2. 延边大学吉林省过敏性常见疾病免疫与靶向研究重点实验室, 吉林 延吉 133002;
3. 延边大学医学院解剖教研室, 吉林 延吉 133002;
4. 延边大学附属医院呼吸与危重症医学科, 吉林 延吉 133000;
5. 延边大学附属医院急诊内科学, 吉林 延吉 133000
2. Jilin Key Laboratory for Immune and Targeting Research on Common Allergic Diseases, Yanbian University, Yanji 133002, China;
3. Department of Anatomy, College of Medicine, Yanbian University, Yanji 133002, China;
4. Respiratory and Critical Care Medicine, Hospital of Yanbian University, Yanji 133000, China;
5. Department of Emergency Medicine, Hospital of Yanbian University, Yanji 133000, China
肥大细胞是主要的免疫细胞, 通过分泌炎症介质诱导过敏性炎症[1]。肥大细胞表面表达多种膜受体, IgE (immunoglobulin E)与FcεRI结合, FcεRI与多价抗原交联导致肥大细胞脱颗粒, 并释放大量生物介质, 包括预形成的分泌颗粒(含组胺和蛋白酶)以及细胞因子[如肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)和白介素-13 (interleukin-13, IL-13)]、生长因子和趋化因子[2]。高迁移率族蛋白B1 (high mobility group protein B1, HMGB1)属于HMG家族的一部分, 是一种非组蛋白, 主要存在于细胞核内, 广泛分布于多种组织细胞中, 是整个HMG家族蛋白中最常表达的蛋白[3], 当其被动释放或主动分泌至细胞外基质时, 其作为促炎细胞因子可介导炎症和免疫反应[4]。在过敏性疾病中, HMGB1起重要作用, 通过抑制HMGB1与TLR4 (toll-like receptor 4)受体结合, 可以抑制哮喘气道炎症反应[5], 但HMGB1在肥大细胞诱导的过敏反应中的具体作用相关报道甚少。
蓝萼甲素(glaucocalyxin A, GLA)是一种具有对映15-氧-16-贝壳杉烯(ent-15-oxo-16-kaurene)骨架结构的二萜类化合物, 其化学结构见图 1。GLA是唇形科香茶菜属植物, 广泛分布在我国的东北和华东等地区, 是蓝萼香茶菜中的主要活性成分, 研究表明, GLA具有抗炎、抗氧化和免疫抑制等多种药理作用, 并且有很高的体内安全性, 主要研究领域是肿瘤和炎性疾病[6, 7]。目前GLA在过敏性疾病中的研究甚少。本实验中, 作者为了评估GLA在过敏性炎症反应中的具体作用机制, 通过体内和体外速发型超敏反应模型, 探讨了蓝萼甲素通过抑制肥大细胞脱颗粒改善过敏性炎症的作用机制。
|
Figure 1 The chemical structure of glaucocalyxin A (GLA).The molecular formula of GLA is C20H28O4, and its molecular weight is332.4339 |
实验动物 雄性清洁级BALB/c小鼠(n=40), 鼠龄4~6周, 体重18±5 g, 雄性Sprague-Dawley大鼠(n=20)购自延边大学健康科学中心(中国延吉), 实验动物合格证号为SCXK (吉) 2017-0003。动物福利和实验过程均遵循延边大学动物伦理委员会的规定。所有小鼠饲养1周, 温度(22±2)℃, 相对湿度55%±5%, 12 h光照-黑暗循环。
药物与试剂 GLA (批号B20698, 上海源叶公司); TNF-α、IL-1β、IL-4和IL-13的酶联免疫吸附剂测定(enzyme-linked immunosorbent assay, ELISA)试剂盒(武汉华美生物工程有限公司); NF-κB (nuclear tran‐scription factor kappa B) p65 (#8242)、phospho-NF-κB p65 (#3039)、PARP (poly ADP-ribose polymerase, 46D11, #9532)、IκBα(inhibitor ofκB alpha, L35A5, #4814)、phospho-IκBα(#2859)、Syk (spleen tyrosine kinase, #13198)、phospho-Syk (#2710)、Lyn (Lck/Yes novel tyrosine kinase, #2796)、phospho-Lyn (#2731)、Gab2(growth-factor receptor-bound protein 2, #3239)、phos‐pho-Gab2 (#3881)、PLC (phospholipase C)γ1 (#2821)、phospho-PLCγ1 (#8713)、HMGB1 (#3935)、TLR4(D8L5W, #14358)、MyD88 (myeloid differentiation factor 88, #3699)、β-actin (#3700)、anti-rabbit Ig G(H+L)(#14708)和anti-mouse Ig G (H+L)(#14709)(美国Cell Signaling Technology公司); anti-DNP(dinitrophenyl)-IgE和DNP-HSA (human serum albumin)(美国Sigma公司); 伊文思蓝(上海如吉生物科技公司)。
仪器 酶标仪EPOCH和细胞成像微孔板检测系统(美国Beckman公司); 15K高速冷冻台式离心机(美国Sigma公司); 透射电镜(日本JEM-1200EX公司); 电泳仪及电泳槽(美国Apparatus Corporation公司); Western blot转膜仪(美国Bio-Rad公司); 分光光度计(Spectra MAX PLUS, 美国Molecular Devices公司); 液体闪烁分析仪(美国Canberra Industries公司)。
实验动物分组 BALB/c小鼠(n=40)分为5组, 即正常组、模型组(IgE+Ag组)和GLA低、中、高剂量组(10、20和40 mg·kg-1), 每组8只。大鼠用于提取腹腔肥大细胞, 不进行分组。
被动皮肤过敏反应(passive cutaneous anaphy‐laxis, PCA) 每只小鼠通过耳部皮内注射含有0.5μg anti-DNP-IgE的50μL磷酸盐缓冲盐水致敏皮肤, 24 h后, 灌胃给予低(10 mg·kg-1)、中(20 mg·kg-1)和高剂量(40 mg·kg-1)的GLA, 1 h后每只小鼠尾静脉注射DNP-HSA (0.1 mg)和4%伊文思蓝(1∶1)混合液激发, 30 min后, 小鼠被人道处死, 双耳被收集, 接着用1 mL甲酰胺在55℃孵育24 h以提取外渗的伊文思蓝染料。染料的吸光度用分光光度计在620 nm测量, 用千分表测量耳厚度。
肥大细胞制备、培养及处理 使用既往实验方法进行大鼠腹腔肥大细胞(rat peritoneal mast cells, RPMCs)的制备及培养[8, 9], 分离的RPMCs细胞培养于含10%胎牛血清、100 u·mL-1青霉素钠和100μg·mL-1链霉素的DMEM (Dulbecco's modified Eagle's medium, 美国Thermo Fisher Scientific公司)培养液中, 在37℃、5%CO2条件下进行培养。RPMCs分为3组, 即对照组、IgE+Ag (antigen)组和IgE+Ag+GLA组。对照组正常培养, 未经任何药物处理; IgE+Ag组用50 ng·mL-1的anti-DNP-IgE处理6 h, 再用100 ng·mL-1的DNP-HSA处理10 min, 诱导肥大细胞脱颗粒; IgE+Ag+GLA组用50 ng·mL-1的anti-DNP-IgE处理6 h, 不同浓度的GLA (纯度≥98%)处理30 min, 100 ng·mL-1的DNP-HSA处理10 min。透射电镜下观察各组细胞形态。
细胞毒性实验 将RPMCs细胞以2×104个/孔接种于96孔板中, 用不同浓度(0.1~10μmol·L-1) GLA处理24 h, 然后于37℃下用1 mg·mL-13-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐(MTT)孵育细胞, 再加入二甲基亚砜溶解甲苯胺晶体, 测定570 nm处的吸光度。
测定组胺释放 向肥大细胞悬液(每毫升1×106个)中加入50 ng·mL-1的anti-DNP-IgE致敏, 并在37℃环境中孵育6 h。然后, 加入或不加GLA (0.1、1.0和5.0μmol·L-1)孵育30 min后洗净, 加入100 ng·mL-1DNP-HSA, 孵育10 min, 离心后取上清液, 采用放射酶法测定组胺含量。
钙摄入量测定 肥大细胞悬液(每毫升1×106个)与含有1.5 mCi·mL-145 Ca2+的HERES-Tyrode缓冲液(无CaCl2和MgCl2)共同孵育。然后用50 ng·mL-1的antiDNP-IgE处理细胞6 h, 添加不同浓度的GLA在37℃下孵育30 min, 用100 ng·mL-1DNP-HSA激发10 min, 用10%Triton-100裂解细胞后, 通过闪烁β计数器测量钙离子的放射性。
ELISA实验 按生产商说明书用ELISA试剂盒测定RPMCs细胞培养液中TNF-α、IL-1β、IL-4和IL-13的水平。
Western blot实验 如前所述方法[10], 提取细胞核和细胞质蛋白后, BCA (bicinchonininc acid)法测定蛋白质量浓度, 取20μg蛋白, 用8%~12%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(dodecyl sulfate, sodium salt-poly‐acrylamide gel electrophoresis, SDS-PAGE)分离蛋白, 然后电转移至聚偏二氟乙烯膜(polyvinylidene fluoride, PVDF), 在5%脱脂奶粉中封闭1 h, 分别加入不同的一抗, 稀释比例为1∶1 000, 4℃过夜, 洗膜, 按照说明书于37℃孵育不同的二抗1 h, 常规洗膜后用高效化学发光(efficient chemiluminescence, ECL)试剂曝光。
统计学分析 采用SPSS 23.0软件进行数据分析, 结果表示为x±s。使用GraphPad Prism 7.0软件进行绘制图表, P < 0.05被视为具有统计学意义。
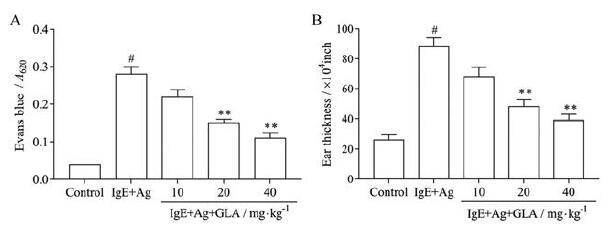
结果 1 GLA对小鼠PCA反应及耳肿胀反应的影响采用PCA模型评价GLA体内抗过敏作用。与IgE+Ag组比较, GLA组均能抑制染料外渗, 其中GLA 20和40 mg·kg-1剂量组有显著性差异(P < 0.01, 图 2A)。用千分表测量耳厚度, IgE+Ag组耳肿胀明显, 经GLA治疗后耳肿胀明显减轻, 与模型组比较有显著差异(P < 0.01, 图 2B)。以上结果提示, 在抗原诱导的变态反应中, GLA能够减轻肥大细胞的脱颗粒, 从而抑制PCA反应。
|
Figure 2 Effect of GLA on anti-dinitrophenyl-immunoglobulin E (DNP-IgE)-induced passive cutaneous anaphylaxis (PCA) in mice.The mice were sensitized with 50 ng·mL-1 anti-DNP-IgE for 6 h, and GLA was administered 1 h before challenging with 100 ng·mL-1 DNP-human serum albumin (HSA).A: The absorbance of dye extravasation is represented; B: Ear thickness is measured with a dial gauge.n=5, x±s.#P < 0.05 vs the control group; **P < 0.01 vs the IgE+antigen (Ag) group |
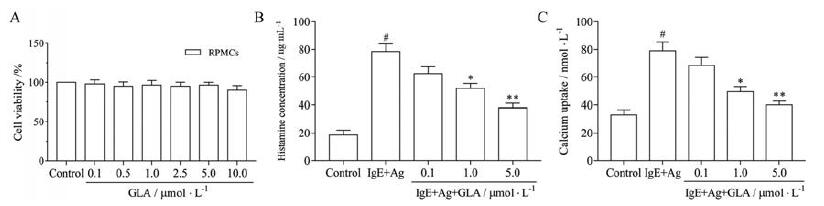
为探索GLA对RPMCs细胞的活性和脱颗粒的影响, 首先用MTT法检测其活性, 用脱颗粒时早期释放介质组胺和细胞内钙离子浓度的测定来评估其脱颗粒作用。MTT实验数据显示, 0.1~10μmol·L-1浓度的GLA对RPMCs细胞活性无明显影响, 其中10μmol·L-1浓度时活性有所下降, 但无统计学意义(图 3A)。与IgE+Ag组比较, GLA处理后明显抑制RPMCs细胞释放组胺及细胞内钙水平(图 3B、C), 以高剂量组作用最为明显(P < 0.01)。
|
Figure 3 Effects of GLA on cell viability and degranulation in rat peritoneal mast cells (RPMCs).RPMCs were stimulated with anti-DNP-IgE (50 ng·mL-1) for 6 h and preincubated at 37℃ for 30 min in the absence or presence of GLA before challenging with DNP-HSA(100 ng·mL-1) for 10 min.A: Cell viability was measured by MTT assay; B: Histamine release rate; C: Calcium uptake.n=5, x±s.#P < 0.05 vs the control group; *P < 0.05, **P < 0.01 vs the IgE+Ag group |
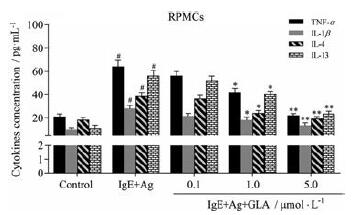
活化的肥大细胞释放促炎细胞因子, 导致过敏性炎症[10], ELISA法检测炎性因子释放水平, 评估GLA对RPMCs分泌促炎细胞因子的影响。结果提示, 与control组相比, IgE+Ag组TNF-α、IL-1β、IL-4和IL-13表达显著升高。与IgE+Ag组相比, IgE+Ag+GLA组的促炎细胞因子水平降低(图 4), 以高剂量组最显著(P < 0.01)。以上结果提示, GLA可以抑制肥大细胞介导的炎性因子的释放。
|
Figure 4 Effects of GLA on the secretion of cytokines in the supernatants of RPMCs.RPMCs were stimulated with anti-DNP-IgE (50 ng·mL-1) for 6 h and preincubated at 37℃ for 30 min in the absence or presence of GLA before challenging with DNP-HSA (100 ng·mL-1) for 10 min.n=5, x±s.#P < 0.05 vs the control group; *P < 0.05, **P < 0.01 vs the IgE+Ag group.TNF-α: Tumor necrosis factor-α; IL: Interleukin |
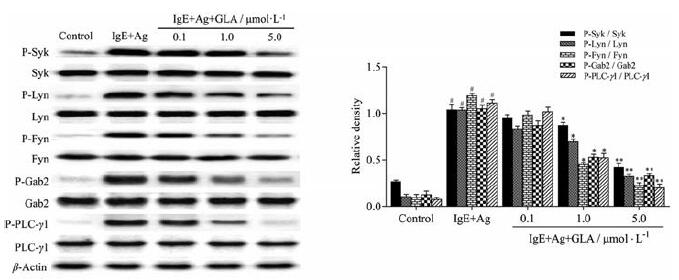
为了预测GLA发挥功能的潜在靶点, 用Western blot法检测RPMCs细胞中FcεRI介导的几个关键蛋白信号通路的影响。如图 5所示, Syk、Lyn、Fyn、Gab2及下游因子PLCγ1信号蛋白的磷酸化水平经anti-DNP-IgE/DNP-HSA刺激后明显增加, 不同浓度的GLA处理后, 可抑制这些信号的蛋白磷酸化水平, 以GLA高剂量组效果最明显, 提示GLA可通过下调FcεRI依赖性信号蛋白磷酸化水平进而抑制肥大细胞脱颗粒。
|
Figure 5 Effects of GLA on FcεRI-mediated signaling pathways in RPMCs.RPMCs were stimulated with anti-DNP-IgE (50 ng·mL-1) for 6 h and preincubated at 37℃ for 30 min in the absence or presence of GLA before challenging with DNP-HSA (100 ng·mL-1) for 10 min.The related proteins and their phosphorylation levels were detected by Western blot assay.n=5, x±s.#P < 0.05 vs the control group; *P < 0.05, **P < 0.01 vs the IgE+Ag group.Syk: Spleen tyrosine kinase; Lyn: Lck/Yes novel tyrosine kinase; Fyn: Tyrosine kinase Fyn; Gab2:Growth-factor receptor-bound protein 2;PLCγ1:Phospholipase Cγ1 |
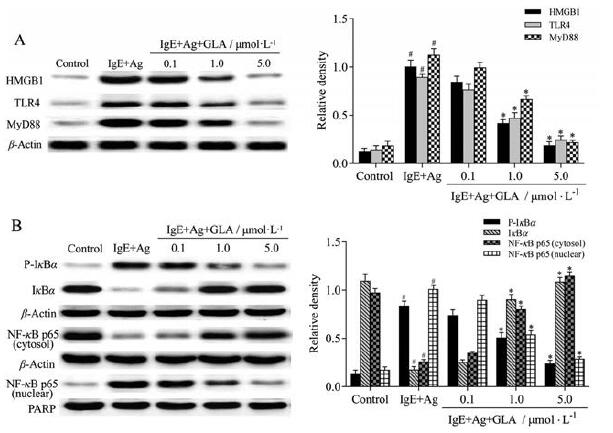
既往研究表明, HMGB1/TLR4信号通路在哮喘和过敏性鼻炎等小鼠模型中异常激活[11, 12]。为了研究GLA是否通过HMGB1/TLR4信号通路来抑制肥大细胞活化, 通过Western blot法测定相关蛋白水平。结果表明, 用anit-DNP-IgE/DNP-HSA刺激后, HMGB1、TLR4及MyD88蛋白表达及IκBα磷酸化水平显著升高, 且NF-κB p65核内转移明显。经过GLA处理后HMGB1、TLR4及MyD88蛋白水平下降, IκBα磷酸化及NF-κB p65核转位水平降低(图 6)。以上结果提示, GLA可以通过抑制HMGB1/TLR4/NF-κB信号通路抑制肥大细胞脱颗粒及NF-κB p65的核转位。
|
Figure 6 GLA on high mobility group box 1 (HMGB1)-related signaling pathways in RPMCs.A: Effect of GLA on toll-like receptor 4(TLR4) and myeloid differentiation factor 88 (MyD88) downstream of HMGB1 in RPMCs.RPMCs were stimulated with anti-DNP-IgE(50 ng·mL-1) for 6 h and preincubated at 37℃ for 30 min in the absence or presence of GLA before challenging with DNP-HSA (100 ng·mL-1)for 10 min, and protein levels were measured by Western blot; B: Effect of GLA on nuclear transcription factor kappa B (NF-κB), a down‐stream factor of MyD88.Cells were treated as above.n=5, x±s.#P < 0.05 vs the control group; *P < 0.05 vs the IgE+Ag group.IκBα: Inhibitor ofκB alpha; P-IκBα: Phospho-IκBα; PARP: Poly ADP-ribose polymerase |
在世界范围内, 过敏性疾病的发病率迅速上升[13]。肥大细胞在过敏性疾病中起关键作用, IgE/FcεRI介导肥大细胞脱颗粒及炎症介质的分泌引起过敏反应[14, 15]。PCA动物模型可在体内评价肥大细胞介导的局限性过敏反应[16, 17]。在IgE/FcεRI介导小鼠PCA反应中, 肥大细胞被激活, 激活的肥大细胞释放组胺引起血管通透性增加, 引起致敏部位血管通透性增加, 血浆渗出和局部肿胀。本实验中, 可以观察GLA能显著抑制PCA小鼠中伊文思蓝的渗漏和耳部肿胀, 表明GLA能明显抑制肥大细胞介导的局限性过敏反应。
肥大细胞的活化依赖于Syk的激活, Syk的激活受FcεRI与Lyn和Fyn因子相互作用的调节[18]。Syk激活下游分子Gab2和PLCγ1通过三磷酸肌醇引起细胞内钙离子变化和PI3K (phosphatidylinositol 3-kinase)/AKT(protein kinase B)途径激活NF-κB, 在肥大细胞脱颗粒中起重要作用[2]。作者数据显示, GLA抑制anti-DNP-IgE/DNP-HSA诱导的Fyn、Lyn、Syk、Gab2和PLCγ1的磷酸化水平。FcεRI介导的肥大细胞活化涉及钙内流[19], 细胞内钙的释放导致肥大细胞脱颗粒。在脱颗粒过程中, 肥大细胞分泌多种炎症介质, 包括组胺、β-氨基己糖苷酶、细胞因子、蛋白酶、趋化因子和生长因子[20]。组胺是过敏性炎症的关键介质[21]。本研究结果发现, GLA显著抑制肥大细胞脱颗粒, 降低肥大细胞内的钙水平及组胺释放。肥大细胞活化后分泌的促炎细胞因子进一步促进过敏性炎症反应进展[22], 关键促炎因子包括TNF-α、IL-1β、IL-4和IL-13[10]。作者结果表明, GLA在anti-DNP-IgE/DNP-HSA刺激的RPMCs细胞中抑制了TNF-α、IL-1β、IL-4和IL-13的水平。这些结果提示, GLA通过阻断IgE/FcεRI途径抑制肥大细胞脱颗粒发挥抗过敏作用。
HMGB1是一种促炎性细胞因子, 作为TLR4配体与之结合后激活MyD88, 导致NF-κB抑制蛋白IκBα磷酸化, NF-κB从复合物上解离, 最后NF-κB进入细胞核内与靶基因结合, 促进炎性因子的生成, 进一步诱导炎症反应[5, 12]。研究表明, 特异性皮炎动物模型中通过抑制肥大细胞中HMGB1的表达, 阻止NF-κB核转位, 减少TNF-α和IL-6等细胞因子的释放, 改善特异性皮炎样症状[23]。本实验结果提示, GLA呈浓度依赖性降低活化的肥大细胞HMGB1、TLR4及MyD88表达, 同时减少了IκBα磷酸化和核内NF-κB表达。这些结果提示, GLA可以通过抑制HMGB1/TLR4/NF-κB信号通路减轻肥大细胞介导的炎症反应。NF-κB为重要的核转录因子, 受多种信号传导的影响。在过敏性疾病中, 抗原等外部刺激因素可通过FcεRI进一步激活下游转录因子NF-κB, 诱导炎症反应。有研究表明, FcεRI通过B细胞淋巴瘤10 (B cell CLL/lymphoma 10, Bcl10)和黏膜相关淋巴组织1 (mucosa-associated lymphoid tissue lymphoma translocation 1, Malt1)蛋白复合体途径, 可以诱导NF-κB活化, 进一步诱导细胞因子的生成[24]。也有报道称, 过敏性炎症最初依赖于FcεRI与Src激酶Lyn和Fyn的相互作用, 随后依赖于磷酸化的Syk, 导致下游信号PLCγ1和Gab2磷酸化, 增加细胞内Ca2+浓度, 进一步激活NF-κB并促进细胞因子合成[25]。
本实验中也证实肥大细胞脱颗粒后可以激活HMGB1, 进一步激活下游因子NF-κB, 引起炎症反应。GLA即可抑制FcεRI相关下游蛋白, 也可抑制HMGB1/TLR4/NF-κB信号通路, 最终减轻过敏性炎症反应(图 7)。

|
Figure 7 Schematic representation of the mechanism of action of GLA on mast cell signaling pathways.GLA inhibits proinflamma‐tory cytokine secretion by inhibiting mast cell FcεRI signaling pathway activation and HMGB1/TLR4/NF-κB signaling pathway |
肥大细胞脱颗粒在变态反应性疾病发病机制的研究越来越受到重视。本研究提供的证据表明, GLA抑制anti-DNP-IgE/DNP-HSA诱导的肥大细胞表面FcεRI受体及下游蛋白表达, 并通过抑制HMGB1/TLR4过表达, 阻止NF-κB核转移减轻过敏性炎症反应。综上, 结合本实验数据, GLA可能是治疗肥大细胞介导的过敏性炎症性疾病的治疗药物。
作者贡献:朴艺花、宋艺兰和李莉负责实验设计; 朴艺花、宋艺兰、王知广和徐畅参与实验并进行数据分析; 朴艺花、姜京植和朴颖负责撰写论文; 朴红梅和延光海负责稿件修改。
利益冲突:所有作者均声明不存在利益冲突。
| [1] |
González-de-Olano D, Álvarez-Twose I. Mast cells as key players in allergy and inflammation[J]. J Investig Allergol Clin Immunol, 2018, 28: 365-378. DOI:10.18176/jiaci.0327 |
| [2] |
Lin W, Su F, Gautam R, et al. Raf kinase inhibitor protein nega‐tively regulates FcεRI-mediated mast cell activation and allergic response[J]. Proc Natl Acad Sci U S A, 2018, 115: E9859-E9868. DOI:10.1073/pnas.1805474115 |
| [3] |
Imbalzano E, Quartuccio S, Di Salvo E, et al. Association between HMGB1 and asthma: a literature review[J]. Clin Mol Allergy, 2017, 15: 12. DOI:10.1186/s12948-017-0068-1 |
| [4] |
Ugrinova I, Pasheva E. HMGB1 protein: a therapeutic target inside and outside the cell[J]. Adv Protein Chem Struct Biol, 2017, 107: 37-76. |
| [5] |
Jiang H, Duan J, Xu K, et al. Resveratrol protects against asthmainduced airway inflammation and remodeling by inhibiting the HMGB1/TLR4/NF-κB pathway[J]. Exp Ther Med, 2019, 18: 459-466. |
| [6] |
Zhu J, Sun Y, Lu Y, et al. Glaucocalyxin A exerts anticancer effect on osteosarcoma by inhibiting GLI1 nuclear translocation via regulating PI3K/Akt pathway[J]. Cell Death Dis, 2018, 9: 708. DOI:10.1038/s41419-018-0684-9 |
| [7] |
Yang F, Cao Y, Zhang J, et al. Glaucocalyxin A improves survival in bleomycin-induced pulmonary fibrosis in mice[J]. Biochem Biophys Res Commun, 2017, 482: 147-153. DOI:10.1016/j.bbrc.2016.11.003 |
| [8] |
Ye J, Piao H, Jiang J, et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways[J]. Sci Rep, 2017, 7: 11895. DOI:10.1038/s41598-017-12252-3 |
| [9] |
Joung EJ, Lee B, Gwon WG, et al. Sargaquinoic acid attenuates inflammatory responses by regulating NF-κB and Nrf2 pathways in lipopolysaccharide-stimulated RAW 264.7 cells[J]. Int Immu‐nopharmacol, 2015, 29: 693-700. DOI:10.1016/j.intimp.2015.09.007 |
| [10] |
Xian Z, Jin G, Li H, et al. Imperatorin suppresses anaphylactic reaction and IgE-mediated allergic responses by inhibiting multi‐ple steps of FcεRI signaling in mast cells: IMP alleviates allergic responses in PCA[J]. Biomed Res Int, 2019, 2019: 7823761. |
| [11] |
Yuan Y, Liu Q, Zhao J, et al. SIRT1 attenuates murine allergic rhinitis by downregulated HMGB1/TLR4 pathway[J]. Scand JImmunol, 2018, 87: e12667. DOI:10.1111/sji.12667 |
| [12] |
Zhu X, Cong J, Yang B, et al. Association analysis of high-mobility group box-1 protein 1 (HMGB1)/toll-like receptor (TLR) 4 with nasal interleukins in allergic rhinitis patients[J]. Cytokine, 2020, 126: 154880. DOI:10.1016/j.cyto.2019.154880 |
| [13] |
Tanno LK, Bierrenbach AL, Simons FER, et al. Critical view of anaphylaxis epidemiology: open questions and new perspectives[J]. Allergy Asthma Clin Immunol, 2018, 14: 12. DOI:10.1186/s13223-018-0234-0 |
| [14] |
Kim YY, Je IG, Kim MJ, et al. 2-Hydroxy-3-methoxybenzoic acid attenuates mast cell-mediated allergic reaction in mice via modulation of the FcεRI signaling pathway[J]. Acta Pharmacol Sin, 2017, 38: 90-99. DOI:10.1038/aps.2016.112 |
| [15] |
Li X, Kwon O, Kim DY, et al. Necro X-5 suppresses IgE/Ag-stimulated anaphylaxis and mast cell activation by regulating the SHP-1-Syk signaling module[J]. Allergy, 2016, 71: 198-209. DOI:10.1111/all.12786 |
| [16] |
Che D, Hou Y, Zeng Y, et al. Dehydroandrographolide inhibits IgE-mediated anaphylactic reactions via calcium signaling path‐way[J]. Toxicol Appl Pharmacol, 2019, 366: 46-53. DOI:10.1016/j.taap.2019.01.019 |
| [17] |
Kim MJ, Kim YY, Choi YA, et al. Elaeocarpusin inhibits mast cell-mediated allergic inflammation[J]. Front Pharmacol, 2018, 9: 591. DOI:10.3389/fphar.2018.00591 |
| [18] |
Kim MJ, Je IG, Song J, et al. SG-SP1 suppresses mast cellmediated allergic inflammation via inhibition of FcεRI signaling[J]. Front Immunol, 2020, 11: 50. DOI:10.3389/fimmu.2020.00050 |
| [19] |
Dhakal H, Lee S, Kim EN, et al. Gomisin M2 inhibits mast cellmediated allergic inflammation via attenuation of FcεRI-mediated Lyn and Fyn activation and intracellular calcium levels[J]. Front Pharmacol, 2019, 10: 869. DOI:10.3389/fphar.2019.00869 |
| [20] |
Espinosa E, Valitutti S. New roles and controls of mast cells[J]. Curr Opin Immunol, 2018, 50: 39-47. DOI:10.1016/j.coi.2017.10.012 |
| [21] |
Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell[J]. Front Immunol, 2016, 6: 620. |
| [22] |
Wang F, Fu X, Wu X, et al. Bone marrow derived M2 macro‐phages protected against lipopolysaccharide-induced acute lung injury through inhibiting oxidative stress and inflammation by modulating neutrophils and T lymphocytes responses[J]. Int Immunopharmacol, 2018, 61: 162-168. DOI:10.1016/j.intimp.2018.05.015 |
| [23] |
Wang Y, Zhang Y, Peng G, et al. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1[J]. Int Immunopharmacol, 2018, 60: 9-17. DOI:10.1016/j.intimp.2018.04.029 |
| [24] |
Klemm S, Ruland J. Inflammatory signal transduction from the FcεRI to NF-κB[J]. Immunobiology, 2006, 211: 815-820. DOI:10.1016/j.imbio.2006.07.001 |
| [25] |
Kang KH, Lee KH, Yoon HM, et al. Rehmannia glutinosa pharmacopuncture solution regulates functional activation, FcεRI expression, and signaling events in mast cells[J]. J Pharmaco‐puncture, 2012, 15: 32-41. DOI:10.3831/KPI.2012.15.015 |
 2021, Vol. 56
2021, Vol. 56


