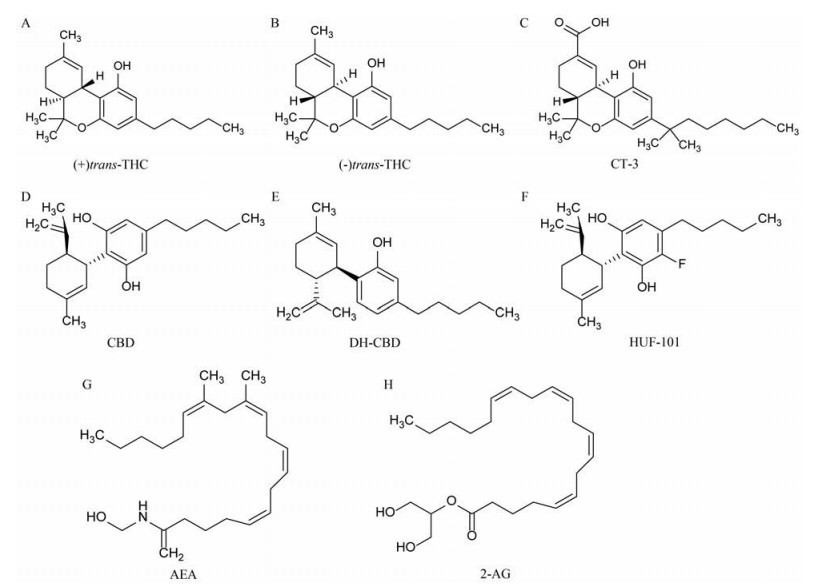
大麻提取物在医学上使用了数千年, 化学类型主要包括Δ9-四氢大麻酚(Δ9-tetrahydrocannabinol, THC)和大麻二酚(cannabidiol, CBD)[1]两类(结构见图 1)。最早的文字记载来源于《神农本草经》[2], 相关记载表明古埃及和印度等将大麻用于治疗感染、疼痛、癫痫和焦虑等疾病[3]。THC具有较强的精神活性, 会引起一些严重的不良反应, 临床上仅用于治疗结肠炎、多发性硬化症引起的疼痛、艾滋病和癌症等少数疾病[1]。CBD作为另一种主要的植物大麻素, 由于不直接激活大麻素1型受体(type 1 cannabinoid receptor, CB1R)和大麻素2型受体(type 2 cannabinoid receptor, CB2R), 因而没有神经精神方面的不良反应[4], 与THC相比安全性更高。临床研究结果显示, CBD能缓解患者烟瘾发作、焦虑、运动障碍及癫痫性发作等症状[5-8]。尽管CBD具有广谱的药理作用, 但其具体的作用机制尚未被完全阐明。本文将总结CBD在癫痫、神经病理性疼痛、焦虑症和抑郁症等几种常见神经精神疾病中的作用及分子机制, 旨在为其类似物的研究及大麻药用价值的开发提供依据。
|
Figure 1 Chemical structures of (+) trans-THC, (-) trans-THC, CT-3, CBD, DH-CBD, HUF-101, AEA and 2-AG (A-H). THC: Δ9-Tetrahydrocannabinol; CT-3: Ajulemic acid; CBD: Cannabidiol; DH-CBD: Dehydroxyl-cannabidiol; HUF-101: 4'-Fluorocannabidiol; AEA: N-Arachidonoyl ethanolamine; 2-AG: 2-Arachidonoyl glycerol |
内源性大麻素系统主要由内源性配体和受体组成, 配体主要包括N-花生四烯酸氨基乙醇(N-arachidonoyl ethanolamine, AEA)和2-花生四烯酸甘油(2-arachidonoyl glycerol, 2-AG) (结构见图 1), 受体包括CB1R和CB2R两种亚型[9]。CB1R主要在中枢神经系统表达, 并通过直接调控内源性大麻素系统而影响谷氨酸、γ-氨基丁酸(γ-aminobutyric acid, GABA)和多巴胺能神经元的功能, 参与认知、情感、奖赏和记忆等多种脑功能调节, 因此受到广泛关注[9-11]。与CB1R不同, CB2R被认为是一种“外周”大麻素受体, 主要分布在脾脏、扁桃体和免疫细胞, 与机体的免疫调节密切相关[12]。近年来, CB2R在外周和中枢免疫调节中的作用得到了越来越多的关注。此外, 在某些病理条件下(如成瘾、炎症、焦虑和癫痫), CB2R在脑中的表达明显上调[13], 说明CB2R可能参与了这些疾病过程。
CBD对CB1R和CB2R的亲和力极低[12], 与经典的激动剂或拮抗剂不同, 其主要通过与受体的其他位点结合发挥作用。研究表明, CBD非竞争性地拮抗CB1R和CB2R各自的激动剂如CP55940与R-(+)-WIN55212的作用, 其中对CB1R表现为负性变构调节, 而对CB2R表现为反向激动作用[14, 15]。CBD对CB1R的负性变构调节提示其在发挥治疗作用的同时, 能避免大麻素样的神经精神方面的不良反应。CBD对CB2R反向激动作用可以抑制免疫细胞迁移, 可能与其抗炎作用有关[16]。虽然CBD与CB1R和CB2R受体结合时表现出拮抗作用, 但它又可通过抑制内源性大麻素的代谢与转运过程间接激活内源性大麻素系统信号转导系统。动物实验研究发现, CB1R拮抗剂AM251可削弱CBD的抗抑郁作用[17, 18], CB2R拮抗剂AM630可逆转CBD对缺血性脑损伤的保护作用, 且可阻碍CBD对可卡因奖赏作用的减弱作用[19, 20]等。究其机制, 这些作用主要与CBD抑制脂肪酰胺水解酶(fatty acid amide hydrolase, FAAH), 增加内源性大麻素AEA的水平进而间接激活内源性大麻素系统信号转导有关[21, 22]。
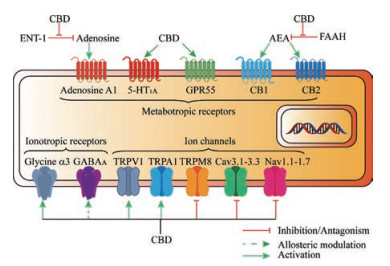
尽管, 研究表明CBD可能参与内源性大麻素系统对各种生理和病理功能的调节, 但由于其对CB1R和CB2R的亲和力低且具体的结合位点尚未明确, 近年来越来越多的研究开始关注内源性大麻素系统之外的其他靶点。有证据[23, 24]表明CBD主要作用于神经精神疾病相关的G蛋白偶联受体和离子通道等, 如五羟色胺(serotonin, 5-HT)受体、甘氨酸受体、腺苷受体及瞬时受体电位离子通道(transient receptor potential, TRP)等, 并且能抑制突触小体对去甲肾上腺素、多巴胺、5-HT和GABA等神经递质的摄取及细胞对内源性大麻素的摄取过程, 同时还可影响线粒体的钙离子存储, 阻断低电压激活的T型钙离子通道。下文将详细介绍CBD在神经精神疾病相关离子通道和受体靶点方面的作用, 主要靶点汇总如下表(表 1)[14, 15, 22, 23, 25-34]和图 2所示。
| Table 1 Affected receptors and ion channels by cannabidiol. IC50: Half inhibitory concentration; EC50: Half effective concentration; ND: Not described; (+): Activation; (-): Inhibition or antagonism. aApparent allosteric modulation; bValues in brackets represent 95% confidence intervals |
|
Figure 2 The molecular targets and potential mechanisms of CBD in neuropsychiatric diseases |
癫痫是大脑神经元突发性异常放电, 导致短暂的大脑功能障碍的一种慢性疾病。据世界卫生组织(World Health Organization, WHO)统计, 癫痫约占全世界疾病负担的1%, 是继抑郁症、酒精成瘾和脑血管疾病后的第四大神经精神疾病[35]。尽管已上市的抗癫痫药物较多, 但是仍有大约1/3的癫痫患者对药物治疗无效[36]。因此, 研发高效、低毒的新型抗癫痫药显得格外重要。
公元前两千多年, 一些亚洲国家就开始使用大麻来治疗癫痫性发作[2, 3]。CBD在毛果芸香碱致癫痫模型[37]和电休克致癫痫模型[38]中均起抗惊厥作用。而且CBD亦可降低Dravet综合征和Lennox-Gastaut综合征等难治性癫痫患者的癫痫发作频率[39, 40]。CBD发挥抗癫痫作用可能主要是通过如下靶点起作用, 主要包括GABAA受体、电压门控性钠通道(Nav)、瞬时受体电位香草酸亚型1 (transient receptor potential vanilloid-1, TRPV1)、G蛋白偶联受体55 (G protein-coupled receptor 55, GPR55)和腺苷A1受体等靶点。
2.1.1 GABAA受体GABAA受体是一类配体门控性离子通道, 激活该通道引起膜电位超极化, 对控制神经元兴奋性极为重要[41]。大脑中GABA的含量减少或GABAA受体功能障碍均可使神经元过度兴奋进而诱发癫痫。临床研究发现, CBD能明显改善Dravet综合征和Lennox-Gastaut综合征等难治性癫痫患者的症状[39, 40]。Dravet综合征主要由编码脑Nav1.1通道的SCN1A基因功能缺失性杂合突变, 选择性降低GABA能中间神经元兴奋性驱动引起[42], 是一种严重的儿童神经精神疾病, 以难治性癫痫发作、自闭症和早期死亡为主要特征[39]。小鼠前脑GABA能神经元SCN1A基因条件性缺失后同样出现了全身强直性痉挛发作和早亡[43]。另有研究发现, 部分Dravet综合征患者还存在GABRA1和GABRG2等基因突变[44, 45]。GABRA1基因编码GABAA受体的α1亚基, 突变后GABA (~1 mmol·L-1)诱导的最大电流幅度下降了63%, 同时GABA的半数有效激活浓度(EC50)也减少到了野生型的1/5[44]。GABRG2基因编码GABA受体的γ2亚基, 突变后GABAA受体功能明显减弱[45]。最新研究表明, 选择性靶向GABAA受体的α2亚基是治疗Dravet综合征的一个潜在选择[46]。Lennox-Gastaut综合征也是一种严重的癫痫性脑病, 在儿童时期发病, 围产期综合征是主要的发病原因, 但部分患者亦存在SCN1A、GABRB3、SLC6A1等基因突变[47, 48]。GABRB3在发育早期脑组织中高表达, 其编码的β3亚基对GABAA受体的组装、转运和胚胎期脑干细胞分化具有重要作用[49]。SLC6A1编码GABA转运体1, 该基因突变后导致GABA的摄取减少进而诱发癫痫[50]。而CBD作为多种GABAA受体亚型的正性变构调节剂, 能显著增强GABA诱发的电流幅度, 特别是对含有α2亚基的亚型(α2β1-3γ2L)效能更强, 且不涉及经典的苯二氮䓬类药物结合位点, 安全性和耐受性更好[34]。此外, 离体脑片钳实验发现, CBD能增加海马脑片中GABAA受体介导的自发性抑制性突触后电流, 减少齿状回兴奋性输出[51]。综上所述, GABAA受体可能是CBD发挥抗癫痫作用的主要靶点之一。
2.1.2 电压门控性钠通道钠通道激活引起神经元动作电位产生, 是维持正常的大脑功能和癫痫性发作的重要基础。钠通道阻滞剂, 如苯妥英钠(phenytoin sodium)、卡马西平(carbamazepine)和拉莫三嗪(lamotrigine)等被广泛用于癫痫的治疗[52]。研究发现, CBD能有效抑制人类重组电压门控性钠通道(hNav1.1~hNav1.7)电流和纹状体神经元钠电流[32, 53]。此外, CBD还能明显抑制Nav1.6通道突变引起的异常复活电流(resurgent)和持续电流(persistent current)[53]。Nav1.6通道突变也发生在某些严重婴儿癫痫性脑病患者中[54, 55], 且在颞叶癫痫动物模型中也发现复活电流增强[56, 57], 而复活电流使神经元兴奋性增强, 提示靶向复活钠电流可能是癫痫治疗的潜在策略。
2.1.3 TPRV1通道TRPV1通道广泛表达于中枢神经系统和外周传入神经纤维, 激活该通道可促进神经元去极化, 增加放电频率和突触活动, 且在内侧颞叶癫痫患者皮质和海马中均发现有该通道过表达[58, 59]。CBD激活TRPV1通道并使其迅速失敏[60], 因而减少了放电频率和突触活动, 进一步抑制神经元过度兴奋。如将TRPV1通道编码基因敲除则CBD的抗惊厥作用明显减弱[61], 提示TRPV1通道可能参与了CBD的抗癫痫作用。
2.1.4 GPR55受体GPR55是一种孤儿G-蛋白偶联受体, 以钙离子依赖的方式调节谷氨酸释放, 该受体在癫痫模型大鼠的海马组织中表达上调, 激活后引起微小兴奋性突触后电流频率和幅度增加[62]。研究发现, CBD对Dravet综合征模型小鼠症状的改善作用可能与它拮抗GPR55有关[25, 51]。CBD通过拮抗GPR55抑制癫痫组织细胞内的钙离子释放和神经元的异常兴奋[51], 如将GPR55编码基因敲除则CBD的抗惊厥作用减弱[61], 部分解释了CBD对耐药癫痫患者的抗惊厥作用。
2.1.5 腺苷A1受体腺苷是机体自身释放的一种内源性物质, 能够调控神经元的兴奋性, 通过与突触前膜和突触后膜上的腺苷A1受体结合, 抑制突触前膜钙离子内流, 进而增强内向整流钾通道活性, 最终引起突触后膜超极化[63]。CBD能直接激活腺苷A1受体, 同时竞争性抑制平衡型转运蛋白1 (equilibrative nucleoside transporter 1, ENT-1)对腺苷的转运, 增加胞外的腺苷水平进而增强腺苷信号转导[29, 64]。
总之, CBD可通过多种作用机制调控神经元兴奋性, 对治疗神经元过度兴奋性疾病具有很好的应用前景。2018年6月25日, 美国FDA批准了首个CBD口服溶液剂(商品名Epidiolex®)用于治疗2岁以上Dravet综合征和Lennox-Gastaut综合征患者的癫痫性发作。Epidiolex®的上市具有里程碑意义, 随着对各种类型癫痫发病机制的深入研究, CBD的抗癫痫机制将会更加明确。
2.2 神经病理性疼痛神经病理性疼痛是一种严重的慢性疼痛, 由影响外周或中枢的躯体感觉神经系统病变或疾病引起, 异常疼痛和痛觉过敏是其主要症状[65, 66]。临床上主要应用三环类抗抑郁药、GABA类似物如普瑞巴林(pregabalin)和阿片类药物缓解患者的症状。然而, 这些药物疗效不明显且存在较多的不良反应, 因此神经病理性疼痛的治疗仍然是医学界面临的一个重要难题。研究表明, CBD不但能改善各种神经病理性疼痛动物模型的痛觉超敏症状[67-70], 而且THC/CBD口腔黏膜喷雾剂(商品名Sativex®)对伴有异常疼痛的外周和中枢神经病理性疼痛患者也有改善作用, 且耐受性良好[71, 72]。CBD对神经病理性疼痛的缓解作用, 可能涉及多种靶点参与, 包括FAAH、TRP通道、T型钙离子通道(Cav3)、电压门控性钠通道(Nav)、5-HT1A受体及甘氨酸α3受体等。
2.2.1 FAAH研究发现, 激活CB1R和CB2R及抑制内源性大麻素失活均显示出镇痛效果[73]。靶向内源性大麻素系统已成为一种具有前景的疼痛治疗策略。FAAH作为一种水解酶, 主要参与内源性大麻素(如AEA)代谢。虽然CBD对CB1R和CB2R的亲和力低, 但能够抑制FAAH的作用, 间接激活内源性大麻素系统信号转导[21, 22]。研究也证明, 在6-羟基多巴胺(6-hydroxydopamine)诱发痛觉过敏模型中, FAAH抑制剂确实增强了CBD的作用, 而CB1R和CB2R反向激动剂则阻止了这一作用[74]。FAAH有望成为疼痛治疗的靶点, 基于该靶点对大麻素进行结构修饰可能有助于研发出更加安全、有效的镇痛药。
2.2.2 TRP通道TRP通道家族是一类在生物体内广泛分布, 能通透钙离子的非选择性阳离子通道, 主要表达于痛觉神经元和初级传入伤害感受器, 被视为神经病理性疼痛治疗的潜在靶点[75, 76]。研究发现, 各种类型大麻素(植物大麻素、合成大麻素和内源性大麻素)均可影响与疼痛调节相关的TRP通道功能, 如TRPV1~TRPV4、TRPA1和TRPM8等6种亚型[77, 78]。CBD主要通过激活TRP通道(如TRPV1和TRPA1), 导致通道失敏, 活性下降, 降低细胞内钙离子水平和神经元兴奋性发挥镇痛作用[79], 继而抑制了相关配体如芥末油(mustard oil)和辣椒素(capsaicin)、热或冷刺激对通道的进一步激活作用[79]。同时, CBD的镇痛作用可被TRP通道特异性拮抗剂所阻止, 如在大鼠坐骨神经分支选择性损伤(spared nerve injury, SNI)模型和坐骨神经慢性挤压性损伤(chronic constriction injury of sciatic nerve, CCI)模型中, CBD的镇痛作用均可被TRPV1通道特异性拮抗剂辣椒平(capsazepine, CPZ)完全阻断[67, 68]。
2.2.3 电压门控性钠通道电压门控性钠通道是一类经典的镇痛药物作用靶点, 该通道阻断剂对各种类型的疼痛均表现出良好的缓解作用。研究发现, CT-3 (一种合成大麻素) (结构见图 1)不但在神经病理性疼痛模型中具有抗炎和镇痛作用[80], 而且在II期临床试验中也可有效缓解神经病理性疼痛患者的疼痛症状, 且未见明显的大麻素样精神活性不良反应[81]。进一步研究发现, 大麻素类药物(包括CT-3和CBD)主要是以状态依赖的方式抑制钠通道起作用, 但无亚型选择性[32, 82]。
2.2.4 T型钙离子通道T型钙离子通道, 又名低电压激活钙离子通道, 主要调节神经元兴奋性[83]。T型钙离子通道共有3种α1亚基, 分别构成Cav3.1、Cav3.2和Cav3.3, 这些通道在痛觉回路的初级传入神经元及脊髓背根神经节(dorsal root ganglion, DRG)神经元中均有表达, 其中Cav3.2通道的表达量最高, 被视为疼痛调节的理想靶点[84-87], 上调T型钙离子通道的活性, 神经元兴奋性增加, 如激动剂ST-101可显著增加DRG神经元的兴奋性[44]。选择性沉默DRG中Cav3.2通道可减少创伤或糖尿病神经病变引起的痛觉过敏[88, 89]。研究发现, CBD能有效抑制T型钙离子通道(Cav3.1~Cav3.3)电流[33], 其中对Cav3.2的效价最强(IC50 = 0.776 μmol·L-1)。
2.2.5 甘氨酸α3受体甘氨酸受体是中枢神经系统中一类抑制性神经递质受体, 主要参与脑干和脊髓对运动及感觉的调节, 在镇痛过程中起重要作用[90]。甘氨酸受体是由α亚基(α1~α4)和β亚基组成的五聚体, 包括多种亚型[91]。由于甘氨酸α3受体主要分布于负责整合疼痛信息的脊髓背角区, 在疼痛调节研究中备受关注[92]。研究发现, CBD及其衍生物DH-CBD (结构见图 1)通过激活甘氨酸α3受体, 显著增强DRG神经元甘氨酸受体电流, 有效抑制炎症性疼痛和神经病理性疼痛, 且未产生明显耐受[26]。
2.2.6 5-HT1A受体5-HT受体分型复杂, 主要分为7个亚家族(5-HT1~5-HT7), 包括15种受体亚型, 其中亚型5-HT1、5-HT2、5-HT3及5-HT7可能参与疼痛调节, 但受体被激活或抑制后如何影响原来的疼痛状态取决于受体的分布、疼痛类型、给药剂量及途径等多个因素[93]。研究发现, CBD能激活5-HT1A、5-HT2A和5-HT3A三种受体亚型[27, 28], 但其镇痛作用可能主要与5-HT1A受体亚型激活有关。在坐骨神经分支选择性损伤模型中, 5-HT能神经元放电活动减少、机械性痛觉异常和焦虑样行为增加, 连续皮下注射CBD一周可缓解这些症状。由于CBD持续作用导致5-HT1A受体失敏, 促进5-HT释放而恢复5-HT神经元正常放电活动, 如果同时给予选择性5-HT1A受体拮抗剂WAY100635, 则能阻止CBD的这些作用[68]。同时, WAY100635还能完全消除CBD对角膜神经病理性疼痛的镇痛作用[94], 逆转CBD对化疗药紫杉醇(paclitaxel)诱导的神经病理性疼痛的抑制作用[69]。在链脲佐菌素诱导的糖尿病神经病理性疼痛模型中, 大鼠脊髓中的5-HT水平显著降低, 每日给予CBD不但可阻止5-HT水平降低, 并且还明显减弱大鼠机械性痛觉异常, 而经WAY100635处理后, CBD的这些作用则被完全阻止[70]。
总之, CBD通过作用于多种受体和离子通道调控痛觉回路神经元兴奋性, 在临床前和临床研究中均表现出良好的镇痛效果, 除了上述靶点之外, CBD的镇痛作用是否还与其他疼痛调节密切相关的靶点(如酸敏感离子通道、超极化激活的核苷酸门控通道、机械门控离子通道等)有关, 尚待进一步研究。
2.3 焦虑症焦虑症是一种常见的精神疾病, 我国焦虑症的终生患病率约为7.6%, 居所有精神疾病之首, 造成了巨大的社会和经济负担[95]。现存药物疗效欠佳并伴有多种不良反应(如失眠、躁动和头痛等), 寻找新型抗焦虑药仍然十分必要。临床前研究表明, CBD全身给药或显微注射到控制恐惧和焦虑的不同脑区时, 均显示出抗焦虑的潜在作用[96-99]。临床研究也表明, 给予CBD单一治疗或辅助治疗均能改善广泛性焦虑障碍、社交焦虑障碍和创伤后应激综合征患者的焦虑症状[100]。此外, CBD还能减轻海洛因依赖者、克罗恩病及帕金森病患者的焦虑症状[101-103]。CBD对焦虑样行为和恐惧记忆处理过程的调节可能主要涉及5-HT1A受体、TRPV1通道和内源性大麻素系统信号转导。
大鼠中脑导水管周围灰质背外侧区(dorsolateral periaqueductal gray, dlPAG)内注射CBD后, 在高架十字迷宫实验中, 中剂量组进入开臂的次数和在开臂中的停留时间显著增加(抗焦虑作用), 用5-HT1A受体拮抗剂WAY100635预处理后, CBD的作用消失[96]。同样, 在大鼠终纹状核以及腹内侧前额叶皮层等脑区, 也可观察到CBD抗焦虑作用被WAY100635阻止的现象[97-99]。另外, 大鼠中脑导水管周围灰质背外侧区内注射CBD后, 发现低剂量组的抗焦虑作用强于高剂量组, 量效曲线呈“钟形”, 当给予TRPV1通道特异性拮抗剂CPZ预处理后, 高剂量组的抗焦虑作用有所增强[104]。这些结果表明, 较低剂量CBD的抗焦虑作用可能涉及5-HT1A受体的激活, 而高剂量CBD可能通过激活TRPV1通道, 促进谷氨酸神经传递和焦虑反应反而使CBD的抗焦虑作用减弱, 这可能是量效曲线呈“钟形”的原因。
研究发现, CB1R选择性拮抗剂AM251也能阻止CBD的抗焦虑作用[105]。该作用可能与AM251阻断CBD对恐惧记忆巩固和再巩固的中断作用有关[106, 107]。由此可见, CBD对焦虑症状和恐惧记忆处理过程的调节至少部分是由大麻素受体介导, 由于CBD对CB1R和CB2R只有轻微的亲和力[12], 提示它可能是通过抑制内源性大麻素代谢和摄取, 间接促进内源性大麻素系统信号转导起作用[23]。
CBD作为一种替代疗法, 在焦虑症的治疗中有很好的应用前景。然而, 还需要更多的研究, 通过标准化的给药方法和临床结果来确定CBD适当的给药策略及其在治疗中的地位。此外, 在抗焦虑方面, 人工合成的CBD类似物HUF-101 (结构见图 1)与CBD相比, 达到相同疗效的同时所使用的剂量更小(3 mg·kg-1 vs 30 mg·kg-1)[105, 108], 提示对CBD进行结构修饰有望得到活性更高的化合物。
2.4 抑郁症抑郁症是一种以显著而持久的心境低落为主要临床特征的情绪障碍, WHO将其列为构成全球疾病负担的第三大因素, 据估计到2030年将排在第一位[109]。抑郁症的发病机制和治疗已成为医学界关注的焦点。药物是目前治疗抑郁症的首要选择。已上市的抗抑郁药起效慢[110, 111], 且仅对1/3~1/2的患者有效。目前, 靶向多种靶点的抗抑郁药物已引起研究人员极大的兴趣[111], 例如CBD类药物。诸多临床前研究表明, CBD全身给药或注入相关脑区均具有抗抑郁作用[18, 112-114]。CBD的抗抑郁机制可能主要与激活5-HT1A受体、影响内源性大麻素系统信号转导及调节突触可塑性有关。
在抑郁症模型中, 给予CBD可显著减少动物在强迫游泳实验中的不动时间, 作用与经典的抗抑郁药丙咪嗪(imipramine)相当, 同时并未影响小鼠对开阔环境的探索行为, 而5-HT1A受体选择性拮抗剂WAY100635阻断了CBD的作用[18, 112]。
此外, 强迫游泳应激可导致大鼠脑内AEA含量显著降低, 如向腹内侧前额叶皮层内注射外源性AEA或AEA水解酶FAAH的抑制剂URB597均可减少大鼠在强迫游泳实验中的不动时间, 增加其游泳时间, 而CB1R拮抗剂AM251和选择性5-HT合成抑制剂对氯苯丙氨酸(p-chlorophenylalanine)均可阻断该作用[18, 115]。同时, AM251也能阻断CBD对强迫游泳实验模型大鼠抑郁状态的改善作用[18]。深入研究发现, 向大鼠腹内侧前额叶皮层内注射URB597可显著增加中缝背核5-HT能神经元的放电频率, 提示应激诱导腹内侧前额叶皮层内源性大麻素系统信号转导发生变化可能最终通过调节5-HT能神经传递产生促抑郁作用[115]。另外, CBD在嗅球切除抑郁症模型中具有快速且持久的抗抑郁作用, 这一效应与CBD显著提高腹内侧前额叶皮层内的5-HT和谷氨酸水平有关, 并且5-HT1A受体阻滞剂WAY100635可阻断这些作用[113], 提示CBD可能通过激活5-HT1A受体、增强大脑皮层5-HT和谷氨酸能信号转导发挥快速且持久的抗抑郁作用。最近研究发现, CBD抗抑郁靶点, 除5-HT1A受体和内源性大麻素系统外, 也可能与BDNF-TrkB-mTOR信号通路激活引起突触可塑性改变有关[114]。
尽管还没有关于CBD用于抑郁症治疗的临床试验数据发表, 但是, 最近一项关于1 483例使用CBD治疗多种疾病的疗效研究发现, 约1/3的患者使用CBD后情绪有所改善[116]。CBD单独或与已有的抗抑郁药合用可能是一个有效的抑郁症干预策略。此外, 进一步确认CBD的抗抑郁机制可能为其用于抑郁症的治疗铺平道路。
3 总结与展望神经精神疾病是一大类疾病, 发病机制复杂, 治愈难度大。本文已经初步阐明了CBD是一个多靶点的药物, 通过靶向离子通道和GPCR受体两大类靶点, 调控5-HT、GABA和谷氨酸等神经递质传递和神经元兴奋性, 在癫痫、神经病理性疼痛、焦虑症和抑郁症等神经精神疾病中均表现出一定药理作用。然而, 大部分的研究尚停留在临床前研究阶段, 仍需开展大量的临床研究来探究CBD的临床价值。但同时, CBD的研究结果为其类似物的研究和大麻药用价值的开发提供了依据, 有望给难治性神经精神疾病的治疗带来希望。
作者贡献:吴军和于海波共同参与了文章的撰写以及修改。
利益冲突:本文作者声明没有利益冲突。
| [1] |
Bonini SA, Premoli M, Tambaro S, et al. Cannabis sativa:a comprehensive ethnopharmacological review of a medicinal plant with a long history[J]. J Ethnopharmacol, 2018, 227: 300-315. DOI:10.1016/j.jep.2018.09.004 |
| [2] |
Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy:ancient times to the 1980s[J]. Epilepsy Behav, 2017, 70: 298-301. DOI:10.1016/j.yebeh.2016.11.033 |
| [3] |
Russo EB. History of cannabis and its preparations in saga, science, and sobriquet[J]. Chem Biodivers, 2007, 4: 1614-1648. DOI:10.1002/cbdv.200790144 |
| [4] |
Mechoulam R, Parker LA, Gallily R. Cannabidiol:an overview of some pharmacological aspects[J]. J Clin Pharmacol, 2002, 42: 11s-19s. DOI:10.1002/j.1552-4604.2002.tb05998.x |
| [5] |
Morgan CJ, Das RK, Joye A, et al. Cannabidiol reduces cigarette consumption in tobacco smokers:preliminary findings[J]. Addict Behav, 2013, 38: 2433-2436. DOI:10.1016/j.addbeh.2013.03.011 |
| [6] |
Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder:a preliminary report[J]. J Psychopharmacol, 2011, 25: 121-130. DOI:10.1177/0269881110379283 |
| [7] |
Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis:a randomized clinical trial[J]. JAMA Psychiatry, 2018, 75: 1107-1117. DOI:10.1001/jamapsychiatry.2018.2309 |
| [8] |
Khan AA, Shekh-Ahmad T, Khalil A, et al. Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model[J]. Br J Pharmacol, 2018, 175: 2097-2115. DOI:10.1111/bph.14202 |
| [9] |
Basavarajappa BS, Shivakumar M, Joshi V, et al. Endocannabinoid system in neurodegenerative disorders[J]. J Neurochem, 2017, 142: 624-648. DOI:10.1111/jnc.14098 |
| [10] |
van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection[J]. Neuromolecular Med, 2005, 7: 37-50. DOI:10.1385/NMM:7:1-2:037 |
| [11] |
Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders[J]. Nat Rev Neurol, 2019, 16: 9-29. |
| [12] |
Tham M, Yilmaz O, Alaverdashvili M, et al. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors[J]. Br J Pharmacol, 2019, 176: 1455-1469. DOI:10.1111/bph.14440 |
| [13] |
Wu J. Cannabis, cannabinoid receptors, and endocannabinoid system:yesterday, today, and tomorrow[J]. Acta Pharmacol Sin, 2019, 40: 297-299. DOI:10.1038/s41401-019-0210-3 |
| [14] |
Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro[J]. Br J Pharmacol, 2007, 150: 613-623. DOI:10.1038/sj.bjp.0707133 |
| [15] |
Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor[J]. Br J Pharmacol, 2015, 172: 4790-4805. DOI:10.1111/bph.13250 |
| [16] |
Lunn CA, Fine JS, Rojas-Triana A, et al. A novel cannabinoid peripheral cannabinoid receptor-selective inverse agonist blocks leukocyte recruitment in vivo[J]. J Pharmacol Exp Ther, 2006, 316: 780-788. DOI:10.1124/jpet.105.093500 |
| [17] |
Hartmann A, Lisboa SF, Sonego AB, et al. Cannabidiol attenuates aggressive behavior induced by social isolation in mice:involvement of 5-HT1A and CB1 receptors[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2019, 94: 109637. DOI:10.1016/j.pnpbp.2019.109637 |
| [18] |
Sartim AG, Guimarães FS, Joca SRL. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex-possible involvement of 5-HT1A and CB1 receptors[J]. Behav Brain Res, 2016, 303: 218-227. DOI:10.1016/j.bbr.2016.01.033 |
| [19] |
Castillo A, Tolón MR, Fernández-Ruiz J, et al. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB2 and adenosine receptors[J]. Neurobiol Dis, 2010, 37: 434-440. DOI:10.1016/j.nbd.2009.10.023 |
| [20] |
Galaj E, Bi GH, Yang HJ, et al. Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms[J]. Neuropharmacology, 2020, 167: 107740. DOI:10.1016/j.neuropharm.2019.107740 |
| [21] |
Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues:effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide[J]. Br J Pharmacol, 2001, 134: 845-852. DOI:10.1038/sj.bjp.0704327 |
| [22] |
De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes[J]. Br J Pharmacol, 2011, 163: 1479-1494. DOI:10.1111/j.1476-5381.2010.01166.x |
| [23] |
Ibeas Bih C, Chen T, Nunn AV, et al. Molecular targets of cannabidiol in neurological disorders[J]. Neurotherapeutics, 2015, 12: 699-730. DOI:10.1007/s13311-015-0377-3 |
| [24] |
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets[J]. Pharmaceuticals (Basel), 2012, 5: 529-552. DOI:10.3390/ph5050529 |
| [25] |
Ryberg E, Larsson N, Sjogren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor[J]. Br J Pharmacol, 2007, 152: 1092-1101. DOI:10.1038/sj.bjp.0707460 |
| [26] |
Xiong W, Cui TX, Cheng KJ, et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors[J]. J Exp Med, 2012, 209: 1121-1134. DOI:10.1084/jem.20120242 |
| [27] |
Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors[J]. Neurochem Res, 2005, 30: 1037-1043. DOI:10.1007/s11064-005-6978-1 |
| [28] |
Yang KH, Galadari S, Isaev D, et al. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine 3A receptor-mediated currents in Xenopus laevis oocytes[J]. J Pharmacol Exp Ther, 2010, 333: 547-554. DOI:10.1124/jpet.109.162594 |
| [29] |
Gonca E, Darici F. The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias:the role of adenosine A1 receptors[J]. J Cardiovasc Pharmacol Ther, 2015, 20: 76-83. DOI:10.1177/1074248414532013 |
| [30] |
De Petrocellis L, Orlando P, Moriello AS, et al. Cannabinoid actions at TRPV channels:effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation[J]. Acta Physiol (Oxf), 2012, 204: 255-266. DOI:10.1111/j.1748-1716.2011.02338.x |
| [31] |
De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8[J]. J Pharmacol Exp Ther, 2008, 325: 1007-1015. DOI:10.1124/jpet.107.134809 |
| [32] |
Ghovanloo MR, Shuart NG, Mezeyova J, et al. Inhibitory effects of cannabidiol on voltage-dependent sodium currents[J]. J Biol Chem, 2018, 293: 16546-16558. DOI:10.1074/jbc.RA118.004929 |
| [33] |
Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by delta9-tetrahydrocannabinol and cannabidiol[J]. J Biol Chem, 2008, 283: 16124-16134. DOI:10.1074/jbc.M707104200 |
| [34] |
Bakas T, van Nieuwenhuijzen PS, Devenish SO, et al. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors[J]. Pharmacol Res, 2017, 119: 358-370. DOI:10.1016/j.phrs.2017.02.022 |
| [35] |
Ali A. Global health:epilepsy[J]. Semin Neurol, 2018, 38: 191-199. DOI:10.1055/s-0038-1646947 |
| [36] |
Tang F, Hartz AMS, Bauer B. Drug-resistant epilepsy:multiple hypotheses, few answers[J]. Front Neurol, 2017, 8: 301. DOI:10.3389/fneur.2017.00301 |
| [37] |
Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures[J]. Seizure, 2012, 21: 344-352. DOI:10.1016/j.seizure.2012.03.001 |
| [38] |
Huizenga MN, Sepulveda-Rodriguez A, Forcelli PA. Preclinical safety and efficacy of cannabidivarin for early life seizures[J]. Neuropharmacology, 2019, 148: 189-198. DOI:10.1016/j.neuropharm.2019.01.002 |
| [39] |
Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome[J]. New Engl J Med, 2017, 376: 2011-2020. DOI:10.1056/NEJMoa1611618 |
| [40] |
Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4):a randomised, double-blind, placebo-controlled phase 3 trial[J]. Lancet, 2018, 391: 1085-1096. DOI:10.1016/S0140-6736(18)30136-3 |
| [41] |
Michels G, Moss SJ. GABAA receptors:properties and trafficking[J]. Crit Rev Biochem Mol Biol, 2007, 42: 3-14. DOI:10.1080/10409230601146219 |
| [42] |
Yu FH, Mantegazza M, Westenbroek RE, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy[J]. Nat Neurosci, 2006, 9: 1142-1149. DOI:10.1038/nn1754 |
| [43] |
Cheah CS, Yu FH, Westenbroek RE, et al. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome[J]. Proc Natl Acad Sci U S A, 2012, 109: 14646-14651. DOI:10.1073/pnas.1211591109 |
| [44] |
Carvill GL, Weckhuysen S, McMahon JM, et al. GABRA1 and STXBP1:novel genetic causes of Dravet syndrome[J]. Neurology, 2014, 82: 1245-1253. DOI:10.1212/WNL.0000000000000291 |
| [45] |
Kang JQ, Macdonald RL. Molecular pathogenic basis for GABRG2 mutations associated with a spectrum of epilepsy syndromes, from generalized absence epilepsy to Dravet syndrome[J]. JAMA Neurol, 2016, 73: 1009-1016. DOI:10.1001/jamaneurol.2016.0449 |
| [46] |
Nomura T, Hawkins NA, Kearney JA, et al. Potentiating alpha2 subunit containing perisomatic GABAA receptors protects against seizures in a mouse model of Dravet syndrome[J]. J Physiol, 2019, 597: 4293-4307. DOI:10.1113/JP277651 |
| [47] |
Camfield PR. Definition and natural history of Lennox-Gastaut syndrome[J]. Epilepsia, 2011, 52(Suppl 5): 3-9. |
| [48] |
Asadi-Pooya AA. Lennox-Gastaut syndrome:a comprehensive review[J]. Neurol Sci, 2018, 39: 403-414. DOI:10.1007/s10072-017-3188-y |
| [49] |
Cai KF, Wang J, Eissman J, et al. A missense mutation in SLC6A1 associated with Lennox-Gastaut syndrome impairs GABA transporter 1 protein trafficking and function[J]. Exp Neurol, 2019, 320: 112973. DOI:10.1016/j.expneurol.2019.112973 |
| [50] |
Shi YW, Zhang Q, Cai KF, et al. Synaptic clustering differences due to different GABRB3 mutations cause variable epilepsy syndromes[J]. Brain, 2019, 142: 3028-3044. DOI:10.1093/brain/awz250 |
| [51] |
Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome[J]. Proc Natl Acad Sci U S A, 2017, 114: 11229-11234. DOI:10.1073/pnas.1711351114 |
| [52] |
Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs[J]. Nat Rev Neurosci, 2004, 5: 553-564. DOI:10.1038/nrn1430 |
| [53] |
Patel RR, Barbosa C, Brustovetsky T, et al. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidio[J]. Brain, 2016, 139(2164): 2181. |
| [54] |
Kong WJ, Zhang YJ, Gao Y, et al. SCN8A mutations in Chinese children with early onset epilepsy and intellectual disability[J]. Epilepsia, 2015, 56: 431-438. DOI:10.1111/epi.12925 |
| [55] |
O'Brien JE, Meisler MH. Sodium channel SCN8A (Nav1.6):properties and de novo mutations in epileptic encephalopathy and intellectual disability[J]. Front Genet, 2013, 4: 213. |
| [56] |
Cruz JS, Silva DF, Ribeiro LA, et al. Resurgent Na+ current:a new avenue to neuronal excitability control[J]. Life Sci, 2011, 89: 564-569. DOI:10.1016/j.lfs.2011.05.016 |
| [57] |
Hargus NJ, Nigam A, Bertram EH 3rd, et al. Evidence for a role of Nav1.6 in facilitating increases in neuronal hyperexcitability during epileptogenesis[J]. J Neurophysiol, 2013, 110: 1144-1157. DOI:10.1152/jn.00383.2013 |
| [58] |
Xing JH, Li JH. TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons[J]. J Neurophysiol, 2007, 97: 503-511. DOI:10.1152/jn.01023.2006 |
| [59] |
Sun FJ, Guo W, Zheng DH, et al. Increased expression of TRPV1 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy[J]. J Mol Neurosci, 2013, 49: 182-193. DOI:10.1007/s12031-012-9878-2 |
| [60] |
Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1(TRPV1) channels in vitro:potential for the treatment of neuronal hyperexcitability[J]. ACS Chem Neurosci, 2014, 5: 1131-1141. DOI:10.1021/cn5000524 |
| [61] |
Gray RA, Stott CG, Jones NA, et al. Anticonvulsive properties of cannabidiol in a model of generalized seizure are transient receptor potential vanilloid 1 dependent[J]. Cannabis Cannabinoid Res, 2019. |
| [62] |
Gray RA, Whalley BJ. The proposed mechanisms of action of CBD in epilepsy[J]. Epileptic Disord, 2020, 22: 10-15. |
| [63] |
Fredholm BB, Chen JF, Cunha RA, et al. Adenosine and brain function[J]. Int Rev Neurobiol, 2005, 63: 191-270. DOI:10.1016/S0074-7742(05)63007-3 |
| [64] |
Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol:a mechanism of cannabinoid immunosuppression[J]. Proc Natl Acad Sci U S A, 2006, 103: 7895-7900. DOI:10.1073/pnas.0511232103 |
| [65] |
Baron R, Binder A, Wasner G. Neuropathic pain:diagnosis, pathophysiological mechanisms, and treatment[J]. Lancet Neurol, 2010, 9: 807-819. DOI:10.1016/S1474-4422(10)70143-5 |
| [66] |
Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain:clinical manifestations and mechanisms[J]. Lancet Neurol, 2014, 13: 924-935. DOI:10.1016/S1474-4422(14)70102-4 |
| [67] |
Comelli F, Giagnoni G, Bettoni I, et al. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain:mechanisms involved[J]. Phytother Res, 2008, 22: 1017-1024. DOI:10.1002/ptr.2401 |
| [68] |
De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain[J]. Pain, 2019, 160: 136-150. DOI:10.1097/j.pain.0000000000001386 |
| [69] |
Ward SJ, McAllister SD, Kawamura R, et al. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy[J]. Br J Pharmacol, 2014, 171: 636-645. DOI:10.1111/bph.12439 |
| [70] |
Jesus CHA, Redivo DDB, Gasparin AT, et al. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors[J]. Brain Res, 2019, 1715: 156-164. DOI:10.1016/j.brainres.2019.03.014 |
| [71] |
Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment[J]. Eur J Pain, 2014, 18: 999-1012. DOI:10.1002/j.1532-2149.2013.00445.x |
| [72] |
Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis[J]. J Neurol, 2013, 260: 984-997. DOI:10.1007/s00415-012-6739-4 |
| [73] |
Woodhams SG, Chapman V, Finn DP, et al. The cannabinoid system and pain[J]. Neuropharmacology, 2017, 124: 105-120. DOI:10.1016/j.neuropharm.2017.06.015 |
| [74] |
do Nascimento GC, Ferrari DP, Guimaraes FS, et al. Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson's disease[J]. Neuropharmacology, 2020, 163: 107808. DOI:10.1016/j.neuropharm.2019.107808 |
| [75] |
Marwaha L, Bansal Y, Singh R, et al. TRP channels:potential drug target for neuropathic pain[J]. Inflammopharmacology, 2016, 24: 305-317. DOI:10.1007/s10787-016-0288-x |
| [76] |
Dai Y. TRPs and pain[J]. Semin Immunopathol, 2016, 38: 277-291. DOI:10.1007/s00281-015-0526-0 |
| [77] |
Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels[J]. Front Mol Neurosci, 2018, 11: 487. |
| [78] |
Cao NK, Lü HN, Wei NN, et al. Natural modulators of transient receptor potential channels[J]. Acta Pharm Sin (药学学报), 2017, 52: 673-684. |
| [79] |
Maione S, Piscitelli F, Gatta L, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action[J]. Br J Pharmacol, 2011, 162: 584-596. DOI:10.1111/j.1476-5381.2010.01063.x |
| [80] |
Dyson A, Peacock M, Chen A, et al. Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat[J]. Pain, 2005, 116: 129-137. DOI:10.1016/j.pain.2005.03.037 |
| [81] |
Karst M, Salim K, Burstein S, et al. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain:a randomized controlled trial[J]. JAMA, 2003, 290: 1757-1762. DOI:10.1001/jama.290.13.1757 |
| [82] |
Foadi N, Berger C, Pilawski I, et al. Inhibition of voltage-gated Na⁺ channels by the synthetic cannabinoid ajulemic acid[J]. Anesth Analg, 2014, 118: 1238-1245. DOI:10.1213/ANE.0000000000000188 |
| [83] |
Bourinet E, Francois A, Laffray S. T-type calcium channels in neuropathic pain[J]. Pain, 2016, 157: S15-S22. DOI:10.1097/j.pain.0000000000000469 |
| [84] |
Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones[J]. Nature, 1984, 310: 501-502. DOI:10.1038/310501a0 |
| [85] |
Fedulova SA, Kostyuk PG, Veselovsky NS. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones[J]. J Physiol, 1985, 359: 431-446. DOI:10.1113/jphysiol.1985.sp015594 |
| [86] |
Talley EM, Cribbs LL, Lee JH, et al. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels[J]. J Neurosci, 1999, 19: 1895-1911. DOI:10.1523/JNEUROSCI.19-06-01895.1999 |
| [87] |
Todorovic SM, Jevtovic-Todorovic V. The role of T-type calcium channels in peripheral and central pain processing[J]. CNS Neurol Disord Drug Targets, 2006, 5: 639-653. DOI:10.2174/187152706779025490 |
| [88] |
Bourinet E, Alloui A, Monteil A, et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception[J]. EMBO J, 2004, 24: 315-324. |
| [89] |
Messinger RB, Naik AK, Jagodic MM, et al. In vivo silencing of the CaV3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy[J]. Pain, 2009, 145: 184-195. DOI:10.1016/j.pain.2009.06.012 |
| [90] |
Lynch JW, Callister RJ. Glycine receptors:a new therapeutic target in pain pathways[J]. Curr Opin Investig Drugs, 2006, 7: 48-53. |
| [91] |
Lynch JW. Molecular structure and function of the glycine receptor chloride channel[J]. Physiol Rev, 2004, 84: 1051-1095. DOI:10.1152/physrev.00042.2003 |
| [92] |
Harvey RJ, Depner UB, Wässle H, et al. GlyR alpha3:an essential target for spinal PGE2-mediated inflammatory pain sensitization[J]. Science, 2004, 304: 884-887. DOI:10.1126/science.1094925 |
| [93] |
Cortes-Altamirano JL, Olmos-Hernandez A, Jaime HB, et al. Review:5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system[J]. Curr Neuropharmacol, 2018, 16: 210-221. |
| [94] |
Thapa D, Cairns EA, Szczesniak AM, et al. The cannabinoids Δ8THC, CBD, and HU-308 act via distinct receptors to reduce corneal pain and inflammation[J]. Cannabis Cannabinoid Res, 2018, 3: 11-20. DOI:10.1089/can.2017.0041 |
| [95] |
Huang YQ, Wang Y, Wang H, et al. Prevalence of mental disorders in China:a cross-sectional epidemiological study[J]. Lancet Psychiatry, 2019, 6: 211-224. DOI:10.1016/S2215-0366(18)30511-X |
| [96] |
Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats[J]. Psychopharmacology (Berl), 2008, 199: 223-230. DOI:10.1007/s00213-008-1168-x |
| [97] |
Gomes FV, Resstel LB, Guimaraes FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors[J]. Psychopharmacology (Berl), 2011, 213: 465-473. DOI:10.1007/s00213-010-2036-z |
| [98] |
Marinho AL, Vila-Verde C, Fogaca MV, et al. Effects of intra-infralimbic prefrontal cortex injections of cannabidiol in the modulation of emotional behaviors in rats:contribution of 5HT(1)A receptors and stressful experiences[J]. Behav Brain Res, 2015, 286: 49-56. DOI:10.1016/j.bbr.2015.02.023 |
| [99] |
Fogaca MV, Reis FM, Campos AC, et al. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior:involvement of 5HT1A receptors and previous stressful experience[J]. Eur Neuropsychopharmacol, 2014, 24: 410-419. DOI:10.1016/j.euroneuro.2013.10.012 |
| [100] |
Skelley JW, Deas CM, Curren Z, et al. Use of cannabidiol in anxiety and anxiety-related disorders[J]. J Am Pharm Assoc, 2020, 60: 253-261. DOI:10.1016/j.japh.2019.11.008 |
| [101] |
Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder:a double-blind randomized placebo-controlled trial[J]. Am J Psychiatry, 2019, 176: 911-922. DOI:10.1176/appi.ajp.2019.18101191 |
| [102] |
Klier CM, de Gier C, Felnhofer A, et al. A case report of cannabidiol treatment of a Crohn's disease patient with anxiety disorder[J]. J Clin Psychopharmacol, 2020, 40: 90-92. DOI:10.1097/JCP.0000000000001152 |
| [103] |
de Faria SM, de Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a simulated public speaking test in patients with Parkinson's disease[J]. J Psychopharmacol, 2020, 34: 189-196. DOI:10.1177/0269881119895536 |
| [104] |
Campos AC, Guimarães FS. Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2009, 33: 1517-1521. DOI:10.1016/j.pnpbp.2009.08.017 |
| [105] |
Campos AC, Ortega Z, Palazuelos J, et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis:involvement of the endocannabinoid system[J]. Int J Neuropsychopharmacol, 2013, 16: 1407-1419. DOI:10.1017/S1461145712001502 |
| [106] |
Stern CAJ, da Silva TR, Raymundi AM, et al. Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors[J]. Neuropharmacology, 2017, 125: 220-230. DOI:10.1016/j.neuropharm.2017.07.024 |
| [107] |
Stern CA, Gazarini L, Takahashi RN, et al. On disruption of fear memory by reconsolidation blockade:evidence from cannabidiol treatment[J]. Neuropsychopharmacology, 2012, 37: 2132-2142. DOI:10.1038/npp.2012.63 |
| [108] |
Breuer A, Haj CG, Fogaça MV, et al. Fluorinated cannabidiol derivatives:enhancement of activity in mice models predictive of anxiolytic, antidepressant and antipsychotic effects[J]. PLoS One, 2016, 11: e0158779. DOI:10.1371/journal.pone.0158779 |
| [109] |
Malhi GS, Mann JJ. Depression[J]. Lancet, 2018, 392: 2299-2312. DOI:10.1016/S0140-6736(18)31948-2 |
| [110] |
Wong ML, Licinio J. From monoamines to genomic targets:a paradigm shift for drug discovery in depression[J]. Nat Rev Drug Discov, 2004, 3: 136-151. DOI:10.1038/nrd1303 |
| [111] |
Ceskova E, Silhan P. Novel treatment options in depression and psychosis[J]. Neuropsychiatr Dis Treat, 2018, 14: 741-747. DOI:10.2147/NDT.S157475 |
| [112] |
Zanelati TV, Biojone C, Moreira FA, et al. Antidepressant-like effects of cannabidiol in mice:possible involvement of 5-HT1A receptors[J]. Br J Pharmacol, 2010, 159: 122-128. DOI:10.1111/j.1476-5381.2009.00521.x |
| [113] |
Linge R, Jiménez-Sánchez L, Campa L, et al. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission:role of 5-HT1A receptors[J]. Neuropharmacology, 2016, 103: 16-26. DOI:10.1016/j.neuropharm.2015.12.017 |
| [114] |
Sales AJ, Fogaca MV, Sartim AG, et al. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex[J]. Mol Neurobiol, 2019, 56: 1070-1081. DOI:10.1007/s12035-018-1143-4 |
| [115] |
McLaughlin RJ, Hill MN, Bambico FR, et al. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway[J]. Eur Neuropsychopharmacol, 2012, 22: 664-671. DOI:10.1016/j.euroneuro.2012.01.004 |
| [116] |
Corroon J, Phillips JA. A cross-sectional study of cannabidiol users[J]. Cannabis Cannabinoid Res, 2018, 3: 152-161. DOI:10.1089/can.2018.0006 |
 2020, Vol. 55
2020, Vol. 55


