2. 上海健康医学院药学院, 上海 201318;
3. 上海皮肤病医院, 上海 200443;
4. 复旦大学药学院, 上海 201203;
5. 上海中医药大学, 上海 201203
2. School of Pharmacy, Shanghai University of Medicine and Health Sciences, Shanghai 201318, China;
3. Shanghai Dermatology Hospital, Shanghai 200443, China;
4. School of Pharmacy, Fudan University, Shanghai 201203, China;
5. Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
自1998年绿色化学的概念确定以来, 可持续发展的需求鼓励人们寻找一种无毒、低成本、无污染和可降解的绿色溶剂作为有机溶剂的替代品[1-3]。离子液体(ionic liquids, ILs)由于其具有低蒸气压、高电导率、强溶解性及高稳定性等特点, 在过去20年中作为绿色溶剂得到了广泛的应用[4-7]。但由于其相对差的生物降解性、生物相容性、可持续性及高昂的制备成本, ILs的应用受到了限制[8-11]。为了克服离子液体的缺点, 同时保持其优良的性能, 低共熔溶剂(deep eutectic solvents, DESs)逐渐替代ILs成为了新一代“可设计”溶剂[12-14]。
DESs的概念于2003年由Abbott等[15]首次提出, 是一种由氢键受体(hydrogen bond acceptor, HBA)和氢键供体(hydrogen bond donor, HBD)以一定化学计量比在一定条件下形成的低共熔混合物。DESs中氢键的相互作用导致电荷离域, 从而使混合物的熔点低于各组成成分自身的熔点, 因此所形成的混合物在室温下呈液态体系[16-21]。这些氢键的强度直接影响溶剂的相变温度、稳定性及独特的物理化学和热力学性质[2, 22]。目前DESs和ILs的定义在文献中经常混淆使用, 如“配位离子液体”、“离子液体类似物”、“低共熔离子液体”和“低共熔混合物”等不同名称[17, 23, 24]。DESs作为一种很有前途的溶剂, 既具备ILs特点之外的自身优势, 又克服了其缺点, 如环境友好、生物降解性高、不可燃性、价廉和易制备等, 且毒性比ILs低[2, 18, 25, 26]。然而, 应用于化工领域的DESs组成成分通常生物相容性不佳, 这限制了在食品和医药工业中的应用。在2011年, Choi等[27]提出自然界中可能存在类似DESs的物质, 如一些氨基酸、有机酸、糖或胆碱衍生物等小分子成分所构成的DESs, 并将其称之为天然低共熔溶剂(natural deep eutectic solvents, NDESs)[28-35]。因NDESs的组分为天然产物, NDESs的毒性明显低于DESs, 且从经济和环境角度看, NDESs的生物降解性、可持续性及制备成本均优于DESs。虽然NDESs的研究刚刚起步, 但其应用领域已非常广泛, 如溶解DNA[27, 34]、作为酶反应的介质[36]、生物转化[37]、生物质加工[38]、色素的稳定[39]和各种化合物的提取[6, 40-42]。NDESs良好的生物相容性使其在医药领域中展现出巨大的潜力。目前已被应用于改善难溶药物的溶解度[43, 44]、增强药物的生物活性[45]及促进药物的跨膜能力[46, 47]等。另外, 通过低共熔技术可直接将药物制成低共熔混合物, 改善其理化或生物学性质[34, 48]。本文通过总结近年来关于NDESs的研究进展, 从NDESs形成的原理、材料、制备方法、理化性质及其药剂学应用进行综述, 为推动NDESs进一步产业化和应用于临床提供参考。
1 NDESs的形成原理及设计 1.1 形成原理NDESs的形成与组分物质间的相互作用密切相关, 由于分子间相互作用极为复杂, 详细的机制仍不明确。大多数观点认为, 卤化盐的阴离子和HBD之间的氢键作用是形成NDESs的主要作用力, 氢键的形成使组成分子的晶格能降低, 导致混合物熔点降低而呈现液态[2, 49-51]。Dai等[34]用核磁共振光谱研究NDESs的分子相互作用, 观察到NDESs中氢键的存在, 并且证明水也参与了NDESs的形成。加入少量的水可以减少NDESs的制备时间、温度及黏度。在水活度值实验中发现, NDESs中的水以键合水的形式存在, 难以将其蒸发除去。另外, 还评价了化合物的结构对NDESs的形成和稳定性的影响, 发现HBD或HBA的数目、基团的空间结构和键的位置对NDESs的形成和稳定性有显著影响。
1.2 分子模拟设计在NDES形成原理的基础上, 为了解NDESs的分子结构和化学性质, 人们从不同的建模角度对其进行研究, 在过去的几年里, 研究者们利用几种分子模拟技术来了解NDESs的分子性质和物质间的相互作用, 以及预测NDESs的相平衡和热力学性质。基于其化学原理, 目前已经发展了几种分子模拟技术用于NDESs及其多组分混合物的研究[52-56]。这些模拟技术可分为4大类[22], 即经验模型、经典热力学、统计热力学和量子化学计算, 如图 1所示。这些模型可用于预测工艺设计和模拟所需的化学信息, 并对控制NDESs形成的分子结构和相互作用以及对热力学性质的影响有了基本的了解。
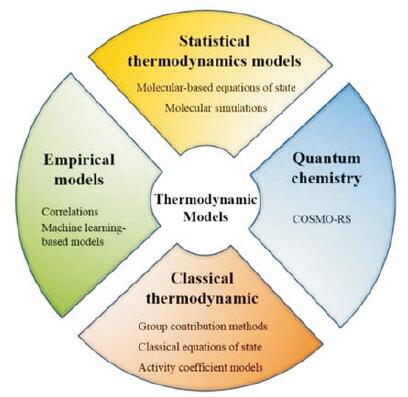
|
Figure 1 The categories and types of the thermodynamic models of natural deep eutectic solvents (NDESs).COSMO-RS: Conductor-like screening model for real solvents |
分子模拟是利用计算机辅助在分子水平上建立模型体系, 能够预测所需的化学信息, 以及对简单和高度非理想系统中的结构特征和分子间相互作用力进行观察[57]。采用分子模拟对NDESs的结构和功能进行研究, 可给出NDESs的构效关系[58-63]。Sun等[19]和Perkins等[20, 21]首次应用分子动力学模拟方法研究了氯化胆碱/尿素NDESs的分子结构和主要分子间相互作用。模拟结果表明, NDESs的形成和熔点降低是由于阴离子和HBD的羟基之间形成了较强的氢键, 随着尿素浓度的降低, 氢键作用减弱, 并得到了实验研究的证实。Altamash等[64]将氯化胆碱与苹果酸、柠檬酸、乳酸和果糖制备成NDESs, 收集其水含量、热量、密度和气体溶解度等各种数据参数, 并利用密度泛函理论和经典分子动力学, 对NDESs的物理化学性质、结构和动力学等进行研究。近年来, 量子化学的兴起为分子模拟提供了更为有力的理论。以量子化学建立起来的导体屏蔽模型(conductor-like screening model for real solvents, COSMO-RS)被广泛地应用于DESs的预测中, 已取得了突出的进展[22, 65-67]。COSMO-RS通过将分子嵌入到虚拟导体环境中来确定分子的电荷分布, 可以计算化学势以及溶解度、活度系数等一系列热力学性质[68, 69]。COSMO-RS模型的主要优势在于只需要研究分子的化学结构, 而不需要实验数据进行参数拟合便能预测分子特性[70, 71]。COSMO-RS模拟技术根据NDESs多组分混合物中的相平衡来预测其物化性质, 如密度、黏度和蒸气压等物化性质[72-74]。Silva等[75]采用COSMO-RS模型估算了含有氯化胆碱和两种糖的新型三元体系的共熔点, 通过实验测定, 验证了COSMO-RS是筛选和设计NDESs的有效工具。尽管COSMO-RS具有预测优势, 但该方法存在局限性, 在大多数情况下, 与实验数据相比, 预测的准确性和相关性较差[22, 55, 76]。因此, 需要根据实验数据对COSMO-RS参数进行微调, 以便准确地描述NDESs的特性。
2 NDESs的常用材料NDESs是由HBA和HBD组合而成的混合物, 且大多数是从非离子物质中获得。NDESs的形式可以用Cat+X-zY通式[17]表示: Cat+原则上为任意铵、磷或硫阳离子, X是路易斯碱, 通常为卤化物阴离子, Cat+X-代表的是盐类; z为Y的分子数, Y为路易斯酸或质子酸。常见的HBA涵盖季铵盐类(氯化胆碱)和两性离子(甜菜碱)等; HBD涵盖可以与HBA中的阴离子形成氢键的有机酸、多元醇和糖等, 如图 2所示。
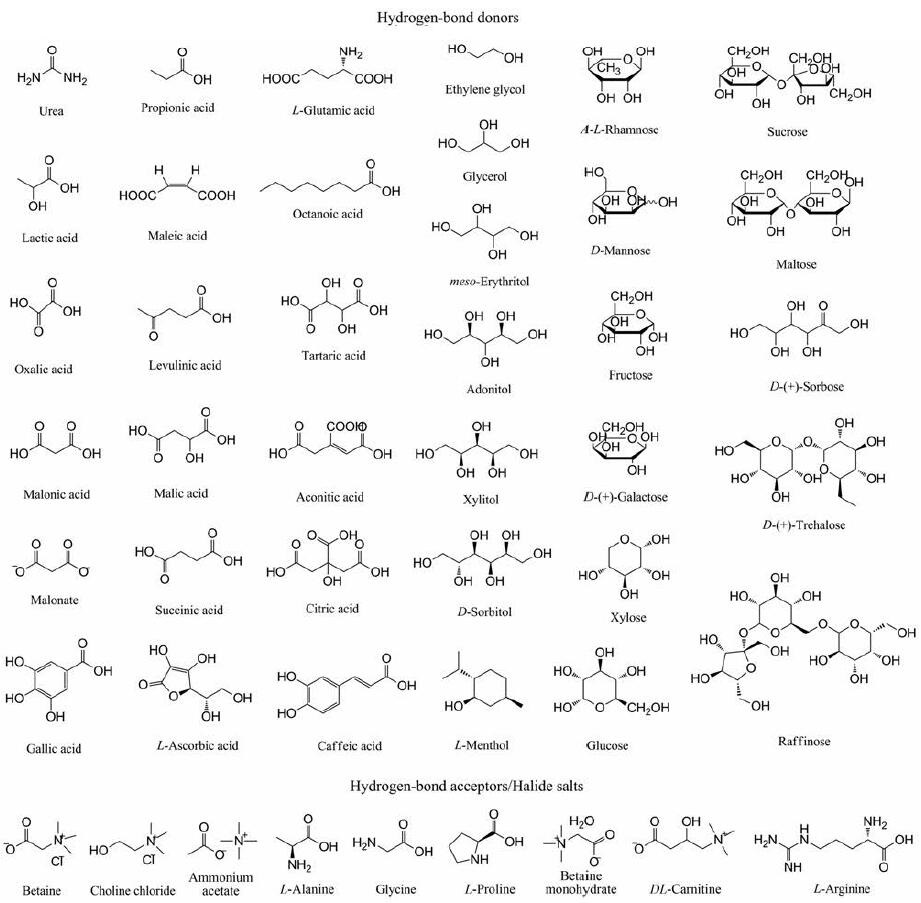
|
Figure 2 Common hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) used in the preparation of NDESs |
随着NDESs被广泛研究, NDESs的组成类型除了HBA和HBD组成的二元组分体系外, 还可以是由HBD组成的一元体系, 如糖和氨基酸、有机酸和多元醇、糖和糖等。大部分NDESs是两组分混合物, 少数NDESs是三组分混合物, 且水分子可作为某些NDESs的组分之一。如Choi等[27]和Francisco等[38]以天然氨基酸为HBA和以天然植物酸为HBD合成了无毒、可生物降解的NDESs。
3 NDESs的制备方法NDESs的制备工艺较为简单, 常见的有以下3种: ①加热法[34, 50, 77, 78]: 若NDESs的组分为干燥化合物且具备较好的热稳定性时, 可采用加热法制备[79]。将用于制备NDESs的HBD和HBA以适当的摩尔比混合, 在一定温度(50~100℃)下, 用磁力搅拌器搅拌, 直到形成均匀的透明液体, 如Roda等[77]将柠檬酸-L-精氨酸-水以1∶1∶7、1∶1∶6、1∶1∶5和1∶1∶4摩尔比混合, 并在50℃和60℃下搅拌, 最后制得呈半透明溶液的NDESs; ②蒸发法[50, 80-82]: 若NDESs的组分为高温下易分解的热敏性材料, 可采用蒸发法制备[34]。将NDESs的各个成分溶于水中, 通过真空蒸发法进行除水, 最常见的是通过离心真空或旋转蒸发器, 除水后将得到的液体放入干燥器中, 直到重量保持不变。如Wikene等[80]将成分溶于温水中, 并在45℃下用旋转蒸发器蒸发15 min制得NDESs; ③冷冻干燥法[37, 83-85]: 冷冻干燥法是基于NDESs的单个对应物的水溶液, 其组分为热不稳定性时, 可采用冷冻干燥法制备[34]。将以适当摩尔比例精确称量的组分混合在一起, 用少量的水溶解, 再将混合物冷冻干燥不少于24 h, 直到重量保持不变。如Gutierrez等[37]将尿素和氯化胆碱的水溶液以2∶1摩尔比混合, 将制得的水溶液冷冻, 然后冷冻干燥, 最后得到透明的黏性液体。
4 NDESs的理化性质 4.1 相行为NDESs是由两种通过氢键相互作用的固体混合而形成的一种新的液相[17, 86]。例如, 熔点为302℃的氯化胆碱和133℃的尿素以1∶2摩尔比混合可获得熔点为12℃的NDESs[15], 室温下呈液态。大多数NDESs的熔点都低于150℃, 而熔点在室温以下的NDESs已作为廉价安全的溶剂应用于各种领域[16, 87]。NDESs的熔点与HBD和HBA之间形成的氢键大小有关[11, 17], 氢键越强, 熔点越低[15]。而氢键的大小与HBA、HBD的性质、结构和摩尔比等因素有关[10, 15, 17, 32, 88]。Abbott等[32]发现熔点与有机酸的分子量有一定的相关性, 分子量越低, 熔点下降幅度越大。Zhang等[16]发现当氯化胆碱与尿素的摩尔比分别为1∶1和1∶2时, 生成的NDESs的熔点分别为50℃以上和12℃。在此基础上, Qin等[10]指出HBA与HBD的摩尔比对NDESs的熔点有显著影响, 但其最低熔点取决于HBD的性质。
4.2 黏度NDESs在室温下是高黏性的, 其黏度大多在0.1~50 Pa·s之间, 是水的20~1 000倍[6]。NDESs的黏度主要受范德华力和氢键的影响, 并与HBA和HBA的性质、摩尔比、温度和含水量等因素有关[25, 32, 48, 78, 89, 90]。目前常用来预测黏度的有Schottky-vacancy、Gas-oriented及空穴理论3种模型, 其中空穴理论由于可进行定量分析而备受关注[91, 92]。Abbott等[32]利用空穴理论解释了NDESs的黏度与离子的流动性、空穴的利用率之间的关系。Gajardo-Parra等[79]发现, 在313K (39.85℃)以下, NDESs的黏度随着温度的升高而显著下降, 但进一步升高温度仅导致溶剂性质的微小变化。Du等[89]研究发现, 当体系中加入水时, 黏度显著降低。如在25℃时, 干燥氯化胆碱-尿素的黏度比水合氯化胆碱-尿素(含6%水)的黏度高13倍。
4.3 表面张力与高温熔盐和ILs相似, NDESs的表面张力较大, 主要与分子间作用力、阳离子类型及温度等因素有关[87, 93]。Garcia等[94]发现阳离子中的羟基由于其氢键能力而导致较高的表面张力, 且实验结果表明, 增加阳离子烷基链长会导致较高的表面张力。因此, 基于葡萄糖的NDESs的表面张力高于基于羧酸的NDESs[95]。Alomar等[96]发现由于NDESs中分子间作用力的减少, 导致NDESs的表面张力随着HBA摩尔分数的增加而降低。Abbott等[32]用空穴理论解释了离子在高温熔盐中迁移率, 可以证明平均空穴半径(r)与液体的表面张力(γ)有关: 4π(r2)=3.5 kT/γ, 其中k是玻尔兹曼常数, T是绝对温度。这与随着温度的升高、表面张力呈线性降低的结果一致。
4.4 电导性由于NDESs的高黏度, 大多数NDESs表现出较低的电导率, 并且电导率的大小与温度和NDESs的组成有关[6, 50]。NDESs的电导率随着温度的升高而显著增加, 这是由于加热产生的动能增加了分子之间碰撞的频率, 导致分子间作用力减弱, 电导率增加[10, 25]。类Arrhenius方程可以用来预测NDESs的电导率[10, 32]:
| $\ln \sigma=\ln \sigma_{0}-\frac{E_{\wedge}}{R T}$ | (1) |
式中, σ是离子电导率, σ0是离子电导率常数, EΛ是电活化能。Abbott等[32]发现通过该公式所得电导率与NDESs的黏度倒数呈线性关系。此外, Abbott等[97]还发现NDESs的电导率随着氯化胆碱含量的增加而增加, 当氯化胆碱的摩尔分数增加到25%时, 氯化胆碱-甘油的电导率达到最大值。水的加入会增加NDESs的电导率, Shah等[90]发现当加入10%的水时, 氯化胆碱: 尿素低共溶剂的电导率提高了3倍, 而黏度降低了80%以上。HBA和HBD的摩尔比和结构对NDESs的电导率也有显著的影响。Zhao等[25]发现含较多羟基的NDESs, 其离子电导性也越强, 因为羟基产生的氢键导致NDESs的离子迁移率变大。
4.5 溶解性NDESs作为萃取剂对很多成分都有很好的溶解性, 包括天然产物、药物、金属氧化物和二氧化碳等[14, 44, 98-100]。NDESs的溶解性可通过改变其组分类型和摩尔比来调节, 并且与温度和含水量有关[34, 54, 101]。因为NDESs中存在广泛的氢键结构, 这导致了高黏性, 使其没有溶解其他溶质的空间, 所以需要水来瓦解键合结构[48]。Abbott等[32]观察到不同氧化物在不同的NDESs中的溶解度不同, 并提出可以设计一种提取特定金属氧化物的溶剂。Dai等[34]研究了水含量对NDESs溶解性的影响, 发现在NDESs中加入少量的水可以增加其溶解性, 这可能与氢键体系的变化有关, 但最佳含水量取决于化合物。此外, 还发现温度对NDESs的溶解性也有很大的影响。当温度从40℃升高至50℃时, 槲皮素在葡萄糖/氯化胆碱中的溶解度增加了2.3倍, 在丙二醇/氯化胆碱中的溶解度增加了1.65倍。Dai等[48]提出NDESs的溶解度与溶质的极性有关, 非极性化合物在纯NDESs中的溶解度最高, 而中极性化合物在含5%~10%水的NDESs中溶解度达到最高。
5 NDESs在药剂学中的应用 5.1 增加药物溶解度NDESs作为新型的绿色溶剂, 能溶解多种难溶性药物, 具有潜在的非水液体给药应用前景[18, 102-104]。Morrison等[105]研究了多种难溶性药物在NDESs中的溶解度, 发现其溶解度比纯水中高出5~22 000倍, 说明药物在NDESs中的高溶解度不是由于药物与溶剂的一个单独组分的缔合, 而是低共熔混合物协同作用的结果。NDESs的增溶作用主要与HBD和药物分子之间形成的氢键有关[106]。Gutierrez等[107]采用密度泛函理论和经典分子动力学方法, 研究了氯化胆碱-乳酸和β-丙氨酸-乳酸, 这两种摩尔比为1∶1的NDESs中利多卡因的溶剂效应。利多卡因分子可以通过N-H位和C-O位, 与NDESs中的HBA和HBD相互作用, 形成较强的氢键, 且从利多卡因到NDESs分子发生少量电荷转移。此外, 还证实体系中存在强大的相互作用力是由于HBA/HBD与利多卡因在没有特定氢键的情况下产生紧密而强烈的范德华力。氢键和非特异性范德华力相互作用的结合, 使NDESs可以有效地将利多卡因溶剂化。在Gutierrez的另一篇文献[106]中, 选择精氨酸作为HBA, 并将其与谷氨酸、草酸和酒石酸3种不同的HBD偶联。利多卡因在NDESs中的溶解伴随着轻微的体积膨胀和轻微的溶剂结构变化。利多卡因分子对精氨酸和HBD分子的亲和力决定了利多卡因在NDESs中的溶解度, 且精氨酸-HBD相互作用随着利多卡因含量的增加而呈线性减少。NDESs可以通过与溶质形成氢键来增加药物溶解度, 同时保持NDESs的大部分性质。因此, 可以通过选择合适的HBD和HBA制备成NDESs来控制活性药物的溶解度。
5.2 增加药物稳定性NDESs不仅能增加药物溶解性, 还能改善药物化学稳定性[108-110]。姜黄素具有降血脂、抗肿瘤、抗炎、利胆和抗氧化等作用, 在许多疾病中具有治疗潜力, 但其水溶性差, 在碱性介质中化学性质不稳定, 在生理pH值下会迅速水解降解, 同时姜黄素还是一种光敏化合物, 易于快速光降解[111-113]。因此, 姜黄素的口服生物利用度低, 限制了其临床疗效。Jelinski等[114]研究发现, 姜黄素在NDESs中的溶解度远大于在水中的溶解度。在室温下, 姜黄素的溶出量与水溶液相比增加了12 000倍。在稳定性实验中, 发现NDESs可以防止姜黄素的光降解。Wikene等[115]合成了几种NDESs, 发现姜黄素在柠檬酸-蔗糖中的水解稳定性比含有环糊精的溶液高2~10倍, 比在pH 8的缓冲液中高1 300倍。另外, 与含环糊精和表面活性剂的制剂相比, 姜黄素在柠檬酸-蔗糖中的光解稳定性提高了5.6~10倍。类似地, β-内酰胺类抗生素[108]、阿司匹林[104, 116]、丹酚酸B[117]及其他酚类化合物[39]等不稳定的化合物在NDESs中的稳定性都有所提高。NDESs的稳定能力可以通过降低含水量和增加黏度来调整, 且其稳定能力是由于药物与NDESs之间形成了较强的氢键。因此, NDESs是一类难溶性药物制剂中具有潜在应用前景的有效溶剂和稳定剂。
5.3 促进药物渗透性在众多给药途径中, 经皮给药具有患者顺应性好、不良反应低且可避免首过效应等优点备受青睐[118]。Zakrewsky等[119]评估了NDESs在破坏生物膜和增强抗生素跨皮肤层输送方面的应用。使用胆碱香叶酸制成的NDESs作为药物的有效渗透促进剂, 使抗生素的释放增加了16倍以上。Cao等[1]通过检测一种胞内酶的含量作为NDESs对大肠杆菌内膜通透性的指标, 证明NDESs可以溶解细胞膜上的磷脂, 破坏细胞膜的结构, 从而增加细胞膜的通透性。Stott等[120]考察了布洛芬与7种萜类透皮促进剂之间共熔体系的形成及其对经皮给药系统熔点下降的影响, 发现氢键作用导致的给药系统的熔点降低与透皮渗透的显著增加相关。Qu等[121]用明胶将含药物成分的NDESs凝胶化, 这种方法使药物活性成分透过皮肤屏障的速度比相应的固体制剂快3倍。Berton等[122]通过将利多卡因制备成离子液体、低共熔溶剂和结晶盐3种形式, 对其进行透皮吸收比较, 研究利多卡因的生物利用度。结果发现, 低共熔形式的利多卡因比另两种的吸收更快, 生物利用度更高。
鼻腔给药已经在临床上使用多年, 是一种快速高效吸收的给药方式, 常用于治疗过敏性疾病、充血和呼吸道感染等局部疾病, 其在临床治疗中的地位及重要性日益突显[123-126]。Roda等[77]针对结核病设计了一种新型的给药系统, 通过超临界CO2技术将基于L-精氨酸的NDESs包裹在脂质基质中, 封装成可吸入给药, 为开发新的、绿色、安全和更高效的药物输送系统提供了新的可能性。Li等[46]用苹果酸和氯化胆碱制备的NDESs, 可以用来改善胰岛素的鼻腔给药。与水凝胶和胰岛素溶液相比, 该NDESs促进了胰岛素在鼻黏膜上皮细胞的渗透, 在不同剂量下均能显著提高胰岛素的降血糖功效, 且与皮下注射胰岛素的降血糖作用相似。
5.4 促进药物口服吸收在医药领域中, 许多药物具有很好的治疗功效, 但由于其口服生物利用度低, 限制了药物的临床使用[127]。而NDESs作为一种具有独特物理性质的绿色溶剂, 通过与药物之间的氢键作用增加药物的溶解度, 以此来提高药物的口服生物利用度[106, 109]。小檗碱具有多方面的治疗潜力, 但药代动力学研究表明, 小檗碱的口服吸收差, 口服后迅速代谢, 因此其血药浓度极低[128]。Sut等[129]选用3种小檗碱NDESs溶液和1种小檗碱水溶液, 以50 mg·kg-1剂量给小鼠灌胃, 采用LC-MS/MS方法测定小檗碱的血药浓度。药代动力学分析显示, NDESs小檗碱的血药浓度增加了2~20倍, 生物利用度明显提升, 且生物利用度的提高主要与不同的NDESs的增溶特性有关。结果表明, NDESs不仅能改善难溶性药物的溶解性, 还能作为口服生物利用度低的天然药物的吸收促进剂。Faggian等[35]以芦丁为模型药物, 以糖、氨基酸和有机酸为原料, 制备了不同的NDESs, 对含芦丁的NDESs进行药代动力学研究, 并与口服水混悬剂的生物利用度进行比较。用LC-MS/MS测定血浆中芦丁的含量, 结果发现, 与水溶液相比, 芦丁在NDESs中的相对生物利用度增加了约100%, NDESs可以促进芦丁在胃肠道的吸收, 使其在动物血浆中的持续时间更长。Chen等[130]比较了丹酚酸B在氯化胆碱-甘油和水中的药代动力学差异, 结果表明氯化胆碱-甘油通过提高膜的穿透力来促进丹酚酸B的吸收, 为NDESs作为口服制剂药物载体的可行性提供了依据。
5.5 药物活性成分低共熔物一般来说, 药物制剂中使用的活性药物成分(active pharmaceutical ingredients API)都是固体结晶形式, 以最大限度地提高其溶解性、纯度、热稳定性和生物利用度[118, 131]。然而, 固体形式的药物有很多局限性, 如多晶型。固体晶体原料药的多晶型限制了药物在给药时的溶解度、吸收和生物利用度[132, 133]。为了解决这些问题, 目前已经采用了从药物配方到给药方法的调整等一系列措施[118, 134]如前药[135, 136]、制成盐[137-139]、结晶工程[140-143]、固体分散体[144, 145]和胶束体系[146]的使用。用于改善药物溶解性的常见极性有机溶剂有吡啶、N, N-二甲基甲酰胺和二甲基亚砜。但许多情况下, 在药物合成反应中使用这些极性溶剂会产生大量的副产物, 由于回收和再利用的困难而引起副产物处理的问题。考虑到这些局限性, 寻找一种潜在的绿色溶剂是非常有必要的[118]。NDESs作为一类可生物降解的绿色溶剂具有解决上述问题的潜力。通过形成包含API的NDESs即API-NDESs, 将原料药转变为液体形式, 在增加原料药生物利用度的同时, 还具有治疗作用[77]。大多数原料药可作为HBD或HBA, 但目前报道的大多数都是HBD, 因为原料药大多存在胺、羧酸和羟基[132]。然而, HBA和HBD的正确选择对API-NDESs的形成, 在室温下产生NDESs液体以及由此产生的治疗特性都起着至关重要的作用[109]。API-NDESs可由大量原料药与多种其他化合物组合而成, 如代谢物[132]或渗透促进剂[120, 147]。NDESs也可以由两种不同的原料药制备形成双功能液体制剂[122, 132]。当包含具有可聚合部分的API时, API-NDESs呈现可聚合特性[148], 从而适当调节药物递送分布。薄荷醇是最近研究较多的萜类化合物, 能够在很大的比例范围内形成共熔溶剂, 常作为药物或辅料的渗透促进剂[149, 150]。薄荷醇可以与辅酶Q10[151]、丹皮酚[152]、布洛芬[153, 154]和阿司匹林[98]结合制成API-NDESs, 可通过促渗及增加药物溶解度提高原料药的生物利用度(表 1)。
| 表 1 Application of menthol as a component in API-NDESs. API: Active pharmaceutical ingredients; NDESs: Natural deep eutectic solvents |
API-NDESs除了可用于提高治疗效果外, 还可作为聚合物生产的单体用于开发控释递送系统[155]。Serrano等[156]以1,8-辛二醇和利多卡因为原料制备成API-NDESs再与柠檬酸为第二聚合物前驱体合成了聚弹性体。该聚弹性体中, NDESs不仅提供了合成所需的大部分成分, 还提供了合成介质。所得弹性聚合物表现出高负载量的利多卡因, 其控制释放取决于聚合物的生物可降解性。通过利用NDESs的聚合能力, Mota-Morales等[148]提出使用丙烯酸和利多卡因的前端聚合的新技术手段。丙烯酸和利多卡因混合而成的API-NDESs具有三重作用, 聚合后能够控制利多卡因的释放。此外, 通过调整HBD和混合物的摩尔比, 可以调节单体的黏度和NDESs的密度, 有利于实现高转化率的前端聚合物。Mano等[157]开发了一种用于快速溶解递送系统的基于明胶的API-NDESs。用明胶膜包裹氯化胆碱-扁桃酸(1∶2)的NDESs在磷酸盐缓冲溶液中迅速溶解, 且还保留了扁桃酸对革兰阴性菌和革兰阳性菌的抗菌特性。
6 结论与展望NDESs具备生物降解性高、毒性低、不可燃、制备简单和成本低等优点, 且其理化性质可以通过HBD和HBA的性质来调节, 从而提供了制备具有特定作用的NDESs的可能性, 促进其在各种领域的应用。在医药领域中, NDESs一方面可以作为药用辅料, 提高药物的溶解性、稳定性和渗透性, 从而提高药物治疗作用; 另一方面NDESs可以作为药物活性成分, 构成API-NDESs的新型给药体系, 将药物液体化可以提高药物的生物利用度, 同时避免了固体药物的多晶型。显然, 这些优势为NDESs在制药行业中的应用提供了巨大的潜力。
NDESs在医药领域中的应用目前还处于起步阶段, 其高黏度是制约因素之一, 并且需要评估NDESs的吸湿性, 因为这可能会影响溶剂的稳定性。虽然NDESs为药物研发提供了新的方向, 但是许多药物在NDESs体系中的作用机制、细胞毒性和药物释放等信息尚不清楚, 还需进一步的研究。另外, 目前极缺乏对NDESs与药物的相互作用及与辅料的相互作用机制研究, 严重阻碍了其在药剂产品开发中的应用, 因此急需开展NDESs与制剂处方相容性的研究, 为其在药剂学中的广泛应用铺平道路。
随着NDESs研究的进一步深入, 其作为新一代绿色溶剂和膜促渗剂必将为药剂学的剂型改革带来重大机遇, 同时为难溶性药物和生物技术药物的非注射给药剂型提供了一种高效的药用材料, 为制药技术带来了新的可能性, 对医药发展做出巨大贡献, 从而造福人类。
作者贡献:宣婧婧负责资料调研和写作; 武喜营参与资料调研; 戚建平和庄婕提出想法、指导并修改论文。
利益冲突:本文作者声明无利益冲突。
| [1] |
Cao J, Wu R, Dong Q, et al. Effective release of intracellular enzymes by permeating the cell membrane with hydrophobic deep eutectic solvents[J]. ChemBioChem, 2020, 21: 672-680. DOI:10.1002/cbic.201900502 |
| [2] |
Francisco M, van den Bruinhorst A, Kroon MC. Low-transitiontemperature mixtures (LTTMs): a new generation of designer solvents[J]. Angew Chem Int Ed Engl, 2013, 52: 3074-3085. DOI:10.1002/anie.201207548 |
| [3] |
Kudłak B, Owczarek K, Namieśnik J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—a review[J]. Environ Sci Pollut Res, 2015, 22: 11975-11992. DOI:10.1007/s11356-015-4794-y |
| [4] |
Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine[J]. Chem Rev, 2017, 117: 7132-7189. DOI:10.1021/acs.chemrev.6b00562 |
| [5] |
Clark JH, Farmer TJ, Hunt AJ, et al. Opportunities for bio-based solvents created as petrochemical and fuel products transition towards renewable resources[J]. Int J Mol Sci, 2015, 16: 17101-17159. DOI:10.3390/ijms160817101 |
| [6] |
Dai YT, van Spronsen J, Witkamp GJ, et al. Ionic liquids and deep eutectic solvents in natural products research: mixtures of solids as extraction solvents[J]. J Nat Prod, 2013, 76: 2162-2173. DOI:10.1021/np400051w |
| [7] |
Cao JP, Mou YX, Chen YY, et al. Applications of ionic liquids in drug research[J]. Acta Pharm Sin(药学学报), 2019, 54: 245-257. |
| [8] |
Paschoal VH, Faria LFO, Ribeiro MCC. Vibrational spectroscopy of ionic liquids[J]. Chem Rev, 2017, 117: 7053-7112. DOI:10.1021/acs.chemrev.6b00461 |
| [9] |
Deetlefs M, Seddon KR. Assessing the greenness of some typical laboratory ionic liquid preparations[J]. Green Chem, 2010, 12: 17-30. DOI:10.1039/B915049H |
| [10] |
Qin H, Hu XT, Wang JW, et al. Overview of acidic deep eutectic solvents on synthesis, properties and applications[J]. Green Energy Environ, 2020, 5: 8-21. DOI:10.1016/j.gee.2019.03.002 |
| [11] |
Makoś P, Słupek E, Gębicki J. Hydrophobic deep eutectic solvents in microextraction techniques-a review[J]. Microchem J, 2020, 152: 104384. DOI:10.1016/j.microc.2019.104384 |
| [12] |
Sheldon RA. Green solvents for sustainable organic synthesis: state of the art[J]. Green Chem, 2005, 7: 267-278. DOI:10.1039/b418069k |
| [13] |
Radosevic K, Bubalo MC, Srcek VG, et al. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents[J]. Ecotoxicol Environ Saf, 2015, 112: 46-53. DOI:10.1016/j.ecoenv.2014.09.034 |
| [14] |
Xie Y, Dong H, Zhang S, et al. Solubilities of CO2, CH4, H2, CO and N2 in choline chloride/urea[J]. Green Energy Environ, 2016, 1: 195-200. DOI:10.1016/j.gee.2016.09.001 |
| [15] |
Abbott AP, Capper G, Davies DL, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chem Commun (Camb), 2003, 9: 70-71. |
| [16] |
Zhang Q, De Oliveira Vigier K, Royer S, et al. Deep eutectic solvents: syntheses, properties and applications[J]. Chem Soc Rev, 2012, 41: 7108-7146. DOI:10.1039/c2cs35178a |
| [17] |
Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications[J]. Chem Rev, 2014, 114: 11060-11082. DOI:10.1021/cr300162p |
| [18] |
Mbous YP, Hayyan M, Hayyan A, et al. Applications of deep eutectic solvents in biotechnology and bioengineering-promises and challenges[J]. Biotechnol Adv, 2017, 35: 105-134. DOI:10.1016/j.biotechadv.2016.11.006 |
| [19] |
Sun H, Li Y, Wu X, et al. Theoretical study on the structures and properties of mixtures of urea and choline chloride[J]. J Mol Model, 2013, 19: 2433-2441. DOI:10.1007/s00894-013-1791-2 |
| [20] |
Perkins SL, Painter P, Colina CM. Molecular dynamic simulations and vibrational analysis of an ionic liquid analogue[J]. J Phys Chem B, 2013, 117: 10250-10260. DOI:10.1021/jp404619x |
| [21] |
Perkins SL, Painter P, Colina CM. Experimental and computational studies of choline chloride-based deep eutectic solvents[J]. J Chem Eng Data, 2014, 59: 3652-3662. DOI:10.1021/je500520h |
| [22] |
Alkhatib III, Bahamon D, Llovell F, et al. Perspectives and guidelines on thermodynamic modelling of deep eutectic solvents[J]. J Mol Liq, 2019, 298: 112183. |
| [23] |
Haerens K, Matthijs E, Binnemansc K, et al. Electrochemical decomposition of choline chloride based ionic liquid analogues[J]. Green Chem, 2009, 11: 1357-1365. DOI:10.1039/b906318h |
| [24] |
Abbott AP, McKenzie KJ. Application of ionic liquids to the electrodeposition of metals[J]. Phys Chem Chem Phys, 2006, 8: 4265-4279. DOI:10.1039/b607329h |
| [25] |
Zhao BY, Xu P, Yang FX, et al. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica[J]. ACS Sustainable Chem Eng, 2015, 3: 2746-2755. DOI:10.1021/acssuschemeng.5b00619 |
| [26] |
Xu GC, Ding JC, Han RZ, et al. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation[J]. Bioresour Technol, 2016, 203: 364-369. DOI:10.1016/j.biortech.2015.11.002 |
| [27] |
Choi YH, van Spronsen J, Dai Y, et al. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology?[J]. Plant Physiol, 2011, 156: 1701-1705. DOI:10.1104/pp.111.178426 |
| [28] |
Gorke JT, Srienc F, Kazlauskas RJ. Hydrolase-catalyzed biotransformations in deep eutectic solvents[J]. Chem Commun, 2008, 10: 1235-1237. |
| [29] |
Imperato G, Eibler E, Niedermaier J, et al. Low-melting sugarurea-salt mixtures as solvents for Diels-Alder reactions[J]. Chem Commun (Camb), 2005(9): 1170-1172. DOI:10.1039/B414515A |
| [30] |
Poletti L, Chiappe C, Lay L, et al. Glucose-derived ionic liquids: exploring low-cost sources for novel chiral solvents[J]. Green Chem, 2007, 9: 337-341. DOI:10.1039/b615650a |
| [31] |
Gore S, Baskaran S, Koenig B. Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid-urea mixtures[J]. Green Chem, 2011, 13: 1009-1013. DOI:10.1039/c1gc00009h |
| [32] |
Abbott AP, Boothby D, Capper G, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids[J]. J Am Chem Soc, 2004, 126: 9142-9147. DOI:10.1021/ja048266j |
| [33] |
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids[J]. J Am Chem Soc, 2005, 127: 2398-2399. DOI:10.1021/ja043451i |
| [34] |
Dai YT, van Spronsen J, Witkamp GJ, et al. Natural deep eutectic solvents as new potential media for green technology[J]. Anal Chim Acta, 2013, 766: 61-68. DOI:10.1016/j.aca.2012.12.019 |
| [35] |
Faggian M, Sut S, Perissutti B, et al. Natural deep eutectic solvents (NADEs) as a tool for bioavailability improvement: pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: possible application in nutraceuticals[J]. Molecules, 2016, 21: 1531. DOI:10.3390/molecules21111531 |
| [36] |
Durand E, Lecomte J, Baréa B, et al. Evaluation of deep eutectic solvent-water binary mixtures for lipase-catalyzed lipophilization of phenolic acids[J]. Green Chem, 2013, 15: 2275-2282. DOI:10.1039/c3gc40899j |
| [37] |
Gutierrez MC, Ferrer ML, Yuste L, et al. Bacteria incorporation in deep-eutectic solvents through freeze-drying[J]. Angew Chem Int Ed Engl, 2010, 49: 2158-2162. DOI:10.1002/anie.200905212 |
| [38] |
Francisco M, van den Bruinhorst A, Kroon MC. New natural and renewable low transition temperature mixtures (LTTMs): screening as solvents for lignocellulosic biomass processing[J]. Green Chem, 2012, 14: 2153-2157. DOI:10.1039/c2gc35660k |
| [39] |
Dai Y, Verpoorte R, Choi YH. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius)[J]. Food Chem, 2014, 159: 116-121. DOI:10.1016/j.foodchem.2014.02.155 |
| [40] |
Gu T, Zhang M, Tan T, et al. Deep eutectic solvents as novel extraction media for phenolic compounds from model oil[J]. Chem Commun (Camb), 2014, 50: 11749-11752. DOI:10.1039/C4CC04661G |
| [41] |
Duan L, Dou LL, Guo L, et al. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products[J]. ACS Sustainable Chem Eng, 2016, 4: 2405-2411. DOI:10.1021/acssuschemeng.6b00091 |
| [42] |
Bakirtzi C, Triantafyllidou K, Makris DP. Novel lactic acid-based natural deep eutectic solvents: efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native greek medicinal plants[J]. J Applied Res Med Aromatic Plants, 2016, 3: 120-127. DOI:10.1016/j.jarmap.2016.03.003 |
| [43] |
Erlund I, Kosonen T, Alfthan G, et al. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers[J]. Eur J Clin Pharmacol, 2000, 56: 545-553. DOI:10.1007/s002280000197 |
| [44] |
Li Z, Lee PI. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives[J]. Int J Pharm, 2016, 505: 283-288. DOI:10.1016/j.ijpharm.2016.04.018 |
| [45] |
Radosevic K, Curko N, Srcek VG, et al. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity[J]. Food Sci Technol, 2016, 73: 45-51. |
| [46] |
Li Y, Wu X, Zhu Q, et al. Improving the hypoglycemic effect of insulin via the nasal administration of deep eutectic solvents[J]. Int J Pharm, 2019, 569: 118584. DOI:10.1016/j.ijpharm.2019.118584 |
| [47] |
Wu X, Chen Z, Li Y, et al. Improving dermal delivery of hydrophilic macromolecules by biocompatible ionic liquid based on choline and malic acid[J]. Int J Pharm, 2019, 558: 380-387. DOI:10.1016/j.ijpharm.2019.01.021 |
| [48] |
Dai YT, Witkamp GJ, Verpoorte R, et al. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications[J]. Food Chem, 2015, 187: 14-19. DOI:10.1016/j.foodchem.2015.03.123 |
| [49] |
Espino M, Fernández MdlÁ, Gomez FJV, et al. Natural designer solvents for greening analytical chemistry[J]. Trends Anal Chem, 2016, 76: 126-136. DOI:10.1016/j.trac.2015.11.006 |
| [50] |
Liu Y, Friesen JB, McAlpine JB, et al. Natural deep eutectic solvents: properties, applications, and perspectives[J]. J Nat Prod, 2019, 81: 679-690. |
| [51] |
Tomé LIN, Baião V, Silva Wd, et al. Deep eutectic solvents for the production and application of new materials[J]. Appl Mater Today, 2018, 10: 30-50. DOI:10.1016/j.apmt.2017.11.005 |
| [52] |
Florindo C, Lima F, Ribeiro BD, et al. Deep eutectic solvents: overcoming 21st century challenges[J]. Green Sustainable Chem, 2019, 18: 31-36. DOI:10.1016/j.cogsc.2018.12.003 |
| [53] |
Shekaari H, Zafarani-Moattar MT, Shayanfar A, et al. Effect of choline chloride/ethylene glycol or glycerol as deep eutecticsolvents on the solubility and thermodynamic propertiesof acetaminophen[J]. J Mol Liq, 2018, 249: 1222-1235. DOI:10.1016/j.molliq.2017.11.057 |
| [54] |
Cysewski P, Jeliński T. Optimization, thermodynamic characteristics and solubility predictions of natural deep eutectic solvents used for sulfonamide dissolution[J]. Int J Pharm, 2019. DOI:10.1016/j.ijpharm.2019.118682 |
| [55] |
deCastilla AG, Bittner JP, Müller S, et al. Thermodynamic and transport properties modeling of deep eutectic solvents: a review on gE-models, equations of state, and molecular dynamics[J]. J Chem Eng Data, 2020, 65: 943-967. DOI:10.1021/acs.jced.9b00548 |
| [56] |
Hizaddin HF, Ramalingam A, Hashim MA, et al. Evaluating the performance of deep eutectic solvents for use in extractive denitrification of liquid fuels by the conductor-like screening model for real solvents[J]. J Chem Eng Data, 2014, 59: 3470-3487. DOI:10.1021/je5004302 |
| [57] |
Xu HF, Peng JJ, Song XM, et al. Review of molecular simulationof deep eutectic solvents[J]. J Qilu Univ Technol(齐鲁工业大学学报: 自然科学版), 2019, 33: 1-9. |
| [58] |
Zhang Y, Ji X, Lu X. Choline-based deep eutectic solvents for CO2 separation: review and thermodynamic analysis[J]. Renewable Sustainable Energy Rev, 2018, 97: 436-455. DOI:10.1016/j.rser.2018.08.007 |
| [59] |
Wagle DV, Adhikari L, Baker GA. Computational perspectives on structure, dynamics, gas sorption, and bio-interactions in deep eutectic solvents[J]. Fluid Phase Equilib, 2017, 448: 50-58. DOI:10.1016/j.fluid.2017.04.018 |
| [60] |
Altamash T, Atilhan M, Aliyan A, et al. Insights into choline chloride-phenylacetic acid deep eutectic solvent for CO2 absorption[J]. RSC Adv, 2016, 6: 109201-109210. DOI:10.1039/C6RA22312E |
| [61] |
Ma C, Laaksonen A, Liu C, et al. The peculiar effect of water on ionic liquids and deep eutectic solvents[J]. Chem Soc Rev, 2018, 47: 8685-8720. DOI:10.1039/C8CS00325D |
| [62] |
Triolo A, Lo Celso F, Russina O. Structural features of betacyclodextrin solvation in the deep eutectic solvent, reline[J]. J Phys Chem B, 2020, 124: 2652-2660. DOI:10.1021/acs.jpcb.0c00876 |
| [63] |
Pal S, Paul S. Understanding the role of reline, a natural des, on temperature-induced conformational changes of c-kit Gquadruplex DNA: a molecular dynamics study[J]. J Phys Chem B, 2020, 124: 3123-3136. DOI:10.1021/acs.jpcb.0c00644 |
| [64] |
Altamash T, Nasser MS, Elhamarnah Y, et al. Gas solubility and rheological behavior of natural deep eutectic solvents (NADEs) via combined experimental and molecular simulation techniques[J]. Chemistryselect, 2017, 2: 7278-7295. DOI:10.1002/slct.201701223 |
| [65] |
Bezold F, Weinberger ME, Minceva M. Assessing solute partitioning in deep eutectic solvent-based biphasic systems using the predictive thermodynamic model COSMO-RS[J]. Fluid Phase Equilib, 2017, 437: 23-33. DOI:10.1016/j.fluid.2017.01.001 |
| [66] |
Mahanta U, Choudhury S, Venkatesh RP, et al. Ionic-liquidbased deep eutectic solvents as novel electrolytes for supercapacitors: COSMO-SAC predictions, synthesis, and characterization[J]. ACS Sustainable Chem Eng, 2020, 8: 372-381. DOI:10.1021/acssuschemeng.9b05596 |
| [67] |
Cheng H, Liu C, Zhang J, et al. Screening deep eutectic solvents for extractive desulfurization of fuel based on COSMO-RS model[J]. Chem Eng Process, 2018, 125: 246-252. DOI:10.1016/j.cep.2018.02.006 |
| [68] |
Klamt A. Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena[J]. J Phys Chem, 1995, 99: 2224-2235. DOI:10.1021/j100007a062 |
| [69] |
Klamt A, Eckert F. COSMO-RS: a novel and efficient method for the a priori prediction of thermophysical data of liquids[J]. Fluid Phase Equilib, 2000, 172: 43-72. DOI:10.1016/S0378-3812(00)00357-5 |
| [70] |
Liu X, Xu D, Diao B, et al. Separation of dimethyl carbonate and methanol by deep eutectic solvents: liquid-liquid equilibrium measurements and thermodynamic modeling[J]. J Chem Eng Data, 2018, 63: 1234-1239. DOI:10.1021/acs.jced.7b00858 |
| [71] |
Vega LF, Vilaseca O, Llovell F, et al. Modeling ionic liquids and the solubility of gases in them: recent advances and perspectives[J]. Fluid Phase Equilib, 2010, 294: 15-30. DOI:10.1016/j.fluid.2010.02.006 |
| [72] |
Abranches DO, Larriba M, Silva LP, et al. Using COSMO-RS to design choline chloride pharmaceutical eutectic solvents[J]. Fluid Phase Equilib, 2019, 497: 71-78. DOI:10.1016/j.fluid.2019.06.005 |
| [73] |
Jelinski T, Cysewski P. Application of a computational model of natural deep eutectic solvents utilizing the COSMO-RS approach for screening of solvents with high solubility of rutin[J]. J Mol Model, 2018, 24: 180. DOI:10.1007/s00894-018-3700-1 |
| [74] |
Aissaoui T, AlNashef IM, Benguerba Y. Dehydration of natural gas using choline chloride based deep eutectic solvents: COSMORS prediction[J]. J Nat Gas Sci Eng, 2016, 30: 571-577. DOI:10.1016/j.jngse.2016.02.007 |
| [75] |
Silva LP, Fernandez L, Conceição JHF, et al. Design and characterization of sugar-based deep eutectic solvents using conductorlike screening model for real solvents[J]. ACS Sustainable Chem Eng, 2018, 6: 10724-10734. DOI:10.1021/acssuschemeng.8b02042 |
| [76] |
Kamgar A, Mohsenpour S, Esmaeilzadeh F. Solubility prediction of CO2, CH4, H2, CO and N2 in choline chloride/urea as a eutectic solvent using NRTL and COSMO-RS models[J]. J Mol Liq, 2017, 247: 70-74. DOI:10.1016/j.molliq.2017.09.101 |
| [77] |
Roda A, Santos F, Matias AA, et al. Design and processing of drug delivery formulations of therapeutic deep eutectic systems for tuberculosis[J]. J Supercrit Fluids, 2020, 161: 104826. DOI:10.1016/j.supflu.2020.104826 |
| [78] |
Achkar TE, Fourmentin S, Greige-Gerges H. Deep eutectic solvents: an overview on their interactions with water and biochemical compounds[J]. J Mol Liq, 2019, 288: 111028. DOI:10.1016/j.molliq.2019.111028 |
| [79] |
Gajardo-Parra NF, Lubben MJ, Winnert JM, et al. Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol[J]. J Chem Thermodyn, 2019, 133: 272-284. DOI:10.1016/j.jct.2019.02.010 |
| [80] |
Wikene KO, Rukke HV, Bruzell E, et al. Physicochemical characterisation and antimicrobial phototoxicity of an anionic porphyrin in natural deep eutectic solvents[J]. Eur J Pharm Biopharm, 2016, 105: 75-84. DOI:10.1016/j.ejpb.2016.06.001 |
| [81] |
Liu Y, Zhang Y, Chen SN, et al. The influence of natural deep eutectic solvents on bioactive natural products: studying interactions between a hydrogel model and Schisandra chinensis metabolites[J]. Fitoterapia, 2018, 127: 212-219. DOI:10.1016/j.fitote.2018.02.024 |
| [82] |
Wikene KO, Bruzell E, Tonnesen HH. Improved antibacterial phototoxicity of a neutral porphyrin in natural deep eutectic solvents[J]. J Photochem Photobiol B, 2015, 148: 188-196. DOI:10.1016/j.jphotobiol.2015.04.022 |
| [83] |
Jeong KM, Zhao J, Jin Y, et al. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media[J]. Arch Pharm Res, 2015, 38: 2143-2152. DOI:10.1007/s12272-015-0678-4 |
| [84] |
Sahin S. Tailor-designed deep eutectic liquids as a sustainable extraction media: an alternative to ionic liquids[J]. J Pharm Biomed Anal, 2019, 174: 324-329. DOI:10.1016/j.jpba.2019.05.059 |
| [85] |
Gutierrez MC, Ferrer ML, Mateo CR, et al. Freeze-drying of aqueous solutions of deep eutectic solvents: a suitable approach to deep eutectic suspensions of self-assembled structures[J]. Langmuir, 2009, 25: 5509-5515. DOI:10.1021/la900552b |
| [86] |
Abbott AP, Capper G, Davies DL, et al. Preparation of novel, moisture-stable, lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains[J]. Chem Commun(Camb), 2001(19): 2010-2011. |
| [87] |
Satlewal A, Agrawal R, Bhagia S, et al. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: recent developments, challenges and novel opportunities[J]. Biotechnol Adv, 2018, 36: 2032-2050. DOI:10.1016/j.biotechadv.2018.08.009 |
| [88] |
Zainal-Abidin MH, Hayyan M, Hayyan A, et al. New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review[J]. Anal Chim Acta, 2017, 979: 1-23. DOI:10.1016/j.aca.2017.05.012 |
| [89] |
Du C, Zhao B, Chen XB, et al. Effect of water presence on choline chloride-2 urea ionic liquid and coating platings from the hydrated ionic liquid[J]. Sci Rep, 2016, 6: 29225. DOI:10.1038/srep29225 |
| [90] |
Shah D, Mjalli FS. Effect of water on the thermo-physical properties of reline: an experimental and molecular simulation based approach[J]. Phys Chem Chem Phys, 2014, 16: 23900-23907. DOI:10.1039/C4CP02600D |
| [91] |
Ru J, Bu J, Wang Z. Effect of temperature on viscosity and conductivity of choline chloride-urea-sb2s3 system[J]. Technol Innov Appl(科技创新与应用), 2019(18): 75-76. |
| [92] |
Abbott AP, Capper G, Gray S. Design of improved deep eutectic solvents using hole theory[J]. ChemPhysChem, 2006, 7: 803-806. DOI:10.1002/cphc.200500489 |
| [93] |
Vigier KD, Chatel G, Jerome F. Contribution of deep eutectic solvents for biomass processing: opportunities, challenges, and limitations[J]. ChemCatChem, 2015, 7: 1250-1260. DOI:10.1002/cctc.201500134 |
| [94] |
Garcia G, Aparicio S, Ullah R, et al. Deep eutectic solvents: physicochemical properties and gas separation applications[J]. Energy Fuels, 2015, 29: 2616-2644. DOI:10.1021/ef5028873 |
| [95] |
Hayyan A, Mjalli FS, AlNashef IM, et al. Glucose-based deep eutectic solvents: physical properties[J]. J Mol Liq, 2013, 178: 137-141. DOI:10.1016/j.molliq.2012.11.025 |
| [96] |
Alomar MK, Hayyan M, Alsaadi MA, et al. Glycerol-based deep eutectic solvents: physical properties[J]. J Mol Liq, 2016, 215: 98-103. DOI:10.1016/j.molliq.2015.11.032 |
| [97] |
Abbott AP, Harris RC, Ryder KS. Application of hole theory to define ionic liquids by their transport properties[J]. J Phys Chem B, 2007, 111: 4910-4913. DOI:10.1021/jp0671998 |
| [98] |
Aroso IM, Silva JC, Mano F, et al. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems[J]. Eur J Pharm Biopharm, 2016, 98: 57-66. DOI:10.1016/j.ejpb.2015.11.002 |
| [99] |
Paiva A, Craveiro R, Aroso I, et al. Natural deep eutectic solvents-solvents for the 21st century[J]. ACS Sustainable Chem Eng, 2014, 2: 1063-1071. DOI:10.1021/sc500096j |
| [100] |
Nerurkar J, Beach JW, Park MO, et al. Solubility of (+/-)-ibuprofen and s (+/-)-ibuprofen in the presence of cosolvents and cyclodextrins[J]. Pharm Dev Technol, 2005, 10: 413-421. |
| [101] |
Jelinski T, Przybylek M, Cysewski P. Solubility advantage of sulfanilamide and sulfacetamide in natural deep eutectic systems: experimental and theoretical investigations[J]. Drug Dev Ind Pharm, 2019, 45: 1120-1129. DOI:10.1080/03639045.2019.1597104 |
| [102] |
Liu Y, Garzon J, Friesen JB, et al. Countercurrent assisted quantitative recovery of metabolites from plant-associated natural deep eutectic solvents[J]. Fitoterapia, 2016, 112: 30-37. DOI:10.1016/j.fitote.2016.04.019 |
| [103] |
Durand E, Lecomte J, Upasani R, et al. Evaluation of the ROS inhibiting activity and mitochondrial targeting of phenolic compounds in fibroblast cells model system and enhancement of efficiency by natural deep eutectic solvent (NADEs) formulation[J]. Pharm Res, 2017, 34: 1134-1146. DOI:10.1007/s11095-017-2124-4 |
| [104] |
Lu C, Cao J, Wanga N, et al. Significantly improving the solubility of nonsteroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration[J]. Med Chem Commun, 2016, 7: 955-959. DOI:10.1039/C5MD00551E |
| [105] |
Morrison HG, Sun CC, Neervannan S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles[J]. Int J Pharm, 2009, 378: 136-139. DOI:10.1016/j.ijpharm.2009.05.039 |
| [106] |
Gutierrez A, Aparicio S, Atilhan M. Design of arginine-based therapeutic deep eutectic solvents as drug solubilization vehicles for active pharmaceutical ingredients[J]. Phys Chem Chem Phys, 2019, 21: 10621-10634. DOI:10.1039/C9CP01408J |
| [107] |
Gutierrez A, Atilhan M, Aparicio S. A theoretical study on lidocaine solubility in deep eutectic solvents[J]. Phys Chem Chem Phys, 2018, 20: 27464-27473. DOI:10.1039/C8CP05641B |
| [108] |
Olivares B, Martínez F, Rivas L, et al. A natural deep eutectic solvent formulated to stabilize β-lactam antibiotics[J]. Sci Rep, 2018, 8: 14900. DOI:10.1038/s41598-018-33148-w |
| [109] |
Pedro SN, Freire MG, Freire CSR, et al. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems[J]. Expert Opin Drug Deliv, 2019, 16: 497-506. DOI:10.1080/17425247.2019.1604680 |
| [110] |
Araya-Sibaja AM, Vega-Baudrit JR, Guillén-Girón T, et al. Drug solubility enhancement through the preparation of multicomponent organic materials: eutectics of lovastatin with carboxylic acids[J]. Pharmaceutics, 2019, 11: 112-128. DOI:10.3390/pharmaceutics11030112 |
| [111] |
Kharat M, Du Z, Zhang G, et al. Physical and chemical stability of curcumin in aqueous solutions and emulsions: impact of pH, temperature, and molecular environment[J]. J Agric Food Chem, 2017, 65: 1525-1532. DOI:10.1021/acs.jafc.6b04815 |
| [112] |
Abdel‑Hafez SM, Hathout RM, Sammour OA. Attempts to enhance the anti‑cancer activity of curcumin as a magical oncological agent using transdermal delivery[J]. Adv Tradit Med, 2020. DOI:10.1007/s13596-020-00439-5 |
| [113] |
Goud NR, Suresh K, Sanphui P, et al. Fast dissolving eutectic compositions of curcumin[J]. Int J Pharm, 2012, 439: 63-72. DOI:10.1016/j.ijpharm.2012.09.045 |
| [114] |
Jelinski T, Przybylek M, Cysewski P. Natural deep eutectic solvents as agents for improving solubility, stability and delivery of curcumin[J]. Pharm Res, 2019, 36: 116. DOI:10.1007/s11095-019-2643-2 |
| [115] |
Wikene KO, Bruzell E, Tonnesen HH. Characterization and antimicrobial phototoxicity of curcumin dissolved in natural deep eutectic solvents[J]. Eur J Pharm Sci, 2015, 80: 26-32. DOI:10.1016/j.ejps.2015.09.013 |
| [116] |
Blessy M, Patel RD, Prajapati PN, et al. Development of forced degradation and stability indicating studies of drugs—a review[J]. J Pharm Anal, 2014, 4: 159-165. DOI:10.1016/j.jpha.2013.09.003 |
| [117] |
Chen J, Li SF, Yao ZF, et al. Improved stability of salvianolic acid B from Radix Salviae miltiorrhizae in deep eutectic solvents[J]. Anal Methods, 2016, 8: 2502-2509. DOI:10.1039/C5AY03351A |
| [118] |
Adawiyah N, Moniruzzaman M, Hawatulailaa S, et al. Ionic liquids as a potential tool for drug delivery systems[J]. MedChemComm, 2016, 7: 1881-1897. DOI:10.1039/C6MD00358C |
| [119] |
Zakrewsky M, Lovejoy KS, Kern TL, et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization[J]. Proc Natl Acad Sci U S A, 2014, 111: 13313-13318. DOI:10.1073/pnas.1403995111 |
| [120] |
Stott PW, Williams AC, Barry BW. Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen[J]. J Control Release, 1998, 50: 297-308. DOI:10.1016/S0168-3659(97)00153-3 |
| [121] |
Qu W, Hakkinen R, Allen J, et al. Globular and fibrous proteins modified with deep eutectic solvents: materials for drug delivery[J]. Molecules, 2019, 24: 3583. DOI:10.3390/molecules24193583 |
| [122] |
Berton P, Di Bona KR, Yancey D, et al. Transdermal bioavailability in rats of lidocaine in the forms of ionic liquids, salts, and deep eutectic[J]. ACS Med Chem Lett, 2017, 8: 498-503. DOI:10.1021/acsmedchemlett.6b00504 |
| [123] |
Tasli H, Yurekli A, Gokgoz MC, et al. Effects of oral isotretinoin therapy on the nasal cavities[J]. Braz J Otorhinolaryngol, 2020, 86: 99-104. DOI:10.1016/j.bjorl.2018.10.004 |
| [124] |
Woensel MV, Wauthoz N, Rosière R, et al. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle gbm?[J]. Cancers (Basel), 2013, 5: 1020-1048. DOI:10.3390/cancers5031020 |
| [125] |
Lobaina Mato Y. Nasal route for vaccine and drug delivery: features and current opportunities[J]. Int J Pharm, 2019, 572: 118813. DOI:10.1016/j.ijpharm.2019.118813 |
| [126] |
Khan AR, Liu M, Khan MW, et al. Progress in brain targeting drug delivery system by nasal route[J]. J Control Release, 2017, 268: 364-389. DOI:10.1016/j.jconrel.2017.09.001 |
| [127] |
Wang B, Liu HR, Chen F, et al. Progress in pharmacokinetics of oral transmucosal drug delivery systems[J]. Acta Pharm Sin(药学学报), 2020, 55: 226-234. |
| [128] |
Liu CS, Zheng YR, Zhang YF, et al. Research progress on berberine with a special focus on its oral bioavailability[J]. Fitoterapia, 2016, 109: 274-282. DOI:10.1016/j.fitote.2016.02.001 |
| [129] |
Sut S, Faggian M, Baldan V, et al. Natural deep eutectic solvents (NADEs) to enhance berberine absorption: an in vivo pharmacokinetic study[J]. Molecules, 2017, 22: 1921. DOI:10.3390/molecules22111921 |
| [130] |
Chen J, Wang Q, Liu M, et al. The effect of deep eutectic solvent on the pharmacokinetics of salvianolic acid B in rats and its acute toxicity test[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2017, 1063: 60-66. DOI:10.1016/j.jchromb.2017.08.016 |
| [131] |
Stoimenovski J, MacFarlane DR, Bica K, et al. Crystalline vs ionic liquid salt forms of active pharmaceutical ingredients: a position paper[J]. Pharm Res, 2010, 27: 521-526. DOI:10.1007/s11095-009-0030-0 |
| [132] |
Abbott AP, Ahmed EI, Prasad K, et al. Liquid pharmaceuticals formulation by eutectic formation[J]. Fluid Phase Equilib, 2017, 448: 2-8. DOI:10.1016/j.fluid.2017.05.009 |
| [133] |
Ferraz R, Branco LC, Prudêncio C, et al. Ionic liquids as active pharmaceutical ingredients[J]. ChemMedChem, 2011, 6: 975-985. DOI:10.1002/cmdc.201100082 |
| [134] |
Marrucho IM, Branco LC, Rebelo LPN. Ionic liquids in pharmaceutical applications[J]. Annu Rev Chem Biomol Eng, 2014, 5: 527-546. DOI:10.1146/annurev-chembioeng-060713-040024 |
| [135] |
Stella VJ. Prodrugs: some thoughts and current issues[J]. J Pharm Sci, 2010, 99: 4755-4765. DOI:10.1002/jps.22205 |
| [136] |
Huttunen KM, Raunio H, Rautio J. Prodrugs-from serendipity to rational design[J]. Pharmacol Rev, 2011, 63: 750-771. DOI:10.1124/pr.110.003459 |
| [137] |
Banerjee R, Bhatt PM, Ravindra NV, et al. Saccharin salts of active pharmaceutical ingredients, their crystal structures, and increased water solubilities[J]. Cryst Growth Des, 2005, 5: 2299-2309. DOI:10.1021/cg050125l |
| [138] |
Serajuddin ATM. Salt formation to improve drug solubility[J]. Adv Drug Deliv Rev, 2007, 59: 603-616. DOI:10.1016/j.addr.2007.05.010 |
| [139] |
Berge SM, Bighley LD, Monkhouse DC. Pharmaceutical salts[J]. J Pharm Sci, 1977, 66: 1-19. DOI:10.1002/jps.2600660104 |
| [140] |
Aitipamula S, Banerjee R, Bansal AK, et al. Polymorphs, salts, and cocrystals: what's in a name?[J]. Cryst Growth Des, 2012, 12: 2147-2152. DOI:10.1021/cg3002948 |
| [141] |
Dean PM, Turanjanin J, Yoshizawa-Fujita M, et al. Exploring an anti-crystal engineering approach to the preparation of pharmaceutically active ionic liquids[J]. Cryst Growth Des, 2009, 9: 1137-1145. DOI:10.1021/cg8009496 |
| [142] |
Elder DP, Holm R, de Diego HL. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility[J]. Int J Pharm, 2013, 453: 88-100. DOI:10.1016/j.ijpharm.2012.11.028 |
| [143] |
Childs SL, Chyall LJ, Dunlap JT, et al. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids[J]. J Am Chem Soc, 2004, 126: 13335-13342. DOI:10.1021/ja048114o |
| [144] |
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions[J]. Eur J Pharm Biopharm, 2000, 50: 47-60. DOI:10.1016/S0939-6411(00)00076-X |
| [145] |
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs[J]. Drug Discov Today, 2007, 12: 1068-1075. DOI:10.1016/j.drudis.2007.09.005 |
| [146] |
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers[J]. Nat Rev Drug Discov, 2005, 4: 145-160. DOI:10.1038/nrd1632 |
| [147] |
Tarate B, Bansal AK. Characterization of CoQ 10-lauric acid eutectic system[J]. Thermochim Acta, 2015, 605: 100-106. DOI:10.1016/j.tca.2015.01.018 |
| [148] |
Mota-Morales JD, Gutiérrez MC, Ferrer ML, et al. Deep eutectic solvents as both active fillers and monomers for frontal polymerization[J]. J Polym Sci Part A Polym Chem, 2013, 51: 1767-1773. DOI:10.1002/pola.26555 |
| [149] |
Phaechamud T, Tuntarawongsa S, Charoensuksai P. Evaporation behavior and characterization of eutectic solvent and ibuprofen eutectic solution[J]. AAPS PharmSciTech, 2016, 17: 1213-1220. DOI:10.1208/s12249-015-0459-x |
| [150] |
Alvarez MS, Zhang YF. Sketching neoteric solvents for boosting drugs bioavailability[J]. J Control Release, 2019, 311: 225-232. |
| [151] |
Nazzal S, Smalyukh II, Lavrentovich OD, et al. Preparation and in vitro characterization of a eutectic based semisolid selfnanoemulsified drug delivery system (SNEDDs) of ubiquinone: mechanism and progress of emulsion formation[J]. Int J Pharm, 2002, 235: 247-265. DOI:10.1016/S0378-5173(02)00003-0 |
| [152] |
Wang WP, Cai YQ, Liu YH, et al. Microemulsions based on paeonol-menthol eutectic mixture for enhanced transdermal delivery: formulation development and in vitro evaluation[J]. Artif Cells Nanomed Biotechnol, 2017, 45: 1241-1246. DOI:10.1080/21691401.2016.1226178 |
| [153] |
Aroso IM, Craveiroc R, Â Rochad, et al. Design of controlled release systems for thedes—therapeutic deep eutectic solvents, using supercritical fluid technology[J]. Int J Pharm, 2015, 492: 73-79. DOI:10.1016/j.ijpharm.2015.06.038 |
| [154] |
Duarte ARC, Ferreira ASD, Barreiros S, et al. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: solubility and permeability studies[J]. Eur J Pharm Biopharm, 2017, 114: 296-304. DOI:10.1016/j.ejpb.2017.02.003 |
| [155] |
Tuntarawongsa S, Phaechamud T. Polymeric eutectic system[J]. Adv Mater Res, 2012, 528: 180-183. DOI:10.4028/www.scientific.net/AMR.528.180 |
| [156] |
Serrano MC, Gutierrez MC, Jimenez R, et al. Synthesis of novel lidocaine-releasing poly(diol-co-citrate)elastomers by using deep eutectic solvents[J]. Chem Commun, 2012, 48: 579-581. DOI:10.1039/C1CC15284J |
| [157] |
Mano F, Martins M, Sá-Nogueira I, et al. Production of electrospun fast-dissolving drug delivery systems with therapeutic eutectic systems encapsulated in gelatin[J]. AAPS PharmSciTech, 2017, 18: 2579-2585. DOI:10.1208/s12249-016-0703-z |
 2021, Vol. 56
2021, Vol. 56


