与HSP90分子伴侣系统相关, 可以通过使用HSP90抑制剂降解。在EGFR外显子20插入NSCLC中, luminespib表现出比第一代或第二代EGFR抑制剂更好的效果[85]。然而, 中位PFS和总生存期都很短, 所以luminespib在EGFR外显子20插入NSCLC患者中是否具有临床应用价值仍不清楚(图 3)。
2.2.3 TarloxotinibTarloxotinib是一种低氧激活的前药(hypoxia-activated prodrug, HAP), 仅在低氧条件下才会释放不可逆的EGFR/HER2抑制剂, 已有许多研究将其作为针对具有EGFR外显子20突变的肿瘤的潜在疗法[86, 87]。在肺癌中, 肿瘤细胞中的低氧状态促进了基因组的不稳定性、侵略性的增强和转移潜力的增加[88]。它还导致了对EGFR抑制剂的耐药性和低存活率[89, 90]。在一项Ⅱ期临床试验中, tarloxotinib在EGFR突变T790M阴性NSCLC患者中效果不佳, 然而, 应用带有内源性EGFR外显子20插入的NSCLC细胞系异种移植的小鼠模型, 显示使用tarloxotinib可使肿瘤显著消退, 而在afatinib中未观察到反应[87]。这些初步数据表明, 需要更集中的临床试验以确定tarloxotinib在EGFR外显子20插入NSCLC患者亚型中的疗效。
2.2.4 Cetuximab和EGFR抑制剂组合Cetuximab是与EGFR胞外域结合并在空间上阻碍二聚体形成的单克隆抗体。抗体部分经过蛋白水解降解后释放DM1[91, 92]。如emtansine也称为T-DM1, 是一种抗体-药物偶联物, 由细胞毒性微管剂DM1与人源化单克隆抗体曲妥珠单抗连接而成。每个抗体T-DM1平均带有3.5个分子的DM1。抗体药物偶联物(antibody-drug conjugates, ADC) 与表面受体HER2结合, 并通过受体介导的内吞作用进入细胞[93, 94]。
另一项研究利用模型证明了某些EGFR外显子20的改变可能促进受体二聚化, 并且可能对靶向该结构域的EGFR抗体敏感[67]。作为治疗方案的一部分, 分别将两名携带EGFR D770_P772delinsKG和EGFR D770 > GY的患者接受cetuximab治疗, 并在6个月和42个月以上实现了持续的部分缓解(表 3)[26, 33, 35, 50, 55, 59, 64, 73, 74, 85, 87, 92, 95-98]。在另一项研究中, 接受cetuximab联合阿法替尼治疗的4名患者中有3名也获得了反应[92]。
| Table 3 Clinical trials in EGFR exon 20 insertion positive NSCLC. Details for trials with NCT numbers can be accessed on https://clinicaltrials.gov/. PFS: Progression-free survival; PR: Partial response; RR: Response rate; ex20ins: Exon 20 insertion; mOS: Median overall survival; mPFS: Median progression-free survival; HSP90: Heat shock protein 90; HER: Human epidermal growth factor |
JNJ-61186372 (JNJ-372) 是一种同时靶向EGFR和cMet受体的双特异性抗体。它阻断配体诱导的EGFR和cMet磷酸化, 并抑制pERK和pAKT, 而且还可以通过依赖Fc的效应子机制介导抗体依赖性细胞毒性诱导受体降解[99]。在一项Ⅰ期临床研究中展现出对部分晚期NSCLC患者的抗肿瘤效果。基于在具有EGFR外显子20突变的NSCLC患者中观察到的活性, 为了更好地了解这种特殊情况下的潜在机制, 已开展了临床前研究[98, 100]。对包含EGFR外显子20突变的Ba/F3细胞系的临床前实验表明, JNJ-61186372通过抑制EGF和cMet受体表达, 下调pERK、pAKT和p-S6的水平来抑制细胞增殖, 并同时上调caspase的表达来促进细胞凋亡[101]。
3 结论NSCLC中的EGFR外显子20突变使其与ATP的结合能力下降, 并使其与EGFR抑制剂的亲和力低于野生型, 这使原有的靶向治疗方法效果不佳。特异的外显子20突变对于不同的TKI的敏感性不同, 通过精确检测目标突变, 为每种突变选择最合适的已上市TKI, 可能是新的临床治疗手段。由于EGFR外显子20插入突变的异质性, 不同的插入位置对药物的响应和耐药性影响可能不同。根据特定的突变类型, 需要进一步来阐明原发性和获得性耐药的机制以及对药物敏感性不同的原因, 选择性靶向这些特异突变位点的特异性疗法也将不断涌现。随着对EGFR外显子20插入研究的不断深入, 靶向EGFR外显子20插入突变的新型药物也不断涌现, 这些药物的临床前和临床方面进展为外显子20突变患者带来了新的希望。但是, 由于药物会抑制野生型EGFR, 安全性问题还需要进一步研究。此外, 这些药物的临床耐药性仍然不清楚。但是可以预见, 这些化合物可能最终成为对抗癌症的武器。正在进行的对类似外显子20插入突变的特定人群的功效和毒性的临床试验将引导研究者进入分子驱动疗法的新时代。
作者贡献: 丁玲和陈羲为文章提供了指导和思路, 李润和郭弘洁进行了文献查阅和写作。
利益冲突: 本文无任何利益冲突。
| [1] |
Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship[J]. Mayo Clin Proc, 2008, 5: 584-594. |
| [2] |
Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer[J]. Signal Transduct Target Ther, 2019, 4: 5. DOI:10.1038/s41392-019-0038-9 |
| [3] |
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer[J]. N Engl J Med, 2014, 23: 2167-2177. |
| [4] |
Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial[J]. Lancet Oncol, 2017, 10: 1307-1316. |
| [5] |
Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer[J]. N Engl J Med, 2014, 21: 1963-1971. |
| [6] |
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study[J]. Lancet Oncol, 2011, 8: 735-742. |
| [7] |
Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors[J]. Acta Pharm Sin B, 2015, 5: 390-401. DOI:10.1016/j.apsb.2015.07.001 |
| [8] |
Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase Ⅱ extension component[J]. J Clin Oncol, 2017, 12: 1288-1296. |
| [9] |
Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study[J]. Lancet Oncol, 2016, 12: 1643-1652. |
| [10] |
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib[J]. N Engl J Med, 2004, 21: 2129-2139. |
| [11] |
Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy[J]. Science, 2004, 5676: 1497-1500. |
| [12] |
Carpenter G, King L, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro[J]. Nature, 1978, 5686: 409-410. |
| [13] |
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network[J]. Nat Rev Mol Cell Biol, 2001, 2: 127-137. DOI:10.1038/35052073 |
| [14] |
Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer[J]. Cancer Sci, 2007, 12: 1817-1824. |
| [15] |
Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases[J]. Cell, 2010, 7: 1117-1134. |
| [16] |
Lowenstein EJ, Daly RJ, Batzer AG, et al. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling[J]. Cell, 1992, 3: 431-442. |
| [17] |
Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications[J]. Lancet Oncol, 2012, 1: e23-e31. |
| [18] |
Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors[J]. Oncogene, 2009, 28 Suppl 1: S24-S31. |
| [19] |
He M, Capelletti M, Nafa K, et al. EGFR exon 19 insertions: a new family of sensitizing EGFR mutations in lung adenocarcinoma[J]. Clin Cancer Res, 2012, 6: 1790-1797. |
| [20] |
Kobayashi Y, Togashi Y, Yatabe Y, et al. EGFR exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first-or third-generation TKIs[J]. Clin Cancer Res, 2015, 23: 5305-5313. |
| [21] |
Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications[J]. Cancer Res, 2004, 24: 8919-8923. |
| [22] |
Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions[J]. J Thorac Oncol, 2013, 2: 179-184. |
| [23] |
Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC[J]. J Thorac Oncol, 2018, 10: 1560-1568. |
| [24] |
Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics[J]. Mol Cancer Ther, 2013, 2: 220-229. |
| [25] |
Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy[J]. Cancer Sci, 2016, 9: 1179-1186. |
| [26] |
Yang M, Xu X, Cai J, et al. NSCLC harboring EGFR exon-20 insertions after the regulatory C-helix of kinase domain responds poorly to known EGFR inhibitors[J]. Int J Cancer, 2016, 1: 171-176. |
| [27] |
Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors[J]. Nature, 2016, 7605: 129-132. |
| [28] |
Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer[J]. Biochim Biophys Acta, 2010, 3: 559-566. |
| [29] |
Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers[J]. J Natl Cancer Inst, 2005, 5: 339-346. |
| [30] |
Murray S, Dahabreh IJ, Linardou H, et al. Somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database[J]. J Thorac Oncol, 2008, 8: 832-839. |
| [31] |
Linardou H, Dahabreh IJ, Bafaloukos D, et al. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC[J]. Nat Rev Clin Oncol, 2009, 6: 352-366. DOI:10.1038/nrclinonc.2009.62 |
| [32] |
Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer[J]. Sci Transl Med, 2013, 216: 216ra177. |
| [33] |
Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase Ⅱ trial in patients with advanced non-small-cell lung cancer[J]. J Clin Oncol, 2010, 18: 3076-3083. |
| [34] |
Voon PJ, Tsui DW, Rosenfeld N, et al. EGFR exon 20 insertion A763-Y764insFQEA and response to erlotinib--letter[J]. Mol Cancer Ther, 2013, 11: 2614-2615. |
| [35] |
Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer[J]. Nat Med, 2018, 5: 638-646. |
| [36] |
Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer[J]. Nat Med, 2013, 11: 1389-1400. |
| [37] |
Patel H, Pawara R, Ansari A, et al. Recent updates on third generation EGFR inhibitors and emergence of fourth generation EGFR inhibitors to combat C797S resistance[J]. Eur J Med Chem, 2017, 142: 32-47. DOI:10.1016/j.ejmech.2017.05.027 |
| [38] |
Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib[J]. Cancer Res, 2006, 16: 8163-8171. |
| [39] |
Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP[J]. Proc Natl Acad Sci U S A, 2008, 6: 2070-2075. |
| [40] |
Mulloy R, Ferrand A, Kim Y, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib[J]. Cancer Res, 2007, 5: 2325-2330. |
| [41] |
Wang S, Song Y, Liu D. EAI045:the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance[J]. Cancer Lett, 2017, 385: 51-54. DOI:10.1016/j.canlet.2016.11.008 |
| [42] |
Wang S, Tsui ST, Liu C, et al. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer[J]. J Hematol Oncol, 2016, 1: 59. |
| [43] |
Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma[J]. Sci Signal, 2009, 87: re6. |
| [44] |
Pines G, Huang PH, Zwang Y, et al. EGFRvIV: a previously uncharacterized oncogenic mutant reveals a kinase autoinhibitory mechanism[J]. Oncogene, 2010, 43: 5850-5860. |
| [45] |
Tan CS, Cho BC, Soo RA. Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer[J]. Lung Cancer, 2016, 93: 59-68. DOI:10.1016/j.lungcan.2016.01.003 |
| [46] |
Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study[J]. J Clin Oncol, 2020, 2: 115-123. |
| [47] |
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial[J]. Lancet Oncol, 2012, 3: 239-246. |
| [48] |
Hirsch FR, Varella-Garcia M, Bunn PA, et al. Molecular predictors of outcome with gefitinib in a phase Ⅲ placebo-controlled study in advanced non-small-cell lung cancer[J]. J Clin Oncol, 2006, 31: 5034-5042. |
| [49] |
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma[J]. N Engl J Med, 2009, 10: 947-957. |
| [50] |
Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib[J]. Cancer, 2015, 18: 3212-3220. |
| [51] |
Yu HA, Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in lung cancers[J]. J Natl Compr Canc Netw, 2013, 2: 161-169. |
| [52] |
Roskoski R. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers[J]. Pharmacol Res, 2019, 139: 395-411. DOI:10.1016/j.phrs.2018.11.014 |
| [53] |
Castellano GM, Aisner J, Burley SK, et al. A novel acquired exon 20 EGFR M766Q mutation in lung adenocarcinoma mediates osimertinib resistance but is sensitive to neratinib and poziotinib[J]. J Thorac Oncol, 2019, 11: 1982-1988. |
| [54] |
Kosaka T, Tanizaki J, Paranal RM, et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors[J]. Cancer Res, 2017, 10: 2712-2721. |
| [55] |
Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6[J]. Lancet Oncol, 2015, 7: 830-838. |
| [56] |
Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase Ⅱb LUX-Lung 7 trial[J]. Ann Oncol, 2017, 2: 270-277. |
| [57] |
Masood A, Kancha RK, Subramanian J. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: focus on afatinib[J]. Semin Oncol, 2019, 3: 271-283. |
| [58] |
Janne PA, Ou SI, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial[J]. Lancet Oncol, 2014, 13: 1433-1441. |
| [59] |
Janne PA, Boss DS, Camidge DR, et al. Phase Ⅰ dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors[J]. Clin Cancer Res, 2011, 5: 1131-1139. |
| [60] |
Sequist LV, Yang JC, Yamamoto N, et al. Phase Ⅲ study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations[J]. J Clin Oncol, 2013, 27: 3327-3334. |
| [61] |
Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer[J]. Cancer Discov, 2014, 9: 1046-1061. |
| [62] |
Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer[J]. N Engl J Med, 2015, 18: 1700-1709. |
| [63] |
Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib[J]. Clin Cancer Res, 2006, 19: 5764-5769. |
| [64] |
Floc'h N, Martin MJ, Riess JW, et al. Antitumor activity of osimertinib, an irreversible mutant-selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR exon 20 insertions[J]. Mol Cancer Ther, 2018, 5: 885-896. |
| [65] |
Chuang JC, Salahudeen AA, Wakelee HA. Rociletinib, a third generation EGFR tyrosine kinase inhibitor: current data and future directions[J]. Expert Opin Pharmacother, 2016, 7: 989-993. |
| [66] |
Ikemura S, Yasuda H, Matsumoto S, et al. Molecular dynamics simulation-guided drug sensitivity prediction for lung cancer with rare EGFR mutations[J]. Proc Natl Acad Sci U S A, 2019, 20: 10025-10030. |
| [67] |
Tsigelny IF, Wheler JJ, Greenberg JP, et al. Molecular determinants of drug-specific sensitivity for epidermal growth factor receptor (EGFR) exon 19 and 20 mutants in non-small cell lung cancer[J]. Oncotarget, 2015, 8: 6029-6039. |
| [68] |
Lee Y, Kim TM, Kim DW, et al. Preclinical modeling of osimertinib for NSCLC With EGFR exon 20 insertion mutations[J]. J Thorac Oncol, 2019, 9: 1556-1566. |
| [69] |
Zhao S, Fang W, Pan H, et al. Conformational landscapes of HER2 exon 20 insertions explain their sensitivity to kinase inhibitors in lung adenocarcinoma[J]. J Thorac Oncol, 2020, 6: 962-972. |
| [70] |
Lee B, Lee T, Lee SH, et al. Clinicopathologic characteristics of EGFR, KRAS, and ALK alterations in 6, 595 lung cancers[J]. Oncotarget, 2016, 17: 23874-23884. |
| [71] |
Li X, Zhang L, Jiang D, et al. Routine-dose and high-dose icotinib in patients with advanced non-small cell lung cancer harboring EGFR exon 21-L858R mutation: the randomized, phase Ⅱ, INCREASE trial[J]. Clin Cancer Res, 2020, 13: 3162-3171. |
| [72] |
Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC[J]. Cancer Discov, 2013, 12: 1404-1415. |
| [73] |
Hasako S, Terasaka M, Abe N, et al. TAS6417, a novel EGFR inhibitor targeting exon 20 insertion mutations[J]. Mol Cancer Ther, 2018, 8: 1648-1658. |
| [74] |
Jang J, Son J, Park E, et al. Discovery of a highly potent and broadly effective epidermal growth factor receptor and HER2 exon 20 insertion mutant inhibitor[J]. Angew Chem Int Ed Engl, 2018, 36: 11629-11633. |
| [75] |
Doebele RC, Riely GJ, Spira AI, et al. First report of safety, PK, and preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK-788(AP32788) in non-small cell lung cancer (NSCLC)[J]. J Clin Oncol, 2018, 36: 9015. DOI:10.1200/JCO.2018.36.15_suppl.9015 |
| [76] |
Gonzalvez F, Zhu XT, Huang WS, et al. AP32788, a potent, selective inhibitor of EGFR and HER2 oncogenic mutants, including exon 20 insertions, in preclinical models[J]. Cancer Res, 2016, 76: 2644. |
| [77] |
Udagawa H, Hasako S, Ohashi A, et al. TAS6417/CLN-081 is a pan-mutation-selective EGFR tyrosine kinase inhibitor with a broad spectrum of preclinical activity against clinically relevant EGFR mutations[J]. Mol Cancer Res, 2019, 11: 2233-2243. |
| [78] |
Cha MY, Lee KO, Kim M, et al. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models[J]. Int J Cancer, 2012, 10: 2445-2454. |
| [79] |
Koga T, Kobayashi Y, Tomizawa K, et al. Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: an in vitro study[J]. Lung Cancer, 2018, 126: 72-79. DOI:10.1016/j.lungcan.2018.10.019 |
| [80] |
Kim E, Kim H, Suh K, et al. Metabolite identification of a new tyrosine kinase inhibitor, HM781-36B, and a pharmacokinetic study by liquid chromatography/tandem mass spectrometry[J]. Rapid Commun Mass Spectrom, 2013, 11: 1183-1195. |
| [81] |
Butler LM, Ferraldeschi R, Armstrong HK, et al. Maximizing the therapeutic potential of HSP90 inhibitors[J]. Mol Cancer Res, 2015, 11: 1445-1451. |
| [82] |
Noor ZS, Goldman JW, Lawler WE, et al. Luminespib plus pemetrexed in patients with non-squamous non-small cell lung cancer[J]. Lung Cancer, 2019, 135: 104-109. DOI:10.1016/j.lungcan.2019.05.022 |
| [83] |
Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors[J]. Nature, 2003, 6956: 407-410. |
| [84] |
Harrison PT, Huang PH. Exploiting vulnerabilities in cancer signalling networks to combat targeted therapy resistance[J]. Essays Biochem, 2018, 4: 583-593. |
| [85] |
Piotrowska Z, Costa DB, Oxnard GR, et al. Activity of the Hsp90 inhibitor luminespib among non-small-cell lung cancers harboring EGFR exon 20 insertions[J]. Ann Oncol, 2018, 10: 2092-2097. |
| [86] |
Hunter FW, Wouters BG, Wilson WR. Hypoxia-activated prodrugs: paths forward in the era of personalised medicine[J]. Br J Cancer, 2016, 10: 1071-1077. |
| [87] |
Salem A, Asselin MC, Reymen B, et al. Targeting hypoxia to improve non-small cell lung cancer outcome[J]. J Natl Cancer Inst, 2018. DOI:10.1093/jnci/djx160 |
| [88] |
Phillips RJ, Mestas J, Gharaee-Kermani M, et al. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha[J]. J Biol Chem, 2005, 23: 22473-22481. |
| [89] |
Murakami A, Takahashi F, Nurwidya F, et al. Hypoxia increases gefitinib-resistant lung cancer stem cells through the activation of insulin-like growth factor 1 receptor[J]. PLoS One, 2014, 1: e86459. |
| [90] |
Lu Y, Liu Y, Oeck S, et al. Hypoxia promotes resistance to EGFR inhibition in NSCLC cells via the histone demethylases, LSD1 and PLU-1[J]. Mol Cancer Res, 2018, 10: 1458-1469. |
| [91] |
Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate[J]. Cancer Res, 2008, 22: 9280-9290. |
| [92] |
van Veggel B, de Langen AJ, Hashemi SMS, et al. Afatinib and cetuximab in four patients with EGFR exon 20 insertion-positive advanced NSCLC[J]. J Thorac Oncol, 2018, 8: 1222-1226. |
| [93] |
von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer[J]. N Engl J Med, 2019, 7: 617-628. |
| [94] |
Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase Ⅱ basket trial[J]. J Clin Oncol, 2018, 24: 2532-2537. |
| [95] |
Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10117 patients: a multicentre observational study by the French ERMETIC-IFCT network[J]. Ann Oncol, 2014, 1: 126-131. |
| [96] |
Wheler JJ, Tsimberidou AM, Falchook GS, et al. Combining erlotinib and cetuximab is associated with activity in patients with non-small cell lung cancer (including squamous cell carcinomas) and wild-type EGFR or resistant mutations[J]. Mol Cancer Ther, 2013, 10: 2167-2175. |
| [97] |
Remon J, Hendriks LEL, Cardona AF, et al. EGFR exon 20 insertions in advanced non-small cell lung cancer: a new history begins[J]. Cancer Treat Rev, 2020, 90: 102105. DOI:10.1016/j.ctrv.2020.102105 |
| [98] |
Yun J, Lee SH, Kim SY, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC[J]. Cancer Discov, 2020, 8: 1194-1209. |
| [99] |
Moores SL, Chiu ML, Bushey BS, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors[J]. Cancer Res, 2016, 13: 3942-3953. |
| [100] |
Vijayaraghavan S, Lipfert L, Chevalier K, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis[J]. Mol Cancer Ther, 2020, 10: 2044-2056. |
| [101] |
Emdal KB, Dittmann A, Reddy RJ, et al. Characterization of in vivo resistance to osimertinib and JNJ-61186372, an EGFR/Met bispecific antibody, reveals unique and consensus mechanisms of resistance[J]. Mol Cancer Ther, 2017, 11: 2572-2585. |
 2021, Vol. 56
2021, Vol. 56


