大量研究证实, 恶性肿瘤中广泛存在乏氧现象(pO2 ≤ 2.5 mmHg), 乏氧是多数实体瘤的固有特征, 严重的肿瘤乏氧会导致肿瘤非手术治疗的疗效明显下降, 甚至导致肿瘤转移及产生耐药性, 因而乏氧已成为肿瘤治疗中的一大难题。
光动力治疗(photodynamic therapy, PDT)是一种治疗恶性肿瘤的新型疗法, 其作用原理是将光敏剂注射到体内, 利用特定波长的光照射光敏剂, 被激发的光敏剂分子与周围组织中的氧气作用生成细胞毒性物质-活性氧, 从而杀死肿瘤细胞。现已被临床应用于治疗各种肿瘤, 如乳腺癌和甲状腺癌[1]等。与传统的手术切除复发率高[2]、化疗易诱导全身性不良反应相比, PDT具有微创、高效和较高的选择性, 且能够诱导抗肿瘤免疫响应等独特的优点。而恶性肿瘤中广泛存在乏氧的现象, 一方面肿瘤乏氧严重限制了PDT效果, 致使PDT治疗不彻底, 使肿瘤存在复发的可能; 另一方面, 由于PDT过程会不断消耗组织氧, 造成肿瘤部位乏氧加剧, 最终可能导致肿瘤的转移及耐药性的产生。
近年来, 人们对肿瘤乏氧所导致的PDT疗效有限及PDT不良预后问题进行了大量的研究。本文简述了近年来克服或利用肿瘤乏氧以增强PDT疗效的研究进展, 以期为肿瘤光动力治疗提供参考和新的研究思路。
1 现有的克服或利用肿瘤乏氧增效PDT的方法现有的克服或利用肿瘤乏氧增效PDT的方法可以大致分为3类:增强氧供给策略, 即通过直接或间接方法, 在PDT之前或过程中提高肿瘤组织中O2含量; 利用肿瘤乏氧加剧增效PDT; 构建非氧依赖型PDT治疗模式。
1.1 增强氧供给策略 1.1.1 直接增强氧供给 1.1.1.1 红细胞携氧在人体内, 红细胞中的血红蛋白将氧从肺运送到其他组织。因此, 将红细胞或血红蛋白及类血红蛋白分子作为光敏剂的运输载体, 可以向肿瘤组织传送足够的氧, 补充氧消耗, 从而克服肿瘤乏氧对PDT的限制。基于这一假设, 红细胞被用于PDT持续供氧研究[3-7]。Wang等[4]将红细胞作为运输载体, 将原卟啉(PpIX)包封于其中, 包封了原卟啉的红细胞能够被肿瘤细胞快速摄取, 光照后, 在红细胞的持续供氧下, 原卟啉产生更好的PDT治疗效果。Shi等[5]通过层层自组装技术, 将具有血红蛋白装载能力的Fe3+纳米粒装载上氧合血红蛋白作为人造红细胞的核, 而透明质酸包裹上光敏剂作为红细胞的细胞膜, 设计出一种人造红细胞, 该人造红细胞到达肿瘤部位后, 细胞膜中的透明质酸, 在透明质酸酶的作用下被降解, 释放出氧气, 从而增效PDT, 如图 1。
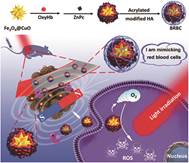
|
Figure 1 A schematic diagram showing the preparation, delivery, hyaluronic acid (HA) shell degradation, O2 release, magnetic field assisted tumor accumulation and photodynamic therapy (PDT) process of a biomimetic red blood cell (BRBC) for the efficient PDT of a hypoxic tumor (Adapted from Ref. 5 with permission. Copyright © 2019 American Chemical Society) |
在一些研究中, 血红蛋白(Hb)分子也被用作单独的氧载体来改善肿瘤乏氧状况[6, 8-12]。Wang等[9]合成了Hb共轭三嵌段共聚物, 并将其自组装成封装了光敏剂酞菁锌(ZnPc)的胶束。结果证明, 相比于没有Hb的胶束, 该胶束在HeLa细胞中能够产生更多的活性氧。Jiang等[12]在共轭聚合物纳米粒(CNPs)表面通过共价连接上Hb, Hb既作为化学发光体系鲁米诺-过氧化氢(H2O2)的催化剂, 又作为氧的载体。鲁米诺在H2O2和Hb存在时发出蓝光, 并将能量转移给CNPs, CNPs被激发从而敏化Hb携带的氧气产生活性氧, 达到杀死肿瘤细胞的目的。
红细胞具有良好的生物相容性, 体内稳定性高, 体循环时间长, 且红细胞去核后可负载各种药物或功能纳米材料, 以上研究表明红细胞或Hb的携氧系统是补充PDT过程中消耗氧气的有效途径。但红细胞尺寸过大(微米级别), 限制了其血管扩散能力, 因而很难到达肿瘤, 且由于这一特点, 也使得基于配体-受体结合的肿瘤靶向PDT难以实现[13]。因此用Hb代替红细胞, 但Hb携氧量有限, 1个Hb只能携带4个O2, 且所携带的O2容易在一些低氧组织中释放(如静脉中), 因而对PDT增效有限; 并且Hb在低氧环境中不稳定, 循环半衰期短。因而部分研究利用透明质酸或脂质体保护Hb克服其不稳定的缺点。
1.1.1.2 全氟化碳(perfluocarbon, PFC)纳米材料携氧PFC是一类人工合成的有机分子, 由碳和氟原子组成, 其结构中氟的高电负性赋予PFC极好的氧亲和力[14]。该类化合物由于具有溶解相当容量氧气的能力及良好的生物相容性而被作为血液代用品, 在临床上应用于创伤、失血性休克和贫血等方面[15, 16]。受此启发, PFC被用于为PDT持续供氧的研究[16-24]。Cheng等[17]制备了一种将PFC包封于核内, 近红外光敏剂(IR780)均匀分散在脂质单层内部的乳化液, 利用PFC的高亲氧能力增强IR780的PDT效果。Yuan等[24]设计了一种负载近红外光敏剂BODIPY的PFC多肽纳米粒, 在乏氧肿瘤环境中的治疗机制见图 2, 该纳米粒具有更高的氧摄取能力, 也具有更强的PDT效果。
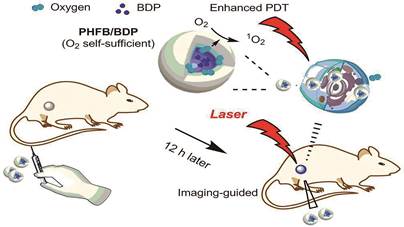
|
Figure 2 Schematic illustration of the mechanism of the near infrared (NIR) imaging-guided photodynamic therapy by the BODIPY-Br2 (BDP) loaded fluorinated amphiphilic polypeptide micelles. PHFB: Fluorinated amphiphilic block copolymers (Adapted from Ref. 24 with permission. Copyright © 2019 American Chemical Society) |
尽管PFC具有强O2亲和力, 且1O2在其中的寿命要比水中或细胞环境中长, 有利于1O2扩散的优点, 由于肿瘤内氧含量低, 使得PFC所能溶解的氧量很少, 其吸附的O2通过简单扩散释放率也很低, 因而对PDT增效有限。目前, 可以利用PFC与红细胞结合, 提高携氧量, 或利用近红外光或超声释放其携带的氧气, 从而提高治疗效果。其他能够增强PFC携氧量, 提高其氧气释放率的方法也值得进一步探索。
1.1.1.3 H2O2分解供氧在生物体内, 过氧化氢酶通过催化H2O2分解生成O2和H2O来保护细胞免受氧化损伤。此外, 研究发现, 与正常细胞相比, 癌细胞中H2O2含量普遍较高, 这可能与癌细胞的异常增殖、转移、血管生成和DNA损伤有关[25, 26]。有学者提出并证明了同时递送过氧化氢酶和光敏剂, 并利用过氧化氢酶催化分解肿瘤组织中过量的H2O2, 可以实现原位供氧, 增强PDT治疗效果[27-33]。Chen等[27]开发了一种H2O2激活型、αvβ3整合素靶向的纳米粒, 该体系以生物相容的聚合物PLGA为载体, 同时包覆光敏剂亚甲基蓝和过氧化氢酶, 并在载体表面修饰环肽肿瘤靶向配体c(RGDfK) (一种高效选择性环肽类抑制剂)。该纳米粒在c(RGDfK)的作用下能特异性地被αvβ3整合素高表达的肿瘤细胞摄取后, 细胞内高水平的H2O2迅速渗入纳米粒内部并在过氧化氢酶的催化下分解产生氧, 大量的氧使纳米粒内压强骤增从而导致壳层破裂并释放出亚甲基蓝。在近红外激光照射下, 亚甲基蓝的光敏性被激活, 产生大量具有细胞毒性的活性氧, 引发肿瘤细胞凋亡。该体系实现了对肿瘤的自供氧、高选择性的光动力治疗。
天然的过氧化氢酶能够高效催化H2O2分解原位产生氧, 但制造成本高、生产复杂、不稳定和苛刻的催化条件限制了其广泛使用[34]。随着纳米技术的快速发展, 具有内在酶活性的纳米材料因其稳定性好[35]、制备简单等优点而受到关注, 如金纳米团簇[36, 37]和铂纳米酶等[38, 39]。因此, 除了过氧化氢酶, 金纳米团簇和铂纳米酶等都被用于PDT相关系统催化H2O2向氧的转化[36-40], 用于对抗癌细胞的乏氧, 而其他新型的纳米酶结构也值得进一步探索。
1.1.1.4 H2O分解供氧绿色植物中的叶绿体吸收光能, 并将光能转化为水, 高效地产生大量的氧气。受到这一自然现象的启发, 研究人员创新性地利用无机、有机或混合材料制成的水分解纳米复合材料用于PDT持续供氧的研究[41-45]。Zheng等[42]将能够裂解水的纳米材料碳氮化合物C3N4应用于提高PDT治疗效果, 首先用碳点(carbon dots)修饰C3N4, 再将PpIX-PEG-RGD吸附在C3N4上, 其中PpIX为光敏剂, 能利用O2产生活性氧; 靶向Argi-Gly-Asp(RGD)的聚合物具有靶向作用; 聚乙二醇(PEG)起到连接PpIX和RGD的作用。经碳点修饰后, C3N4能够吸收红光(630 nm)裂解H2O产生O2, 随后PpIX将一部分O2转化为活性氧。这种材料不仅能够提高肿瘤中O2水平以提高PDT疗效, 还能够降低肿瘤扩散的风险。Jiang等[43]报道了一种红细胞膜包被的氧化石墨炔纳米片, 在近红外光照射下, 该纳米片良好的肿瘤富集及其优异的光催化水分解产生氧, 缓解了肿瘤扩散型乏氧; 与此同时, 其光热转化功能有效扩张肿瘤血管, 增强血液灌注, 从而有效克服了灌注型乏氧。该纳米片同时改善扩散型乏氧与灌注型乏氧, 显著提高了肿瘤PDT效果。Li等[44]设计了一种集光动力治疗、光热治疗和多模式成像于一体的FeS2@C-PEG纳米结构(200 nm), 在近红外光照射下, 该纳米材料可将H2O氧化, 产生O2, 从而减轻肿瘤乏氧并增效PDT。Li等[45]设计了双光子激发的纳米复合材料(FCRH), 首次使用氮化碳(Fe-C3N4)激发双光子辐射引发H2O分解, 介导产生O2。
水是生物体内最丰富的材料, 与以往的产氧材料相比, 以水为来源将为体内产氧提供无限材料。因此在缺氧微环境下, 利用H2O产生O2, 是一种有前景的方法。但各类水分解材料对可见光和近红外的响应较差, 催化效率有待提高。
1.1.1.5 氧化锰(MnO2)分解供氧最近, 作为创新材料MnO2得到了广泛的关注, 它可以在肿瘤的微酸环境中和过量的H2O2作用下产生O2, 其降解原理可用以下反应表示。
| $ {{\rm{Mn}}{{\rm{O}}_2} + 2{{\rm{H}}^ + } \to {\rm{M}}{{\rm{n}}^{2 + }} + {{\rm{H}}_{\rm{2}}}{\rm{O}} + 1/2{{\rm{O}}_2} \uparrow } $ | (1) |
| $ {{\rm{Mn}}{{\rm{O}}_{\rm{2}}} + {{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}} + {\rm{2}}{{\rm{H}}^ + } \to {\rm{M}}{{\rm{n}}^{2 + }} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}} + {{\rm{O}}_{\rm{2}}} \uparrow } $ | (2) |
基于此, 研究人员利用各种组成不同的MnO2材料作为光敏剂的载体, 为光动力治疗提供O2 [46-48]。Fan等[46]设计了基于上转换纳米探针(UCSMs)的智能MnO2纳米片, 该纳米材料可同时pH响应和H2O2响应, 实现高分辨率上转换发光成像和氧生成, 协助PDT。当MnO2在酸性条件下被分解, UCSMs将恢复被淬灭上转换发光从而进行诊断, 同时, MnO2分解所产生O2可以显著促进PDT疗效。Zhao等[47]开发了一种基于氮杂-BODIPY/MnO2新型纳米复合材料。该纳米材料中MnO2可产生热量并催化内源性H2O2产生O2, 以提高PDT效率。
尽管MnO2生物相容性好, 既可作为药物载体, 又可催化H2O2分解产O2, 但其潜在的风险、长期毒性、细胞摄取机制和代谢途径尚不清楚。在保持其治疗效果的前提下, 应充分重视降低或减少其在生物体内的毒性, 结合生物可降解材料[49], 加速其生物降解性, 从而促进MnO2在PDT领域的应用。
氧载体(Hb和PFC)可以通过向肿瘤内加载额外的氧分子来增加肿瘤内O2。氧发生器(MnO2和过氧化氢酶)可以催化肿瘤中过表达的H2O2生成O2, 因为肿瘤中H2O2含量高于正常组织。尽管这些策略前景看好, 但仍存在载氧率低、漏氧快和H2O2有限表达等缺陷。因此, 开发克服氧限制的新型作用机制非常必要。
1.1.2 间接提高氧供给PDT过程中肿瘤血管的异常和血管的闭合共同降低了肿瘤的供氧量, 从而影响PDT的疗效。因此, 改善肿瘤血管异常化和避免血管闭合是改善肿瘤组织乏氧状况的有效途径。基于以上认知, 许多研究小组致力于通过改变肿瘤微环境, 间接提高肿瘤氧供给, 从而提高PDT疗效。
研究表明, 通过温和加热(约43 ℃)提高局部温度会导致肿瘤血流量增加从而提高肿瘤内氧水平[50-52]。因此, 利用温和光热对肿瘤进行预热处理是减少乏氧、增效PDT的有效方法。基于此原理, 光热疗法(photothermal therapy, PTT)被广泛用于肿瘤热疗[53-60]。Zhang等[57]将具有高热转化率的金纳米片(GNS)用作PTT的核心, 其外壳由负载Ce6光敏剂的介孔二氧化硅组成。结果表明, 相较于单一PDT和PTT, 联合使用PTT, 减轻了乏氧并增加了PDT效果。Zhu等[58]构建了基于牛血清白蛋白(BSA)、磺胺类药物(SAs)和铁卟啉纳米级金属有机骨架(NMOFs, 图 3), 可用于协同PDT和PTT。体外活性氧检测和光热温度检测结果表明, 该BSA/SAs-NMOF纳米平台可以在肿瘤细胞中表现出更好的PDT效果。Liu等[59]将生物相容性铜铁氧体纳米球(CFNs), 经直接电子转移和光增强的Fenton反应后, 在650 nm激光辐照下生成活性氧, 并且该纳米球暴露于808 nm激光时具有高的光热转换效率, 从而显著提高PDT效果。Feng等[60]制备了含有光敏剂己胺偶联氯(e6)和光热剂3'-四甲基吲哚并花青碘化物(DiR)的脂质体体系。在此体系中, 通过785 nm激光激发DiR, 可产生温和的光热效应, 同时使用660 nm激光激发光敏剂。结果表明, 该方法可显著改善肿瘤内血流, 使肿瘤乏氧减轻, 从而产生更好的PDT效果。虽然以上研究表明可有效改善肿瘤内血流, 使肿瘤乏氧减轻, 但光热转换效率低、由热疗引起的正常细胞损伤和炎性反应可能导致包括肿瘤复发和治疗抗性等不良反应仍有待解决。
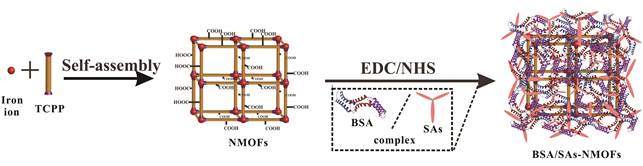
|
Figure 3 Schematic illustration of the synthesis procedure of BSA/SAs-NMOFs. BSA: Bovine serum albumin; SAs: Sulfonamides; NMOFs: Iron-porphyrin; TCPP: Tetrakis (4-carboxyphenyl)-porphyrin; EDC: Crystalline N-ethylcarbodiimide hydrochloride; NHS: N-Hydroxysuccinimide (Adapted from Ref. 58 with permission. Copyright © 2019 American Chemical Society) |
除了使用温和加热来暂时改善肿瘤内血流, 化学药物(紫杉烷、吉西他滨和顺铂等)最近被用于调节肿瘤微血管系统的混乱结构, 使血液灌注率更高, 从而提高肿瘤内氧含量[30, 61-65]。Wang等[64]开发了一种交联了光敏剂(THPP)和肿瘤治疗前药(顺铂)的共价有机聚合物, 用于癌症联合治疗。将该聚合物静脉注射4T1荷瘤小鼠体内后, 顺铂能显著降低血管内皮生长因子的表达, 使血管恢复正常, 缓解肿瘤乏氧, PDT疗效随之提高。Yang等[65]使用肝素, 增强抗凝血酶Ⅲ的活性, 减轻血管内凝血和血栓形成, 从而增强PDT效果。但是, 用这些抑制剂调节血管异常状态和预防血管闭合是暂时的, 导致氧供给量有限, PDT效果受到限制。利用化学药物诱导血管正常化, 可更有效地供氧和给药, 但用药过量反而会使血管退化严重, 阻碍O2和药物运输, 且血管正常化只能持续一定时间, 故化学药物的用药量和作用时间的控制仍需进一步探索。
在肿瘤微环境中其他异常, 如过表达缺氧诱导因子(HIF-1)和存在致密的细胞外基质, 均为导致肿瘤乏氧的重要原因[66-70]。因此, 通过降低肿瘤组织中HIF-1的水平减轻乏氧, 是提高PDT效果的有效方法。Chen等[69]开发了以茴香酰胺为靶点的脂质磷酸钙纳米粒(LCP), 将HIF-1αsiRNA (siRNA, HIF-1α的干扰RNA)输送至SAS细胞(人舌鳞状细胞癌贴壁细胞)和SCC4细胞(鳞状细胞癌)中, 然后给予PDT。结果表明, 由于RNA的干扰作用, HIF-1α表达被有效降低, 且联合疗法比单独的HIF-1α siRNA或PDT更有效, 实现增效PDT。Liu等[70]通过疏水性光敏剂Ce6和雷帕霉素(RAP)自组装形成了双药物纳米核, RAP的持续释放抑制了HIF-1α表达, 从而缓解了肿瘤乏氧, 实现增效PDT。降低肿瘤组织中HIF-1水平可一定程度缓解肿瘤乏氧状况, 对提高PDT有一定作用, 但缓解乏氧效果有限, 因而对PDT增效有限, 且肿瘤微环境的复杂性对该方法的影响也需进一步探索。
最近研究发现, 抑制肿瘤细胞线粒体呼吸和减少内源性氧消耗取得了新进展[71-73]。Mai等[71]以血小板膜(PM)作为纳米载体, 包封二甲双胍(Met)和光敏剂IR780 (PM-IR780-Met NP), Met的引入可抑制线粒体呼吸从而降低肿瘤耗氧量, 缓解肿瘤乏氧, 实现增效PDT的目的。Yang等[73]将IR780和Met包装在PEG-PCL脂质体中。Met可以直接抑制线粒体电子传输链, 从而有效地抑制细胞呼吸。减少内源性氧消耗的这一概念提供了新的方式, 解决了乏氧限制PDT效果的重要难题。尽管抑制肿瘤细胞线粒体呼吸可减少内源性氧消耗, 间接促使肿瘤氧含量提高, 但受限于肿瘤组织本身氧含量低的状况, 其对提高肿瘤中氧含量效果有限。结合其他提高肿瘤组织氧含量的方法(PFC为载体)或许是解决上述问题的有效方法。
1.2 利用肿瘤乏氧加剧增效PDT与其他侧重于缓解肿瘤乏氧的方法不同, 也有研究变劣势为优势, 巧妙利用光动力反应加剧的肿瘤乏氧, 释放其他治疗药物或活化生物还原药, 实现联合治疗。
1.2.1 利用乏氧加剧控制药物释放研究表明, 硝基基团、醌、脂族氮氧化物和芳族氮氧化物等均为乏氧敏感结构。将光敏剂和具有乏氧敏感结构的药物载体相结合, 光动力反应加剧了肿瘤乏氧, 活化乏氧敏感结构, 进而促进了药物的释放, 整个治疗可以被认为是一种自反馈过程, 提高了治疗效果[74-78]。Qian等[76]报道了2-硝基咪唑(NI)缀合, 含二噻吩-苯并三唑的聚合物(NI-CP), 通过复乳法将多柔比星(DOX)封装成核壳(DOX/NI-CP)结构。肿瘤区域富集了DOX/NI-CP纳米粒后, 近红外光光敏剂(二噻吩-苯并三唑)产生高水平的细胞毒性活性氧和更严重的肿瘤组织乏氧状态, 从而触发疏水性NI基团被生物还原形成亲水性2-氨基咪唑类化合物, 导致纳米粒塌陷并释放DOX, 最终发挥DOX和PDT的协同抗癌作用。Wang等[77]将PDT与缺氧反应性化疗(简称为Ce6-PEG-Azo-PCL)相结合研发了一种乏氧响应性输送系统。在该纳米系统中, 光敏剂Ce6在照射过程中持续消耗氧, 进一步放大肿瘤的缺氧状况, 并触发肿瘤部位乏氧响应性偶氮苯的分解, 从而释放负载的DOX, 实现了PDT和化疗的协同治疗, 增强了PDT效果。Zhang等[78]将低氧响应药物递送系统与PDT整合在一起, 构建了一种新型偶氮苯(偶氮)纳米载体(CPs-CPT-Ce6 NPs), 该载体负载了光敏剂Ce6和化疗药物喜树碱(CPT), 通过PDT增强肿瘤区域的乏氧状态, 从而激活乏氧响应, 释放CPT, 实现PDT与化疗的结合。且体外和体内研究均证实该载药系统可以增效PDT。
1.2.2 乏氧加剧激活生物还原药近年来, 针对乏氧引起的肿瘤生物学变化发展的生物还原药成为研究的热点。生物还原药是指“无毒”的前药在乏氧环境中被相关的还原酶还原激活为相应的细胞毒剂的一类制剂, 典型的生物还原药包括硝基咪唑类化合物、醌类化合物和氮氧化物等。目前已进入临床试验的该类药物有巴诺蒽醌(AQ4N)和替拉扎明(tirapazamine, TPZ)。将PDT同光敏剂与生物还原药联合使用, 利用PDT加剧的肿瘤乏氧活化生物还原药, 可起到联合治疗的作用[79-81]。Liu等[79]在PDT后, 瘤内直接注射生物还原前药TPZ, 利用PDT加剧的肿瘤乏氧活化TPZ, 达到PDT与生物还原药的协同治疗。Feng等[80]制备了一种包裹了光敏剂Ce6和生物还原药AQ4N的脂质体, 在光照条件下, Ce6消耗瘤内氧并产生光动力效应, 加剧肿瘤乏氧, 肿瘤乏氧的加剧导致AQ4N活化, 产生细胞毒性, 从而进一步杀伤肿瘤细胞。Zhang等[81]创新使用2-硝基咪唑衍生物共轭PEG制备了脂质体, 并在其中封装Ce6和低氧激活的前药TPZ。Ce6介导的PDT在激光照射下会引起缺氧导致脂质体分解并激活TPZ的抗肿瘤活性, 从而增强对癌细胞的杀伤力。体外和体内研究均表明, 与常规PDT相比, 该脂质体抗癌活性更高。
利用乏氧控制药物释放及激活乏氧响应药物都是充分利用了肿瘤本身的乏氧状况及PDT导致的肿瘤乏氧加剧, 在提高PDT靶向性的同时实现了协同治疗。然而, 面对肿瘤微环境的复杂性和个体差异引起的异质性, 该方法很多问题仍需进一步探讨和明晰。
1.3 构建非氧依赖型PDT由于传统的PDT依靠氧产生具有细胞毒性的活性氧, 因而实体瘤的乏氧状态极大地限制了其疗效。而设计非氧依赖的新型光敏剂是克服这一限制的有效方法[82-85]。Babii等[82]开发了一种由二芳基乙烯衍生的拟肽, 在无氧条件下, 光照即可产生活性氧, 因此可以通过这种非氧依赖PDT进行肿瘤治疗。Han等[84]设计了一种肿瘤pH响应性聚合物胶束。该胶束在808 nm近红外光辐射下产生高温, 同时诱导单线态氧供体(二苯基蒽内过氧化物, DPAE)的热裂解, 从而实现不需要氧参与即可产生大量PDT用单线态氧。该纳米体系将光热触发的非氧依赖PDT和PTT结合, 实现了有效的肿瘤消融, 且没有明显的毒副作用。Huang等[85]设计报道了一种新型的聚合物单线态氧载体, 该聚合物由1, 4-二甲基萘的衍生物(DMN)、氮杂氟化硼二吡咯化物(B1)和水溶性PEG构成, 其中, DMN能与单线态氧反应生成过氧化物且在受热时释放出单线态氧, 实现了乏氧肿瘤内单线态氧的高效、可控释放, 成功抑制肿瘤的生长。目前, 非氧依赖的新型光敏剂仍较少见报道, 因而构建更多非氧依赖的新型光敏剂的研究仍需进一步开展。
综上所述, 3类基于肿瘤乏氧增效PDT的治疗策略: “克服乏氧”策略(增强氧供给策略)、“规避乏氧”策略(构建非氧依赖型PDT治疗模式)及“利用乏氧”策略(乏氧应激控释及乏氧激活乏氧响应药物)的优势和缺点见表 1[3-13, 16-24, 27-33, 41-45, 46-48, 50-90]。
| Table 1 A summary of the advantages and disadvantages of the methods |
实体瘤乏氧对PDT介导的癌症治疗是一个严峻挑战, 本文对克服PDT乏氧的各种策略进行了概述, 虽然所列的方法都被证明了具有提高肿瘤组织氧含量的能力, 但是肿瘤氧含量与PDT效率之间的精确相关性仍需要精密监测, 以便进一步合理设计PDT供氧载体, 提高其携氧能力。PDT还面临其他重要挑战, 如光的穿透有限和光敏剂对正常组织的毒性。因此, 需要开发智能和多功能的载体, 协同克服这些系统性障碍, 以获得最大抗癌效果, 还需要注意PDT诱导的肿瘤侵袭和转移等不良反应。此外, 为了满足临床应用, PDT载体需要满足生物相容性、生物降解性、简单、可控性和可重复性等要求。随着这些问题的解决, 利用或克服乏氧的系统将为PDT开辟一条新的途径。
作者贡献: 尹小杰负责整理文献并撰写文章初稿; 王晓倩负责绘图、制作表格; 张凤玲负责文章设计、把握文章方向以及稿件修改。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Muhanna N, Chan HHL, Townson JL, et al. Photodynamic therapy enables tumor-specific ablation in preclinical models of thyroid cancer[J]. Endocr Relat Cancer, 2020, 27: 41-53. DOI:10.1530/ERC-19-0258 |
| [2] |
Wang GA, Wu HC. Clinical application of photodynamic therapy in respiratory tract tumors[J]. Mod Pract Med (现代实用医学), 2020, 32: 7-8. |
| [3] |
Liu WL, Liu T, Zou MZ, et al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy[J]. Adv Mater, 2018, 30: 1802006. DOI:10.1002/adma.201802006 |
| [4] |
Wang L, Shi X, Yang C, et al. Versatile RBC-derived vesicles as nanoparticle vector of photosensitizers for photodynamic therapy[J]. Nanoscale, 2013, 5: 416-421. DOI:10.1039/C2NR32506C |
| [5] |
Shi X, Zhan Q, Yan X, et al. Oxyhemoglobin nano-recruiter preparation and its application in biomimetic red blood cells to relieve tumor hypoxia and enhance photodynamic therapy activity[J]. J Mater Chem B, 2020, 8: 534-545. DOI:10.1039/C8TB02430H |
| [6] |
Tang W, Zhen Z, Wang M, et al. Red blood cell-facilitated photodynamic therapy for cancer treatment[J]. Adv Funct Mater, 2016, 26: 1757-1768. DOI:10.1002/adfm.201504803 |
| [7] |
Liu L, Li TW, Ruan Z, et al. Polypeptide-based artificial erythrocytes conjugated with near infrared photosensitizers for imaging-guided photodynamic therapy[J]. J Mater Sci, 2018, 53: 9368-9381. DOI:10.1007/s10853-018-2276-6 |
| [8] |
Wang P, Li X, Yao C, et al. Orthogonal near-infrared upconversion co-regulated site-specific O2 delivery and photodynamic therapy for hypoxia tumor by using red blood cell microcarriers[J]. Biomaterials, 2017, 125: 90-100. DOI:10.1016/j.biomaterials.2017.02.017 |
| [9] |
Wang S, Yuan F, Chen K, et al. Synthesis of hemoglobin conjugated polymeric micelle:a ZnPc carrier with oxygen self-compensating ability for photodynamic therapy[J]. Biomacromolecules, 2015, 16: 2693-2700. DOI:10.1021/acs.biomac.5b00571 |
| [10] |
Luo Z, Zheng M, Zhao P, et al. Self-monitoring artificial red cells with sufficient oxygen supply for enhanced photodynamic therapy[J]. Sci Rep, 2016, 6: 23393. DOI:10.1038/srep23393 |
| [11] |
Xu X, Cui Y, Bu H, et al. A photosensitizer loaded hemoglobin-polymer conjugate as a nanocarrier for enhanced photodynamic therapy[J]. J Mater Chem B, 2018, 6: 1825-1833. DOI:10.1039/C7TB03109B |
| [12] |
Jiang LY, Bai HT, Liu LB, et al. Luminescent, oxygen-supplying, hemoglobin-linked conjugated polymer nanoparticles for photodynamic therapy[J]. Angew Chem Int Ed Engl, 2019, 58: 10660-10665. DOI:10.1002/anie.201905884 |
| [13] |
Wang C, Sun X, Cheng L, et al. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer[J]. Adv Mater, 2014, 26: 4794-4802. DOI:10.1002/adma.201400158 |
| [14] |
Kaneda MM, Caruthers S, Lanza GM, et al. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics[J]. Ann Biomed Eng, 2009, 37: 1922-1933. DOI:10.1007/s10439-009-9643-z |
| [15] |
Chen J, Pan H, Lanza GM, et al. Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy[J]. Adv Chronic Kidney Dis, 2013, 20: 466-478. DOI:10.1053/j.ackd.2013.08.004 |
| [16] |
Song X, Feng L, Liang C, et al. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies[J]. Nano Lett, 2016, 16: 6145-6153. DOI:10.1021/acs.nanolett.6b02365 |
| [17] |
Cheng Y, Cheng H, Jiang C, et al. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy[J]. Nat Commun, 2015, 6: 8785. DOI:10.1038/ncomms9785 |
| [18] |
Que Y, Liu Y, Tan W, et al. Enhancing photodynamic therapy efficacy by using fluorinated nanoplatform[J]. ACS Macro Lett, 2016, 5: 168-173. DOI:10.1021/acsmacrolett.5b00935 |
| [19] |
Song G, Ji C, Liang C, et al. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy[J]. Biomaterials, 2017, 112: 257-263. DOI:10.1016/j.biomaterials.2016.10.020 |
| [20] |
Song G, Liang C, Yi X, et al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer[J]. Adv Mate, 2016, 28: 2716-2723. DOI:10.1002/adma.201504617 |
| [21] |
Spahn D. Blood substitutes artificial oxygen carriers:perfluorocarbon emulsions[J]. Critical Care, 1999, 3: R93. DOI:10.1186/cc364 |
| [22] |
Day RA, Estabrook DA, Logan JK, et al. Fluorous photosensitizers enhance photodynamic therapy with perfluorocarbon nanoemulsions[J]. Chem Commun, 2017, 53: 13043-13046. DOI:10.1039/C7CC07038A |
| [23] |
Wang J, Liu L, You Q, et al. All-in-one theranostic nanoplatform based on hollow MoS(x) for photothermally-maneuvered oxygen self-enriched photodynamic therapy[J]. Theranostics, 2018, 8: 955-971. DOI:10.7150/thno.22325 |
| [24] |
Yuan P, Ruan Z, Jiang W, et al. Oxygen self-sufficient fluorinated polypeptide nanoparticles for NIR imaging-guided enhanced photodynamic therapy[J]. J Mater Chem B, 2018, 6: 2323-2331. DOI:10.1039/C8TB00493E |
| [25] |
López-Lázaro M. Dual role of hydrogen peroxide in cancer:possible relevance to cancer chemoprevention and therapy[J]. Cancer Lett, 2007, 252: 1-8. DOI:10.1016/j.canlet.2006.10.029 |
| [26] |
Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems[J]. Acc Chem Res, 2011, 44: 793-804. DOI:10.1021/ar200126t |
| [27] |
Chen H, Tian J, He W, et al. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells[J]. J Am Chem Soc, 2015, 137: 1539-1547. DOI:10.1021/ja511420n |
| [28] |
Cheng H, Zhu JY, Li SY, et al. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy[J]. Adv Funct Mater, 2016, 26: 7847-7860. DOI:10.1002/adfm.201603212 |
| [29] |
Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy[J]. ACS Nano, 2017, 11: 7006-7018. DOI:10.1021/acsnano.7b02533 |
| [30] |
Chen Q, Chen J, Liang C, et al. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy[J]. J Control Release, 2017, 263: 79-89. DOI:10.1016/j.jconrel.2016.11.006 |
| [31] |
Tang L, Yang X, Yin Q, et al. Investigating the optimal size of anticancer nanomedicine[J]. Proc Natl Acad Sci U S A, 2014, 111: 15344-15349. DOI:10.1073/pnas.1411499111 |
| [32] |
Zhang Y, Shen TT, Zhang HL, et al. A multifunctional nanocomposite for luminescence resonance energy transfer-guided synergistic monitoring and therapy under single near infrared light[J]. Chem Commun, 2016, 52: 4880-4883. DOI:10.1039/C6CC00010J |
| [33] |
He Y, Cong C, He Y, et al. Tumor hypoxia relief overcomes multidrug resistance and immune inhibition for self-enhanced photodynamic therapy[J]. Chem Eng J, 2019, 375: 122079. DOI:10.1016/j.cej.2019.122079 |
| [34] |
Zhang X, Li G, Wu D, et al. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy[J]. Bionsens Bioelectron, 2019, 137: 178-198. DOI:10.1016/j.bios.2019.04.061 |
| [35] |
Shang Y, Liu F, Wang Y, et al. Enzyme mimic nanomaterials and their biomedical applications[J]. ChemBioChem, 2020. DOI:10.1002/cbic.202000123 |
| [36] |
Dan Q, Hu D, Ge Y, et al. Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy[J]. Biomater Sci, 2020, 8: 973-987. DOI:10.1039/C9BM01742A |
| [37] |
Liu CP, Wu TH, Liu CY, et al. Self-supplying O2 through the catalase-like activity of gold nanoclusters for photodynamic therapy against hypoxic cancer cells[J]. Small, 2017, 13: 1700278. DOI:10.1002/smll.201700278 |
| [38] |
Gao Z, Li Y, Zhang Y, et al. Biomimetic platinum nanozyme immobilized on 2D metal-organic frameworks for mitochondrion-targeting and oxygen self-supply photodynamic therapy[J]. ACS Appl Mater Inter, 2020, 12: 1963-1972. DOI:10.1021/acsami.9b14958 |
| [39] |
Ma YC, Zhu YH, Tang XF, et al. Au nanoparticles with enzyme-mimicking activity-ornamented ZIF-8 for highly efficient photodynamic therapy[J]. Biomater Sci, 2019, 7: 2740-2748. DOI:10.1039/C9BM00333A |
| [40] |
Zhang Y, Wang F, Liu C, et al. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy[J]. ACS Nano, 2018, 12: 651-661. DOI:10.1021/acsnano.7b07746 |
| [41] |
Hu D, Sheng Z, Gao G, et al. Activatable albumin-photosensitizer nanoassemblies for triple-modal imaging and thermal-modulated photodynamic therapy of cancer[J]. Biomaterials, 2016, 93: 10-19. DOI:10.1016/j.biomaterials.2016.03.037 |
| [42] |
Zheng DW, Li B, Li CX, et al. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting[J]. ACS Nano, 2016, 10: 8715-8722. DOI:10.1021/acsnano.6b04156 |
| [43] |
Jiang W, Zhang Z, Wang Q, et al. Tumor reoxygenation and blood perfusion enhanced photodynamic therapy using ultrathin graphdiyne oxide nanosheets[J]. Nano Lett, 2019, 19: 4060-4067. DOI:10.1021/acs.nanolett.9b01458 |
| [44] |
Li M, Lin H, Qu F. FeS2@C-ICG-PEG nanostructure with intracellular O2 generation for enhanced photo-dynamic/thermal therapy and imaging[J]. Biochem Eng J, 2020, 384: 123374. |
| [45] |
Li RQ, Zhang C, Xie BR, et al. A two-photon excited O2-evolving nanocomposite for efficient photodynamic therapy against hypoxic tumor[J]. Biomaterials, 2019, 194: 84-93. DOI:10.1016/j.biomaterials.2018.12.017 |
| [46] |
Fan W, Bu W, Shen B, et al. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-responsive UCL imaging and oxygen-elevated synergetic therapy[J]. Adv Mater, 2015, 27: 4155-4161. DOI:10.1002/adma.201405141 |
| [47] |
Zhao M, Xie M, Guo J, et al. Facile phototherapeutic nanoplatform by integrating a multifunctional polymer and MnO2 for enhancing tumor synergistic therapy[J]. Adv Healthc Mater, 2019, 8: 1900414. DOI:10.1002/adhm.201900414 |
| [48] |
Liu Y, Pan Y, Cao W, et al. A tumor microenvironment responsive biodegradable CaCO3/MnO2-based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy[J]. Theranostics, 2019, 9: 6867-6884. DOI:10.7150/thno.37586 |
| [49] |
Luo L, Liu JB, Yin Z, et al. Biodegradable micelles for two-photon photodynamic therapy in a mouse model of breast cancer[J]. Acta Pharm Sin (药学学报), 2019, 54: 927-936. |
| [50] |
Song CW, Rhee JG, Levitt SH. Effect of hyperthermia on hypoxic cell fraction in tumor[J]. Int J Radiat Oncol, 1982, 8: 851-856. DOI:10.1016/0360-3016(82)90088-8 |
| [51] |
Chen Q, Chen H, Shapiro H, et al. Sequencing of combined hyperthermia and photodynamic therapy[J]. Radiat Res, 1996, 146: 293-297. DOI:10.2307/3579459 |
| [52] |
Brizel DM, Scully SP, Harrelson JM, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas[J]. Cancer Res, 1996, 56: 5347-5350. |
| [53] |
Jin CS, Lovell JF, Chen J, et al. Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly[J]. ACS Nano, 2013, 7: 2541-2550. DOI:10.1021/nn3058642 |
| [54] |
Jaque D, Martínez Maestro L, del Rosal B, et al. Nanoparticles for photothermal therapies[J]. Nanoscale, 2014, 6: 9494-9530. DOI:10.1039/C4NR00708E |
| [55] |
Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy[J]. Nat Commun, 2016, 7: 13193. DOI:10.1038/ncomms13193 |
| [56] |
Li XS, Peng XH, Zheng BD, et al. New application of phthalocyanine molecules:from photodynamic therapy to photothermal therapy by means of structural regulation rather than formation of aggregates[J]. Chem Sci, 2018, 9: 2098-2104. DOI:10.1039/C7SC05115H |
| [57] |
Zhang L, Yang XQ, Wei JS, et al. Intelligent gold nanostars for in vivo CT imaging and catalase-enhanced synergistic photodynamic & photothermal tumor therapy[J]. Theranostics, 2019, 9: 5424-5442. DOI:10.7150/thno.33015 |
| [58] |
Zhu W, Liu Y, Yang Z, et al. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites:targeting tumor hypoxia by carbonic anhydrase Ⅸ inhibition and T1-T2 dual mode MRI guided photodynamic/photothermal therapy[J]. J Mater Chem B, 2018, 6: 265-276. DOI:10.1039/C7TB02818K |
| [59] |
Liu Y, Zhen W, Jin L, et al. All-in-one theranostic nanoagent with enhanced reactive oxygen species generation and modulating tumor microenvironment ability for effective tumor eradication[J]. ACS Nano, 2018, 12: 4886-4893. DOI:10.1021/acsnano.8b01893 |
| [60] |
Feng LZ, Tao DL, Dong ZL, et al. Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection[J]. Biomaterials, 2017, 127: 13-24. DOI:10.1016/j.biomaterials.2016.11.027 |
| [61] |
Jain RK. Normalization of tumor vasculature:an emerging concept in antiangiogenic therapy[J]. Science, 2005, 307: 58-62. DOI:10.1126/science.1104819 |
| [62] |
Cham KKY, Baker JHE, Takhar KS, et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma[J]. Br J Cancer, 2010, 103: 52-60. DOI:10.1038/sj.bjc.6605727 |
| [63] |
Mpekris F, Baish JW, Stylianopoulos T, et al. Role of vascular normalization in benefit from metronomic chemotherapy[J]. Proc Natl Acad Sci U S A, 2017, 114: 1994-1999. DOI:10.1073/pnas.1700340114 |
| [64] |
Wang H, Zhu W, Feng L, et al. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer[J]. Nano Res, 2018, 11: 3244-3257. DOI:10.1007/s12274-017-1858-y |
| [65] |
Yang L, Wei Y, Xing D, et al. Increasing the efficiency of photodynamic therapy by improved light delivery and oxygen supply using an anticoagulant in a solid tumor model[J]. Laser Surg Med, 2010, 42: 671-679. DOI:10.1002/lsm.20951 |
| [66] |
Jahanban-Esfahlan R, de la Guardia M, Ahmadi D, et al. Modulating tumor hypoxia by nanomedicine for effective cancer therapy[J]. J Cell Physiol, 2018, 233: 2019-2031. DOI:10.1002/jcp.25859 |
| [67] |
Broekgaarden M, Weijer R, Krekorian M, et al. Inhibition of hypoxia-inducible factor 1 with acriflavine sensitizes hypoxic tumor cells to photodynamic therapy with zinc phthalocyanine-encapsulating cationic liposomes[J]. Nano Res, 2016, 9: 1639-1662. DOI:10.1007/s12274-016-1059-0 |
| [68] |
Gong H, Chao Y, Xiang J, et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy[J]. Nano Lett, 2016, 16: 2512-2521. DOI:10.1021/acs.nanolett.6b00068 |
| [69] |
Chen WH, Lecaros RLG, Tseng YC, et al. Nanoparticle delivery of HIF1α siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer[J]. Cancer Lett, 2015, 359: 65-74. DOI:10.1016/j.canlet.2014.12.052 |
| [70] |
Liu P, Xie X, Shi X, et al. Oxygen-self-supplying and HIF-1α-inhibiting core-shell nanosystem for hypoxia-resistant photodynamic therapy[J]. ACS Appl Mater Inter, 2019, 11: 48261-48270. DOI:10.1021/acsami.9b18112 |
| [71] |
Mai X, Zhang Y, Fan H, et al. Integration of immunogenic activation and immunosuppressive reversion using mitochondrial-respiration-inhibited platelet-mimicking nanoparticles[J]. Biomaterials, 2020, 232: 119699. DOI:10.1016/j.biomaterials.2019.119699 |
| [72] |
Zhao LP, Zheng RR, Chen HQ, et al. Self-delivery nanomedicine for O2-economized photodynamic tumor therapy[J]. Nano Lett, 2020, 20: 2062-2071. DOI:10.1021/acs.nanolett.0c00047 |
| [73] |
Yang Z, Wang J, Liu S, et al. Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway[J]. Biomaterials, 2020, 229: 119580. DOI:10.1016/j.biomaterials.2019.119580 |
| [74] |
Park W, Bae BC, Na K. A highly tumor-specific light-triggerable drug carrier responds to hypoxic tumor conditions for effective tumor treatment[J]. Biomaterials, 2016, 77: 227-234. DOI:10.1016/j.biomaterials.2015.11.014 |
| [75] |
Kim K, Lee CS, Na K. Light-controlled reactive oxygen species (ROS)-producible polymeric micelles with simultaneous drug-release triggering and endo/lysosomal escape[J]. Chem Commun, 2016, 52: 2839-2842. DOI:10.1039/C5CC09239F |
| [76] |
Qian C, Yu J, Chen Y, et al. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy[J]. Adv Mater, 2016, 28: 3313-3320. DOI:10.1002/adma.201505869 |
| [77] |
Wang W, Lin L, Ma X, et al. Light-iduced hypoxia-triggered living nanocarriers for synergistic cancer therapy[J]. ACS Appl Mater Inter, 2018, 10: 19398-19407. DOI:10.1021/acsami.8b03506 |
| [78] |
Zhang X, Wu M, Li J, et al. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemo-therapy[J]. ACS Appl Mater Inter, 2018, 10: 21909-21919. DOI:10.1021/acsami.8b06491 |
| [79] |
Liu Y, Liu Y, Bu W, et al. Hypoxia induced by upconversion-based photodynamic therapy:towards highly effective synergistic bioreductive therapy in tumors[J]. Angew Chem Int Ed Engl, 2015, 54: 8105-8109. DOI:10.1002/anie.201500478 |
| [80] |
Feng L, Cheng L, Dong Z, et al. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy[J]. ACS Nano, 2017, 11: 927-937. DOI:10.1021/acsnano.6b07525 |
| [81] |
Zhang K, Zhang Y, Meng X, et al. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy[J]. Biomaterials, 2018, 185: 301-309. DOI:10.1016/j.biomaterials.2018.09.033 |
| [82] |
Babii O, Afonin S, Garmanchuk LV, et al. Direct photocontrol of peptidomimetics:an alternative to oxygen-dependent photodynamic cancer therapy[J]. Angew Chem Int Ed Engl, 2016, 55: 5493-5496. DOI:10.1002/anie.201600506 |
| [83] |
Kolemen S, Ozdemir T, Lee D, et al. Remote-controlled release of singlet oxygen by the plasmonic heating of endoperoxide-modified gold nanorods:towards a paradigm change in photodynamic therapy[J]. Angew Chem Int Ed Engl, 2016, 55: 3606-3610. DOI:10.1002/anie.201510064 |
| [84] |
Han Y, Chen Z, Zhao H, et al. Oxygen-independent combined photothermal/photodynamic therapy delivered by tumor acidity-responsive polymeric micelles[J]. J Control Release, 2018, 284: 15-25. DOI:10.1016/j.jconrel.2018.06.012 |
| [85] |
Huang T, Zhao M, Yu Q, et al. De novo design of polymeric carrier to photothermally release singlet oxygen for hypoxic tumor treatment[J]. Research, 2019, 2019: 1-11. |
| [86] |
Zheng RR, Zhao LP, Chen HQ, et al. Tumor microenvironment responsive biomimetic nanoparticles for photodynamic tumor therapy[J]. Acta Pharm Sin (药学学报), 2020, 55: 1672-1679. |
| [87] |
Hu HM, Yan XF, Wang H, et al. Perfluorocarbon-based O2 nanocarrier for efficient photodynamic therapy[J]. J Mater Chem B, 2019, 7: 1116-1123. DOI:10.1039/C8TB01844H |
| [88] |
Yang D, Yang G, Sun Q, et al. Carbon-dot-decorated TiO2 nanotubes toward photodynamic therapy based on water-splitting mechanism[J]. Adv Healthc Mater, 2018, 7: 1800042. DOI:10.1002/adhm.201800042 |
| [89] |
Chen Y, Cong H, Shen Y, et al. Biomedical application of manganese dioxide nanomaterials[J]. Nanotechnology, 2020, 31: 202001. DOI:10.1088/1361-6528/ab6fe1 |
| [90] |
Hu JJ, Cheng YJ, Zhang XZ. Recent advances in nanomaterials for enhanced photothermal therapy of tumors[J]. Nanoscale, 2018, 10: 22657-22672. DOI:10.1039/C8NR07627H |
 2020, Vol. 55
2020, Vol. 55


