慢性肾病(chronic kidney disease, CKD)是指肾脏结构或功能出现异常, 且持续时间超过3个月[1], 肾脏功能在几个月甚至几年内缓慢衰竭的一类肾脏疾病。随着病程不断发展, CKD最终会演化成终末期肾病(end stage renal disease, ESRD)[2]。
CKD发病率高、死亡率高, 但是知晓率低、防治率低, 已经成为危害全球公众健康的重大公共卫生问题[3]。2017年第23届世界肾脏大会发布的全球肾病健康报告显示, CKD在全球的发病率已经超过10%, 其中, 中国的CKD发病率为10.8%, 占全球CKD患者的17.29%[4-6]。随着人口老龄化以及糖尿病和高血压患病率的不断增加, CKD的发病率还在不断攀升[7, 8]。
CKD目前缺乏有效的治疗措施, 现有治疗方案主要包括: ①对CKD病因的治疗。如对可能引起肾脏损伤的慢性肾炎、糖尿病肾病和高血压等疾病进行积极治疗; ②避免或消除引起CKD加重的因素。如严格控制血压、血糖、血脂和蛋白尿等; ③对CKD并发症的控制。如对贫血、矿物质和骨代谢异常、心血管疾病等CKD并发症进行纠正和治疗; ⑤调整CKD患者生活方式和饮食结构。如适当进行锻炼和控制体重, 同时采取低蛋白饮食方案, 减少盐的摄入; ⑥终末期肾病患者采取血液透析、腹膜透析及肾脏移植等替代治疗[9]。国内外肾脏病学界一直致力于探索延缓CKD进展的治疗策略, 已经取得了重要进展, 但仍然存在一些尚未解决的问题。
一直以来, 人们只关注宿主本身, 通过调控宿主细胞代谢通路来防治疾病。近年来, 随着肠道菌群在疾病中的作用被逐渐发现, 靶向肠道菌群中的代谢通路, 调控肠道菌群-宿主共代谢产物的生物合成, 已经成为干预宿主疾病的新策略[10-12]。肠道菌群-宿主共代谢产物是指那些由肠道菌群和宿主共同参与合成的代谢产物, 即由肠道菌群合成前体, 之后在宿主细胞内代谢形成最终产物。当调控宿主细胞代谢通路防治疾病出现困境时, 靶向肠道菌群中的代谢通路就成为了新的后备策略。
2015年美国克利夫兰医学中心的研究团队首次报道[13], 小分子化合物3, 3-二甲基丁醇通过靶向肠道菌群中三甲胺代谢通路, 抑制氧化三甲胺的生物合成, 可以有效延缓动脉粥样硬化的进程, 证实靶向肠道菌群中的代谢通路可干预宿主疾病进程。氧化三甲胺是一种代表性的肠道菌群-宿主共代谢产物, 肠道菌群可以将饮食中的胆碱等物质转化为三甲胺, 三甲胺随后会在宿主肝脏中被代谢成为氧化三甲胺, 与人类的动脉粥样硬化等疾病密切相关[13]。2018年该团队再次证实[14], 靶向肠道菌群中的三甲胺裂解酶可抑制氧化三甲胺的生物合成, 可以有效降低血栓形成。同年, 北京大学研究团队的一项研究成果表明[15], 降糖药二甲双胍通过抑制肠道脆弱拟杆菌中胆汁酸水解酶活性, 调控肠道菌群中胆汁酸代谢通路, 增加特定胆汁酸水平, 可以有效发挥降糖作用。2017年中国医学科学院研究团队发表的一项研究成果证实[3], 通过调控肠道菌群中小檗碱代谢相关的硝基还原酶, 可以对高血脂进行有效的个性化治疗。这些最前沿的研究均提示, 靶向肠道菌群代谢通路干预宿主疾病的新策略已经成为近年来的研究热点, 在防治疾病中显示出巨大的潜力。
肠道菌群是生活在人体消化道内的微生物组成的复杂群落, 与宿主维持着相对稳定的平衡状态, 对宿主营养、代谢和免疫等生理过程具有重要作用。本世纪的肠道菌群研究热潮始于1996年戈登实验室对肠道菌群的开创性工作, Bry等[16]首先揭示了肠道菌群对宿主肥胖、发育及营养的影响, 指出了肠道菌群在宿主生理病理过程中的关键地位。随后的20年里肠道菌群与宿主疾病的关系被进一步揭示, 证实了肠道菌群与肥胖、糖尿病、免疫系统失衡、肠道疾病、代谢性疾病和心血管疾病的发生发展密切相关[17]。除了肠道菌群, 人体肠道还存在肠黏膜屏障, 是肠道抵御外界侵袭、阻止有害物质进入血液并维持肠道微环境的重要结构。肠道菌群和肠黏膜屏障共同构成肠道稳态系统, 共同维护着宿主稳态与健康。
在CKD的发生发展过程中, 常常伴随着肠道菌群及其代谢产物代谢紊乱、肠道屏障功能受损等现象, 因此, 基于肠道菌群及其代谢产物的代谢调控进而干预慢性肾病进展, 具有重要的应用前景及科学价值。
1 肠道稳态与宿主健康密切相关人体肠道内定居着种类繁多的微生物, 包括细菌、真菌、病毒和支原体等, 其中绝大部分是细菌, 称作肠道菌群。正常情况下, 肠道菌群维持动态平衡, 保持肠道微环境的稳定。
1.1 肠道菌群肠道菌群可以分为共生菌、条件致病菌和致病菌3类, 由于胃和小肠不适合细菌定植, 因此肠道细菌主要分布在结肠部位, 以双歧杆菌和乳酸杆菌等共生菌占优势, 同时还存在一些肠杆菌和肠球菌等条件致病菌[18]。正常人体肠道内细菌是处于相对稳定的平衡状态, 其中绝大部分属于厚壁菌门和拟杆菌门两大优势门[19], 但是肠道菌群也会受遗传、年龄、饮食和地理环境等影响存在差异。
在长期的共同进化中, 肠道菌群和宿主形成了互利共生的关系, 宿主给肠道菌群提供生长环境和营养, 而肠道菌群则帮助人体营养吸收、物质代谢、抵御病原体、调节免疫、生成短链脂肪酸和维生素K等营养物质, 维持肠道稳态[20], 并且可能影响药物在人体内的药代动力学[21-23]。然而不合理使用抗生素、疾病、营养不良或者外来病菌入侵等将会打破这种平衡, 出现肠道微生态失调[24, 25]。
1.2 肠黏膜屏障肠黏膜屏障由机械屏障、化学屏障、免疫屏障和生物屏障4部分组成[26], 其中肠黏膜上皮细胞和上皮细胞间的紧密连接构成的机械屏障是肠黏膜屏障最重要的组成因素[27]。化学屏障由肠道分泌的溶菌酶和消化酶等化学物质构成, 免疫屏障由肠内淋巴组织和免疫细胞组成, 肠道菌群相互作用则形成了生物屏障。完整的黏膜屏障系统可以阻止肠道细菌及其产物向血液易位[28], 一旦黏膜屏障受损, 肠通透性增加, 则细菌及其产物内毒素等会易位, 激活单核巨噬细胞系统, 促进大量炎症因子如白细胞介素-6 (interleukin-6, IL-6)和肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)等产生, 最终形成慢性微炎症状态[29, 30]。
2 慢性肾病和肠道微生态的关联性分析(肠-肾轴理论)早在2011年的国际透析大会上, 就有学者提出“肠肾综合征”的概念[31], 认为肠道和肾脏之间存在着密切的联系。随着肠道微生态和肾脏联系的研究逐渐深入, Meijers等[32]于同年提出了对CKD进展和肠道进行关联的肠-肾轴理论(the theory of gut-kidney axis), 认为肠道和肾脏的病变可以相互影响, 一方受损, 则另一方的结构和功能也会受到影响, 此理论已成为近年研究的热点。
2.1 伴随着慢性肾病的发生发展, 肠道菌群稳态失调CKD患者常伴随有肠道菌群失调, 肠道菌群的数量、组成、分布和结构等都发生了改变, 具体表现为有益菌减少, 而致病菌和条件致病菌异常增殖, 原本相对无菌的小肠中细菌数量显著上升。在人体实验中, Vaziri等[29, 33]研究发现, ESRD患者与健康人对照相比有190种细菌丰度有显著差异, 其中短状杆菌科、肠杆菌科、多囊黏菌科、盐单胞菌科、莫拉氏菌科和假单胞菌科等显著增多, 而普雷沃氏菌科和乳杆菌科等则明显减少。细菌代谢功能关联分析发现, 含有尿素酶、尿酸酶、吲哚和对甲酚形成酶的细菌占主导地位, 产短链脂肪酸的细菌减少甚至消失。动物实验也有类似的发现, 在5/6肾切致慢性肾衰模型大鼠中发现, 变形菌门的细菌增多, 尤其是肠杆菌属和变形杆菌属增多, 乳酸菌属和双歧菌属减少[34]。
导致CKD患者肠道菌群失调的原因主要包括以下方面: ① CKD患者肾脏排泄功能下降, 很多代谢产物如尿素不能及时排出体外, 不断在体内蓄积, 高浓度的尿素会经肠壁血管进入肠腔被细菌分解成氨, 改变肠道pH值, 引起肠道菌群失调并破坏肠黏膜; ② CKD患者的饮食结构发生改变, 膳食纤维摄入不足, 使得蛋白质利用率提高, 加之肠道转运能力下降, 有毒物质保留时间增加, 使得菌群发生改变; ③ CKD患者因出现的全身炎症而使用抗生素, 自身肠壁充血水肿和终末期血液透析引起的肠道缺血低氧会破坏肠屏障而引起菌群失调等。
2.2 伴随着慢性肾病的发生发展, 肠黏膜屏障受到破坏CKD的进展还会导致肠黏膜屏障受损。在正常状态下, 由肠黏膜上皮细胞和紧密连接组成的肠上皮屏障可以有效阻挡肠内的细菌和内毒素等有害产物进入血液。然而CKD模型大鼠结肠上皮细胞中的紧密连接蛋白claudin-1、occludin和ZO-1 (zonula occludens1)表达显著降低, 肠上皮屏障被破坏, 肠道通透性增加, 形成渗漏性肠道(leaky gut)[35], 作者推测可能与尿素的不断积累有关[36]。同时失调的肠道菌群会产生大量肠源性尿毒素, 使得肠黏膜出现水肿、萎缩和炎症等变化, 肠黏膜屏障受损。
2.3 随着肠道菌群失调, 肠源性尿毒素合成增加及体内蓄积, 进一步加剧慢性肾病的进展随着CKD的进展以及肾脏功能的丧失, 体内代谢产生的氮质废物不能及时排出体外, 在体内不断堆积形成的对机体有毒性的物质称作尿毒素。2003年, 欧洲尿毒素工作组邀请了数十位专家, 对已经确认的尿毒素进行分类整理, 按照分子量将尿毒素分为3类: 第1类是不与蛋白质结合的水溶性小分子物质(45种, 如肌酐和尿素氮); 第2类是中分子物质(22种, 如甲状旁腺素); 第3类则是蛋白结合型尿毒素(25种, 如硫酸吲哚酚和硫酸对甲酚, 因与血浆蛋白结合率高而命名)[37]。
蛋白结合尿毒素的前体多在肠道内由肠道微生物代谢生成, 故也称其为肠源尿毒素。肠源尿毒素是一类肠道来源的, 由肠道细菌与机体共代谢生成的产物, 即首先由肠道细菌合成前体, 之后被机体特定酶代谢生成。过去很长一段时间, 研究者都致力于小分子量及中分子量尿毒素的研究, 对肠源尿毒素并未给予充分重视, 直到最近几年, 研究者才开始认识到肠源尿毒素的重要性。
临床研究证实, 肠源尿毒素能够损伤肾脏和心血管系统, 是引起CKD进展加快, 诱发CKD患者发生心血管并发症(如胰岛素抵抗和动脉粥样硬化), 影响CKD患者预后, 导致CKD患者死亡率升高的独立风险因素[38-41]。实验研究证实, 肠源尿毒素可诱导模型动物体内的氧化应激反应, 产生各种炎症因子, 引起肾小管损伤, 诱导心肌细胞凋亡并加重心脏功能失调[39-46]。以下列出几种危害较大, 且目前研究较多的肠源尿毒素分子(图 1)。
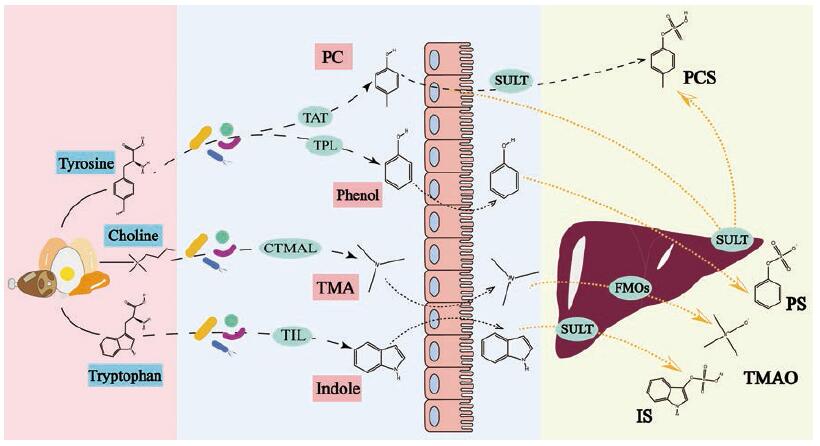
|
Figure 1 Generation pathways of gut-derived uremic toxins.TAT: Tyrosine aminotransferase; TPL: Tyrosine phenol-lyase; CTMAL: Choline trimethylamine lyase; TIL: Tryptophan indole-lyase; SULT: Sulfotransferase; FMOs: Flavin monooxygenases; PC: p-Cresol; TMA: Trimethylamine; PCS: p-Cresyl sulfate; PS: Phenyl sulfate; TMAO: Trimethylamine-N-oxide; IS: Indoxyl-sulfate |
硫酸吲哚酚(indoxyl-sulfate, IS)是典型的肠道菌群-宿主共代谢产物, 食物中的色氨酸首先在肠道菌群作用下转化为吲哚, 吲哚进入宿主细胞后在肝脏细胞色素P450酶及磺基转移酶作用下转化为IS[47]。IS和白蛋白非共价结合, 蛋白结合率高达90%以上[48]。IS经肾脏排泄, 主要是通过肾小管的有机阴离子转运系统(organic anion transporter, OAT)摄取和排泄[49]。
IS属于蛋白结合型尿毒素, 是促进CKD进展的关键性尿毒素。动物实验研究表明[47, 50], IS会诱导肾小管细胞进入氧化应激状态, 加速肾小管细胞损伤凋亡, 导致肾间质纤维化以及肾小球硬化。临床研究也表明[47], 进入透析治疗阶段的CKD患者体内IS水平是正常人体的20倍, 并且CKD患者血浆中的IS水平与其肾功能呈负相关, IS水平越高则肾功能越弱。IS的毒性作用具体表现见图 2。
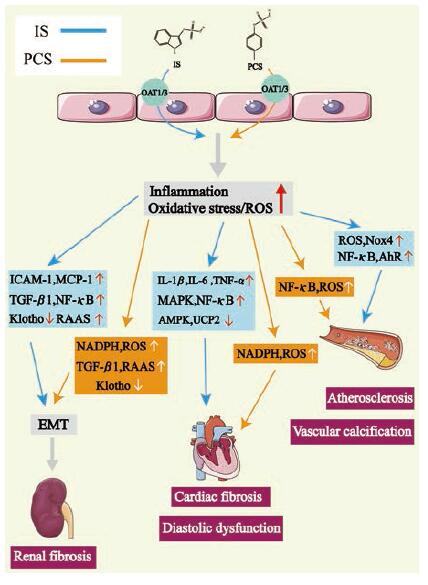
|
Figure 2 The role of IS and PCS in cardiorenal syndrome and related pathways.IS and PCS can up-regulate the level of inflam‐matory factors and promote oxidative stress in patients with chronic kidney disease (CKD), and eventually cause renal fibrosis and a variety of cardiovascular diseases through multiple pathways.OAT: Organic anion transporter; ROS: Reactive oxygen species; ICAM-1:Intercellular adhesion molecule-1;MCP-1:Monocyte chemoattractant protein-1;NADPH: Nicotinamide adenine dinu‐cleotide phosphate; Nox4:NADPH oxidase 4;NF-κB: Nuclear factor kappa-B; RAAS: Renin-angiotensin-aldosterone system; IL: Interleukin; TNF-α: Tumor necrosis factor-α; MAPK: Mitogenactivated protein kinase; AMPK: AMP-activated protein kinase; UCP2:Uncoupling protein 2;AhR: Aryl hydrocarbon receptor; TGF-β1:Transforming growth factor-β1;EMT: Epithelial-to-mes‐enchymal transition |
IS通过OAT1或OAT3进入肾小管细胞, 刺激肾小管细胞释放TGF-β1 (transforming growth factor-β1)和其他趋化因子, 例如细胞间黏附分子-1 (intercellular adhesion molecule-1, ICAM-1)、单核细胞趋化蛋白-1 (monocyte chemoattractant protein-1, MCP-1)、骨桥蛋白(osteopontin)和内皮素-1 (endothe‐lin-1), 从而促进巨噬细胞增殖并分泌TGF-β1[51]。TGF-β1可以刺激金属蛋白酶组织抑制因子-1 (tissue inhibitor of metalloproteinases-1, TIMP-1)和胶原蛋白的产生, 引起肾脏纤维化[52]; IS还能诱导自由基(free radicals)生成, 进而增强NF-κB (nuclear factor kappa-B)和TIMP-1的活性, 从而增强纤溶酶原激活抑制剂-1(plasminogen activator inhibitor-1, PAI-1)的活性, 导致肾脏纤维化[53]; IS还可以通过激活肾素-血管紧张素-醛固酮系统(renin-angiotensin-aldosterone system, RAAS)和TGF-β/Smad途径, 诱导肾小管发生上皮细胞间充质转化(epithelial-to-mesenchymal transition, EMT), 导致肾脏纤维化[54]。
Klotho蛋白在抗衰老、矿物质代谢和维生素D代谢中起重要作用[55], 还对肾脏有保护作用, 减少肾纤维化和血管钙化[56, 57]。体外实验发现IS可以促进DNA甲基转移酶(DNA methyltransferase, DNMT) 1、3a和3b的表达, 使得Klotho基因超甲基化而失去其生理功能[58]。IS还可以通过产生活性氧(reactive oxygen species, ROS)和激活近端小管细胞中的NF-κB而下调肾脏Klotho的表达, 加重肾脏损伤[59]。IS还可以通过ROS/NF-κB/p53途径促进肾近端小管细胞衰老[60]。
2.3.1.2 IS的心血管毒性IS可以显著提高新生大鼠心肌成纤维细胞胶原蛋白的合成和心肌细胞的肥大, 上调人单核细胞中IL-1β、IL-6和TNF-α等炎性细胞因子的mRNA表达, 通过促进心脏纤维化和左心室肥大参与心脏重塑, 并且以上作用都与p38、p42/p44 MAPK(mitogen-activated protein kinase)和NF-κB通路有关[61]。IS还通过抑制AMP活化蛋白激酶(AMP-activated protein kinase, AMPK)/解偶联蛋白2 (uncoupling protein 2, UCP2)途径, 增加线粒体ROS诱导心肌肥大[62]。有研究发现IS还可以通过氧化应激引起兔子心律失常[63]。
IS可促进动脉粥样硬化。动脉粥样硬化的病理标志包括内皮细胞损伤、血管炎症和血管平滑肌细胞(vascular smooth muscle cell, VSMC)增殖[64]。研究显示IS可以通过增加ROS的产生和提高p53活性诱导内皮细胞衰老[65], 通过还原型烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate, NADPH)氧化酶Nox4 (NADPH oxidase 4)诱导自由基的产生, 从而减少一氧化氮的产生, 并可能导致内皮细胞的过度氧化[66]。IS还可以抑制内皮细胞增殖和修复, 并诱导内皮微粒释放, 这是一种内皮细胞损伤的标记物[67]。IS通过ROS诱导内皮细胞NF-κB活化而上调ICAM-1和MCP-1的表达, 诱导白细胞黏附于血管内皮细胞[68], 通过上调E-选择素(E-selectin)[可能是通过JNK (c-Jun N-terminal kinase)和NF-κB依赖性途径]来增强白细胞与内皮的相互作用[69], 同时IS能刺激血管平滑肌细胞增殖[70]。IS还是VSMC上芳烃受体(aryl hydrocarbon receptor, AhR)的内源性激动剂, AhR的活化不仅诱导了MCP-1的表达[71], 还抑制了组织因子降解, 使其水平升高, 而组织因子可以启动凝血反应[72-74]。
IS可以促进血管钙化。越来越多的证据表明尿毒症的血管钙化是人主动脉平滑肌细胞(human aortic smooth muscle cells, HASMCs)介导的主动过程, 而不是被动的钙磷沉淀, HASMC的成骨作用是关键。Adijiang等[75]证明IS可以促进高血压大鼠主动脉钙化和动脉壁增厚。体外实验也证明IS通过上调Nox4诱导ROS生成, 在HASMC中表达成骨细胞特异性蛋白, 如核心结合因子1 (core binding factor 1, Cbfa1)、碱性磷酸酶(alkaline phosphatase, ALP)和骨桥蛋白等[76]。
2.3.1.3 IS的其他毒性IS还被认为可以损害成骨细胞功能, 下调成骨细胞中甲状旁腺素受体(parathyroid hormone receptor, PTHR)的表达[77], 阻止破骨细胞分化和骨吸收[78], 降低骨骼质量。血液透析患者认知功能降低也被认为与IS有关[79]。
2.3.2 吲哚-3-乙酸吲哚-3-乙酸(indole-3-acetic acid, IAA)是一种由肠道细菌代谢色氨酸产生的蛋白结合型尿毒素, IAA与白蛋白结合率约为80%, 不易被透析清除。IAA在CKD患者中其血清水平升高, 可以独立预测CKD患者的死亡风险和心血管事件。IAA和IS一样都属于吲哚类尿毒素家族, IAA还是AhR的激动剂, 外源性配体激活AhR可以促进血管炎症、氧化应激和动脉粥样硬化, 并在心血管疾病中发挥作用[80]。有研究发现IAA可以诱导内皮组织因子表达, 导致促凝作用, 并通过激活AhR/p38MAPK/NF-κB等途径诱导内皮细胞氧化应激和炎症, 从而导致环氧合酶-2的上调, 该酶主要负责炎症类的前列腺素合成[81]。IAA还被证实可以增加接受血液透析患者的认知功能受损的风险[82]。
2.3.3 硫酸对甲酚硫酸对甲酚(p-cresyl sulfate, PCS)是当前研究最多的一种肠源尿毒素。肠道和肝脏是PCS体内代谢生成的重要部位, 肠道内的厌氧菌可将食物中的蛋白质分解为酪氨酸(tyrosine), 并将酪氨酸进一步转变为4-羟基苯乙酸(4-OH phenylacetic acid), 4-羟基苯乙酸在肠道细菌如艰难梭菌(Clostridium difficile)的作用下脱羧生成PCS的前体对甲酚(p-cresol, PC), 大部分对甲酚经肠道黏膜吸收, 在肠道上皮或肝脏内经磺基转移酶(sulfotransferase, SULT)转化为PCS[42], PCS主要通过肾小管基底膜侧的OAT分泌到肾小管管腔, 经尿液排出[83]。
在肾功能正常的情况下, PCS能被肾脏完全排泄, 血液中PCS含量极低。但是, CKD患者由于肾脏排泄功能受损, PCS无法充分被肾脏排泄, 血液循环中PCS浓度显著增加。体内大量堆积的PCS分子在肾脏有机阴离子转运体(OAT1和OAT3)作用下转运进入肾小管细胞, 激活PKC/PI3K-Nox4-ROS信号通路, 产生大量氧自由基, 诱发肾脏的氧化应激反应, 造成肾小管损伤及肾间质纤维化[44, 45]。临床研究证实, PCS体内堆积会引起CKD进展加快, 诱发CKD患者发生心血管并发症, 影响CKD患者预后, 导致CKD患者死亡率升高[38-42]。PCS的毒性作用见图 2。
2.3.3.1 PCS的肾脏毒性PCS通过激活NADPH氧化酶, 增加ROS水平, 从而上调与纤维化有关的炎性细胞因子和TGF-β1等的m RNA水平, 促进肾纤维化发展, 也可通过ROS降低人肾小管上皮细胞(HK-2细胞)活力[84]。与IS类似, PCS也可以激活RAAS和TGF-β1/Smad途径, 通过增加纤连蛋白和α-平滑肌肌动蛋白的表达, 降低E-钙黏蛋白的表达, 诱导肾小管EMT, 从而导致肾脏损伤和纤维化[54]。PCS同样可以增加DNMT表达, 使Klotho基因的Cp G过度甲基化, 降低肾小管细胞中Klotho的表达, 加重肾损伤[55]。
2.3.3.2 PCS的心血管毒性PCS可以诱导白细胞的氧化应激, 使自由基产生增加[85], 同时增加血管内皮中滚动的白细胞数量, 引发血管疾病[86]。PCS还能刺激内皮微粒释放, 并在内皮细胞和血管平滑肌细胞中诱导氧化应激[87, 88]。PCS通过激活NADPH氧化酶, 产生ROS, 促进心肌细胞凋亡, 并引起心脏舒张功能障碍[89]。在人动脉平滑肌细胞中, PCS通过触发细胞内ROS、pERK MAPK (phosphorylation of extracellular signal-regulated kinases MAPK)途径和NF-κB核易位来诱导成骨作用[90]。
2.3.3.3 PCS的其他毒性近来有研究发现CKD患者发生瘙痒症伴随有PCS总水平升高, 表明总PCS可能在CKD瘙痒症中发挥作用[91]。
2.3.4 苯基硫酸盐苯基硫酸盐(phenyl sulfate, PS)是由肠道细菌代谢酪氨酸产生的, 也是一种肠道菌群衍生的尿毒素。首先饮食中的酪氨酸在肠道中被表达酪氨酸苯酚裂解酶(tyrosine phenol-lyase, TPL)的肠道微生物分解成PS的前体苯酚(phenol), 苯酚在肝脏中被代谢为PS, 其随后通过人类肾脏中唯一的有机阴离子转运多肽(the organic anion transporting polypeptide, OATP)SLCO4C1的作用, 由近端肾小管细胞分泌[92]。
有研究证实PS可以剂量依赖性地降低猪肾小管细胞的总谷胱甘肽水平, 而谷胱甘肽被认为是一种非常有效的抗氧化物质。过氧化氢是一种活性氧, 据报道, 在接受血液透析的患者的血浆中, 过氧化氢含量比健康对照者含量升高, 而对比正常细胞, PS处理过的细胞更容易受到氧化应激, 过氧化氢诱导的细胞死亡率更高[93]。PS被证明对足细胞有毒性, 可以导致足细胞肥大和足突消失, 使肾小球基底膜增厚, 引起血管周围纤维化和炎症, 还能降低线粒体的功能。PS的这些多重毒性可能导致蛋白尿增加, 抑制PS产生可以减少蛋白尿。在微量白蛋白尿患者中, PS的水平与基础蛋白尿和糖尿病肾病(diabetic kidney disease, DKD)两年进展具有显著相关性, PS可以作为DKD的早期诊断标志物和治疗靶点[94]。
2.3.5 氧化三甲胺氧化三甲胺(trimethylamine-N-oxide, TMAO)来自于食物中的胆碱、卵磷脂和左旋肉碱等, 它们在肠道细菌的作用下生成三甲胺(trimethylamine, TMA), 然后在肝脏单加氧酶作用下生成TMAO[95]。TMAO也已经被证实可以影响肾小球的滤过率和肾功能[96], 可能导致进行性肾纤维化和功能损伤, TMAO水平也和CKD死亡率显著相关, 可以预测CKD患者五年死亡风险[97]。此外TMAO水平也和心血管疾病相关, TMAO能促进动脉粥样硬化, 增加血栓形成风险[98]。
3 靶向肠道菌群调控肠源尿毒素代谢通路干预CKD进展的治疗策略 3.1 目前肠源尿毒素的清除方式肠源尿毒素是引起CKD进展加快, 导致CKD患者死亡率升高的独立风险因素, 然而目前临床缺乏清除肠源尿毒素的安全有效方式或药物。目前清除肠源尿毒素的方法主要包括生活方式干预、益生菌调节、吸附剂吸附、中医方剂灌肠、血液透析、腹膜透析和肾脏移植等(图 3)。在CKD初期, 低蛋白饮食、益生菌调节、吸附剂吸附和中医方剂灌肠是清除肠源尿毒素的主要方法。但是, 这些初期的清除方式均存在不同的局限性。例如, 多孔类活性炭吸附剂如AST-120通过在肠道内吸附肠源尿毒素前体, 减少肠源尿毒素的肠道吸收和体内堆积, 来延缓CKD进展, 改善CKD并发心血管病变[99-101]。但是, 这些吸附剂的吸附选择性较差[102], 在吸附肠源尿毒素前体的同时会吸附肠道内的其他药物, 最终影响其他药物的临床疗效。再例如, 中医临床会采用类似于肠道透析的方式, 用含有大黄等泄下药物的方剂灌肠, 促进肠源尿毒素前体从肠道排出, 以此来延缓CKD进展[103, 104]。但是, 这种方法如果长期使用, 会导致患者出现肠道内菌群失调及电解质紊乱等不良反应。

|
Figure 3 Clinical methods for the clearance of gut-derived uremic toxins.TCM: Traditional Chinese medicine; HD: Hemodialysis; PD: Peritoneal dialysis; PBUT: Protein-bound uremic toxin |
进入CKD后期, 血液透析、腹膜透析是清除患者体内尿毒素的主要方式。透析利用半透膜原理将体内多余的尿毒素移出体外, 但无论是人工合成的半透膜还是人体自身的腹膜, 都只允许小分子和部分中分子物质通过, 蛋白质和血细胞等大分子都无法透过。所以, 透析仅对小分子尿毒素的清除效果较好, 而肠源尿毒素在体内多与血浆蛋白结合, 分子量较大, 不易通过半透膜。因此, 透析对肠源尿毒素的清除效率并不能令人满意[105, 106], 透析患者5年存活率低于50%[107], 且生活质量低。肾脏移植是目前最好的治疗方式, 但由于肾源不足, 以及手术并发死亡风险及终身使用免疫抑制剂, 均极大限制了它的使用。
3.2 基于肠源尿毒素代谢调控的CKD治疗策略目前临床缺乏可以特异性抑制肠源尿毒素生物合成的方法, 当肠源尿毒素生物合成后又缺乏有效的清除手段, 当前的治疗策略无法缓解CKD防治工作所面临的严峻形势, 必须寻求新的干预方法。
IS和PCS等肠源尿毒素作为肠道菌群-宿主共代谢产物, 与肠道菌群的关系密不可分, 通过肠道菌群来调控肠源尿毒素的合成有巨大的前景。近年发表的一项研究表明[47], 通过重塑肠道菌群结构或者操纵肠道菌群中吲哚代谢酶的编码基因, 可以调控肠道菌群合成吲哚, 减轻IS在宿主体内的蓄积, 为基于肠道菌群调控肠源尿毒素提供了依据, 为CKD治疗提供了新的思路。
Gryp等[108]最新研究发现, 随着CKD的进展, 患者血浆肠源尿毒素水平不断升高, 而粪便和尿液中这些肠源尿毒素及其前体水平保持不变。体外实验结果也显示, 健康对照者、CKD1期和5期患者粪便样品厌氧培养后对甲酚、吲哚和吲哚-3-乙酸的生成水平没有差异, 因此作者推测CKD患者血浆中肠源尿毒素水平升高主要是由于肾脏功能障碍, 而不是肠道微生物生成对甲酚、吲哚和IAA速度的改变引起的。Gryp等[109]从CKD患者粪便样品中分离得到148种细菌, 其中92种细菌可以生成肠源尿毒素的前体, 提示这些细菌有可能成为降低CKD患者血浆肠源尿毒素水平的潜在靶标, 其进一步将这些细菌分为产酚类化合物(对甲酚和苯酚)细菌和产吲哚类化合物(吲哚和IAA)细菌, 发现酚类化合物主要在厌氧条件下生成, 而吲哚类化合物则在有氧和厌氧条件下均可生成。作者还发现CKD患者的肠道微生物组成发生了改变: 随着肾功能的降低, 产短链脂肪酸细菌如双歧杆菌和链球菌丰度下降, 而好氧菌如肠杆菌科和大肠杆菌等丰度增加。这些研究使得通过调控肠源尿毒素代谢治疗慢性肾病向前进了一步。
IS和PCS等在体内形成需要经过一系列过程, 有学者提出可以分别通过调控其前体吲哚和对甲酚的生成及转运以及IS和PCS的合成和作用通路等来减轻它们在CKD患者体内的蓄积[42-44]。有研究发现鼠李糖乳杆菌可以有效减少血液透析患者血清尿毒素苯酚和对甲酚水平[110]; 唾液乳杆菌BP121可以调节肠道环境并抑制IS和PCS生成, 从而防止顺铂引起的急性肾损伤[111]; 多酚作为一种新型的益生元, 也在慢性肾病的治疗中有广泛的应用[112]。然而, 除益生菌和益生元之外, 至今仍没有发现可以抑制肠道内IS和PCS前体生成, 减少IS和PCS转运, 或是能调控IS和PCS作用信号通路, 最终延缓CKD进展的有效药物。通过药物来调控IS和PCS代谢途径和作用通路, 减轻IS和PCS诱导的肾脏损伤的相关研究也相对缺乏。对于AST-120研究相对较多, 然而AST-120的效果目前只在小规模临床研究中得到证实, 一项大规模的EPPIC(Evaluating Prevention of Progression in CKD)研究发现CKD患者使用AST-120效果并不理想[113]。
因此, 开展药物调控IS和PCS等肠源尿毒素的代谢途径和作用通路以及延缓CKD进展的作用机制研究刻不容缓。调控肠源尿毒素的生成, 减少它们在体内的堆积和/或阻断肠源尿毒素的转运环节及作用通路, 减轻肠源尿毒素诱导的肾脏和心血管损伤的具体作用路径包括(图 4): ①针对肠源尿毒素前体分子在肠道内合成环节, 调控肠道菌群及特异细菌, 减少它们前体分子在肠道内的生成; ②针对肠源尿毒素在肝脏或肠黏膜内合成的环节, 抑制肝脏或肠黏膜中SULT和FMOs的酶活力和/或表达水平, 减少前体分子在肝脏或肠黏膜内转化为肠源尿毒素; ③针对肠源尿毒素在肾小管细胞内的转运环节, 抑制肾小管细胞膜上的OAT和OATP等的转运功能和/或表达水平, 减少肠源尿毒素向肾小管细胞内转运; ④针对肠源尿毒素在肾脏和心血管的作用信号通路, 比如调控肾小管细胞内的NADPH氧化酶4 (Nox4)及相关信号通路, 减轻PCS诱导的肾小管细胞内ROS生成及继发的肾小管细胞损伤。

|
Figure 4 CKD treatment strategy based on gut-derived uremic toxins metabolism regulation.OATP: Organic anion transporting polypeptide |
越来越多研究认为中医里的“脾”很可能与肠道菌群相关。在中医理论里, 脾主运化, 为气血生化之源, 负责将水谷化为精微并输布到全身, 这与肠道菌群的吸收代谢和生成营养物质功能相似; “四季脾旺不受邪”和“脾为之卫”等说法则与肠道菌群的免疫和防御功能相关; “内伤脾胃, 百病由生”, 现代研究也发现肠道菌群与多种疾病如肥胖、糖尿病、心血管疾病和胃肠疾病等相关。当脾虚时, 会出现纳差、便溏和消瘦等消化道症状, 而肠道菌群失调时, 也会出现各种胃肠道疾病。对脾虚腹泻和非脾虚腹泻患者的肠道菌群进行分析, 发现脾虚型患者肠道菌群严重失调。Wang等[114]发现大鼠造脾虚模型后肠道菌群紊乱, 经四君子汤治疗后恢复。因此, 脾与肠道菌群之间存在着密切联系。
中医认为慢性肾病的病机属于“脾肾亏虚、浊毒内蕴”, 本虚邪实, 虚实夹杂。而肾为先天之本, 脾为后天之本, “后天之气得先天之气, 则生生而不息; 先天之气得后天之气, 始化化而不穷也”, 肾得脾濡养才能保证生机, 脾需肾温煦才能运化得力。因此, 当任何一方出现问题都会影响另一方, 与肠-肾轴理论相符, 因此基于中医药理论对CKD的治疗取得了明显的效果。
黄葵四物方由黄蜀葵花、黄芪、虎杖和姜黄4味中药组成, 具有清利活血、通淋消肿、补泻同施、标本兼顾之功。本课题组最新研究发现, 黄葵四物方通过调控肠道菌群抑制了PCS的前体分子PC的生成, 减少了肠源尿毒素PCS在体内的蓄积[115]。黄蜀葵花是锦葵科秋葵属植物黄蜀葵的花朵, 据《嘉祐本草》记载, 黄蜀葵花的主要功效为清热除湿和消肿解毒。黄蜀葵花的单方制剂黄葵胶囊作为临床治疗CKD的一线药物, 应用广泛, 疗效确切。本课题组研究发现黄葵可以显著降低CKD模型大鼠体内肠源尿毒素IS的含量, 并且明确了它是通过干扰肠道菌群转运色氨酸来抑制IS的前体吲哚的生成来发挥作用[116]。大黄素灌洗结肠可以调节CKD大鼠肠道菌群, 减少与尿素和IS呈正相关的肠道菌群, 增加有益菌, 从而减少CKD大鼠尿素和IS水平, 改善肾功能[117]。丹参中的丹参乙酸镁也被发现可以通过改变肠道菌群来改善糖尿病肾病小鼠的肾功能, 并且可以改善胆汁酸代谢[118]。以上这些发现验证了基于肠道菌群调控肠源尿毒素的可行性, 并且为中医药治疗CKD提供了更多研究思路。
5 小结与展望肠道菌群已经被证明与多种疾病存在联系, 比如溃疡性结肠炎、非酒精性脂肪肝和化疗性肠黏膜炎等[119-121], 从肠道菌群入手可能是解决许多疾病的突破口。越来越多证据表明靶向肠道菌调控肠源尿毒素也许是治疗CKD的一项新选择, 也应注意到, 除了IS和PCS之外, 肠源尿毒素的种类还有很多[37], 可以将肠源尿毒素中最具代表性的IS和PCS作为切入点, 揭示药物调控肠源尿毒素代谢途径和作用通路延缓CKD进展的作用机制。对于进一步寻找可调控其他肠源尿毒素类成分的创新药物, 探索调控肠源尿毒素, 延缓CKD进展的临床治疗新策略, 建立靶向肠源尿毒素以延缓CKD进展的药物发现新模式等具有重要的启示意义。同时, 中医药是一个巨大的宝库, 应不断发掘, 从中寻找到具有临床转化价值的中药复方及有效成分, 通过有效调控肠源尿毒素的代谢过程和作用通路来延缓CKD进展, 为我国公共卫生健康事业做出贡献。
作者贡献:彭印为本文主要撰写者; 徐雪君和李建萍负责文献查阅和内容修改; 李成曦和尹佳婷负责绘图; 段金廒为本文提出修改意见; 郭建明提出本文思路并参与文章撰写及修改。
利益冲突:所有作者均声明不存在利益冲突。
| [1] |
Levey AS, Coresh J. Chronic kidney disease[J]. Lancet, 2012, 379: 165-180. DOI:10.1016/S0140-6736(11)60178-5 |
| [2] |
Chen T, Harris DC. Challenges of chronic kidney disease prevention[J]. Med J Aust, 2015, 203: 209-210. DOI:10.5694/mja15.00241 |
| [3] |
Wang Y, Tong Q, Shou JW, et al. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine[J]. Theranostics, 2017, 7: 2443-2451. DOI:10.7150/thno.18290 |
| [4] |
Bello AK, Levin A, Tonelli M, et al. Assessment of global kidney health care status[J]. JAMA, 2017, 317: 1864-1881. DOI:10.1001/jama.2017.4046 |
| [5] |
Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey[J]. Lancet, 2012, 379: 815-822. DOI:10.1016/S0140-6736(12)60033-6 |
| [6] |
Cai GY, Chen XM. Complications of chronic kidney disease: current management and challenge[J]. Chin J Pract Intern Med(中国实用内科杂志), 2010, 30: 102-103. |
| [7] |
Hakemi MS. Chronic kidney disease epidemiology[J]. Iran J Kidney Dis, 2014, 8: 261-262. |
| [8] |
Parrish AR. Advances in chronic kidney disease[J]. Int J Mol Sci, 2016, 17: 1314. DOI:10.3390/ijms17081314 |
| [9] |
Gao X, Mei CL. Guideline for screening, diagnosis, prevention and treatment of chronic kidney disease[J]. Chin J Pract Intern Med(中国实用内科杂志), 2017, 37: 28-34. |
| [10] |
Jonsson AL, Backhed F. Drug the bug![J]. Cell, 2015, 163: 1565-1566. DOI:10.1016/j.cell.2015.12.005 |
| [11] |
Garber K. Drugging the gut microbiome[J]. Nat Biotechnol, 2015, 33: 228-231. DOI:10.1038/nbt.3161 |
| [12] |
Brown JM, Hazen SL. Targeting of microbe-derived metabolites to improve human health: the next frontier for drug discovery[J]. J Biol Chem, 2017, 292: 8560-8568. DOI:10.1074/jbc.R116.765388 |
| [13] |
Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis[J]. Cell, 2015, 163: 1585-1595. DOI:10.1016/j.cell.2015.11.055 |
| [14] |
Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential[J]. Nat Med, 2018, 24: 1407-1417. DOI:10.1038/s41591-018-0128-1 |
| [15] |
Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin[J]. Nat Med, 2018, 24: 1919-1929. DOI:10.1038/s41591-018-0222-4 |
| [16] |
Bry L, Falk PG, Midtvedt T, et al. A model of host-microbial interactions in an open mammalian ecosystem[J]. Science, 1996, 273: 1380-1383. DOI:10.1126/science.273.5280.1380 |
| [17] |
Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota[J]. Nature, 2012, 489: 220-230. DOI:10.1038/nature11550 |
| [18] |
Guarner F. Enteric flora in health and disease[J]. Digestion, 2006, 73 Suppl 1: 5-12. |
| [19] |
Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora[J]. Science, 2005, 308: 1635-1638. DOI:10.1126/science.1110591 |
| [20] |
Mafra D, Lobo JC, Barros AF, et al. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease[J]. Future Microbiol, 2014, 9: 399-410. DOI:10.2217/fmb.13.165 |
| [21] |
Sun YM, Zhang YT, Zhang JH, et al. Advances in the study of gut pharmacomicrobiomics[J]. Acta Pharm Sin(药学学报), 2020, 55: 2314-2321. |
| [22] |
Wang Y, Jiang JD. A new research mode of drug PK-PD mediated by the gut microbiota: insights into the pharmacokinetics of berberine[J]. Acta Pharm Sin(药学学报), 2018, 53: 659-666. |
| [23] |
Zhang JH, Zhang JM, Wang R, et al. Interaction of amoxicillin and nifedipine mediated by intestinal flora[J]. Acta Pharm Sin(药学学报), 2018, 53: 1721-1725. |
| [24] |
Yin JX, Lin DR. Intestinal flora and desease[J]. Bull Biol(生物学通报), 2004, 39: 26-28. |
| [25] |
Kanbay M, Onal EM, Afsar B, et al. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus[J]. Int Urol Nephrol, 2018, 50: 1453-1466. DOI:10.1007/s11255-018-1873-2 |
| [26] |
Turner JR. Intestinal mucosal barrier function in health and disease[J]. Nat Rev Immunol, 2009, 9: 799-809. DOI:10.1038/nri2653 |
| [27] |
Catalioto RM, Maggi CA, Giuliani S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions[J]. Curr Med Chem, 2011, 18: 398-426. DOI:10.2174/092986711794839179 |
| [28] |
Sabatino A, Regolisti G, Brusasco I, et al. Alterations of intestinal barrier and microbiota in chronic kidney disease[J]. Nephrol Dial Transplant, 2015, 30: 924-933. DOI:10.1093/ndt/gfu287 |
| [29] |
Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment[J]. Nephrol Dial Transplant, 2016, 31: 737-746. DOI:10.1093/ndt/gfv095 |
| [30] |
Andersen K, Kesper MS, Marschner JA, et al. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation[J]. J Am Soc Nephrol, 2017, 28: 76-83. DOI:10.1681/ASN.2015111285 |
| [31] |
Ritz E. Intestinal-renal syndrome: mirage or reality?[J]. Blood Purif, 2011, 31: 70-76. DOI:10.1159/000321848 |
| [32] |
Meijers BK, Evenepoel P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression[J]. Nephrol Dial Transplant, 2011, 26: 759-761. DOI:10.1093/ndt/gfq818 |
| [33] |
Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora[J]. Kidney Int, 2013, 83: 308-315. DOI:10.1038/ki.2012.345 |
| [34] |
Zhang P, Wei M, Jiang HL, et al. Gut bacterial translocation contributes to microinflammationin experimental uremia[J]. Chin J Nephrol(中华肾脏病杂志), 2013, 29: 611-615. |
| [35] |
Vaziri ND, Yuan J, Rahimi A, et al. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKDassociated inflammation[J]. Nephrol Dial Transplant, 2012, 27: 2686-2693. DOI:10.1093/ndt/gfr624 |
| [36] |
Vaziri ND, Goshtasbi N, Yuan J, et al. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium[J]. Am J Nephrol, 2012, 36: 438-443. DOI:10.1159/000343886 |
| [37] |
Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability[J]. Kidney Int, 2003, 63: 1934-1943. DOI:10.1046/j.1523-1755.2003.00924.x |
| [38] |
Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease[J]. Nephrol Dial Transplant, 2011, 26: 938-947. DOI:10.1093/ndt/gfq580 |
| [39] |
Jing YJ, Ni JW, Ding FH, et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE-/-mice[J]. Kidney Int, 2016, 89: 439-449. DOI:10.1038/ki.2015.287 |
| [40] |
Han H, Zhu J, Zhu Z, et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes[J]. J Am Heart Assoc, 2015, 4: e001852. |
| [41] |
Koppe L, Pillon NJ, Vella RE, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD[J]. J Am Soc Nephrol, 2013, 24: 88-99. DOI:10.1681/ASN.2012050503 |
| [42] |
Gryp T, Vanholder R, Vaneechoutte M, et al. p-Cresyl sulfate[J]. Toxins(Basel), 2017, 9: 52. |
| [43] |
Watanabe H, Miyamoto Y, Otagiri M, et al. Update on the pharmacokinetics and redox properties of protein-bound uremic toxins[J]. J Pharm Sci, 2011, 100: 3682-3695. DOI:10.1002/jps.22592 |
| [44] |
Gorin Y. Nox4 as a potential therapeutic target for treatment of uremic toxicity associated to chronic kidney disease[J]. Kidney Int, 2013, 83: 541-543. DOI:10.1038/ki.2012.434 |
| [45] |
Watanabe H, Miyamoto Y, Honda D, et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase[J]. Kidney Int, 2013, 83: 582-592. DOI:10.1038/ki.2012.448 |
| [46] |
Hung SC, Kuo KL, Huang HL, et al. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization[J]. Kidney Int, 2016, 89: 574-585. DOI:10.1016/j.kint.2015.11.020 |
| [47] |
Devlin AS, Marcobal A, Dodd D, et al. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota[J]. Cell Host Microbe, 2016, 20: 709-715. DOI:10.1016/j.chom.2016.10.021 |
| [48] |
de Loor H, Meijers BK, Meyer TW, et al. Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography[J]. J Chromatogr A, 2009, 1216: 4684-4688. DOI:10.1016/j.chroma.2009.04.015 |
| [49] |
Deguchi T, Ohtsuki S, Otagiri M, et al. Major role of organic anion transporter 3 in the transport of indoxyl sulfate in the kidney[J]. Kidney Int, 2002, 61: 1760-1768. DOI:10.1046/j.1523-1755.2002.00318.x |
| [50] |
Vanholder R, Schepers E, Pletinck A, et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review[J]. J Am Soc Nephrol, 2014, 25: 1897-1907. DOI:10.1681/ASN.2013101062 |
| [51] |
Niwa T. Indoxyl sulfate is a nephro-vascular toxin[J]. J Ren Nutr, 2010, 20: S2-S6. DOI:10.1053/j.jrn.2010.05.002 |
| [52] |
Liu WC, Tomino Y, Lu KC. Impacts of indoxyl sulfate and pcresol sulfate on chronic kidney disease and mitigating effects of AST-120[J]. Toxins(Basel), 2018, 10: 367. |
| [53] |
Motojima M, Hosokawa A, Yamato H, et al. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells[J]. Kidney Int, 2003, 63: 1671-1680. DOI:10.1046/j.1523-1755.2003.00906.x |
| [54] |
Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition[J]. PLoS One, 2012, 7: e34026. DOI:10.1371/journal.pone.0034026 |
| [55] |
Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation[J]. Kidney Int, 2012, 81: 640-650. DOI:10.1038/ki.2011.445 |
| [56] |
Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects[J]. J Am Soc Nephrol, 2014, 25: 2169-2175. DOI:10.1681/ASN.2013111209 |
| [57] |
Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease[J]. J Am Soc Nephrol, 2011, 22: 124-136. DOI:10.1681/ASN.2009121311 |
| [58] |
Young GH, Wu VC. Klotho methylation is linked to uremic toxins and chronic kidney disease[J]. Kidney Int, 2012, 81: 611-612. DOI:10.1038/ki.2011.461 |
| [59] |
Shimizu H, Bolati D, Adijiang A, et al. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-κB[J]. Am J Nephrol, 2011, 33: 319-324. DOI:10.1159/000324885 |
| [60] |
Shimizu H, Bolati D, Adijiang A, et al. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells[J]. Am J Physiol Cell Physiol, 2011, 301: C1201-C1212. DOI:10.1152/ajpcell.00471.2010 |
| [61] |
Lekawanvijit S, Adrahtas A, Kelly DJ, et al. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes?[J]. Eur Heart J, 2010, 31: 1771-1779. DOI:10.1093/eurheartj/ehp574 |
| [62] |
Yang K, Xu X, Nie L, et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway[J]. Toxicol Lett, 2015, 234: 110-119. DOI:10.1016/j.toxlet.2015.01.021 |
| [63] |
Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis[J]. J Cardiovasc Electrophysiol, 2015, 26: 203-210. DOI:10.1111/jce.12554 |
| [64] |
Lekawanvijit S. Cardiotoxicity of uremic toxins: a driver of cardiorenal syndrome[J]. Toxins(Basel), 2018, 10: 352. |
| [65] |
Adelibieke Y, Shimizu H, Muteliefu G, et al. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity[J]. J Ren Nutr, 2012, 22: 86-89. DOI:10.1053/j.jrn.2011.10.027 |
| [66] |
Tumur Z, Niwa T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells[J]. Am J Nephrol, 2009, 29: 551-557. DOI:10.1159/000191468 |
| [67] |
Ryu JH, Kim SJ. Clopidogrel effectively suppresses endothelial microparticle generation induced by indoxyl sulfate via inhibition of the p38 mitogen-activated protein kinase pathway[J]. Blood Purif, 2011, 32: 186-194. DOI:10.1159/000326297 |
| [68] |
Tumur Z, Shimizu H, Enomoto A, et al. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation[J]. Am J Nephrol, 2010, 31: 435-441. DOI:10.1159/000299798 |
| [69] |
Ito S, Osaka M, Higuchi Y, et al. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin[J]. J Biol Chem, 2010, 285: 38869-38875. DOI:10.1074/jbc.M110.166686 |
| [70] |
Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells[J]. Kidney Int, 2006, 69: 1780-1785. DOI:10.1038/sj.ki.5000340 |
| [71] |
Watanabe I, Tatebe J, Namba S, et al. Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells[J]. Circ J, 2013, 77: 224-230. DOI:10.1253/circj.CJ-12-0647 |
| [72] |
Gondouin B, Cerini C, Dou L, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway[J]. Kidney Int, 2013, 84: 733-744. DOI:10.1038/ki.2013.133 |
| [73] |
Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor[J]. Circulation, 2013, 127: 365-376. DOI:10.1161/CIRCULATIONAHA.112.118174 |
| [74] |
Shivanna S, Kolandaivelu K, Shashar M, et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia[J]. J Am Soc Nephrol, 2016, 27: 189-201. DOI:10.1681/ASN.2014121241 |
| [75] |
Adijiang A, Goto S, Uramoto S, et al. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats[J]. Nephrol Dial Transplant, 2008, 23: 1892-1901. DOI:10.1093/ndt/gfm861 |
| [76] |
Muteliefu G, Enomoto A, Jiang P, et al. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells[J]. Nephrol Dial Transplant, 2009, 24: 2051-2058. DOI:10.1093/ndt/gfn757 |
| [77] |
Nii-Kono T, Iwasaki Y, Uchida M, et al. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells[J]. Kidney Int, 2007, 71: 738-743. DOI:10.1038/sj.ki.5002097 |
| [78] |
Mozar A, Louvet L, Godin C, et al. Indoxyl sulphate inhibits osteoclast differentiation and function[J]. Nephrol Dial Transplant, 2012, 27: 2176-2181. DOI:10.1093/ndt/gfr647 |
| [79] |
Lin YT, Wu PH, Liang SS, et al. Protein-bound uremic toxins are associated with cognitive function among patients undergoing maintenance hemodialysis[J]. Sci Rep, 2019, 9: 20388. DOI:10.1038/s41598-019-57004-7 |
| [80] |
Brito JS, Borges NA, Anjos JSD, et al. Aryl hydrocarbon receptor and uremic toxins from the gut microbiota in chronic kidney disease patients: is there a relationship between them?[J]. Biochemistry, 2019, 58: 2054-2060. DOI:10.1021/acs.biochem.8b01305 |
| [81] |
Dou L, Sallee M, Cerini C, et al. The cardiovascular effect of the uremic solute indole-3 acetic acid[J]. J Am Soc Nephrol, 2015, 26: 876-887. DOI:10.1681/ASN.2013121283 |
| [82] |
Lin YT, Wu PH, Lee HH, et al. Indole-3 acetic acid increased risk of impaired cognitive function in patients receiving hemodialysis[J]. Neurotoxicology, 2019, 73: 85-91. DOI:10.1016/j.neuro.2019.02.019 |
| [83] |
Miyamoto Y, Watanabe H, Noguchi T, et al. Organic anion transporters play an important role in the uptake of p-cresyl sulfate, a uremic toxin, in the kidney[J]. Nephrol Dial Transplant, 2011, 26: 2498-2502. DOI:10.1093/ndt/gfq785 |
| [84] |
Watanabe H, Miyamoto Y, Honda D, et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase[J]. Kidney Int, 2013, 83: 582-592. DOI:10.1038/ki.2012.448 |
| [85] |
Schepers E, Meert N, Glorieux G, et al. p-Cresyl sulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production[J]. Nephrol Dial Transplant, 2007, 22: 592-596. |
| [86] |
Pletinck A, Glorieux G, Schepers E, et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall[J]. J Am Soc Nephrol, 2013, 24: 1981-1994. DOI:10.1681/ASN.2012030281 |
| [87] |
Meijers BK, Van Kerckhoven S, Verbeke K, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial damage[J]. Am J Kidney Dis, 2009, 54: 891-901. DOI:10.1053/j.ajkd.2009.04.022 |
| [88] |
Gross P, Massy ZA, Henaut L, et al. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling[J]. J Cell Physiol, 2015, 230: 2927-2935. DOI:10.1002/jcp.25018 |
| [89] |
Han H, Zhu J, Zhu Z, et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes[J]. J Am Heart Assoc, 2015, 4: e001852. |
| [90] |
Chang JF, Hsieh CY, Liou JC, et al. Scavenging intracellular ROS attenuates p-cresyl sulfate-triggered osteogenesis through MAPK signaling pathway and NF-κB activation in human arterial smooth muscle cells[J]. Toxins(Basel), 2020, 12: 472. |
| [91] |
Wang CP, Lu YC, Tsai IT, et al. Increased levels of total p-cresyl sulfate are associated with pruritus in patients with chronic kidney disease[J]. Dermatology, 2016, 232: 363-370. DOI:10.1159/000445429 |
| [92] |
Fiaccadori E, Cosola C, Sabatino A. Targeting the gut for early diagnosis, prevention, and cure of diabetic kidney disease: is the phenyl sulfate story another step forward?[J]. Am J Kidney Dis, 2020, 75: 144-147. DOI:10.1053/j.ajkd.2019.07.001 |
| [93] |
Edamatsu T, Fujieda A, Itoh Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells[J]. PLoS One, 2018, 13: e0193342. DOI:10.1371/journal.pone.0193342 |
| [94] |
Kikuchi K, Saigusa D, Kanemitsu Y, et al. Gut microbiomederived phenyl sulfate contributes to albuminuria in diabetic kidney disease[J]. Nat Commun, 2019, 10: 1835. DOI:10.1038/s41467-019-09735-4 |
| [95] |
Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation[J]. Cell Metab, 2013, 17: 49-60. DOI:10.1016/j.cmet.2012.12.011 |
| [96] |
Missailidis C, Hallqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease[J]. PLoS One, 2016, 11: e0141738. DOI:10.1371/journal.pone.0141738 |
| [97] |
Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease[J]. Circ Res, 2015, 116: 448-455. DOI:10.1161/CIRCRESAHA.116.305360 |
| [98] |
Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk[J]. Cell, 2016, 165: 111-124. DOI:10.1016/j.cell.2016.02.011 |
| [99] |
Fujii H, Yonekura Y, Yamashita Y, et al. Anti-oxidative effect of AST-120 on kidney injury after myocardial infarction[J]. Br J Pharmacol, 2016, 173: 1302-1313. DOI:10.1111/bph.13417 |
| [100] |
Schulman G, Berl T, Beck GJ, et al. The effects of AST-120 on chronic kidney disease progression in the United States of America: a post hoc subgroup analysis of randomized controlled trials[J]. BMC Nephrol, 2016, 17: 141. DOI:10.1186/s12882-016-0357-9 |
| [101] |
Yamamoto S, Kazama JJ, Omori K, et al. Continuous reduction of protein-bound uraemic toxins with improved oxidative stress by using the oral charcoal adsorbent AST-120 in haemodialysis patients[J]. Sci Rep, 2015, 5: 14381. DOI:10.1038/srep14381 |
| [102] |
Koya Y, Uchida S, Machi Y, et al. Prediction of drug interaction between oral adsorbent AST-120 and concomitant drugs based on the in vitro dissolution and in vivo absorption behavior of the drugs[J]. Eur J Clin Pharmacol, 2016, 72: 1353-1361. DOI:10.1007/s00228-016-2102-5 |
| [103] |
Zou C, Wu YC, Lin QZ, et al. Effects of Chinese herbal enema therapy combined basic treatment on BUN, SCr, UA, and IS in chronic renal failure patients[J]. Chin J Integr Tradit West Med(中国中西医结合杂志), 2012, 32: 1192-1195. |
| [104] |
Lu Z, Zeng Y, Lu F, et al. Rhubarb enema attenuates renal tubulointerstitial fibrosis in 5/6 nephrectomized rats by alleviating indoxyl sulfate overload[J]. PLoS One, 2015, 10: e0144726. DOI:10.1371/journal.pone.0144726 |
| [105] |
Sirich TL, Fong K, Larive B, et al. Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the frequent hemodialysis network daily trial[J]. Kidney Int, 2017, 91: 1186-1192. DOI:10.1016/j.kint.2016.11.002 |
| [106] |
Martinez AW, Recht NS, Hostetter TH, et al. Removal of pcresol sulfate by hemodialysis[J]. J Am Soc Nephrol, 2005, 16: 3430-3436. DOI:10.1681/ASN.2005030310 |
| [107] |
Meyer TW, Hostetter TH. Uremia[J]. N Engl J Med, 2007, 357: 1316-1325. DOI:10.1056/NEJMra071313 |
| [108] |
Gryp T, De Paepe K, Vanholder R, et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease[J]. Kidney Int, 2020, 97: 1230-1242. DOI:10.1016/j.kint.2020.01.028 |
| [109] |
Gryp T, Huys GRB, Joossens M, et al. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients[J]. Int J Mol Sci, 2020, 21: 1986. DOI:10.3390/ijms21061986 |
| [110] |
Eidi F, Poor-Reza Gholi F, Ostadrahimi A, et al. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and p-cresol) in hemodialysis patients: a double blind randomized clinical trial[J]. Clin Nutr ESPEN, 2018, 28: 158-164. DOI:10.1016/j.clnesp.2018.08.010 |
| [111] |
Lee TH, Park D, Kim YJ, et al. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis[J]. Int J Mol Med, 2020, 45: 1130-1140. |
| [112] |
Bao N, Chen F, Dai D. The regulation of host intestinal microbiota by polyphenols in the development and prevention of chronic kidney disease[J]. Front Immunol, 2020, 10: 2981. DOI:10.3389/fimmu.2019.02981 |
| [113] |
Schulman G, Berl T, Beck GJ, et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD[J]. J Am Soc Nephrol, 2015, 26: 1732-1746. DOI:10.1681/ASN.2014010042 |
| [114] |
Wang Z, Peng Y, Li XB. Effect of sijunzi decoction on the intestinal flora disturbance in two rat models of Pi-deficiency syndrome[J]. Chin J Integr Tradit West Med(中国中西医结合杂志), 2009, 29: 825-829. |
| [115] |
Lu JB, Wang YY, Zhang S, et al. Huang-Kui-Si-Wu Formula decreases uremic toxin production by modulating intestinal microbial metabolic pathways[J]. Acta Pharm Sin(药学学报), 2020, 55: 1229-1236. |
| [116] |
Wang YY, Li JP, Lu JB, et al. Effect and mechanism of Huangkui capsule on reduction of uremic toxin accumulation in an animal model of chronic kidney disease[J]. Acta Pharm Sin(药学学报), 2019, 54: 2267-2276. |
| [117] |
Zeng YQ, Dai Z, Lu F, et al. Emodin via colonic irrigation modulates gut microbiota and reduces uremic toxins in rats with chronic kidney disease[J]. Oncotarget, 2016, 7: 17468-17478. DOI:10.18632/oncotarget.8160 |
| [118] |
Zhao J. Interaction Between Magnesium Lithospermate B and Gut Microbiota(丹参乙酸镁与肠道菌群的相互作用)[D]. Shanghai: Shanghai Institute of Materia Medica, University of Chinese Academy of Sciences, 2018.
|
| [119] |
Gao XJ, Li T, Wei B, et al. Regulatory mechanisms of gut microbiota on intestinal CYP3A and P-glycoprotein in rats with dextran sulfate sodium-induced colitis[J]. Acta Pharm Sin(药学学报), 2017, 52: 34-43. |
| [120] |
Li XL, Jiang W, Fan WM, et al. Role of gut microbiota in the treatment of nonalcoholic fatty liver disease with traditional Chinese medicine[J]. Acta Pharm Sin(药学学报), 2020, 55: 15-24. |
| [121] |
Wang R, Wang L, Wei GY, et al. The effect and mechanism of baicalein on regulating gut microbiota and improving chemotherapy-induced intestinal mucositis in mice[J]. Acta Pharm Sin(药学学报), 2020, 55: 868-876. |
 2021, Vol. 56
2021, Vol. 56


