2. 华中科技大学同济医学院附属同济医院药学部, 湖北 武汉 430000;
3. 北京市药品检验所, 北京 102206
2. Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430000, China;
3. Beijing Institute for Drug Control, Beijing 102206, China
甘草是我国最常用的大宗药材之一, 《中华人民共和国药典》[1]明确规定甘草药材的质量标准为三萜类化合物甘草酸的含量不低于2.0%、黄酮类化合物甘草苷的含量不低于0.5%。近年来, 针对甘草药材有效成分生物合成途径的研究多集中于三萜生物合成途径。现代药理学研究表明, 甘草黄酮类化合物具有重要的药理活性, 是甘草发挥抗炎[2]、抗病毒[3]、抗癌[4]等作用不可替代的成分。因此, 甘草黄酮生物合成途径亦具有重要的研究价值。
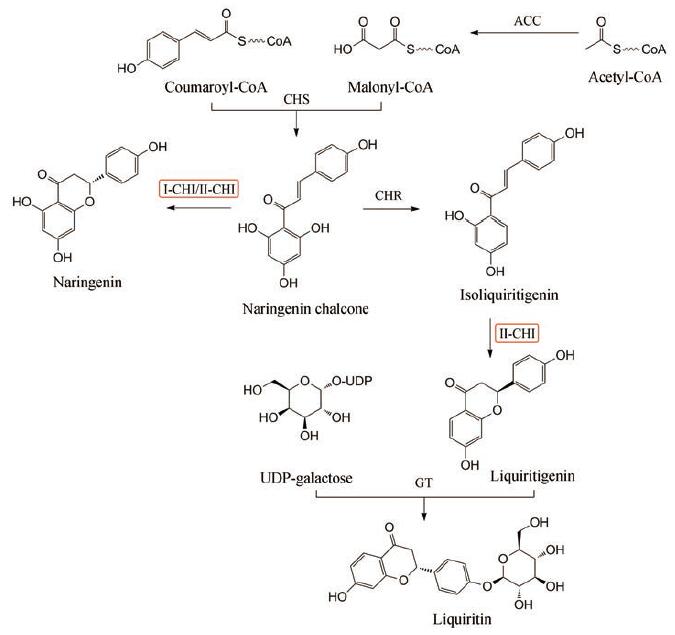
甘草黄酮生物合成途径受到多个酶的调控, 其中查尔酮异构酶(chlacone isomerase, CHI)是最早被认识的类黄酮物质合成相关酶[5], 也是甘草苷生物合成途径上的第二个限速酶。CHI可分为2种类型, Ⅰ型CHI只能以查耳酮为底物, Ⅱ型CHI既可以查耳酮为底物, 也可以6′-脱氧查耳酮为底物[6]。图 1所示为甘草苷生物合成途径, CHI催化分子内环化反应, 将双环查尔酮转化为三环(2S)-黄烷酮[7]。开展CHI功能的相关研究对于解析甘草苷生物合成的分子调控机制具有重要意义。
|
Figure 1 The biosynthetic pathway of flavonoids in licorice.ACC: Acetyl-Co A carboxylase, CHS: Chalcone synthase, CHI: Chalcone isomerase, CHR: Chalcone reductase, GT: Galactosyltransferase |
自1987年首次从法国豌豆(Pisum sativum L.) 中克隆得到CHI基因以来[8], 已陆续从矮牵牛(Petunia hybrida Vilmorin.)[9]、菜豆(Phaseolus vulgaris L.)[10]、玉米(Zea mays L.)[11]、紫花苜蓿(Medicago sativa L.)[12]、水母雪莲(Saussurea medusa Maxim.)[13]等多种植物中克隆得到该基因。目前GenBank数据库中已注册了3 000多条植物CHI基因cDNA序列。CHI基因对于黄酮生物合成具有重要的调控作用, Muir等[14]在番茄(Lycopersicon esculentum Miller.) 中过表达CHI基因, 使得番茄果皮中黄酮含量增加了76倍, 果肉中黄酮醇含量增加了21倍。Kim等[15]发现CHI基因缺失的大麦(Hordeum vulgare L.) 和洋葱(Allium cepa L.) 突变体中黄酮类化合物含量急剧下降。目前, 甘草中CHI基因的报道相对较少, 且主要集中于基因克隆和生物信息学分析[16]。
毛状根是发根农杆菌(Agrobacterium rhizogenes)侵染植物受伤部位而产生的一种病理表现。由于毛状根能合成植物的特征次生代谢产物, 因此毛状根培养技术在药用植物领域受到了较多关注。目前, 黄芩[17]、丹参[18]、西洋参[19]等药用植物毛状根的研究均取得了较好的成就。近年来, 越来越多的学者通过毛状根聚焦植物基因的功能研究, 如: 在丹参毛状根中过表达SmMYB9b可使丹参酮含量增加2.2倍[20]; 在桔梗毛状根中过表达HMGR可提高植物甾醇和三萜类化合物的积累[21]; 利用CRISPR/Cas9定向敲除丹参SmCPS1基因可使丹参毛状根中的丹参酮, 尤其是隐丹参酮、丹参酮IIA和丹参酮I在纯合突变体中完全缺失[22]。因此, 本文也将采用毛状根培养技术解析甘草CHI基因的功能, 为阐明甘草黄酮类化合物生物合成的分子调控机制奠定基础。
材料与方法材料 健康饱满的甘草种子。
目标基因 本课题组前期开展了CHI基因多态性与甘草黄酮生物合成的相关性研究[23], 确定了黄酮高含量甘草的特异CHI单倍型(Gen Bank注册号: KY115232), 将其保存于大肠杆菌中, 本文以此基因作为目标基因, 开展相关研究。
试剂与耗材 植物双元表达载体pCAMBIA1305.1购于北京华越洋生物科技有限公司; DNA Marker(BM2000、BM5000、BM15000)、高纯质粒小量快速提取试剂盒(离心柱型)均购于北京博迈德科技发展有限公司; 限制性内切酶Bgl II、Spe I、rTaqDNA聚合酶、dNTP mixture等购于Ta Ka Ra公司; Fast-Fusion克隆试剂盒购于广州复能基因有限公司; 甘草素(liquiritigenin)、异甘草素(isoliquiritigenin)、甘草苷(liquiritin)、异甘草苷(isoliquiritin)批号分别为MUST-15021104、MUST-15042010、MUST-15082811、MUST-15031204, 纯度分别为99.07%、98%、98.5%、99.99%, 均购于成都曼思特生物科技有限公司。
仪器 TC-3000 PCR扩增仪(英国Techne公司); 1-13000型离心机(美国Sigma公司); DYY-8型稳压稳流电泳仪(上海琪特分析仪器有限公司); BG-gds AUTO510型凝胶成像系统(上海培清科技有限公司); Gene Pulser MXcell电穿孔仪(美国BIO-RAD公司); LRH-250生化培养箱(上海一恒科技有限公司)。
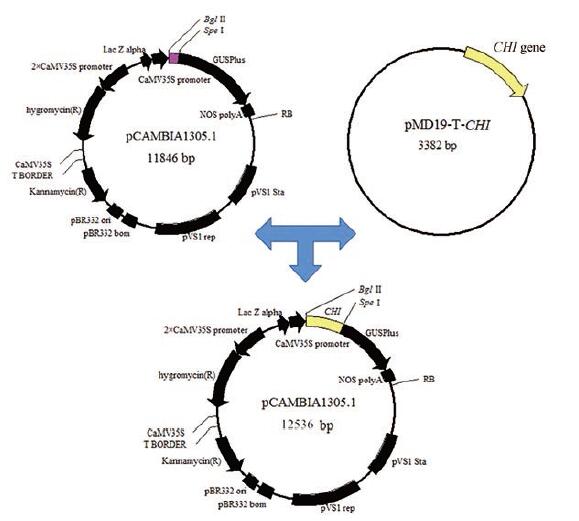
过表达甘草CHI基因植物双元表达载体的构建 图 2为过表达甘草CHI基因植物双元表达载体的构建策略。将含甘草特异CHI基因的大肠杆菌工程菌进行活化并提取质粒。按照Fusion重组酶引物设计原理设计引物CHIF和CHIR (表 1), 并进行PCR扩增, 50 μL反应体系如下: cDNA 2.0 μL、10×Buffer 5.0 μL、dNTP (2.5 mmol·L-1) 5.0 μL、CHIF (5μmol·L-1)与CHIR(5μmol·L-1)各2.0 μL、rTaq酶1.0 μL、RNase-free Water33.0 μL, 反应程序如下: 95 ℃ 2 min; 95 ℃ 20 s, 58 ℃ 30 s, 72 ℃ 45 s, 35个循环; 72 ℃ 5 min, 对PCR产物进行胶回收纯化。采用限制性内切酶Bgl II和Spe I对pCAMBIA1305.1进行双酶切, 37 ℃, 5 h, 50 μL酶切体系如下: Bgl II (10 U· μL-1)与Spe I (10 U· μL-1)各2.5 μL、10×H Buffer 5.0 μL、载体DNA 30.0 μL、ddH2O 10.0 μL, 将载体大片段胶回收纯化。利用Fast-Fusion克隆试剂盒, 在25 ℃将载体大片段与PCR产物连接30 min, 10 μL连接体系如下: PCR产物2.0 μL、载体片段5.0 μL、FastFusion Clonase 1.0 μL、10×Clonase Buffer 1.0 μL、ddH2O 1.0 μL。连接产物转化大肠杆菌DH5α感受态细胞, 在含有50 mg·L-1卡那霉素的LB平板上筛选阳性克隆并进行PCR验证及测序验证(由上海生工生物工程有限公司完成), 将验证正确的质粒命名为pCA-CHI。
|
Figure 2 The construction of the recombinant plant expression vector pCA-CHI |
| 表 1 Primers used in this study. a: Primers for the verification of recombinant A.rhizogenes; b: Primers for the verification of CHI in hairy roots; c: Primers for qRT-PCR |
转甘草CHI基因发根农杆菌工程菌的构建 采用电转法(C: 25μF, PC: 200Ω, U: 2 400 V)将重组质粒pCA-CHI导入发根农杆菌ACCC10060感受态细胞。采用含卡那霉素(50 mg·L-1)的YEB平板筛选阳性克隆。设计引物HF、HR (表 1)对工程菌中的CHI进行PCR验证, PCR条件如“过表达甘草CHI基因植物双元表达载体的构建”项下所示。设计引物RF、RR (表 1)对发根农杆菌rolC基因进行PCR验证, PCR反应程序如下: 94 ℃ 5 min; 94 ℃ 30 s, 55 ℃ 30 s, 72 ℃ 1 min, 35个循环; 72 ℃ 10 min。将PCR产物送上海生工生物工程有限公司进行测序。
过表达CHI基因甘草毛状根的诱导及培养 对甘草种子进行表面灭菌, 接种于MS培养基, 4 500 lx光照培养7~10 d可获得无菌苗。在无菌条件下将其胚轴及子叶切下, 浸泡于“转甘草CHI基因发根农杆菌工程菌的构建”项下构建的发根农杆菌工程菌菌液(OD600为0.5左右)中20~30 min, 然后转接于6, 7-V固体培养基, 黑暗条件下共培养2~3 d。采用500 mg·L-1头孢噻肟钠(cefotaxime sodium, Cef)水溶液浸泡共培养的外植体材料5 min, 转接至含500 mg·L-1Cef的6, 7-V固体培养基上, 诱导生根。野生型毛状根的诱导则采用活化后的发根农杆菌ACCC10060浸泡, 其他条件均相同。每7天转接继代除菌, 并逐步降低Cef的浓度, 直至除净。提取各甘草毛状根系DNA进行PCR及测序验证, 将验证正确的毛状根系转接至6, 7-V液体培养基中, 25 ℃、110 r·min-1条件下震荡培养。
转基因甘草毛状根中外源CHI基因拷贝数的测定 提取毛状根DNA, 以Actin基因为内标基因, 根据CHI (Gen Bank注册号: KY115232)和Actin (Gen Bank注册号: EU190972.1)序列信息, 设计qRT-PCR特异性引物IF、IR和AF、AR (表 1)。qRT-PCR反应程序为95 ℃ 2 min; 95 ℃ 20 s, 58 ℃ 30 s, 72 ℃ 45 s, 35个循环; 72 ℃ 5 min。根据本课题组前期建立的拷贝数测定方法[24], 分析各转基因甘草毛状根样品中CHI基因的拷贝数。
UPLC法测定甘草毛状根中黄酮类成分含量 在无菌条件下称取过表达CHI基因甘草毛状根5个样品(I6、I7、I20、I41、I80)及野生型甘草毛状根样品(K15)各2.0 g, 每个样品4个重复, 传代于6, 7-V液体培养基中, 置于25 ℃、110 r·min-1条件下培养20 d。将液体培养的毛状根洗净、60 ℃烘干至恒重、打粉、过60目筛。精密称定各甘草毛状根样品粉末0.1 g, 置于50 mL量瓶中, 加入50%甲醇水溶液定容, 密塞, 超声(频率50 kHz, 功率250 W)提取30 min, 放冷, 用50%甲醇水溶液补至刻度线。采用0.45μm微孔滤膜对超声提取后的溶液进行过滤处理, 取续滤液作为UPLC分析的样品, 采用本课题组前期已建立的UPLC方法对毛状根中4种黄酮类化合物进行含量测定[25]。采用ACQUITY UPLC BEH C18色谱柱(2.1 mm×100 mm, 1.7μm); 以乙腈(A)-0.05%磷酸溶液(B)为流动相, 梯度洗脱, 洗脱程序见表 2; 柱温为40 ℃、流速为0.3 mL·min-1、进样量为1 μL; 在波长276 nm检测甘草素、甘草苷, 360 nm检测异甘草苷, 370 nm检测异甘草素。
| 表 2 UPLC gradient elution procedure |
图 3显示了重组质粒pCA-CHI及重组发根农杆菌工程菌的PCR验证结果, 条带1~3为重组质粒中扩增得到的690 bp片段, 与CHI序列长度一致, 测序结果表明此片段与CHI (Gen Bank注册号: KY115232)具有100%的一致性; 条带7~9为重组工程菌中扩增得到的580 bp片段, 与发根农杆菌rolC基因长度一致, 测序结果表明此序列与发根农杆菌rolC基因(Gen Bank注册号: DQ 160187.1)有99%的一致性; 条带4~6为重组工程菌中扩增得到的1 030 bp片段, 通过Editseq分析, 此片段包含690 bp和340 bp两个片段, 通过DNAMAN分析, 690 bp片段与CHI (Gen Bank注册号: KY115232)具有100%的一致性, 而340 bp片段与GUS基因有100%的一致性(Gen Bank注册号: KT985054.1), 从而表明CHI基因确为外源电转转入而非自身携带。以上结果表明成功构建了含CHI基因的发根农杆菌工程菌。

|
Figure 3 The verification of the recombinant plant expression vector pCA-CHI and the recombinant A.rhizogenes containing CHI gene.M is DNA marker, No.1-3 shows the CHI in pCA-CHI, No.4-6 shows the CHI together with GUS in the recombinant A.rhizogenes, and No.7-9 shows the rol C in the recombinant A.rhizogenes |
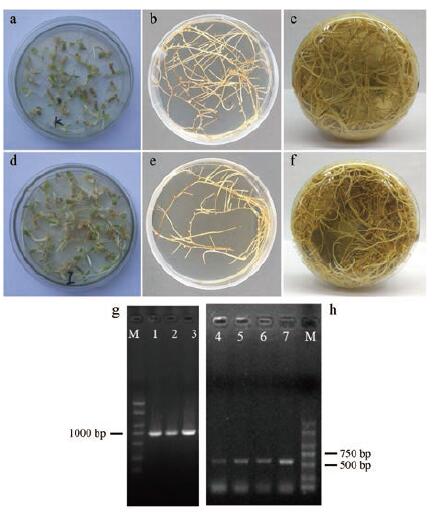
图 4a~4f是侵染后10、20和40天的野生型甘草毛状根和转CHI基因甘草毛状根的生长情况, 均长势良好。图 4g为转基因毛状根中CHI基因的PCR验证结果, 获得了长度约为1 000 bp的条带, 测序结果表明片段长度为1 030 bp, 其中690 bp片段与CHI (Gen Bank注册号: KY115232)具有100%的一致性, 340 bp片段与GUS基因有99%的一致性(Gen Bank注册号: KT985054.1)。图 4h为转基因毛状根中rolC基因的PCR验证结果, 获得了长度约600 bp的条带, 测序结果表明片段长度为580 bp, 与发根农杆菌rolC基因(Gen Bank注册号: DQ 160187.1)具有99%的一致性。以上结果证明已成功获得转CHI基因甘草毛状根。
|
Figure 4 The transgenic hairy roots overexpressing CHI and the wild hairy roots of G. uralensis. a-c: 10, 20, and 40 d old wild type hairy roots culturing on the solid 6, 7-V medium, respectively. d-f: 10, 20, and 40 d old transgenic hairy roots overexpressing CHI culturing on the solid 6, 7-V medium, respectively. g: PCR verification results of the CHI in the transgenic hairy roots. h: PCR verification results of the rolC in the transgenic hairy root samples (No. 4-6) and the wild type hairy root sample (No. 7) |
Actin基因的qRT-PCR标准曲线为: Y = -3.133X + 38.17 (R2 = 0.999 2); CHI基因的qRT-PCR标准曲线为: Y = -3.250X + 40.44 (R2 = 0.998 8)。CHI基因的拷贝数计算结果列于表 3, 各毛状根系中CHI的拷贝数分别为1、1、1、5和1。
| 表 3 Copy number of CHI in different transgenic hairy root lines detected by qRT-PCR |
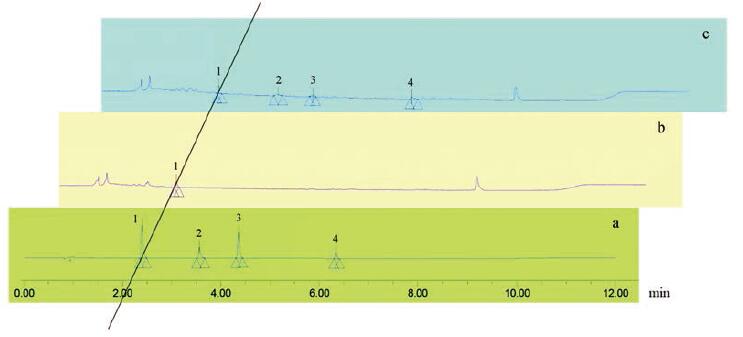
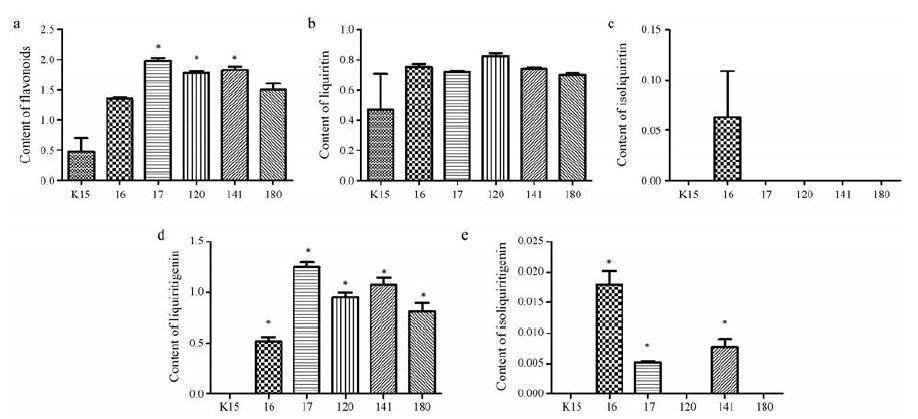
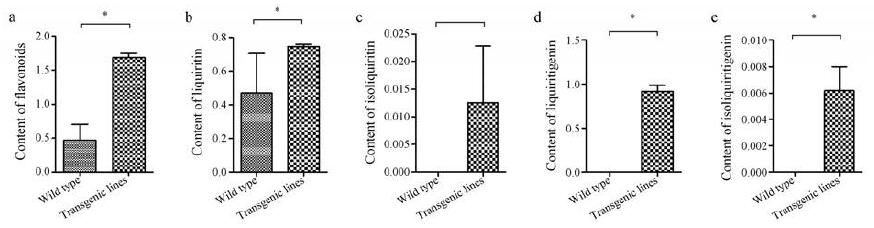
UPLC标准曲线、线性范围、定量限(LOQ)、检测限(LOD)和峰面积相对标准偏差(RSD)值列于表 4中。UPLC色谱图如图 5所示, 甘草苷、异甘草苷、甘草素和异甘草素的保留时间分别为2.447、3.705、4.377和6.378 min。图 6为5个过表达CHI基因甘草毛状根系(I6、I7、I20、I41、I80)及野生型甘草毛状根系(K15)中总黄酮、甘草苷、异甘草苷、甘草素、异甘草素的含量分析柱状图, 结果显示: 5个转CHI甘草毛状根系中总黄酮的含量均高于野生型毛状根, 其中样品I7、I20、I41与野生型毛状根具有显著性差异; 5个转CHI甘草毛状根系中甘草素的含量均显著高于野生型毛状根; 在转CHI毛状根系I6、I7、I41中检测到异甘草素, 而其他根系中未检测到; 5个转CHI甘草毛状根系中甘草苷的含量均高于野生型, 但无显著性差异; 仅在转CHI毛状根系I6中检测到异甘草苷。图 7则直观展示了转CHI基因甘草毛状根与野生型甘草毛状根组间各黄酮类化合物含量的比较分析, 运用SAS 9.4软件对2组样品中各黄酮类化合物及总黄酮含量进行非参数检验, 结果表明转CHI甘草毛状根样品组的总黄酮、甘草苷、甘草素和异甘草素的含量均显著高于野生型毛状根样品。
| 表 4 The UPLC standard curves, linearity range, LOQ, LOD, and RSD of peak area |
|
Figure 5 The UPLC chromatograms of reference substances (a), wild hairy root sample (b), and transgenic hairy root sample (c).Peak 1-4represents liquiritin, isoliquiritin, liquiritigenin, and isoliquiritigenin, respectively |
|
Figure 6 The contents of total flavonoids (a), liquiritin (b), isoliquiritin (c), liquiritigenin (d), and isoliquiritigenin (e) in different transgenic and wild type hairy root lines.Y axis shows the contents of flavonoids (mg·g-1).Paired T test was applied to calculate the content difference among samples.*P < 0.05 vs K15 |
|
Figure 7 Difference in the contents of flavonoids (mg·g-1)between wild type and transgenic hairy roots analyzed by Non-parametric test.Total flavonoids (a), liquiritin (b), isoliquiritin (c), liquiritigenin (d), and isoliquiritigenin (e).*P < 0.05 vs wild type |
功能基因对于药用植物次生代谢产物的积累具有重要的调控作用, 本课题组前期研究发现, 3-羟基-3-甲基戊二酰CoA还原酶(3-hydroxy-3methylglutary Co A reductase, HMGR)[26]以及鲨烯合酶(squalene synthase, SQS)[27]的拷贝数多态性对甲羟戊酸代谢途径(mevalonate pathway, MVA)可产生明确的影响; 还发现过表达β-AS基因的甘草毛状根中甘草酸含量明显增加[28]。因此, 有理由推测CHI作为甘草黄酮类化合物生物合成途径中的关键酶, 也势必会对黄酮类成分的积累发挥重要的调控作用。Zhou等[29]将牡丹CHI基因导入烟草高效遗传转化体系后, 转基因烟草的总黄酮含量比野生型提高了3倍; Park等[30]研究表明CHI基因过表达可增加黄芩毛状根中黄酮类有效成分的含量。因此, 对甘草CHI基因开展相关研究, 也将有助于解析甘草黄酮类化合物生物合成的分子调控机制。
本文通过农杆菌转化法成功构建了5个转甘草CHI基因的毛状根系, 对黄酮类化合物含量进行组间差异分析, 结果显示过表达CHI可有效增加毛状根中总黄酮、甘草苷、甘草素和异甘草素的含量。由于毛状根是植物病理状态下产生的根器官, 与正常生长中的植物根系具有一定的差异, 并非所有的毛状根中均可产生植物特征性次生代谢产物, 因此本文在野生型毛状根中未检测到异甘草苷、甘草素和异甘草素。然而, 在转CHI基因的甘草毛状根系中, 如I6中检测到了全部4种黄酮化合物, I41和I7中检测到了3种, 这表明过表达CHI基因有助于提高甘草黄酮类化合物的生物合成。就黄酮整体水平而言, 样品I7、I20和I41具有更为明显的优势, 可作为后期大规模培养的实验材料, 用于开展离体积累甘草黄酮类化合物的相关研究。然而, 转基因毛状根系中CHI的拷贝数与黄酮含量之间并无直接相关性, I7和I20携带有单拷贝CHI, I41携带有5拷贝CHI, 而它们在黄酮含量方面并无显著性差异, 这可能与基因的表达水平以及酶活水平均具有一定的相关性。
此外, 其他学者研究发现高蔗糖、高生长素的培养基配方[31]、细胞提取物[32]、真菌多糖[33]、重金属离子[34]、光和紫外线辐射[35]等因素对于毛状根的生长均会产生不同程度的影响。因此, 今后将陆续开展毛状根培养条件的优化研究, 以进一步增加转基因甘草毛状根中黄酮类化合物的含量, 为甘草毛状根的大规模培养和黄酮类化合物的离体积累奠定基础。
作者贡献:侯嘉铭撰写了论文; 刘颖和李文东构思并设计了实验方案; 侯嘉铭、尹彦超和田少凯完成了实验; 张智新和杨林分析了相关实验数据; 所有作者均阅读并参与修改了这篇文章。
利益冲突:本文作者均没有利益冲突。
| [1] |
Chinese Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China(中华人民共和国药典)[M]. Part 1 Beijing: China Medical Science Press, 2015: 86-87.
|
| [2] |
Kim YW, Zhao RJ, Park SJ, et al. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-κB-dependent iNOS and proinflammatory cytokines production[J]. Br JPharmacol, 2008, 154: 165-173. |
| [3] |
Grienke U, Braun H, Seidel N, et al. Computer-guided approach to access the anti-influenza activity of licorice constituents[J]. JNat Prod, 2014, 77: 563-570. |
| [4] |
Zhou Y, Ho WS. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 nonsmall cell lung cancer cells[J]. Oncol Rep, 2014, 31: 298-304. DOI:10.3892/or.2013.2849 |
| [5] |
Boland MJ, Wong E. Purification and kinetic properties of chalcone-flavanone isomerase from soya bean[J]. Eur J Biochem, 1975, 50: 383-389. DOI:10.1111/j.1432-1033.1975.tb09814.x |
| [6] |
Shimada N. A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume specific 5-deoxy (iso) flavonoids in Lotus japonicas[J]. Plant Physiol, 2003, 131: 941-951. DOI:10.1104/pp.004820 |
| [7] |
Jez JM, Bowman ME, Dixon RA, et al. Structure and mechanism of the evolutionarily unique plant enzyme chalcone isomerase[J]. Nat Struct Biol, 2000, 7: 786-791. DOI:10.1038/79025 |
| [8] |
Mehdy MC, Lamb CJ. Chalcone isomerase cDNA cloning and mRNA induction by fungal elicitor, wounding and infection[J]. EMBO J, 1988, 86: 1527-1533. |
| [9] |
Van TAJ, Koes RE, Spelt CE, et al. Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light-regulated and differential expression of flavonoid genes[J]. EMBO J, 1988, 7: 1257-1263. DOI:10.1002/j.1460-2075.1988.tb02939.x |
| [10] |
Blyden ER, Doerner PW, Lamb CJ, et al. Sequence analysis of a chalcone isomerase cDNA of Phaseolus vulgaris L.[J]. Plant Mol Biol, 1991, 16: 167-169. DOI:10.1007/BF00017927 |
| [11] |
Grotwold E, Peterson T. Isolation and characterization of a maize gene encoding chalcone flavonone isomerase[J]. Mol Gen Genet, 1994, 242: 1-8. DOI:10.1007/BF00277341 |
| [12] |
Mckhann HI, Hirsch AM. Isolation of chalcone synthase and chalcone isomerase cDNAs from alfalfa (Medicago sativa L.): highest transcript levels occur in young roots and root tips[J]. Plant Mol Biol, 1994, 24: 767-777. DOI:10.1007/BF00029858 |
| [13] |
Li F, Jin Z, Qu W, et al. Cloning of a cDNA encoding the Saussurea medusa chalcone isomerase and its expression in transgenic tobacco[J]. Plant Physiol Biochem, 2006, 44: 455-461. DOI:10.1016/j.plaphy.2006.08.006 |
| [14] |
Muir SR, Collins GJ, Robinson S, et al. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols[J]. Nat Biotechnol, 2001, 19: 470-474. DOI:10.1038/88150 |
| [15] |
Kim S, Jones R, Yoo K S, et al. Gold color in onions (Allium cepa): a natural mutation of the chalcone isomerase gene resulting in a premature stop codon[J]. Mol Genet Genomics, 2004, 272: 411-419. DOI:10.1007/s00438-004-1076-7 |
| [16] |
Li JH. Study on Flavonoid Prenyltransferases from Glycyrrhiza uralensis(乌拉尔甘草黄酮异戊烯基转移酶的研究)[D]. Beijing: Peking Union Medical College, 2014.
|
| [17] |
Elkin YN, Kulesh NI, Stepanova AY, et al. Methylated flavones of the hairy root culture Scutellaria baicalensis[J]. J Plant Physiol, 2018, 231: 277-280. DOI:10.1016/j.jplph.2018.10.009 |
| [18] |
Wang XY, Cui HG, Huang LQ, et al. A full length cDNA of 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol kinase cloning and analysis of introduced gene expression in Salvia miltiorrhiza[J]. Acta Pharm Sin(药学学报), 2008, 43: 1251-1257. |
| [19] |
Kochan E, Nowak A, Zakłos-Szyda M, et al. Panax quinquefolium L. ginsenosides from hairy root cultures and their clones exert cytotoxic, genotoxic and pro-apoptotic activity towards human colon adenocarcinoma cell line Caco-2[J]. Molecules, 2020, 25: E2262. DOI:10.3390/molecules25092262 |
| [20] |
Zhang J, Zhou L, Zheng X, et al. Overexpression of SmMYB9b enhances tanshinone concentration in Salvia miltiorrhiza hairy roots[J]. Plant Cell Rep, 2017, 36: 1297-1309. DOI:10.1007/s00299-017-2154-8 |
| [21] |
Kim YK, Kim JK, Kim YB, et al. Enhanced accumulation of phytosterol and triterpene in hairy root cultures of Platycodon grandiflorum by overexpression of Panax ginseng 3-hydroxy-3-methylglutaryl-coenzyme A reductase[J]. J Agric Food Chem, 2013, 61: 1928-1934. DOI:10.1021/jf304911t |
| [22] |
Li B, Cui G, Shen G, et al. Targeted mutagenesis in the medicinal plant Salvia miltiorrhiza[J]. Sci Rep, 2017, 7: 43320. DOI:10.1038/srep43320 |
| [23] |
Zhang XD. Molecular Mechanism of Licorice Flavonoid Biosynthesis Based on CHI Gene Polymorphism(基于CHI基因多态性的甘草黄酮类化合物生物合成分子机制研究)[D]. Beijing: Beijing University of Chinese Medicine, 2019.
|
| [24] |
Yin YC, Hou JM, Tian SK, et al. Overexpressing chalcone synthase (CHS) gene enhanced flavonoids accumulation in Glycyrrhiza uralensis hairy roots[J]. Bot Lett, 2020, 167: 219-231. DOI:10.1080/23818107.2019.1702896 |
| [25] |
Hu T, Gao ZQ, Yin YC, et al. Determination of seven flavonoids in Glycyrrhiza uralensis Fisch. and Glycyrrhiza glabra L. by UPLC[J]. Chin J Pharm Anal(药物分析杂志), 2019, 39: 763-771. |
| [26] |
Liu Y, Xu QX, Xi PY, et al. Cloning and characterization of a cDNA coding 3-hydroxy-3-methylglutary Co A reductase involved in glycyrrhizic acid biosynthesis in Glycyrrhiza uralensis[J]. Acta Pharm Sin(药学学报), 2013, 48: 773-779. |
| [27] |
Liu Y, Zhang N, Wang XY, et al. Study on the effects of Glycyrrhiza uralensis Fisch squalene synthase gene polymorphism on its enzyme catalytic efficiency[J]. China J Chin Mater Med(中国中药杂志), 2012, 37: 3777-3783. |
| [28] |
Yin YC, Zhang XD, Gao ZQ, et al. Enhancing glycyrrhizic acid accumulation by over-expressingβ-amyrin synthase gene (GuBAS) root-specifically in hairy roots of Glycyrrhiza uralensis[J]. Chin Herb Med, 2019, 11: 192-199. DOI:10.1016/j.chmed.2019.03.001 |
| [29] |
Zhou L, Wang Y, Ren L, et al. Overexpression of Ps-CHI1, a homologue of the chalcone isomerase gene from tree peony (Paeonia suffruticosa), reduces the intensity of flower pigmentation in transgenic tobacco[J]. Plant Cell Tissue Organ Culture, 2013, 116: 285-295. DOI:10.1007%2Fs11240-013-0403-2 |
| [30] |
Park NI, Xu H, Li X, et al. Enhancement of flavone levels through overexpression of chalcone isomerase in hairy root cultures of Scutellaria baicalensis[J]. Funct Integr Genomics, 2011, 11: 491-496. DOI:10.1007/s10142-011-0229-0 |
| [31] |
Gao Y, Wu CH, Piao XC, et al. Optimization of culture medium components and culture period for production of adventitious roots of Echinacea pallida (Nutt.)[J]. Plant Cell Tissue Organ Culture, 2018, 135: 299-307. DOI:10.1007/s11240-018-1464-z |
| [32] |
Jisha S, Gouri PR, Anith KN, et al. Piriformospora indica cell wall extract as the best elicitor for asiaticoside production in Centella asiatica (L.)Urban, evidenced by morphological, physiological and molecular analyses[J]. Plant Physiol Biochem, 2018, 125: 106-115. DOI:10.1016/j.plaphy.2018.01.021 |
| [33] |
Mercier L, Lafitte C, Borderies G, et al. The algal polysaccharide carrageenans can act as an elicitor of plant defence[J]. New Phytol, 2010, 149: 43-51. |
| [34] |
Zhou X, Yang Y. Differential expression of rice Nramp genes in response to pathogen infection, defense signal molecules and metal ions[J]. Physiol Mol Plant Pathol, 2004, 65: 235-243. DOI:10.1016/j.pmpp.2005.02.007 |
| [35] |
Logemann E, Wu SC, Schröder J, et al. Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes[J]. Plant J Cell Mol Biol, 2010, 8: 865-876. |
 2021, Vol. 56
2021, Vol. 56


