肝性脑病(hepatic encephalopathy, HE) 是指由严重肝病引起的、以代谢紊乱为基础的中枢神经系统功能失调综合征, 30%~40%的肝硬化患者会发生HE。HE在全球范围内有较高的发病率和死亡率, 约影响8.44亿人, 每年造成200万人死亡, 其中100万人与肝硬化及其并发症相关[1, 2]。
HE的发生发展是一个连续过程, 根据HE的不同病理进程, 可以将HE分为不同的阶段。目前国际上对于HE的分级应用较为广泛的是West-Haven分级标准, 将HE分为0~4级。0级为轻微型肝性脑病(minimal hepatic encephalopathy, MHE)[3], 0级和1级HE也被归类为“隐匿性肝性脑病”, 2~4级HE为“显性肝性脑病”[4]。HE患者1年内死亡率高达54%~85%, 3年生存率仅为23%[5]。在美国, HE患者占所有住院患者的0.33%, 在2000年至2015年, 与HE相关的住院费用增加了197.2%[6]。一项采用多中心横断面研究方法的调查结果显示, 我国住院肝硬化患者中MHE患病率为39.9%, 并且MHE高发生率与肝硬化患者健康相关生活质量损害程度成正比[7], 给我国的医疗系统和社会经济带来了巨大负担。
目前HE的发病机制尚未完全明确, 很大程度上可归因于高氨血症和与肝脏损伤相关的炎症反应过程。此外, 神经炎性反应、胆汁酸(bile acid, BA)代谢紊乱、氧化应激和神经递质功能障碍等也是HE的重要病因[8]。有证据表明, HE的发生发展与肠道菌群及其代谢产物, 例如氨基酸代谢物(氨、吲哚类和奥昔多尔类)和内毒素有关。例如, 慢性肝病发生时, 由于患者肝脏代谢功能受损, 肠道来源的含氮毒素无法被肝脏分解, 从而导致大量的氨进入大脑。大脑中高浓度的氨会引起脑细胞炎症、脑谷氨酰胺升高和星形胶质细胞肿胀, 导致脑功能紊乱[9], 这些因素与肠道屏障渗漏和免疫功能紊乱一起参与HE的发病机制。
目前对于HE的临床治疗方法主要集中在减少氨的产生、减轻全身和中枢炎症及调节神经递质合成[10], 但这些方法的治疗效果仍不令人满意, 或是会出现较大的不良反应。越来越多的研究表明, 肠–肝–脑轴是HE病理生理过程的重要组成部分, 肠道菌群在HE的进程中起到了不可忽视的作用[11], 通过调节肠道菌群来干预HE进程的治疗策略越来越引起人们的关注。本文阐述了肠道菌群在HE不同发病机制中所发挥的作用, 以及通过调节肠道菌群治疗HE的最新研究进展, 为进一步探索HE的干预靶标和治疗方案提供新思路(图 1)。
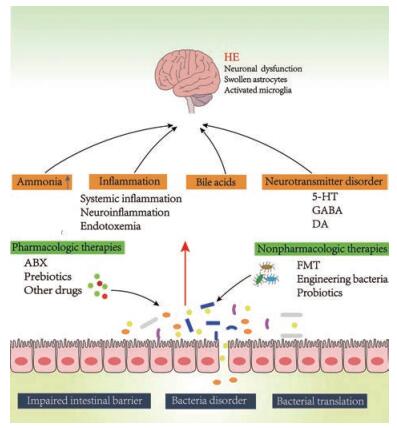
|
Figure 1 Impaired intestinal barrier, gut microbiota disorder, bacterial translation will result in the increased production of ammonia, bile acid metabolism disorders, inflammation, and neurotransmitter dysfunction, which further lead to nerve function disorders. Intestinal flora participates in and influences the pathogenesis of hepatic encephalopathy (HE), therefore, regulating gut microbiota can play a therapeutic role in the treatment of HE. Pharmacologic management of the gut bacteria, including ABX, probiotics, prebiotics, other drugs, and nonpharmacologic management, such as FMT, engineering bacteria, have been used in the treatment of HE. ABX: Antibiotic; FMT: Fecal microbiota transplantation; 5-HT: 5-Hydroxytryptamine; GABA: Gamma aminobutyric acid; DA: Dopamine |
肠道微生物作为一个复杂的器官系统, 由10~100万亿个细菌、真菌和病毒组成, 在健康状态下, 肠道菌群有助于人体合成、吸收营养、增强免疫功能和维护神经系统的稳定性, 使机体保持动态平衡状态[12, 13]。在机体受到疾病及其他不良干扰时, 就会出现肠道菌群的紊乱。肠道菌群的紊乱与肝硬化密切相关。肝硬化患者肠道功能缺陷和微生物改变有多种机制, 其中包括小肠运动受损、肠道通透性增加、抗菌防御受损和小肠细菌过度生长。此外, 由于胆汁酸的合成减少和肝肠循环缺陷, 也会导致肠道微生物改变[14]。有研究采用宏基因组分析方法发现, 大多数肝硬化患者54%的肠道失调菌群种类起源于颊部, 这表明口腔菌大量侵入肠道也是引起肠道菌群失调的重要原因, 导致疾病的恶化[15]。
肝硬化患者肠道菌群的失调主要表现为专性厌氧菌丰度降低, 而需氧菌及兼性厌氧菌丰度相对增高。相对于健康人群, 肝硬化患者肠道中类杆菌的相对丰度明显降低, 而变形杆菌和梭杆菌的比例明显升高。其中肠道杆菌科和链球菌科等潜在致病菌的增加, 以及毛螺菌科等有益菌群的减少可能会影响预后[16]。而Bajaj等[17]的实验表明显性HE患者和非显性HE患者在乙状结肠黏膜区菌落出现较大差异。同时, 肠球菌、巨型球菌和伯克霍尔德氏菌被证明与认知能力差和炎症水平升高有关。
在HE患者体内, 肠道菌群已经被证明其与氨水平、胆汁酸水平、炎症、神经递质分泌等HE的发病机制密切相关, 并且这4种发病机制之间存在一定的内在关系, 互相有影响与交叉。例如氨的增加可引起兴奋性与抑制性神经递质失衡, 干扰正常脑活动[18]。触发氧化或氮化应激反应可导致炎症因子表达增加和炎症反应发生[19]。炎症反应还可破坏血脑屏障, 使其通透性增加, 导致氨等毒性物质和炎症因子进入脑内, 引起神经紊乱[20]。炎症反应也会抑制肝脏胆汁酸的合成, 肠道胆汁酸的减少会使卟啉单胞菌科和肠杆菌科等促炎肠道菌群过度生长, 进一步加重炎症反应[21]。HE的发病机制尚未明确, 可能共同作用并最终导致HE的发生。肠道菌群在HE各个病理环节中发挥了不同作用并产生了不同影响。与肠道菌群直接相关的代谢产物氨与胆汁酸的紊乱是HE非常重要的发病机制。肠道菌群参与HE炎症反应也受到越来越多的关注与研究。另外, 肠道菌群影响了神经递质代谢的分泌, 神经递质代谢紊乱在HE发生过程中的作用也不可忽略。本文将按顺序阐述肠道菌群与氨、胆汁酸、炎症反应和神经递质之间的关系, 以及肠道菌群在HE进程中所发挥的作用。
2.1 肠道菌群与HE发生时体内氨水平异常增加密切相关尽管与HE发病有关的确切因素尚不清楚, 但血氨和脑氨水平的升高所导致的高氨血症通常被认为是HE发病的关键因素[22]。而血氨的升高与肝-肠轴有关[23]。机体的氨主要来源于三方面: ①肠道吸收产生(尿素水解与肠内氨基酸分解); ②肾小管分泌产生(谷氨酰胺被催化水解); ③组织中氨基酸脱氨基分解产生[24]。氨在体内主要经过两个转运过程, 一是谷氨酸与氨合成谷氨酰胺转到肝脏进行分解, 二是氨以无毒丙氨酸转运到肝脏, 最终氨主要经肝脏分解代谢为尿素经尿液排出体外[25]。肝损伤、慢性肝病或尿素循环缺陷患者的全身氨水平升高, 这些患者的肝脏功能异常, 肝脏中特异性谷氨酰胺合成酶(氨代谢中的关键酶) 的缺失使得氨无法在内循环分解而蓄积, 最终引起神经系统的功能紊乱而引发HE[26] (图 2)。
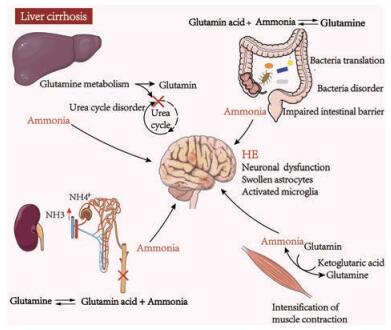
|
Figure 2 Production and transport of ammonia in liver cirrhosis. In patients of liver cirrhosis, intestinal damage and gut dysbacteriosis can lead to the increased production of ammonia. Additionally, increased muscle contractions as well as renal insufficiency also produce more ammonia. Liver damage can cause dysfunction of the urea cycle and reduce ammonia excretion. Eventually, the accumulation of ammonia in the body can lead to brain dysfunction. The transport of ammonia in the body mainly consists of alanine and glutamine. Ammonia can be transported mainly in the form of alanine in the muscle and glutamine in the liver, kidney, and gut |
肠道是产生氨的重要场所。肠道中氨的产生按数量重要性顺序为: ①细菌脲酶水解尿素产生氨; ②细菌蛋白脱氨反应产生氨; ③肠黏膜谷氨酰胺代谢产生氨[27]。肠道中产脲酶细菌主要由革兰阴性杆菌、厌氧菌和革兰阳性细菌产生, 如结肠型细菌包括梭杆菌、产碱杆菌、链球菌和韦荣球菌等可以催化尿素产生氨气[28]。参与蛋白质分解的细菌的种类主要有肠杆菌、变形杆菌、梭杆菌和磺胺化细菌[29]。有实验表明, 链球菌科、肠杆菌科、乳杆菌科和消化链球菌科与氨、终末期肝病模型评分和脑磁共振波谱表现呈正相关, 卟啉单胞菌科与神经元功能障碍和脑水肿有关, 但与氨水平无关[30]。
2.2 肠道菌群与HE发生时体内胆汁酸水平异常增加密切相关除了氨以外, 肠道菌群的另一代谢产物-胆汁酸的代谢紊乱也是HE的重要病因。正常生理情况下, 胆汁酸(BAs) 在外周循环只少量存在, 但在肝损伤过程中, 受损肝细胞释放BA增多并且BA从肠道再吸收增加, 胆汁酸的代谢紊乱而在循环中大量积累[31, 32]。循环中BA的积累可能有助于HE的发展, 降低血清BA浓度已被证实可降低肝衰竭相关神经并发症的严重程度[33], 但其机制尚不清楚, 可能与通过激活法尼酯X受体(farnesoid X receptor, FXR) 和促进肠道铵向氨的转化有关。钠依赖的BAs转运体(apical sodium-dependent BA transporter, ASBT) 介导的BA重吸收增加了肠腔pH, 促进了肠道铵向氨的转化, 导致血液和大脑中神经毒性氨和细胞毒性BA的异常增高。使用ASBT抑制剂SC-435可以有效地清除血液中的神经毒性物质和氨[34]。随后, Jia等[35]提出“肠道菌群-胆汁酸-脑”关系轴可能是阿尔茨海默病/肝性脑病发病的干预靶点, 为治疗HE提供了新的思路。BA代谢产物主要通过孕烷受体(pregnane X receptor, PXR) 和FXR调节肠道菌群的分布[36]。在HE患者体内, 胆汁酸代谢异常通常也会引起肠道菌群结构的紊乱, 肠道菌群物种的多样性和丰度均会发生改变。药物通过作用于潜在靶点包括胆汁BA受体、FXR和G蛋白偶联胆汁酸受体1 (G-protein-coupled bile acid receptor 1, GPBAR1/TGR5), 可以减少胆汁淤积[37, 38], 例如FXR激动剂可以在肝细胞和肠细胞中同时激活FXR受体, 但在肠细胞中需要成纤维细胞生长因子19 (fibroblast growth factor 19, FGF19) 的信号传导来完成, 最后通过抑制胆固醇7α-羟化酶的表达减少胆汁酸合成[39] (图 3), 改善相关代谢和炎症性疾病, 也是治疗HE有效的方法。
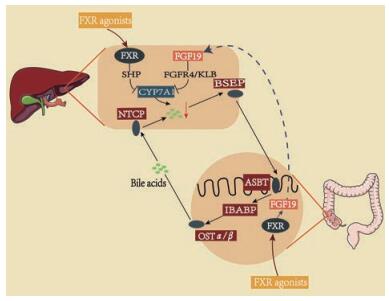
|
Figure 3 The reduction of bile acid synthesis by FXR agonist. Bile acids (BAs) are synthesized in the liver by CYP7A1 enzyme and transported to the small intestine by bile salt outlet pump (BSEP). Most BAs can be re-absorbed into the intestinal epithelial cells in the ileum by the apical sodium-dependent bile acid transporter (ASBT). Then, they are transported from the ileal enterocyte and actively absorbed into the liver cells. In intestinal and hepatic epithelial cells, BAs bind to FXR in the nucleus and stimulate transcription of proteins such as SHP and FGF19. FXR agonist indirectly inhibits the expression of CYP7A1 by activating FXR and reduces the synthesis of liver bile acids. CYP7A1: Cholesterol 7α-hydroxylase; FXR: Farnesoid X receptor; FGF19: Fibroblast growth factor 19; SHP: Short heterodimer partner; FGFR4: Fibroblast growth factor receptor 4; KLB: Klotho-β; NTCP: Na+-taurocholate polypeptide; BSEP: Bile salt export pump; IBABP: Ileal bile acid binding protein; OSTα/β: Organic solute transporter α/β |
近年来越来越多的研究表明, 炎症(包括全身炎症、神经炎症和内毒素血症) 与氨在HE患者的发病进程中起协同作用[40]。肠道稳态失调是全身炎症发生和发展的重要因素。肠道微生物群多样性对先天性和适应性免疫系统的发展和调节至关重要[41]。在肝硬化患者体内, 小肠细菌过度生长、细菌移位(bacterial translocation, BT)、肠道通透性增大等会导致内毒素(包括脂多糖、鞭毛蛋白、肽聚糖和细菌DNA) 的增多[42], 产生直接的神经毒性或是引起外周机制包括免疫功能紊乱和炎症。内毒素能够损伤脑血管内皮细胞, 进而破坏血脑屏障, 或是通过激活Toll样受体(Toll-like receptor, TLR), 肝巨噬细胞会被激活进而产生炎性细胞因子如肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)、白介素(interleukin, IL)-6和IL-8, 导致肝损伤和全身炎症, 进一步使免疫功能紊乱, 诱发HE的发展[43, 44] (图 4)。漆球菌科、瘤胃球菌科和梭菌属XIV的减少以及葡萄球菌科、肠杆菌科和肠球菌科的过度生长和内毒素血症相关[45]。

|
图 4 Pathogenesis of inflammation due to gut dysbacteriosis in HE. The intestinal flora disorder will increase the production of endotoxin. Through activating TLR, liver macrophages will be activated and produce inflammatory cytokines, such as TNF-α, IL-6, NF-κB, and IL-8. Further, it will result in the edema of astrocytes, microglia activation and neurotoxin in the brain. At the same time, there is a decrease in the beneficial products of gut microbiota, especially butyrate, which aggravates inflammation. Butyrate can inhibit inflammatory factors including IL-10 and IFN-γ. Inflammatory factors can be reduced by regulating gut microbiota. TLR: Toll-like receptor; TNF-α: Tumor necrosis factor-α; IL: Interleukin; NF-κB: Nuclear factor kappa B; IFN-γ: Interferon-γ; SCFA: Short-chain fatty acid |
肠道微生物区的改变也推动了肝硬化动物神经炎症和全身性炎症反应的发生。Kang等[46]发现无菌肝硬化小鼠并无全身炎症的升高, 而常规肝硬化小鼠表现出肠道失调和全身炎症, 这与神经炎症和胶质或小胶质细胞活化有关。全身炎症与金葡菌科、乳杆菌科和链球菌科呈负相关。肠杆菌科细菌与血清炎性细胞因子呈正相关。肠道菌群的代谢产物短链脂肪酸(short-chain fatty acid, SCFA) 包括乙酸、丙酸和丁酸, 丁酸盐是结肠形成细胞的主要营养来源, 并且可以通过调节紧密连接蛋白和黏蛋白的表达来改善黏膜完整性, 保护肠道屏障功能[47], 可以减轻炎症。由大肠杆菌、卵形拟杆菌和双温梭状芽孢杆菌产生的吲哚降低了肠上皮细胞中TNF-α介导的活化, 有抗炎作用并且也能增强肠的黏膜屏障功能[48]。而吲哚可进一步转化为奥昔多尔, 其与氨对大脑具有协同毒性作用[49]。
2.4 肠道菌群与HE神经递质的分泌密切相关氨的增加与炎症反应升高会影响神经递质的分泌, 神经递质功能障碍在HE的发生发展中也不可忽略。神经递质功能与γ-氨基丁酸(gamma aminobutyric acid, GABA) 有关, HE患者体内出现明显的GABA浓度升高和GABA受体表达增加[50]。微生物群的变化可以改变神经活性分子的水平, 如一氧化氮(nitric oxide, NO)、P物质和内源性大麻素, 它们有可能影响肠道运动活性。细菌可以产生和/或消耗多种哺乳动物神经递质, 包括多巴胺(dopamine, DA)、去甲肾上腺素(noradrenaline, NA)、5-羟色胺(5-hydroxytryptamine, 5-HT) 或GABA[51]。人体内超过90%的5-HT是在肠内合成的, 它激活肠细胞、肠神经元和免疫系统细胞中14种不同类型的受体[52]。研究显示, 无特殊病原体(specific pathogen free, SPF) 小鼠的血浆5-HT水平约是无菌小鼠的3倍[53]。5-HT一直被认为在中枢神经系统的发育和功能中起着关键作用, 进一步了解肠源性5-HT对脑肠道疾病的具体影响可能为治疗HE创造新的靶点[54]。肠道微生物直接向内皮细胞传递代谢信号, 乳酸杆菌和双歧杆菌等参与DA、NA和乙酰胆碱等兴奋性神经递质的产生, 链球菌和大肠埃希菌可以通过影响宿主结肠肠嗜铬细胞调节5-HT代谢[55]。乳酸杆菌还可提高吲哚胺-2, 3-双加氧酶的活性, 参与色氨酸的分解代谢, 并形成血尿和喹啉酸的神经活性化合物[56]。肠道菌群的代谢产物Ω-3脂肪酸, 特别是二十二碳六烯酸, 在发育和衰老过程中具有神经保护作用[57]。
3 菌群干预HE进展的治疗策略分析肝功能衰竭导致脑功能障碍的病理生理机制复杂, 涉及高氨血症、胆汁酸代谢紊乱、炎性反应、神经递质功能障碍等, 肠道菌群参与并影响了HE发病机制的各个进程, 下文主要通过综述了药物与非药物两种途径来调控肠道菌群减轻HE的病理进程, 从而发挥治疗作用。
3.1 调控肠道菌群降低体内氨水平 3.1.1 药物调控药物靶向调控肠道菌群减少氨的生成已经体现出一定的有效性。生物黏附素A是一种天然存在的类黄酮化合物, 存在于红三叶草和苜蓿等植物中, 最新研究发现, 生物素A通过抑制蛋白分解菌以及抑制肠道中尿素和氨基酸的分解来减少氨的产生[58]。在体外结肠模型系统中, Wang等[59]发现菊粉型果聚糖可以抑制肠道细菌的蛋白水解, 减少了蛋白代谢产物支链脂肪酸和氨的产生, 同时结肠双歧杆菌和乳酸杆菌增多, 脱硫弧菌的丰度降低。柑橘提取物可增强黏膜免疫动态平衡, 显著增加了巴氏杆菌和布劳特氏菌属的丰度, 而降低了另枝菌属和拟杆菌的丰度, 减少了氨基酸的发酵产物(氨、胺、对甲酚和吲哚)[60]。有研究发现肠道菌群可以促进绿茶多酚(-)-表没食子儿茶素没食子酸酯[(-)-epigallocatechin-3-gallate, EGCG)] 与氨快速反应生成EGCG的胺化代谢物, 减少氨在体内的蓄积, 此研究证明EGCG在体内具有清除有毒代谢产物的能力, 并且饮茶可能是预防一些慢性病的潜在策略[61]。
也有研究证实, 采用临床上常用的药物如抗生素(antibiotic, ABX)、乳果糖改变肠道菌群的结构和功能, 可以在一定程度上降低氨的产生[62]。传统上用于治疗HE的ABX, 包括氨基糖苷类和甲硝唑, 受到耳毒性、肾毒性和周围神经病变等不良反应和安全性的限制[63]。利福昔明可以降低肝硬化患者血清可溶性CD163和甘露糖受体水平, 并且部分改变肠道微生物, 特别是维管菌在利福昔明处理后显著减少, 改善了肠道高通透性, 减轻了肝硬化患者的HE和内毒素血症[64]。尽管利福昔明是一种基本不被肠道吸收的ABX, 在预防和治疗HE方面已显示出有效性, 但长期使用可能产生的抗药性仍然令人担忧[65]。双效抗菌剂TNP-2092是一种独特的多靶向药物结合物, 具有极低的耐药倾向, 研究发现其对大鼠肠道菌群的影响与利福昔明相似, 并且可以抑制其中一组脲酶产生菌的活性。该药物目前正在临床开发中, 可以改善肝硬化和肝性脑病相关症状[66]。乳果糖用于酸化粪便, 并以铵的形式分离氨, 但乳果糖耐受性差, 导致患者依附性差[67]。
3.1.2 非药物调控除了药物调控以外, 粪菌移植(fecal microbiota transplantation, FMT)、生物工程菌、益生菌等非药物调控方式在高氨血症的治疗中也有越来越多的应用(表 1[58-61, 68-73])。Zhang等[74]基于16S核糖体RNA的焦磷酸测序技术, 发现唾液链球菌数量的变化与MHE伴有肝硬化的患者中氨气蓄积呈正相关, 肠道增氨菌唾液葡萄球菌有望成为肝硬化MHE患者降氨治疗的潜在生物标志物。Shen等[68]设计了小鼠肠道微生物群来降低脲酶活性, 先通过ABX清除小鼠体内的肠道微生物群, 然后接种改变的Schaedler菌群(altered Schaedler flora, ASF), 这是一个由8个脲酶基因含量最低的细菌组成的联合体, 包括副杆菌属和毛螺菌属等。通过重建一个新的肠道细菌群落, 提高了粪便脲酶活性并且使氨氮产量长期下降。此外, 在硫代乙酰胺诱导的小鼠肝损伤模型中, 通过ASF移植也可以降低发病率和死亡率。这些研究证明宿主通过接种一个确定的肠道微生物群可以发挥持久的治疗作用。Nicaise等[75]发现基因工程技术修饰的乳酸杆菌可通过直接消耗氨来降低体内血氨水平和小鼠的死亡率。随着基因工程技术的不断进步与发展, Kurtz等[69]又通过改造口服益生菌大肠杆菌Nissle 1917, 创造了一种能将NH3转化为L-精氨酸的菌株SYNB1020。该实验上调了SYNB1020中精氨酸的生物合成。在体外系统中, SYNB1020也可以对胃肠道厌氧环境做出反应, 将氨转化为L-精氨酸等氨基酸。SYNB1020减轻了高氨血症, 提高了鸟氨酸转氨酶缺乏的spf-ash小鼠的存活率。Ⅰ期临床试验研究发现其菌株在人体中耐受性良好并呈现出剂量依赖性, 该菌株在临床前高氨血症模型和临床上表现出一定的可行性。
| Table 1 Current methods of regulating gut flora to reduce ammonia level |
益生菌虽在临床上的使用正在增加, 但在应用上缺乏具体和统一的标准, 并且大多数研究都为短期试验, 不能反映长期治疗的益处[76-78]。利用生物工程技术对细菌进行改造也能在未来微生物群介导的治疗中发挥重要作用, 但目前临床开发较少[79]。尽管FMT前景看好[80], 但对于FMT仍存在方法学上的局限性, 这些菌类药物的临床前和转化特征包括安全性、排泄曲线、剂量反应和显示人类菌株活性的生物标记物, 对其研究都是有限的。如何标准化FMT, 如量化移植细菌和建立标准化粪菌库等方面是目前发展FMT的方向[81]。
3.2 调控肠道菌群减轻胆汁酸盐代谢紊乱 3.2.1 药物调控胆汁酸G蛋白偶联受体靶向代谢组学分析显示[82], 华中五味子乙醇提取物五指片可促进血清和肝脏向肠道和粪便排泄BA。同时, 五指片中含有的活性木脂素化合物是PXR激动剂, 因此, 五指片可能通过调节PXR来介导BA和肠道微生物群的稳态。五指片能显著改善小鼠肠道的菌落复杂度和丰度, 减少类杆菌科细菌, 将石胆酸诱导的肠道菌群紊乱逆转至正常水平。强肝方是我国治疗肝病的常用中药方剂, 其提取物可以通过调节BA代谢和肠道菌群改善小鼠非酒精性脂肪性肝炎(non-alcoholic steatohepatitis, NASH), 其机制可能与调节肠道微生物区介导的粪便石胆酸产生, 促进TGR5表达, 以及抑制核因子κB (nuclear factor kappa B, NF-κB) 活化有关。此方主要增加了NASH小鼠石胆酸产生菌类杆菌和梭状芽孢杆菌的数量[83]。热塑性弹性体富含膳食中的黄酮及其糖基衍生物, 通过调节肝肠轴相关的FXR/FGF15通路和FXR靶向蛋白, 对ABX所致的肠道菌群组成的恢复、屏障完整性的重塑和BA在肝肠轴的稳态具有保护作用[84]。树莓渣膳食制剂可以改变高脂饮食的大鼠盲肠微生物的活性, 降低盲肠氨, 减少了次级BA[85]。肝硬化患者的黏液、上皮层以及肠血管屏障被破坏, 使细菌能够进入门静脉循环, 从而进入肠肝轴。两种FXR激动剂OCA和Fex都能减少大肠杆菌向肝脏的病理性转位, 通过门静脉途径降低病理性PBT, 减少管腔BA的蓄积, 调节了肠-血管屏障, 该实验研究提出了FXR对黏液和血管屏障影响肠肝轴的新的调节作用[86]。考利韦仑可增加小鼠粪便中BA的排泄, 促进BA向继发性BA的转化, 从而刺激结肠分泌胰高血糖素样肽-1, 减轻肝、胆管损伤。微生物区系分析显示, 经考利韦仑处理后, δ-变形杆菌门的数量有所增加, 而厚壁菌门由原来的梭状芽孢杆菌向乳酸杆菌转变[87]。
3.2.2 非药物调控目前对于减轻胆汁酸代谢紊乱的非药物调控方式主要集中在益生菌上。鼠李糖乳杆菌(Lactobacillus rhamnosus GG, LGG) 可以减轻胆管结扎小鼠的肝脏炎症、损伤和纤维化, 并显著降低肝BAs。补充LGG可增强肠道FXR/FGF15通路信号, 抑制BA合成, 并促进BA排泄, 预防BA过度蓄积所致的肝损伤。由肠道细菌衍生的胆汁盐水解酶(bile salt hydrolase, BSH) 是能够产生非结合BAs的一种主要酶。LGG本身具有较高的BSH活性, 同时也增加了肠道中含有BSH的菌群, 增加了BA粪便排泄[88]。益生菌VSL#3通过抑制经典的BA合成途径、诱导选择性BA途径和激活回肠TGR5调节的信号通路, 有助于逆转BA合成失调和生物合成障碍引起的NASH。此外, 通过VSL#3治疗, 类杆菌科、卟啉单胞菌科和螺杆菌科减少, 毛螺菌科增加, 能够产生丁酸盐的瘤胃球菌和粪细菌的丰度增加, 重建了肠道菌群[89], 并且Ⅰ期临床试验研究显示其对于HE患者是安全的, 可以减少患者的住院时间[90]。
3.3 调控肠道菌群减轻炎症水平 3.3.1 药物调控天然药物以及肠道有益代谢产物对于肠道菌群的调控能够缓解炎症反应。白藜芦醇可以改善肠道微环境, 减轻了高脂饮食喂养小鼠的非酒精性脂肪性肝病[91]。白藜芦醇可以修复肠黏膜形态, 也可调节肠道细菌组成, 使有害细菌脱硫弧菌、毛螺旋菌科NK4A316和另枝菌属的数量减少, 以及SCFA产生菌的数量增加, 如异杆菌、拟杆菌和布劳特氏菌属, 并减轻炎症。最新研究发现[92], 天然配体银杏内酯-A (ginkgolide-A, GA) 激活PXR可改善肝硬化患者紧密连接蛋白的表达并减轻PBT。GA可能通过PXR介导的NF-κB的拮抗作用减弱了炎性细胞因子的表达, 减轻了炎症。
肠道菌群有益代谢产物也能减轻炎症水平。SCFAs参与维持肠道屏障的完整性和宿主的免疫反应。肝硬化患者血液中SCFA水平下降, 并且丁酸循环水平与门脉高压、内毒素血症和全身炎症呈负相关[93]。研究表明, 产丁酸细菌如厚壁菌门中的柔嫩梭菌增多, 可以减轻宿主全身炎症反应[94]。丁酸盐干预提高了粪便SCFAs浓度, 降低了粪便和血清中的内毒素水平。此外, 丁酸干预可抑制肝组织IL-1β、IL-6和单核细胞趋化蛋白1 (monocyte chemoattractant protein 1, MCP1) 的表达。丁酸的抗炎效应通过选择性地调节肠道微生物群, 如增加SCFAs产生菌和减少内毒素分泌菌的数量。相关分析表明, 内毒素水平与脱硫弧菌科丰度呈正相关[95]。肠道微生物区产生的丙酸对雷公藤甲素诱导的肝毒性有保护作用, 可以减轻炎症水平(TNF-α、IL-6和COX-2)、ATP、丙二醛和肝组织学改变, 补充丙酸可能是改善雷公藤甲素对肝脏毒性的临床策略[96]。
3.3.2 非药物调控有新的研究表明[97], 与肠道菌群相关的小鼠肝硬化的神经炎症可以通过FMT减轻。肝硬化患者粪便微生物定植导致无菌小鼠神经炎症程度增高, GABA能神经元活化。而通过FMT治疗后患者的粪便微生物样本来定植无菌小鼠, 可以减少神经炎症。也有研究发现[98]通过口服FMT胶囊治疗HE的Ⅰ期临床试验是安全的, 这会减轻肠道失调和PBT, 十二指肠黏膜中瘤胃球菌科、双歧杆菌科、链球菌科和细脉杆菌科菌增加, 菌群多样性增加。目前, Ⅱ期临床试验正在进行, 以验证其对神经功能的疗效。补充吲哚-3-乙酸或者工程化的IL-22产生菌可恢复IL-22诱导的抗菌素C型凝集素再生胰岛衍生3γ的表达, 对乙醇诱导的脂肪性肝炎有保护作用, 从而防止细菌移位到肝脏[99]。用唾液酸菌LI01和戊糖酵母菌LI05治疗后, 大鼠血清内毒素、PBT和肠黏膜超微结构的破坏减少, 可以保护肠道屏障; 降低肝脏炎症细胞因子TNF-α、IL-6、IL-17A和肝脏TLR2、TLR4、TLR5、TLR9, 则有益细菌如淋菌和普氏菌增加, 而致病细菌如大肠杆菌减少[100]。益生菌母牛分枝杆菌通过调节性T细胞依赖机制加强免疫调节过程, 增加中枢神经系统中的抗炎细胞因子, 是抑制神经炎症的一种非常有效的策略[101]。
3.4 调控肠道菌群减轻神经递质功能障碍 3.4.1 药物调控经过人参皂苷Rb1 (ginsenoside 1, Rb1) 治疗后, 神经功能损伤减轻和促炎细胞因子表达减少, 特异性益生菌特别是瑞士乳杆菌相对丰度显著提高。Rb1主要是通过调节GABA受体表达和丰度发挥神经保护作用[102]。银杏叶的水溶性多糖可以减轻应激引起的5-HT和DA水平, 并且增加了乳酸杆菌物种的丰富度[103]。Chen等[104]发现, 巴戟天低聚果糖的摄入可以改善炎症和氧化应激障碍, 组织学研究发现其能减轻脑组织肿胀和神经元凋亡, 并调节神经递质的合成和分泌, 如NA、DA、5-HT和5-羟基吲哚乙酸。神经保护剂P7C3-A20通过以LKB1 (liver kinase B1)/AMPK (adenosine monophosphate activated protein kinase)/CRTC2 (CREB regulated transcription coactivator 2) 依赖的方式刺激FGF21和FGF1并改善肠道微生物区来减轻非酒精性脂肪性肝病。P7C3-A20提高了阿克曼属、乳杆菌和普氏菌的比例, 降低了肠杆菌科、大肠杆菌和副杆菌的比例[105]。
3.4.2 非药物调控益生菌在减轻神经递质功能障碍中有一定的作用。益生菌可以维持免疫系统的动态平衡, 益生菌菌株NS8对高氨血症大鼠的认知功能减退和焦虑样行为有一定的治疗作用, 可明显降低炎症标志物水平, 降低5-HT代谢, 恢复认知功能, 可能成为治疗高血氨症介导的HE的潜在药物[106]。低聚半乳糖和富含低聚半乳糖的益生菌抑制了星形胶质细胞和小胶质细胞的激活, 并调节一些与炎症和凋亡相关的因子, 对HE的治疗也有一定的帮助[107]。
4 总结近年来随着肠-肝轴概念的提出, 在肝脏疾病发生发展过程中, 人们越来越重视其与肠道内环境稳态的关系, 肠道微生物在肝脏疾病中作用已经成为近年来的研究热点。利用宏基因组学和代谢组学来研究肠道菌群及其代谢产物如何影响HE及肝硬化的临床过程仍需进一步研究。在临床方面要致力于通过恰当的手段来调整肠道菌群, 就目前以肠道菌群为靶点的治疗手段来看, FMT、微生态制剂、对细菌的生物工程改造等在HE的治疗和预防中也取得了一定进展, 但需要更多的研究来证明其使用的合理性以及临床应用的可行性。在今后的研究工作中, 精确定位到具体哪一种细菌对HE的病理进程产生有利或是有害的影响仍需要进一步探索。如何能进一步明确肠-肝-脑轴之间的确切机制, 从多学科、多角度阐述其中的病理生理模式, 将会给HE的治疗提供新的靶点和治疗手段。
作者贡献: 尹佳婷为本文主要撰写者; 彭印负责文献查阅及文章部分内容撰写和修改; 徐文浩和毛梦菲负责文献整理及部分绘图; 段金廒为本文提出修改意见; 郭建明提出本文的思路并参与文章撰写及修改。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world[J]. J. Hepatol, 2019, 70: 151-171. DOI:10.1016/j.jhep.2018.09.014 |
| [2] |
Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening[J]. Liver Int, 2018, 38(Suppl 1): 2-6. |
| [3] |
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the american association for the study of liver diseases and the european association for the study of the liver[J]. Hepatology, 2014, 60: 715-735. |
| [4] |
Bajaj JS, Cordoba J, Mullen KD, et al. Review article: the design of clinical trials in hepatic encephalopathy——an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement[J]. Aliment Pharmacol Ther, 2011, 33: 739-747. DOI:10.1111/j.1365-2036.2011.04590.x |
| [5] |
Zuo Z, Fan H, Tang XD, et al. Effect of different treatments and alcohol addiction on gut microbiota in minimal hepatic encephalopathy patients[J]. Exp Ther Med, 2017, 14: 4887-4895. |
| [6] |
Elsaid MI, John T, Li Y, et al. The health care burden of hepatic encephalopathy[J]. Clin Liver Dis, 2020, 24: 263-275. DOI:10.1016/j.cld.2020.01.006 |
| [7] |
Wang JY, Zhang NP, Chi BR, et al. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China[J]. World J Gastroenterol, 2013, 19: 4984-4991. DOI:10.3748/wjg.v19.i30.4984 |
| [8] |
Ferenci P. Hepatic encephalopathy[J]. Gastroenterol Rep, 2017, 5: 138-147. DOI:10.1093/gastro/gox013 |
| [9] |
Fiati Kenston SS, Song X, Li Z, et al. Mechanistic insight, diagnosis, and treatment of ammonia-induced hepatic encephalopathy[J]. J Gastroenterol Hepatol, 2019, 34: 31-39. DOI:10.1111/jgh.14408 |
| [10] |
Butterworth RF. Hepatic encephalopathy in cirrhosis: pathology and pathophysiology[J]. Drugs, 2019, 79: 17-21. DOI:10.1007/s40265-018-1017-0 |
| [11] |
Weir V, Reddy KR. Nonpharmacologic management of hepatic encephalopathy: an update[J]. Clin Liver Dis, 2020, 24: 243-261. DOI:10.1016/j.cld.2020.01.003 |
| [12] |
Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review[J]. Hepatol Int, 2018, 12: 135-147. DOI:10.1007/s12072-017-9812-3 |
| [13] |
Lin Z, Zu XP, Xie HS, et al. Research progress in mechanism of intestinal microorganisms in human diseases[J]. Acta Pharm Sin (药学学报), 2016, 51: 843-852. |
| [14] |
Acharya C, Bajaj J. Altered microbiome in patients with cirrhosis and complications[J]. Clin Gastroenterol Hepatol, 2019, 17: 307-321. DOI:10.1016/j.cgh.2018.08.008 |
| [15] |
Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis[J]. Nature, 2014, 513: 59-64. DOI:10.1038/nature13568 |
| [16] |
Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis[J]. Hepatology, 2011, 54: 562-572. DOI:10.1002/hep.24423 |
| [17] |
Bajaj JS, Hylemon PB, Ridlon JM, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 303: G675-G685. DOI:10.1152/ajpgi.00152.2012 |
| [18] |
Bobermin L, Arús B, Leite M, et al. Gap junction intercellular communication mediates ammonia-induced neurotoxicity[J]. Neurotox Res, 2016, 29: 314-324. DOI:10.1007/s12640-015-9581-5 |
| [19] |
Kurmi K, Haigis M. Nitrogen metabolism in cancer and immunity[J]. Trends Cell Biol, 2020, 30: 408-424. DOI:10.1016/j.tcb.2020.02.005 |
| [20] |
Lv XY, Li L. Clinical effect of rifaximin in treatment of complications associated with liver cirrhosis[J]. J Clin Hepatol (临床肝胆病杂志), 2018, 34: 1551-1554. |
| [21] |
Ridlon J, Alves J, Hylemon P, et al. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship[J]. Gut Microbes, 2013, 4: 382-387. DOI:10.4161/gmic.25723 |
| [22] |
Jayakumar AR, Norenberg MD. Hyperammonemia in hepatic encephalopathy[J]. J Clin Exp Hepatol, 2018, 8: 272-280. DOI:10.1016/j.jceh.2018.06.007 |
| [23] |
Ohtani N, Kawada N. Role of the gut-liver axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship[J]. Hepatol Commun, 2019, 3: 456-470. DOI:10.1002/hep4.1331 |
| [24] |
Liu J, Lkhagva E, Chung HJ, et al. The pharmabiotic approach to treat hyperammonemia[J]. Nutrients, 2018, 10: 140. DOI:10.3390/nu10020140 |
| [25] |
Kurmi K, Haigis M. Nitrogen metabolism in cancer and immunity[J]. Trends Cell Biol, 2020, 30: 408-424. DOI:10.1016/j.tcb.2020.02.005 |
| [26] |
Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation[J]. J Clin Exp Hepatol, 2015, 5: S7-S20. |
| [27] |
Levitt DG, Levitt MD. A model of blood-ammonia homeostasis based on a quantitative analysis of nitrogen metabolism in the multiple organs involved in the production, catabolism, and excretion of ammonia in humans[J]. Clin Exp Gastroenterol, 2018, 11: 193-215. DOI:10.2147/CEG.S160921 |
| [28] |
Sawhney R, Jalan R. Liver: the gut is a key target of therapy in hepatic encephalopathy[J]. Nat Rev Gastroenterol Hepatol, 2015, 12: 7-8. DOI:10.1038/nrgastro.2014.185 |
| [29] |
Gilbert MS, Ijssennagger N, Kies AK, et al. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry[J]. Am J Physiol Gastrointest Liver Physiol, 2018, 315: G159-G170. DOI:10.1152/ajpgi.00319.2017 |
| [30] |
Ahluwalia V, Betrapally NS, Hylemon PB, et al. Impaired gut-liver-brain axis in patients with cirrhosis[J]. Sci Rep, 2016, 6: 26800. DOI:10.1038/srep26800 |
| [31] |
DeMorrow S. Bile acids in hepatic encephalopathy[J]. J Clin Exp Hepatol, 2019, 9: 117-124. DOI:10.1016/j.jceh.2018.04.011 |
| [32] |
Wei LG, Wang XH, Niu M, et al. Metabolomic screening for diagnostic biomarkers of drug-induced chronic liver injury related cirrhosis[J]. Acta Pharm Sin (药学学报), 2019, 54: 1449-1456. |
| [33] |
McMillin M, Frampton G, Quinn M, et al. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure[J]. Am J Pathol, 2016, 186: 312-323. DOI:10.1016/j.ajpath.2015.10.005 |
| [34] |
Xie G, Wang X, Jiang R, et al. Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure[J]. EBioMedicine, 2018, 37: 294-306. DOI:10.1016/j.ebiom.2018.10.030 |
| [35] |
Jia W, Rajani C, Kaddurah-Daouk R, et al. Expert insights: the potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer's disease and hepatic encephalopathy[J]. Med Res Rev, 2020, 40: 1496-1507. DOI:10.1002/med.21653 |
| [36] |
Ridlon JM, Bajaj JS. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics[J]. Acta Pharm Sin B, 2015, 5: 99-105. DOI:10.1016/j.apsb.2015.01.006 |
| [37] |
Trauner M, Fuchs CD, Halilbasic E, et al. New therapeutic concepts in bile acid transport and signaling for management of cholestasis[J]. Hepatology, 2017, 65: 1393-1404. DOI:10.1002/hep.28991 |
| [38] |
Yu HC, Hou SC, Cui B, et al. Research progress on the role of bile acids in regulating glycolipid metabolism[J]. Acta Pharm Sin (药学学报), 2020, 55: 1419-1430. |
| [39] |
Hegyi P, Maléth J, Walters JR, et al. Guts and gall: bile acids in regulation of intestinal epithelial function in health and disease[J]. Physiol Rev, 2018, 98: 1983-2023. DOI:10.1152/physrev.00054.2017 |
| [40] |
Luo M, Guo JY, Cao WK. Inflammation: a novel target of current therapies for hepatic encephalopathy in liver cirrhosis[J]. World J Gastroenterol, 2015, 21: 11815-11824. DOI:10.3748/wjg.v21.i41.11815 |
| [41] |
Nicoletti A, Ponziani FR, Biolato M, et al. Intestinal permeability in the pathogenesis of liver damage: from non-alcoholic fatty liver disease to liver transplantation[J]. World J Gastroenterol, 2019, 25: 4814-4834. DOI:10.3748/wjg.v25.i33.4814 |
| [42] |
Woodhouse CA, Patel VC, Singanayagam A, et al. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease[J]. Aliment Pharmacol Ther, 2018, 47: 192-202. DOI:10.1111/apt.14397 |
| [43] |
Tranah TH, Vijay GK, Ryan JM, et al. Systemic inflammation and ammonia in hepatic encephalopathy[J]. Metab Brain Dis, 2013, 28: 1-5. DOI:10.1007/s11011-012-9370-2 |
| [44] |
Li XL, Jiang W, Fan WM, et al. Role of gut microbiota in the treatment of nonalcoholic fatty liver disease with traditional Chinese medicine[J]. Acta Pharm Sin (药学学报), 2020, 55: 15-24. |
| [45] |
Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications[J]. J Hepatol, 2014, 60: 940-947. DOI:10.1016/j.jhep.2013.12.019 |
| [46] |
Kang DJ, Betrapally NS, Ghosh SA, et al. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice[J]. Hepatology, 2016, 64: 1232-1248. |
| [47] |
Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases?[J]. Obes Rev, 2013, 14: 950-959. DOI:10.1111/obr.12068 |
| [48] |
Bansal T, Alaniz RC, Wood TK, et al. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation[J]. Proc Natl Acad Sci U S A, 2010, 107: 228-233. DOI:10.1073/pnas.0906112107 |
| [49] |
Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases[J]. Gut, 2016, 65: 2035-2044. DOI:10.1136/gutjnl-2016-312729 |
| [50] |
Groiss SJ, Butz M, Baumgarten TJ, et al. GABA-ergic tone hypothesis in hepatic encephalopathy-revisited[J]. Clin Neurophysiol, 2019, 130: 911-916. DOI:10.1016/j.clinph.2019.03.011 |
| [51] |
Strandwitz P. Neurotransmitter modulation by the gut microbiota[J]. Brain Res, 2018, 1693: 128-133. DOI:10.1016/j.brainres.2018.03.015 |
| [52] |
Bermúdez-Humarán LG, Salinas E, Ortiz GG, et al. From probiotics to psychobiotics: live beneficial bacteria which act on the brain-gut axis[J]. Nutrients, 2019, 11: 890. DOI:10.3390/nu11040890 |
| [53] |
Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites[J]. Proc Natl Acad Sci U S A, 2009, 106: 3698-3703. DOI:10.1073/pnas.0812874106 |
| [54] |
Gao K, Mu CL, Farzi A, et al. Tryptophan metabolism: a link between the gut microbiota and brain[J]. Adv Nutr, 2020, 11: 709-723. DOI:10.1093/advances/nmz127 |
| [55] |
Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis[J]. Cell, 2015, 161: 264-276. DOI:10.1016/j.cell.2015.02.047 |
| [56] |
Forsythe P, Sudo N, Dinan T, et al. Mood and gut feelings[J]. Brain Behav Immun, 2010, 24: 9-16. DOI:10.1016/j.bbi.2009.05.058 |
| [57] |
Rönnemaa E, Zethelius B, Vessby B, et al. Serum fatty-acid composition and the risk of Alzheimer's disease: a longitudinal population-based study[J]. Eur J Clin Nutr, 2012, 66: 885-890. DOI:10.1038/ejcn.2012.63 |
| [58] |
Harlow BE, Flythe MD, Kagan IA, et al. Isoflavone supplementation, via red clover hay, alters the rumen microbial community and promotes weight gain of steers grazing mixed grass pastures[J]. PLoS One, 2020, 15: e0229200. DOI:10.1371/journal.pone.0229200 |
| [59] |
Wang X, Gibson GR, Sailer M, et al. Prebiotics inhibit proteolysis by gut bacteria in a host diet-dependent manner: a three-stage continuous in vitro gut model experiment[J]. Appl Environ Microbiol, 2020, 86: e02730-19. |
| [60] |
Yu M, Li Z, Chen W, et al. Dietary supplementation with citrus extract altered the intestinal microbiota and microbial metabolite profiles and enhanced the mucosal immune homeostasis in yellow-feathered broilers[J]. Front Microbiol, 2019, 10: 2662. DOI:10.3389/fmicb.2019.02662 |
| [61] |
Zhang S, Zhao Y, Ohland C, et al. Microbiota facilitates the formation of the aminated metabolite of green tea polyphenol (-)-epigallocatechin-3-gallate which trap deleterious reactive endogenous metabolites[J]. Free Radic Biol Med, 2019, 131: 332-344. DOI:10.1016/j.freeradbiomed.2018.12.023 |
| [62] |
Alimirah M, Sadiq O, Gordon SC. Novel therapies in hepatic encephalopathy[J]. Clin Liver Dis, 2020, 24: 303-315. DOI:10.1016/j.cld.2020.01.009 |
| [63] |
Leise MD, Poterucha JJ, Kamath PS, et al. Management of hepatic encephalopathy in the hospital[J]. Mayo Clin Proc, 2014, 89: 241-253. DOI:10.1016/j.mayocp.2013.11.009 |
| [64] |
Kaji K, Saikawa S, Takaya H, et al. Rifaximin alleviates endotoxemia with decreased serum levels of soluble CD163 and mannose receptor and partial modification of gut microbiota in cirrhotic patients[J]. Antibiotics (Basel), 2020, 9: 145. DOI:10.3390/antibiotics9040145 |
| [65] |
Coronel-Castillo CE, Contreras-Carmona J, Frati-Munari AC, et al. Efficacy of rifaximin in the different clinical scenarios of hepatic encephalopathy[J]. Rev Gastroenterol Mex, 2020, 85: 56-68. |
| [66] |
Yuan Y, Wang X, Xu X, et al. Evaluation of a dual-acting antibacterial agent, TNP-2092, on gut microbiota and potential application in the treatment of gastrointestinal and liver disorders[J]. ACS Infect Dis, 2020, 6: 820-831. DOI:10.1021/acsinfecdis.9b00374 |
| [67] |
Wijdicks EFM. Lactulose: a simple sugar in a complex encephalopathy[J]. Neurocrit Care, 2018, 28: 154-156. DOI:10.1007/s12028-017-0494-4 |
| [68] |
Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia[J]. J Clin Invest, 2015, 125: 2841-2850. DOI:10.1172/JCI79214 |
| [69] |
Kurtz CB, Millet YA, Puurunen MK, et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans[J]. Sci Transl Med, 2019, 11: eaau7975. DOI:10.1126/scitranslmed.aau7975 |
| [70] |
Rivera-Flores R, Morán-Villota S, Cervantes-Barragán L, et al. Manipulation of microbiota with probiotics as an alternative for treatment of hepatic encephalopathy[J]. Nutrition, 2020, 73: 110693. DOI:10.1016/j.nut.2019.110693 |
| [71] |
Bajaj J, Fagan A, Gavis E, et al. Long-term outcomes after fecal microbiota transplant in cirrhosis[J]. Gastroenterology, 2019, 15: 1921-1923. |
| [72] |
Wang JY, Bajaj JS, Wang JB, et al. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: a multicenter, randomized controlled trial[J]. J Dig Dis, 2019, 20: 547-556. DOI:10.1111/1751-2980.12816 |
| [73] |
Ponziani FR, Scaldaferri F, Petito V, et al. The role of antibiotics in gut microbiota modulation: the eubiotic effects of rifaximin[J]. Dig Dis, 2016, 34: 269-278. DOI:10.1159/000443361 |
| [74] |
Zhang Z, Zhai H, Geng J, et al. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing[J]. Am J Gastroenterol, 2013, 108: 1601-1611. DOI:10.1038/ajg.2013.221 |
| [75] |
Nicaise C, Prozzi D, Viaene E, et al. Control of acute, chronic, and constitutive hyperammonemia by wild-type and genetically engineered Lactobacillus plantarum in rodents[J]. Hepatology, 2008, 48: 1184-1192. DOI:10.1002/hep.22445 |
| [76] |
Sharma BC, Singh J. Probiotics in management of hepatic encephalopathy[J]. Metab Brain Dis, 2016, 31: 1295-1301. DOI:10.1007/s11011-016-9826-x |
| [77] |
Peng D, Hu ZW, Zhang XW. Therapeutic perspectives of intestinal probiotics A. muciniphila in metabolic disorders[J]. Acta Pharm Sin (药学学报), 2019, 54: 768-777. |
| [78] |
Sun YM, Zhang YT, Zhang JH, et al. Advances in the study of gut pharmacomicrobiomics[J]. Acta Pharm Sin (药学学报), 2020, 55: 2314-2321. |
| [79] |
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease[J]. N Engl J Med, 2016, 375: 2369-2379. DOI:10.1056/NEJMra1600266 |
| [80] |
Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis[J]. J Hepatol, 2020, 72: 1003-1027. DOI:10.1016/j.jhep.2020.01.017 |
| [81] |
Fuentes S, de Vos WM. How to manipulate the microbiota: fecal microbiota transplantation[J]. Adv Exp Med Biol, 2016, 902: 143-153. |
| [82] |
Li DS, Huang QF, Guan LH, et al. Targeted bile acids and gut microbiome profiles reveal the hepato-protective effect of WZ tablet (Schisandra sphenanthera extract) against LCA-induced cholestasis[J]. Chin J Nat Med, 2020, 18: 211-218. |
| [83] |
Li Q, Li M, Li F, et al. Qiang-Gan formula extract improves non-alcoholic steatohepatitis via regulating bile acid metabolism and gut microbiota in mice[J]. J Ethnopharmacol, 2020, 258: 112896. DOI:10.1016/j.jep.2020.112896 |
| [84] |
Liu L, Liu Z, Li H, et al. Naturally occurring TPE-CA maintains gut microbiota and bile acids homeostasis via FXR signaling modulation of the liver-gut axis[J]. Front Pharmacol, 2020, 11: 12. DOI:10.3389/fphar.2020.00012 |
| [85] |
Fotschki B, Juśkiewicz J, Jurgoński A, et al. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high-fat diet[J]. J Nutr Biochem, 2017, 46: 13-20. DOI:10.1016/j.jnutbio.2017.03.004 |
| [86] |
Sorribas M, Jakob MO, Yilmaz B, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis[J]. J Hepatol, 2019, 71: 1126-1140. DOI:10.1016/j.jhep.2019.06.017 |
| [87] |
Fuchs CD, Paumgartner G, Mlitz V, et al. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2-/- mice by modulating composition, signalling and excretion of faecal bile acids[J]. Gut, 2018, 67: 1683-1691. DOI:10.1136/gutjnl-2017-314553 |
| [88] |
Liu Y, Chen K, Li F, et al. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice[J]. Hepatology, 2020, 71: 2050-2066. DOI:10.1002/hep.30975 |
| [89] |
Jena PK, Sheng L, Li Y, et al. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model[J]. Hepatobiliary Surg Nutr, 2019, 9: 170-182. |
| [90] |
Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial[J]. Gastroenterology, 2014, 147: 1327-1337. DOI:10.1053/j.gastro.2014.08.031 |
| [91] |
Wang P, Wang J, Li D, et al. Targeting the gut microbiota with resveratrol: a demonstration of novel evidence for the management of hepatic steatosis[J]. J Nutr Biochem, 2020, 81: 108363. DOI:10.1016/j.jnutbio.2020.108363 |
| [92] |
Mohandas S, Vairappan B. Pregnane X receptor activation by its natural ligand ginkgolide-A improves tight junction proteins expression and attenuates bacterial translocation in cirrhosis[J]. Chem Biol Interact, 2020, 315: 108891. DOI:10.1016/j.cbi.2019.108891 |
| [93] |
Juanola O, Ferrusquía-Acosta J, García-Villalba R, et al. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis[J]. FASEB J, 2019, 33: 11595-11605. DOI:10.1096/fj.201901327R |
| [94] |
Duan Y, Wang Y, Dong H, et al. Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation[J]. Front Microbiol, 2018, 9: 2191. DOI:10.3389/fmicb.2018.02191 |
| [95] |
Zhai S, Qin S, Li L, et al. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice[J]. FEMS Microbiol Lett, 2019, 366: fnz153. |
| [96] |
Huang JF, Zhao Q, Dai MY, et al. Gut microbiota protects from triptolide-induced hepatotoxicity: key role of propionate and its downstream signalling events[J]. Pharmacol Res, 2020, 155: 104752. DOI:10.1016/j.phrs.2020.104752 |
| [97] |
Liu R, Kang JD, Sartor RB, et al. Neuroinflammation in murine cirrhosis is dependent on the gut microbiome and is attenuated by fecal transplant[J]. Hepatology, 2020, 71: 611-626. DOI:10.1002/hep.30827 |
| [98] |
Bajaj JS, Salzman NH, Acharya C, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial[J]. Hepatology, 2019, 70: 1690-1703. DOI:10.1002/hep.30690 |
| [99] |
Hendrikx T, Duan Y, Wang Y, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice[J]. Gut, 2019, 68: 1504-1515. DOI:10.1136/gutjnl-2018-317232 |
| [100] |
Shi D, Lv L, Fang D, et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4-induced liver cirrhosis by protecting the intestinal barrier in rats[J]. Sci Rep, 2017, 7: 6927. DOI:10.1038/s41598-017-07091-1 |
| [101] |
Frank MG, Fonken LK, Watkins LR, et al. Could probiotics be used to mitigate neuroinflammation?[J]. ACS Chem Neurosci, 2019, 10: 13-15. DOI:10.1021/acschemneuro.8b00386 |
| [102] |
Chen H, Shen J, Li H, et al. Ginsenoside Rb1 exerts neuroprotective effects through regulation of Lactobacillus helveticus abundance and GABAA receptor expression[J]. J Ginseng Res, 2020, 44: 86-95. DOI:10.1016/j.jgr.2018.09.002 |
| [103] |
Chen P, Hei M, Kong L, et al. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome[J]. Food Funct, 2019, 10: 8161-8171. DOI:10.1039/C9FO01178A |
| [104] |
Chen D, Yang X, Yang J, et al. Prebiotic effect of fructooligosaccharides from Morinda officinalis on Alzheimer's disease in rodent models by targeting the microbiota-gut-brain axis[J]. Front Aging Neurosci, 2017, 9: 403. DOI:10.3389/fnagi.2017.00403 |
| [105] |
Hua X, Sun DY, Zhang WJ, et al. P7C3-A20 alleviates fatty liver by shaping gut microbiota and inducing FGF21/FGF1, via the AMP-activated protein kinase/CREB regulated transcription coactivator 2 pathway[J]. Br J Pharmacol, 2020. DOI:10.1111/bph.15008 |
| [106] |
Luo J, Wang T, Liang S, et al. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat[J]. Sci China Life Sci, 2014, 57: 327-335. DOI:10.1007/s11427-014-4615-4 |
| [107] |
Song L, Gao Y, Zhang X, et al. Galactooligosaccharide improves the animal survival and alleviates motor neuron death in SOD1G93A mouse model of amyotrophic lateral sclerosis[J]. Neuron, 2013, 246: 281-290. |
 2021, Vol. 56
2021, Vol. 56


