绿原酸(chlorogenic acid, CGA)是由咖啡酸的羧基和奎尼酸的羟基缩合而成的缩酚酸, 是植物细胞通过莽草酸途径合成的一种苯丙素类物质, 其分子结构中有酯键、不饱和双键、多元酚和邻二酚羟基[1, 2]。绿原酸在杜仲、金银花、绿咖啡豆、土豆、苹果以及茶叶等多种植物中含量很高[3-5]。具有抗氧化、抗菌、抗病毒、抗肿瘤、降血脂、降血糖和免疫调节等多方面的药理作用[6-8], 在食品、医药和化工等领域都有广泛的应用[9] (图 1)。随着科技的进步和研究的深入, 绿原酸药理作用机制逐渐被重视。现对绿原酸的药理作用和作用机制进行综述, 以期为绿原酸的药用研究与开发提供参考。
|
Figure 1 The application of chlorogenic acid from green coffee beans in weight loss |
大量研究证明, 绿原酸具有广谱抗真菌和细菌的作用[7]。Martíne等[10]在2017年报道了绿原酸对多种植物致病真菌有抗真菌活性, 具有生物杀真菌剂的潜力, 其机制是通过抑制真菌孢子的早期透膜化来控制不同的植物病原真菌的生长。而绿原酸的抗菌作用研究, 已有大量文献报道, 其主要是通过破坏肺炎链球菌(Streptococcus pneumoniae)、金葡球菌(Staphylococcus aureus)和痢疾志贺氏菌(Shigella dysenteriae)的细胞膜, 增加外膜和质膜通透性, 导致细菌的屏障功能丧失, 进而发挥其抗菌活性[11, 12]。近些年, 关于绿原酸新的抗菌机制被报道, 通过降低细菌细胞壁的硬度, 减慢细菌的迁移, 影响细菌细胞膜的稳定性和诱导活性氧(reactive oxygen species, ROS)的产生[13, 14]。由此可见, 随着绿原酸的抗菌机制深入研究将有助于新型的抗菌药物的研发。
1.2 抗病毒已有多篇文献指出, 绿原酸及其衍生物作为天然化合物, 对多种类型的病毒有很好的抗病毒活性, 其中包括艾滋病病毒(human immunodeficiency virus, HIV)、甲型流感病毒、单纯疱疹病毒(herpes simplex virus, HSVs)和乙型肝炎病毒(hepatitis B virus, HBV)等[15, 16]。1997年, Robinson等[17]报道了绿原酸可以抑制HIV-1整合酶。随后, Tamura等[18]在人淋巴瘤细胞株MT-2上验证了绿原酸可以有效地抑制HIV病毒。近年来, 研究发现绿原酸及其衍生物可有效抑制甲型流感病毒(H1N1/H3N2)的感染, 其机制是下调了病毒核蛋白的表达和抑制神经氨酸酶活性[19, 20]。绿原酸还可以通过有效地降低炎症反应和抑制病毒感染产生来治疗病毒感染。Zhao课题组[21]实验结果表明, 加入绿原酸显著提高HSV-1感染的小胶质细胞(BV2)的存活率, 其机制是抑制感染细胞中Toll样受体2、Toll样受体9和髓样分化因子88的增加, 降低炎症因子肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)和白细胞介素-6 (interleukin-6, IL-6)释放。Wang等[22]以HepG2.2.15细胞和鸭乙型肝炎病毒感染模型证明了绿原酸可以抑制HBV, 其机制是通过抑制HBV-DNA复制以及乙型肝炎表面蛋白抗原的产生。综上所述, 绿原酸有望成为一个潜在的广谱抗病毒药物。
1.3 抗肿瘤20世纪80年代, 绿原酸被发现具有抑制肿瘤的作用, 从而引起了人们的广泛关注[23]。2017年本课题组首次报道了绿原酸通过介导巨噬细胞的M1/M2极化, 即促进M1型巨噬细胞和抑制M2表型巨噬细胞, 进而抑制脑胶质瘤生长[24]。Jiang课题组[25]发现绿原酸对肝癌、肺癌和胶质瘤都有明显抑制作用, 其机制不是通过直接的肿瘤细胞杀伤作用, 而是通过诱导其分化来抑制肿瘤, 并指出绿原酸有望成为一种安全有效的肿瘤分化诱导剂。Sapio等[26]报道绿原酸可有效抑制骨肉瘤其机制是抑制磷酸化的信号传导及转录激活蛋白3, 同时指出当绿原酸与细胞外信号调节激酶1/2的抑制剂PD98059联用时可显著增强抑瘤效果。然而, 一些文献报道绿原酸是通过诱导ROS的产生进而抑制结肠癌和通过影响细胞凋亡相关基因的表达, 从而发挥抑制肺癌细胞生长的作用[27, 28]。尽管大量文献报道了绿原酸抗肿瘤作用机制, 但看法不尽一致。因此, 潜在靶点的寻找和作用的机制有待进一步探索。
1.4 抗氧化和抗炎绿原酸是一种广泛存在植物中的多酚类次生代谢产物, 具有很强抗氧化和抗炎特性。儿茶酚结构的存在为自由基提供了结合位点[29, 30], 绿原酸能够螯合金属离子和清除自由基[超氧阴离子(O2-)、过氧化氢(H2O2)、羟基自由基(•OH)、次氯酸(HOCl)、过氧亚硝酸盐阴离子(ONOO-)和一氧化氮(NO)][31-33]。Lu等[34]研究显示绿原酸具有很强的抗氧化活性, 其清除1, 1-二苯基-2-三硝基苯肼(DPPH)自由基的活性是维生素C和E的2~3倍, 清除超氧阴离子自由基的活性是维生素C和E的10~30倍。近年来, 绿原酸的抗炎作用也备受关注。2006年, Dos Santos等[35]在角叉菜胶致大鼠足跖肿胀炎症模型上首次报道了绿原酸具有抗炎活性。随后, 有很多文献对其抗炎机制进行了深入地探讨。绿原酸及其异构体可通过清除细胞内ROS, 抑制p38级联磷酸化和上调核因子κB (nuclear factor kappa-B, NF-κB)信号通路来抑制白介素-8 (interleukin-8, IL-8)的产生, 从而起到抗炎作用[36, 37]。然而在硫酸葡聚糖诱导的小鼠溃疡性结肠炎模型中, 发现绿原酸是通过调控丝裂原活化蛋白激酶/ERK/c-Jun氨基末端激酶信号通路来降低组织的炎症反应[38]。文献报道, 绿原酸有望成为治疗类风湿关节炎的新型治疗剂, 其机制是通过降低了NF-κB与B细胞活化因子启动子区域的DNA结合能力, 进而抑制了通过NF-κB途径介导的B细胞活化因子表达[39]。绿原酸具有较强的肝功能保护作用, 研究发现绿原酸可以有效降低CCl4诱导的急性肝损伤产生TNF-α、IL-6和IL-1β炎症因子, 其机制是通过核因子E-2相关因子2 (nuclear factor erythroid 2-related factor 2, Nrf2)介导的抗氧化和抑制Nod样受体家族热蛋白结构域3炎症小体的激活来进行急性肝损伤的保护作用[40]。绿原酸也可以通过增加B淋巴细胞瘤-2 (B-cell lymphoma-2, Bcl2)表达并抑制环氧化酶-2、诱导型一氧化氮合酶、Bax (Bcl2 associated X, Bax)和caspases 3、9介导的炎症反应来降低甲氨蝶呤(methotrexate, MTX)诱导的肝毒性[41]。绿原酸还可以通过影响肝星状LX2细胞系中IL-13/microRNA-21 (miR-21)/Smad7信号传导相互作用, 抑制血吸虫病诱导的肝纤维化, 因此, 绿原酸有望成为用于治疗血吸虫病肝纤维化的抗肝纤维化类药物[42]。神经退行性疾病阿尔茨海默病和帕金森病的病理学研究表明, 慢性氧化应激和炎症反应会导致神经元受损[43]。基于绿原酸具有较强的抗氧化和抗炎作用, 很多学者发现它具有很好的神经系统保护作用[44]。一些临床和临床前研究表明, 咖啡提取物(主要成分绿原酸)对阿尔茨海默病和帕金森病展现出很好的治疗效果[45, 46]。Hermawati等[47]发现绿原酸可以改善记忆力减退和海马细胞短暂性全脑缺血后死亡, 其机制是通过增加Bcl2、超氧化物歧化酶2和血小板-内皮细胞黏附分子CD31表达, 并降低内皮素-1表达来改善空间记忆, 防止双侧颈总动脉闭塞后CA1锥体细胞死亡。由于绿原酸作为一种有效的自由基清除剂, 可以显著保护PC12细胞免受氧化损伤。其机制包括直接的ROS淬灭活性和通过激活Nrf2诱导内源性抗氧化酶[48]。因此, 绿原酸可以作为潜在的神经保护药物。近年来, 文献报道绿原酸还可以通过调节线粒体的功能进而发挥其抗氧化和抗炎的功能。Tsai等[49]在用绿原酸预处理的情况下, 用氧化型低密度脂蛋白(oxidized low density, oxLDL)处理HUVEC细胞。结果表明, 绿原酸预处理可提高NAD-依赖性去乙酰化酶(sirtuin 1, SIRT1)活性水平, 逆转了由oxLDL刺激造成SIRT1的损伤和AMP激活的蛋白激酶(AMP-activated protein kinase, AMPK)/过氧化物酶体增殖物受体γ共激活因子1的活性, 并减轻了oxLDL诱导的氧化应激和线粒体生物发生的功能障碍。其机制是通过调节SIRT1和AMPK/过氧化物酶体增殖物受体γ共激活因子1功能来抑制oxLDL诱导的内皮细胞凋亡。Yang等[50]报道了绿原酸通过激活SIRT1调节的线粒体功能来预防饱和游离脂肪酸(free fatty acid, FFA)诱导的脂毒性。其机制是通过减少ROS的产生以及增加线粒体质量和线粒体膜电位, 减轻了氧化应激和线粒体功能障碍; 显著降低促细胞凋亡蛋白Bax表达, 从而减少线粒体介导的caspase依赖性细胞凋亡。
1.5 治疗代谢性疾病随着社会的进步和人类物质生活水平的提高, 代谢相关性疾病已成为全球影响人类健康的重要问题。其中包括血脂异常、高血压、高空腹血糖水平、胰岛素抵抗、慢性炎症和血栓形成等[51]。随着天然产物在治疗代谢性疾病优势的逐渐展现, 人们又重新燃起了对天然产物的兴趣。许多研究已经评估绿原酸对代谢类疾病的影响, 其中包括肥胖、血脂异常、糖尿病、高血压、代谢综合征和保护心血管等[52]。
在治疗肥胖方面, 研究表明绿原酸主要通过调控糖代谢和脂代谢来控制肥胖。Wang等[53]喂食高脂饮食(high fat diet, HFD)小鼠用绿原酸治疗6周。结果表明, 给予绿原酸可显著降低小鼠的体重, 降低血浆中的脂质水平, 并改变脂肪组织中脂肪生成和脂肪分解相关基因的mRNA表达。此外, 绿原酸改善HFD诱发的肠道菌群失调, 也有助于改善HFD诱发的肥胖。同时, 绿原酸可以通过促进葡萄糖的摄取和线粒体的功能来刺激褐色脂肪细胞的生热。其机制增强了棕色脂肪细胞的生热和质子泄漏, 上调葡萄糖转运蛋白2和磷酸果糖激酶来促进葡萄糖的吸收, 增加线粒体的数量和功能[54]。绿原酸也可以通过激活AMPK, 抑制3-羟基3-甲基戊二酰辅酶A还原酶, 增强肉碱棕榈酰转移酶的活性控制肥胖[55, 56]。Cho等[57]研究了绿原酸对高脂饮食诱导肥胖小鼠的体重、体脂和肥胖相关激素的影响。与高脂饮食对照组相比, 绿原酸和咖啡酸均显著降低体重、内脏脂肪量、血浆瘦素和胰岛素水平、肝脏和心脏中的甘油三酸酯以及脂肪组织和心脏中的胆固醇。在大鼠实验中获得了相似的结果, 绿原酸以剂量依赖性方式抑制体内和内脏脂肪的增加和高脂饮食诱导的游离脂肪酸。同样临床研究也显示, 富含绿原酸的食物具有抗肥胖作用[58]。给予30名超重受试者每天饮用5杯普通速溶咖啡或Coffee Slender® (富含绿原酸)。饮用Coffee Slender®参与者明显减少了体重, 其中80%的减少是体内脂肪, 而普通速溶咖啡的参与者减少体重和体内脂肪的作用并不显著[59]。此外, Soga等[60]研究了绿原酸对人体能量代谢的影响。18名健康男性受试者每天食用185 mL含或不含绿原酸的测试饮料(329 mg), 持续4周, 与对照饮料相比, 含有绿原酸的饮料可显著提高餐后能量的利用, 并且饮用含绿原酸饮料的受试者餐后脂肪利用率更高。
在控制血脂异常方面, 绿原酸和咖啡酸均显著降低小鼠血浆中的游离脂肪酸、甘油三酸酯、胆固醇和血清脂质水平, 显著提高高密度脂蛋白胆固醇/总胆固醇的比率[57, 58]。同时在大鼠高胆固醇血症模型中, 发现绿原酸可显著降低总胆固醇和低密度脂蛋白胆固醇, 并增加高密度脂蛋白胆固醇; 此外, 还改善了动脉粥样硬化指数和心脏危险因素[61]。
在治疗糖尿病方面, 在动物水平上, 研究发现绿原酸可有效抑制糖尿病小鼠的肝中葡萄糖6-磷酸酶表达和活性, 减少肝脂肪变性, 改善脂质分布和骨骼肌葡萄糖摄取, 从而改善空腹血糖水平、葡萄糖耐量、胰岛素敏感性和血脂异常[62]。在链脲霉素诱导糖尿病大鼠模型中, 研究发现白桑树的叶提取物、绿原酸和芦丁的非空腹血糖水平呈剂量依赖性降低, 而异槲皮素则未见[63]。Jin等[64]研究了绿原酸对晚期糖尿病小鼠葡萄糖和脂质代谢的影响。与对照组相比, 绿原酸组的体脂、空腹血糖和糖基化血红蛋白百分比显著降低。Kim等[65]通过建立大鼠半乳糖性白内障模型来确定绿原酸对糖性白内障的影响。实验显示, 持续2周口服绿原酸可以有效阻止糖性白内障的发展。同时在研究绿原酸对大鼠糖尿病伤口愈合的影响时, 发现绿原酸加速伤口愈合[66]。在临床研究中, 通过对咖啡中绿原酸抗糖尿病作用的评估, 实验结果表明, 绿原酸可以显著降低葡萄糖依赖性促胰岛素多肽分泌, 显著增加胰高血糖素样肽1的分泌, 有效降低肠道葡萄糖吸收率, 对葡萄糖转运具有拮抗作用[67]。同样Iwai等[68]通过对45位受试者评估了富含绿原酸的不含咖啡因的绿咖啡豆提取物的降血糖作用。发现摄入含有绿原酸饮料后血浆葡萄糖显著降低, 但未观察到血浆胰岛素谱有显著变化。Ahrens等[69]研究了EmulinTM (绿原酸、杨梅素和槲皮素的专利混合物)的抗糖尿病作用。对40位2型糖尿病患者进行治疗, 结果显示, 如果定期食用EmulinTM, 不仅具有降低餐后血糖的作用, 而且可以长期降低2型糖尿病患者的血糖水平。基于绿原酸对糖代谢障碍具有调节作用, Gao等[70]通过绿原酸修饰的功能化磁微球蛋白发现绿原酸的靶点为蛋白激酶B。并通过绿原酸分子探针的免疫荧光进一步证明了绿原酸与AKT的共定位。同时阐明了其作用机制是通过直接靶向AKT的PH结构域, 激活AKT在Ser-473上的磷酸化, 诱导下游分子糖原合酶激酶3和叉头框转录因子O亚族1的磷酸化, 从而发挥降低血糖的作用。
在治疗代谢综合征方面, 研究发现绿原酸的预防和治疗对肥胖症及与肥胖有关的小鼠肝脏脂肪变性和胰岛素抵抗有很好的疗效。绿原酸有效防止体重增加, 抑制肝脂肪变性的发展, 并减低高脂饮食诱导的胰岛素抵抗[71]。通过评价一个含有绿原酸的天然补品对代谢综合征患者的影响, 发现78名患有代谢综合征的患者持续服用4个月, 其体重、体重指数、腰围、空腹血糖和体重显著降低, 也观察到总胆固醇得到有效的降低[52]。
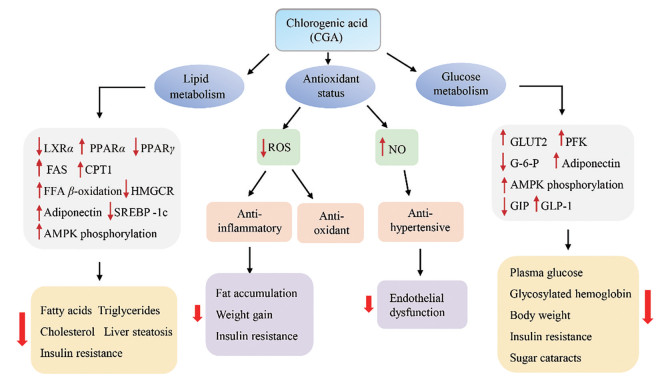
在治疗心血管系统和血栓栓塞性疾病方面, 绿原酸显现出很好的疗效。在细胞水平, 用200 mmol·L-1绿原酸处理3T3-L1脂肪细胞后, 细胞内钙浓度相对于对照增加了9倍, 同时也促进了胰岛素的分泌, 显著提高了过氧化物酶体增殖剂激活受体γ (150%)、GLUT4 (220%)、过氧化物酶体增殖剂激活受体α (40%)和脂肪酸转运蛋白(25%)的mRNA表达。因此, 可作为刺激胰岛素的增敏剂和降脂剂[72]。在整体动物中, 绿原酸可有效减低自发性高血压大鼠的血压和改善血管功能。其机制通过抑制血管系统中ROS过量的产生来降低氧化应激和提高一氧化氮的生物利用度, 进而减轻了自发性高血压大鼠的内皮功能障碍、血管肥大和高血压[73]。绿原酸可用于预防糖尿病引发的心血管系统损害[74]。研究表明绿原酸也作为抗凝剂, 当糖尿病大鼠的血小板在激动剂二磷酸腺苷刺激下, 绿原酸治疗30天后血小板聚集可明显减少[75]。此外, 绿原酸还可以通过激活血小板A2A受体/腺苷酸环化酶/环磷酸腺苷/蛋白激酶A信号通路进而抑制小鼠动脉血栓形成[76]。在临床研究中, 纯绿原酸和绿原酸含量很高的食物可以对血压产生积极影响。Kozuma等[77]针对117名轻度的高血压健康男性进行研究, 分组给予28天不同浓度的绿原酸, 发现绿原酸可以有效降低收缩压和舒张压, 其原因为绿原酸具有抗氧化特性, 改善内皮功能障碍进而降低了血压。同时研究表明, 绿原酸有效降低患有高血压患者的血压[78]。Mubarak等[79]研究了绿原酸对一氧化氮状态、内皮功能和血压的急性影响。结果显示, 给予绿原酸的受试者的收缩压和舒张压均明显降低, 一氧化氮的状态和内皮功能未受到明显影响。综上所述, 现对绿原酸治疗代谢性疾病的作用机制进行总结(图 2)。
|
Figure 2 Mechanisms of CGA on improving metabolic diseases. AMPK: AMP-activated protein kinase; CPT1: Carnitine palmitoyltransferase 1; FAS: Fatty acid synthase; FFA: Free fatty acid; GLUT2: Glucose transporter type 2; G-6-P: Glucose-6-phosphatase; GIP: Glucose-dependent insulinotropic polypeptide; GLP-1: Glucagon-like peptide-1; HMGCR: 3-Hydroxy-3-methylglutaryl CoA reductase; LXR: Liver X receptor; NO: Nitric oxide; PFK: Phosphofructokinase; PPAR: Peroxisome proliferator-activated receptor; ROS: Reactive oxygen species; SREBP-1c: Sterol regulatory element-binding protein 1c |
近年来, 有关绿原酸的研究报道越来越多, 业已成为天然产物领域研究热点之一。绿原酸作为金银花、杜仲、咖啡豆、茵陈等许多中草药的主要有效成分之一, 随着其生物活性的不断深入研究, 它的应用愈加广泛。在国外, 已将绿原酸作为减肥的保健品出售。在我国, 绿原酸也作为抗肿瘤药物进行晚期复发脑胶质母细胞瘤的Ⅱ期临床研究。但值得注意的是, 虽然我国对含有绿原酸及其衍生物的中药的使用有着悠久的历史, 但是研究者对绿原酸分子机制的探索和靶点验证仍然存在很大的不足, 这也是今后研究着力解决的问题。
作者贡献:王庆华完成了论文的撰写, 杜婷婷和张智慧协助完成了本论文的文献收集和论文校对, 季明和胡海宇提供了论文写作思路的建议并对论文进行了校对, 通讯作者陈晓光为本论文进行了修改和指导
利益冲突:无任何利益冲突。
| [1] |
Kühnl T, Koch U, Heller W, et al. Chlorogenic acid biosynthesis:characterization of a light-induced microsomal 5-O-(4-coumaroyl)-D-quinate/shikimate 3'-hydroxylase from carrot (Daucus carota L.) cell suspension cultures[J]. Arch Biochem Biophys, 1987, 258: 226-232. DOI:10.1016/0003-9861(87)90339-0 |
| [2] |
Lallemand LA, Zubieta C, Lee SG, et al. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee[J]. Plant Physiol, 2012, 160: 249-260. DOI:10.1104/pp.112.202051 |
| [3] |
Lepelley M, Cheminade G, Tremillon N, et al. Chlorogenic acid synthesis in coffee:an analysis of CGA content and real-time RT-PCR expression of HCT, HQT, C3H1, and CCoAOMT1 genes during grain development in C. canephora[J]. Plant Sci, 2017, 172: 978-996. |
| [4] |
Duarte GS, Pereira AA, Farah A. Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods[J]. Food Chem, 2010, 118: 851-855. DOI:10.1016/j.foodchem.2009.05.042 |
| [5] |
Wang Z, Clifford MN. Comparison of the profiles of chlorogenic acids and their derivatives from three Chinese traditional herbs by LC-MSn[J]. Acta Pharm Sin (药学学报), 2008, 43: 185-190. |
| [6] |
Naveed M, Hejazi V, Abbas M, et al. Chlorogenic acid (CGA):a pharmacological review and call for further research[J]. Biomed Pharmacother, 2018, 97: 67-74. DOI:10.1016/j.biopha.2017.10.064 |
| [7] |
Bagdas D, Gul Z, Meade JA, et al. Pharmacologic overview of chlorogenic acid and its metabolites in chronic pain and inflammation[J]. Curr Neuropharmacol, 2020, 18: 216-228. DOI:10.2174/1570159X17666191021111809 |
| [8] |
Li Y, Wang Q, Yao X, et al. Induction of CYP3A4 and MDR1 gene expression by baicalin, baicalein, chlorogenic acid, and ginsenoside Rf through constitutive androstane receptor-and pregnane X receptor-mediated pathways[J]. Eur J Pharmacol, 2010, 640: 46-54. DOI:10.1016/j.ejphar.2010.05.017 |
| [9] |
Santana-Gálvez J, Luis CZ, Jacobo-Velázquez DA. Chlorogenic acid:recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome[J]. Molecules, 2017, 22: 358. DOI:10.3390/molecules22030358 |
| [10] |
Martínez G, Regente M, Jacobi S, et al. Chlorogenic acid is a fungicide active against phytopathogenic fungi[J]. Pestic Biochem Physiol, 2017, 140: 30-35. DOI:10.1016/j.pestbp.2017.05.012 |
| [11] |
Lou Z, Wang H, Zhu S, et al. Antibacterial activity and mechanism of action of chlorogenic acid[J]. J Food Sci, 2011, 76: M398-M403. DOI:10.1111/j.1750-3841.2011.02213.x |
| [12] |
Li G, Wang X, Xu Y, et al. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus[J]. Eur Food Res Technol, 2014, 238: 589-596. DOI:10.1007/s00217-013-2140-5 |
| [13] |
Ren S, Wu M, Guo J, et al. Sterilization of polydimethylsiloxane surface with Chinese herb extract:a new antibiotic mechanism of chlorogenic acid[J]. Sci Rep, 2015, 5: 10464. DOI:10.1038/srep10464 |
| [14] |
Lee B, Lee DG. Depletion of reactive oxygen species induced by chlorogenic acid triggers apoptosis-like death in Escherichia coli[J]. Free Radic Res, 2018, 52: 1-11. DOI:10.1080/10715762.2017.1402304 |
| [15] |
Neamati N, Hong H, Sunder S, et al. Potent inhibitors of human immunodeficiency virus type 1 integrase:identification of a novel four-point pharmacophore and tetracyclines as novel inhibitors[J]. Mol Pharmacol, 1997, 52: 1041. DOI:10.1124/mol.52.6.1041 |
| [16] |
Utsunomiya H, Ichinose M, Uozaki M, et al. Antiviral activities of coffee extracts in vitro[J]. Food Chem Toxicol, 2008, 46: 1919-1924. DOI:10.1016/j.fct.2008.01.031 |
| [17] |
Robinson WE, Cordeiro M, Abdel-Malek S, et al. Dicaffeoylquinic acid inhibitors of human immunodeficiency virus integrase:inhibition of the core catalytic domain of human immunodeficiency virus integrase[J]. Mol Pharmacol, 1996, 50: 846-855. |
| [18] |
Tamura H, Akioka T, Ueno K, et al. Anti-human immunodeficiency virus activity of 3, 4, 5-tricaffeoylquinic acid in cultured cells of lettuce leaves[J]. Mol Nutr Food Res, 2006, 50: 396-400. DOI:10.1002/mnfr.200500216 |
| [19] |
Karar MGE, Matei MF, Jaiswal R, et al. Neuraminidase inhibition of dietary chlorogenic acids and derivatives-potential antivirals from dietary sources[J]. Food Funct, 2016, 7: 2052-2059. |
| [20] |
Ding Y, Cao Z, Cao L, et al. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase[J]. Sci Rep, 2017, 7: 45723. DOI:10.1038/srep45723 |
| [21] |
Guo YJ, Luo T, Wu F, et al. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia[J]. Life Sci, 2015, 127: 12-18. DOI:10.1016/j.lfs.2015.01.036 |
| [22] |
Wang GF, Shi LP, Ren YD, et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro[J]. Antiviral Res, 2009, 83: 186-190. DOI:10.1016/j.antiviral.2009.05.002 |
| [23] |
Huang MT, Smart RC, Wong CQ, et al. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate[J]. Cancer Res, 1988, 48: 5941-5946. |
| [24] |
Xue N, Zhou Q, Ji M, et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype[J]. Sci Rep, 2017, 7: 39011. DOI:10.1038/srep39011 |
| [25] |
Huang S, Wang LL, Xue NN, et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation[J]. Theranostics, 2019, 9: 6745-6763. DOI:10.7150/thno.34674 |
| [26] |
Sapio L, Salzillo A, Illiano M, et al. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells[J]. J Cell Physiol, 2020, 235: 3741-3752. DOI:10.1002/jcp.29269 |
| [27] |
Hou N, Liu N, Han J, et al. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells[J]. Anticancer Drugs, 2017, 28: 59-65. DOI:10.1097/CAD.0000000000000430 |
| [28] |
Yamagata K, Izawa Y, Onodera D, et al. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells[J]. Mol Cell Biochem, 2018, 441: 9-19. DOI:10.1007/s11010-017-3171-1 |
| [29] |
Bouayed J, Rammal H, Dicko A, et al. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects[J]. J Neurol Sci, 2007, 262: 77-84. DOI:10.1016/j.jns.2007.06.028 |
| [30] |
Riceevans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids[J]. Free Radic Biol Med, 1996, 20: 933-956. DOI:10.1016/0891-5849(95)02227-9 |
| [31] |
Sato Y, Itagaki S, Kurokawa T, et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caeic acid[J]. Int J Pharm, 2011, 403: 136-138. DOI:10.1016/j.ijpharm.2010.09.035 |
| [32] |
Kono Y, Kobayashi K, Tagawa S, et al. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen[J]. Biochim Biophys Acta, 1997, 1335: 335. DOI:10.1016/S0304-4165(96)00151-1 |
| [33] |
Zhang LY, Cosma G, Gardner H, et al. Effect of chlorogenic acid on hydroxyl radical[J]. Mol Cell Biochem, 2003, 247: 205-210. DOI:10.1023/A:1024103428348 |
| [34] |
Lu Y, Foo LY. Antioxidant and radical scavenging activities of polyphenols from apple pomace[J]. Food Chem, 2000, 68: 81-85. DOI:10.1016/S0308-8146(99)00167-3 |
| [35] |
Dos Santos MD, Almeida MC, Lopes NP, et al. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid[J]. Biol Pharm Bull, 2006, 29: 2236-2240. DOI:10.1248/bpb.29.2236 |
| [36] |
Shin HS, Satsu H, Bae MJ, et al. Catechol groups enable reactive oxygen species scavenging-mediated suppression of PKD-NFkappaB-IL-8 signaling pathway by chlorogenic and caffeic acids in human intestinal cells[J]. Nutrients, 2017, 9: 165. DOI:10.3390/nu9020165 |
| [37] |
Liang N, Kitts DD. Chlorogenic acid (CGA) isomers alleviate interleukin 8(IL-8) production in Caco-2 cells by decreasing phosphorylation of p38 and increasing cell integrity[J]. Int J Mol Sci, 2018, 19: 3873. DOI:10.3390/ijms19123873 |
| [38] |
Gao W, Wang C, Yu L, et al. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway[J]. BioMed Res Internat, 2019, 2019: 6769789. |
| [39] |
Fu X, Lyu X, Liu H, et al. Chlorogenic acid inhibits BAFF expression in collagen-induced arthritis and human synoviocyte MH7A cells by modulating the activation of the NF-κB signaling pathway[J]. J Immunol Res, 2019, 2019: 8042097. |
| [40] |
Shi A, Shi H, Wang Y, et al. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury[J]. Int Immunopharmacol, 2018, 54: 125-130. DOI:10.1016/j.intimp.2017.11.007 |
| [41] |
Ali N, Rashid S, Nafees S, et al. Protective effect of chlorogenic acid against methotrexate induced oxidative stress inflammation and apoptosis in rat liver:an experimental approach[J]. Chem Biol Interact, 2017, 272: 80-91. DOI:10.1016/j.cbi.2017.05.002 |
| [42] |
Wang Y, Yang AF, Xue BJ, et al. Antischistosomiasis liver fibrosis effects of chlorogenic acid through IL-13/miR-21/Smad7 signaling interactions in vivo and in vitro[J]. Antimicrob Agents Chemother, 2016, 61: e01347-16. |
| [43] |
Nabavi SM, Manayi A, Daglia M, et al. Chlorogenic acid and mental diseases:from chemistry to medicine[J]. Curr Neuropharmacol, 2017, 15: 471-479. DOI:10.2174/1570159X14666160325120625 |
| [44] |
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases[J]. Nature, 2006, 443: 787-795. DOI:10.1038/nature05292 |
| [45] |
Ascherio A, Chen H, Schwarzschild MA, et al. Caffeine, postmenopausal estrogen, and risk of Parkinsons disease[J]. Neuro-logy, 2003, 60: 790-795. |
| [46] |
Arendash GW, Schleif W, Rezai-Zadeh K, et al. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production[J]. Neuroscience, 2006, 142: 941-952. DOI:10.1016/j.neuroscience.2006.07.021 |
| [47] |
Hermawati E, Arfian N, Mustofa, et al. Chlorogenic acid ameliorates memory loss and hippocampal cell death after transient global ischemia[J]. Eur J Neurosci, 2020, 51: 651-669. DOI:10.1111/ejn.14556 |
| [48] |
Juan Y, Peng S, Xu J, et al. Reversing ROS-mediated neurotoxi-city by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway[J]. Biofactors, 2019, 45: 616-626. |
| [49] |
Tsai KL, Hung CH, Chan SH, et al. Chlorogenic acid protects against oxLDL-induced oxidative damage and mitochondrial dysfunction by modulating SIRT1 in endothelial cells[J]. Mol Nutr Food Res, 2018, 62: e1700928. DOI:10.1002/mnfr.201700928 |
| [50] |
Yang L, Wei J, Sheng F, et al. Attenuation of palmitic acid-induced lipotoxicity by chlorogenic acid through activation of SIRT1 in hepatocytes[J]. Mol Nutr Food Res, 2019, 63: 1801432. DOI:10.1002/mnfr.201801432 |
| [51] |
Gregory K, Panagiota P, Eva K, et al. Metabolic syndrome:definitions and controversies[J]. BMC Med, 2011, 9: 48. DOI:10.1186/1741-7015-9-48 |
| [52] |
Patti AM, Al-Rasadi K, Katsiki N, et al. Effect of a natural supplement containing Curcuma longa, Guggul, and chlorogenic acid in patients with metabolic syndrome[J]. Angiology, 2015, 66: 856-861. DOI:10.1177/0003319714568792 |
| [53] |
Wang Z, Lam KL, Hu J, et al. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice[J]. Food Sci Nutr, 2019, 7: 579-588. DOI:10.1002/fsn3.868 |
| [54] |
Han X, Zhang Y, Guo J, et al. Chlorogenic acid stimulates the thermogenesis of brown adipocytes by promoting the uptake of glucose and the function of mitochondria[J]. J Food Sci, 2019, 84: 3815-3824. DOI:10.1111/1750-3841.14838 |
| [55] |
Xu M, Yang L, Zhu Y, et al. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice[J]. Food Funct, 2019, 10: 7489-7497. DOI:10.1039/C9FO00502A |
| [56] |
Kumar R, Sharma A, Iqbal MS, et al. Therapeutic promises of chlorogenic acid with special emphasis on its anti-obesity property[J]. Curr Mol Pharmacol, 2020, 13: 7-16. DOI:10.2174/1874467212666190716145210 |
| [57] |
Cho AS, Jeon SM, Kim MJ, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice[J]. Food Chem Toxicol, 2010, 48: 937-943. DOI:10.1016/j.fct.2010.01.003 |
| [58] |
Huang K, Liang XC, Zhong YL, et al. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription[J]. J Sci Food Agric, 2015, 95: 1903-1910. DOI:10.1002/jsfa.6896 |
| [59] |
Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people[J]. J Int Med Res, 2007, 35: 900-908. DOI:10.1177/147323000703500620 |
| [60] |
Soga S, Ota N, Shimotoyodome A. Stimulation of postprandial fat utilization in healthy humans by daily consumption of chlorogenic acids[J]. Biosci Biotechnol Biochem, 2013, 77: 1633-1636. DOI:10.1271/bbb.130147 |
| [61] |
Wan CW, Wong CN, Pin WK, et al. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet[J]. Phytother Res, 2013, 27: 545-551. DOI:10.1002/ptr.4751 |
| [62] |
Ong KW, Hsu A, Tan BKH. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation[J]. Biochemn Pharmacol, 2013, 85: 1341-1351. DOI:10.1016/j.bcp.2013.02.008 |
| [63] |
Attila H, Ana M, Tusty-Jiuan H, et al. Chlorogenic acid and rutin play a major role in the in vivo antidiabetic activity of Morus alba leaf extract on type Ⅱ diabetic rats[J]. PLoS One, 2012, 7: e50619. DOI:10.1371/journal.pone.0050619 |
| [64] |
Jin S, Chang C, Zhang L, et al. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice[J]. PLoS One, 2015, 10: e0120842. DOI:10.1371/journal.pone.0120842 |
| [65] |
Kim CS, Kim J, Lee YM, et al. Inhibitory effects of chlorogenic acid on aldose reductase activity in vitro and cataractogenesis in galactose-fed rats[J]. Arch Pharm Res, 2011, 34: 847-852. DOI:10.1007/s12272-011-0519-z |
| [66] |
Bagdas D, Etoz BC, Gul Z, et al. In vivo systemic chlorogenic acid therapy under diabetic conditions:wound healing effects and cytotoxicity/genotoxicity profile[J]. Food Chem Toxicol, 2015, 81: 54-61. DOI:10.1016/j.fct.2015.04.001 |
| [67] |
Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans:glycemic effects of chlorogenic acid and caffeine[J]. Am J Clin Nutr, 2003, 78: 728-733. DOI:10.1093/ajcn/78.4.728 |
| [68] |
Iwai K, Narita Y, Fukunaga T, et al. Study on the postprandial glucose responses to a chlorogenic acid-rich extract of decaffeinated green coffee beans in rats and healthy human subjects[J]. Food Sci Technol Res, 2012, 18: 849-860. DOI:10.3136/fstr.18.849 |
| [69] |
Ahrens MJ, Thompson DL. Effect of emulin on blood glucose in type 2 diabetics[J]. J Med Food, 2013, 16: 1-6. DOI:10.1089/jmf.2013.1601.ed |
| [70] |
Gao J, He X, Ma Y, et al. Chlorogenic acid targeting of the AKT PH domain activates AKT/GSK3β/FOXO1 signaling and improves glucose metabolism[J]. Nutrients, 2018, 10: 1366. DOI:10.3390/nu10101366 |
| [71] |
Ma Y, Gao M, Liu D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice[J]. Pharm Res, 2015, 32: 1200-1209. DOI:10.1007/s11095-014-1526-9 |
| [72] |
Sancheza MB, Miranda-Pereza E, Gomez JC, et al. Potential of the chlorogenic acid as multitarget agent:insulin-secretagogue and PPAR α/γ dual agonist[J]. Biomed Pharmacother, 2017, 94: 169. DOI:10.1016/j.biopha.2017.07.086 |
| [73] |
Suzuki A, Yamamoto N, Jokura H, et al. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats[J]. J Hypertens, 2006, 24: 1065-1073. DOI:10.1097/01.hjh.0000226196.67052.c0 |
| [74] |
Stefanelloa N, Spanevellob RM, Passamontic S, et al. Coffee, caffeine, chlorogenic acid, and the purinergic system[J]. Food Chem Toxicol, 2019, 123: 298-313. DOI:10.1016/j.fct.2018.10.005 |
| [75] |
Stefanello N, Schmatz R, Pereira LB, et al. Effects of chlorogenic acid, caffeine and coffee on components of the purinergic system of streptozotocin-induced diabetic rats[J]. J Nutr Biochem, 2016, 38: 145-153. DOI:10.1016/j.jnutbio.2016.08.015 |
| [76] |
Fuentes E, Caballero J, Alarcón M, et al. Chlorogenic acid inhibits human platelet activation and thrombus formation[J]. PLoS One, 2014, 9: e90699. DOI:10.1371/journal.pone.0090699 |
| [77] |
Kozuma K, Tsuchiya S, Kohori J, et al. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects[J]. Hypertens Res, 2005, 28: 711-718. DOI:10.1291/hypres.28.711 |
| [78] |
Watanabe T, Arai Y, Mitsui Y, et al. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension[J]. Clin Exp Hypertens, 2006, 28: 439-449. DOI:10.1080/10641960600798655 |
| [79] |
Mubarak A, Bondonno CP, Liu AH, et al. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers:a randomized trial[J]. J Agric Food Chem, 2012, 60: 9130-9136. DOI:10.1021/jf303440j |
 2020, Vol. 55
2020, Vol. 55


