2. 解放军总医院第五医学中心全军中医药研究所, 北京 100039;
3. 湖南中医药大学附属常德医院, 湖南 常德 415000
2. China Military Institute of Chinese Medicine, Fifth Medical Center of Chinese PLA General Hospital, Beijing 100039, China;
3. Changde Hospital Affiliated to Hunan University of Chinese Medicine, Changde 415000, China
顺铂(cisplatin, DDP)是一种铂(Pt)的配位络合物, 自首次批准用于膀胱癌与睾丸癌治疗, 在临床上已使用40余年, 其对癌症治疗医学产生了巨大的影响, 改变了如卵巢癌和睾丸癌等恶性肿瘤的治疗方法[1, 2]。顺铂在结构上类似于双功能烷化剂, 由二价铂、两个氨基配体与两个氯离子构成, 顺铂通过水解1个氯离子而在细胞内激活并与DNA共价结合形成Pt-DNA加合物[3, 4]。DNA加合物形成形式主要为两个相邻嘌呤碱基之间形成的链内交联, 少部分为链间交联。Pt-DNA加合物形成之后引起细胞周期阻滞, 阻止DNA复制和转录最终导致肿瘤细胞凋亡[5]。然而, 一旦顺铂引起DNA损伤便会触发相应修复机制, 从而提高癌细胞存活率, 导致化疗失败, 引起顺铂耐药。
顺铂耐药性产生的一个重要原因是铂类化合物未达到有效所需的细胞内水平或在与DNA反应之前便已失活[6, 7], 如肿瘤细胞内药物摄取减少、内皮细胞药物外流增加等都可能降低到达肿瘤药物的疗效水平, 顺铂共价结合谷胱甘肽和含硫醇的蛋白质致药物失活; 亦可增加DNA损伤修复机制或抑制细胞凋亡以提高肿瘤细胞存活率, 从而产生顺铂耐药性; 肿瘤细胞外的微环境和肿瘤干细胞也对耐药性的产生有所贡献。其耐药性的分子机制可见图 1。耐药性的产生极大地限制了顺铂的治疗效果, 为了逆转顺铂耐药性, 国内外学者在多方面进行了尝试。如研发卡铂和奥沙利铂等新型铂类抗癌药物以规避顺铂耐药性, 识别与耐药性有关基因和蛋白质并利用RNA干扰、小分子抑制剂或特定抗体使其沉寂[8], 靶向肿瘤微环境等均对顺铂耐药性的逆转有一定成效。有研究发现, 顺铂与中药联用可在一定程度上恢复耐药肿瘤细胞对顺铂的敏感性, 达到逆转耐药性的效果。本文就顺铂耐药性机制与中药逆转顺铂抗性的研究进行综述。
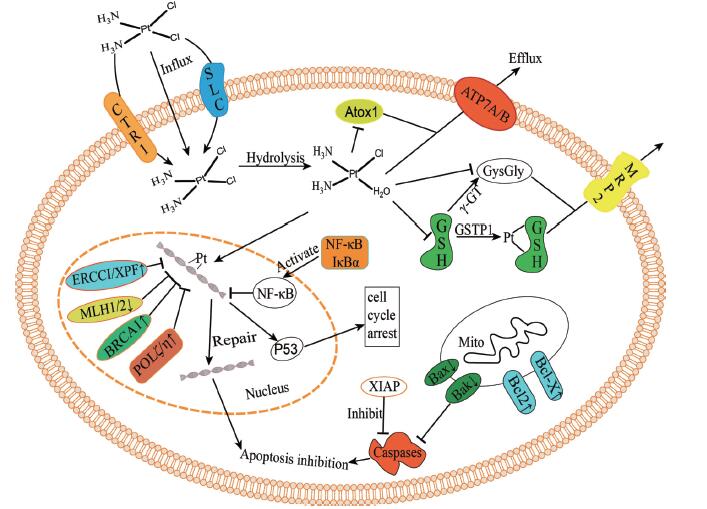
|
Figure 1 Molecular mechanisms of cisplatin resistance. CTR1: Copper transporter 1; SLC: Solute carriers; Atox1: Antioxidant protein 1; ATP7A/B: Copper-transporting P-type adenosine triphosphatase 1 and 2; MRP2: Multidrug resistance protein 2; GSH: Glutathione; GSTP1: Glutathione S-transferase pi-1; γ -GT: γ -Glutamyltransferase; GysGly: Cysteinylglycine; ERCC1: Excision repair cross-complementing-1; XPF: Xeroderma pigmentosum group F; MLH1/2: MutL homolog 1 and 2; BRCA1: Breast cancer susceptibility proteins 1; POLζ/η: DNA polymerase ζ and η; NF- κB: Nuclearfactor- κB; IκBα: Inhibitor of NF- κB α; XIAP: X-linked inhibitor of apoptosis protein; Bcl-2: B-cell lymphoma-2; Bcl-X: B-cell lymphoma-X; Bax: Bcl-2-associated protein X; Bak: Bcl-2 homologous antagonist/killer |
有充分证据表明, 肿瘤细胞内药物积累降低是顺铂耐药的一个重要机制。这种积累降低可能是源于药物摄取量减少或药物外排增加, 也可能两者兼而有之。
1.1.1 药物摄取量减少与其他类型的小分子抗癌药物相比, 顺铂具有较高极性, 进入细胞的速度相对较慢, 过去一直认为在质膜上被动扩散是其进入细胞唯一途径[9]。近年来, 研究表明顺铂摄取主要由高亲和力铜转运蛋白1 (copper transporter 1, CTR1)调控, 其下调与铂类药物的耐药性有关[10]。对小鼠胚胎成纤维细胞和酵母菌的CTR1基因进行敲除, 均表现为顺铂胞内积累量大大减少, 形成细胞顺铂耐药[11, 12]。CTR1转运体蛋白通过调节细胞内铜稳态而发挥作用[13], 临床试验表明, 使用铜螯合剂可以使耐铂类药物上皮性卵巢癌患者重新接受铂类化疗, 使得肿瘤细胞恢复顺铂敏感度[14]。此外, 一种顺铂结合蛋白即铜伴侣蛋白(antioxidant protein 1, Atox1)也被证实与顺铂耐药相关, 顺铂与细胞质中Atox1结合, 顺铂转运到DNA的过程被阻断从而形成耐药, 在耐药细胞中也发现Atox1的表达水平上升[15]。过度表达Atox1会导致顺铂失活, 也会促使更多的顺铂被铜转运P型腺苷三磷酸酶2 (copper-transporting P-type adenosine triphosphatase 2, ATP7B)泵出细胞[16]。然而Atox1的减少又会减弱CTR1对顺铂的摄取和随后在囊泡室和DNA中的积累[17]。值得注意的是, 顺铂亦可通过位于细胞外膜的溶质载体(solute carriers, SLC)进入细胞, 人体内的SLC22亚家族由25种转运蛋白组成, 包括有机阳离子、阴离子和两性离子转运体[18]。在临床前研究中, 有机阳离子溶质载体SLC22A2可促进顺铂的转运并调控其细胞内摄取和细胞毒性, 在铂类转运中起关键作用[19]。
1.1.2 药物外排增加另一种耐药性表达机制是顺铂在到达其细胞内靶点之前已被主动泵出, 主要是由ATP驱动的跨膜转运蛋白过度表达所导致。虽然顺铂通常不是多药耐药表型的一部分, 但部分多药耐药相关转运体仍与顺铂耐药有关。例如, 多药耐药相关蛋白2 (multidrug resistance protein 2, MRP2)参与顺铂的转运[20], 特别是, MRP2的表达在临床上决定了顺铂在肝细胞癌患者中的化疗疗效[21]。此外, 铜转运P型腺苷三磷酸酶1 (copper-transporting P-type adenosine triphosphatase 1, ATP7A)和ATP7B已被证明在调节顺铂的外排中起主要作用[22], 对顺铂有较为显著的耐药性, 且其过度表达与顺铂治疗卵巢癌的耐药性相关[23]。在顺铂耐药肿瘤细胞中, 沉默ATP7A在一定程度上恢复了顺铂敏感性, 增强了细胞凋亡[24]。同时, 转染ATP7A的人卵巢癌细胞显示, ATP7A表达仅小幅度增加(约1.5倍)便导致顺铂用药出现耐药性, ATP7A的增加导致肿瘤细胞内负责转运的囊泡数量增多也增加了顺铂在囊泡中的含量[25]。
1.2 硫醇分子灭活顺铂活性金属与内源性硫醇分子如谷胱甘肽(glutathione, GSH)和金属硫蛋白(metallothionein, MT)的相互作用是细胞解除金属毒性的主要机制之一, 且硫醇分子对顺铂有高度反应性, 顺铂分子在到达肿瘤细胞核DNA之前会有部分与该类硫醇分子在细胞质中相互结合[26]。该类硫醇分子的过度表达, 增加了顺铂在细胞质内的蛋白质结合而降低了顺铂到达细胞核的水平, 也因此被认为在肿瘤细胞顺铂耐药性方面起决定性作用。
1.2.1 谷胱甘肽GSH是一种含有三肽的硫醇分子, 与顺铂在细胞内的解毒和清除有关。硫醇分子对顺铂的高反应性使得顺铂能与GSH直接共价结合, 同时这种结合反应也可通过谷胱甘肽巯基转移酶1 (glutathione S-transferase pi-1, GSTP1)催化形成Pt-GSH结合物[27]。使得化合物更容易被MRP2泵出细胞, 由此可知, 参与GSH结合的酶表达增加也与顺铂耐药性有关。对耐药性发生前后同一患者衍生的两种卵巢癌细胞系进行研究, 耐药肿瘤细胞中谷胱甘肽巯基转移酶(glutathione S-transferase, GST)含量高出不耐药肿瘤细胞2.9倍[28]。在顺铂耐药肿瘤细胞中还观察到γ-谷氨酰转移酶(γ-glutamyltransferase, γ-GT)的过度表达[29]。γ-GT用于维持GSH稳态, 参与GSH的合成与代谢, 可在GSH分解代谢过程中生成半胱氨酰甘氨酸(cysteinylglycine, GysGly), 其对顺铂的反应性比GSH高10倍, 因此γ-GT的过度表达可能主导了GSH引起的顺铂耐药性。另有研究表明, 谷氨酸/半胱氨酸转运系统水平的升高也与细胞内GSH高含量及顺铂耐药性有关[30]。细胞内半胱氨酸的摄取在很大程度上是由该系统调控, Okuno等[31]对比人类卵巢癌细胞系(A2780)与它的顺铂耐药变异系(A2780DDP), 半胱氨酸在耐药细胞中表达水平约为敏感细胞中的4.2倍, A2780DDP细胞内GSH含量增加是由于半胱氨酸的含量增加而导致的, 半胱氨酸是GSH合成的限速底物。
1.2.2 金属硫蛋白MT是一种低分子量富含半胱氨酸的蛋白质, 负责调节细胞内金属稳态以及细胞内重金属解毒, 其与顺铂的相互作用, 在很大程度上与GSH反应相同[32]。MT也已被证明导致顺铂耐药性, 在小鼠细胞或人卵巢癌细胞中过度表达致使顺铂耐药性分别增加4和7倍[33]。另一方面, 使用顺铂治疗也会相应导致MT的表达增加[34]。在生殖细胞肿瘤中, MT在细胞系和肿瘤中的表达较高, 但在对顺铂治疗耐药患者与不耐药患者之间并不存在差异[35]。因此, 推断MT并不是导致顺铂耐药性的主要因素, 而仅仅与部分肿瘤顺铂耐药性有关联。
1.3 DNA损伤修复增加Pt-DNA加合物的形成和稳定是诱导细胞凋亡的重要因素, 因此, 加合物DNA损伤修复增加将减弱细胞凋亡过程, 引发顺铂耐药性[2]。在睾丸癌治疗中发现其对顺铂治疗高度敏感, 已被证实是DNA修复缺陷所致[36], 多种顺铂耐药细胞株的DNA修复能力与敏感细胞株对比, 耐药细胞株具有更强的修复能力。核苷酸切除修复(nucleotide excision repair, NER)、错配修复(mismatch repair, MMR)、同源重组修复(homologous recombination repair, HR)和跨损伤合成(translesion synthesis, TLS)均被证实与顺铂耐药的DNA损伤修复有关[37]。
1.3.1 核苷酸切除修复NER是去除Pt-DNA加合物和DNA损伤修复的主要途径, 细胞NER缺失导致对顺铂治疗的高度敏感性, 而细胞恢复NER完整性其对顺铂的敏感度即恢复正常水平[38]。NER过程由17种以上不同的蛋白质构成, 在细胞系和临床活检标本中, 归属NER内切酶蛋白的切除修复交叉互补蛋白1 (excision repair cross-complementing-1, ERCC1)是检测的重要指标[39]。ERCC1通过与着色性干皮病基因组F (xeroderma pigmentosum group F, XPF)形成异二聚体, 从DNA 5′端切除损伤的DNA以修复DNA[40]。在顺铂耐药卵巢癌细胞内发现NER增加与ERCC1和XPF表达增加有关, 敲除ERCC1可增强细胞对顺铂的敏感性, 同时顺铂所导致的DNA损伤NER修复能力降低[41]。对卵巢癌患者的临床研究也证明ERCC1 mRNA水平升高与铂类化疗的临床耐药性呈正相关性。
1.3.2 错配修复MMR的主要作用是纠正DNA复制错误, 抑制重组, 与其他DNA修复途径相互作用, 细胞周期检查点调控和调控细胞凋亡[42]。MMR也负责调控顺铂对肿瘤细胞的细胞毒性, 这种类型的DNA修复功能障碍可能导致肿瘤细胞凋亡减少因而形成顺铂耐药[43]。在MMR修复过程中, MMR试图通过修复DNA错配病变来维持基因组的完整性, 但它并不能修复Pt-DNA加合, 反而随着几次修复的失败最终导致细胞凋亡[44]。MMR通路由系统蛋白MLH1 (mutL homolog 1)、MSH2 (mutS homolog 2)和MSH6 (mutS homolog 6)等多种蛋白组成, 在顺铂耐药细胞中, 可观察到MMR蛋白MLH1或MSH2的下调或突变, 实际上, 细胞内顺铂耐药往往伴随着MMR蛋白的丢失[45]。
1.3.3 同源重组修复HR主要负责细胞周期S期DNA双链的断裂修复, 而顺铂诱导的DNA加合物可产生双链断裂, 顺铂引起的双链断裂通常在DNA合成过程中通过HR修复[46]。其中, HR通路中的乳腺癌易感蛋白1和2 (breast cancer susceptibility proteins 1 and 2, BRCA1/2)在乳腺癌和卵巢癌中经常发生突变[47]。正常情况下, BRCA1/2缺失的癌细胞通过同源重组进行DNA修复, 对顺铂等链间DNA交联剂敏感[48]。然而, 在肿瘤化疗过程中, BRCA1或BRCA2突变之后进行逆转性突变恢复了HR功能, 从而导致顺铂耐药性[49]。研究也发现, BRCA1在对顺铂耐药的人乳腺癌和卵巢癌细胞株中过表达可导致顺铂的耐药性增强, 相反的, 抑制BRCA1的表达增强了顺铂的敏感性[50]。此外, 还发现BRCA1 mRNA的表达水平与非小细胞肺癌中顺铂的敏感性呈负相关[51], mRNA的低表达也会导致接受顺铂治疗的乳腺癌、卵巢癌和食管鳞状细胞癌存活时间延长[52]。总之, BRCA1蛋白突变与mRNA水平的降低表现出对基于DNA损伤的化疗方案有益。
1.3.4 跨损伤合成TLS能为存在复制阻塞加合物的细胞提供继续复制的途径, 有证据表明, 该类细胞修复机制会影响顺铂的抗肿瘤效果[53]。TLS通常被认为是抗DNA损伤的途径, 因为其允许细胞以增加突变和重组频率为代价完成原有的复制和有丝分裂[54]。使用酿酒酵母的菌株进行研究发现, 缺失TLS的菌株表现出对顺铂高度敏感, 这也意味着TLS可能参与Pt-DNA加合物的清除。此外, 在NER通路激活而TLS通路受损的情况下, 细胞仍表现对顺铂治疗的高敏感度, 这一发现验证了TLS可能是肿瘤细胞顺铂产生耐药性的机制[55]。TLS通路由一组DNA聚合酶(DNA polymerase, POL)调控, 该组聚合酶包含POL ζ、POL η、POL μ、POL κ和POL τ[56]。在一些耐药细胞中观察到, POL ζ表达降低使细胞对顺铂的细胞毒性作用更加敏感, 显著降低了细胞对顺铂的耐药性, 同时也显著降低了其致突变性, POL ζ 的表达也被认为是对顺铂耐药的预测因子[57]。缺乏POL η的人细胞也表现出相应的对顺铂治疗的高度敏感性[58]。
1.4 细胞凋亡失调顺铂诱导的细胞毒性作用主要是由多分支凋亡信号通路的激活进行调控, 以响应不可修复的DNA损伤[59]。顺铂导致的细胞凋亡依赖于凋亡信号通路的正常表达, 编码这些通路关键介质的遗传或表观遗传改变一直被认为与耐药性有关[60]。当这些改变产生可能导致细胞上游生存信号增强、下游凋亡信号减少或细胞对凋亡信号的敏感性降低, 由此导致耐药性的产生。在该类凋亡信号传导通路中涉及多种蛋白质反应, 目前研究较多的包含p53蛋白、核转录因子κB (nuclearfactor-κB, NF-κB)与其他的抗凋亡蛋白[61]。
1.4.1 抑癌蛋白p53的失活p53蛋白是调节DNA损伤后细胞存活的主要途径之一, 在细胞周期阻滞和凋亡反应中起着重要作用, 其失活或突变与顺铂耐药相关[62]。研究对比含有野生型p53的细胞与缺乏p53功能的细胞, 含有野生型p53的细胞表现出对顺铂治疗的高度敏感[63], 野生型p53发现顺铂引起的DNA损伤后即过度表达, DNA损伤细胞被p53滞留在G1/S期, 随后被诱导凋亡, 以此来保证细胞对顺铂的敏感性。而p53的失活则避免了该过程, 使本该凋亡的细胞得以继续存活, 细胞因此获得对顺铂治疗的耐药性。
1.4.2 转录因子NF-κB的激活另一个调节DNA损伤后细胞存活的关键蛋白是转录因子NF-κB。一些研究表明, 顺铂的抗癌活性受到NF- κB激活的影响。NF-κB通常以灭活形式存在于与NF-κB抑制蛋白α (inhibitor of NF-κB α, IκBα)结合的细胞质中, 而当上游激酶磷酸化IκBα使其降解后, NF-κB处于激活态并开始向细胞核内转移, 与核内特定DNA序列结合, 激活参与适应性抗氧化和抗凋亡反应基因的转录, 最终诱导肿瘤细胞产生相应耐药性[64, 65]。Mabuchi等[66]研究发现顺铂耐药Caov-3细胞IκBα磷酸化水平和NF-κB活性显著高于对顺铂敏感的A2780细胞。同样, 使用NF-κB活性抑制剂也表现出肿瘤细胞对凋亡信号通路敏感, 例如: IκBα磷酸化抑制剂BAY 11-7085增强了对IκBα磷酸化和NF-κB活性的抑制作用, 提高顺铂在体内与体外卵巢癌模型中的敏感度[67]。
1.4.3 调控细胞凋亡蛋白调控细胞凋亡的蛋白质表达的变化也可影响细胞对顺铂的敏感性, B淋巴瘤-2 (B-cell lymphoma-2, Bcl-2)蛋白家族促凋亡和抗凋亡蛋白、半胱天冬氨酸蛋白酶(caspases)、X-连锁细胞凋亡抑制剂(X-linked inhibitor of apoptosis protein, XIAP)、钙蛋白和线粒体膜间蛋白等表达的变化均被证实与顺铂耐药性有关[68, 69]。例如, Bcl-2家族抗凋亡成员Bcl-2和Bcl-XL的高内源性表达或促凋亡蛋白Bax (Bcl-2- associated protein X)和Bak (Bcl-2 homologous antagonist/ killer)的丢失均增强了顺铂耐药性[70, 71]。而XIAP可能是耐药性的主导者, XIAP能够抑制caspases凋亡蛋白的表达与活化, 抑制细胞凋亡, 研究也证实了敲除或抑制XIAP蛋白可逆转顺铂耐药性。
1.5 肿瘤微环境近年来, 肿瘤微环境在肿瘤细胞耐药性方面的作用受到了越来越多的关注。事实上, 细胞与基质或细胞与细胞之间的相互作用可以影响癌细胞对凋亡的敏感性, 进而导致耐药性[72]。例如, 肿瘤细胞与基质之间的相互作用会导致产生良好的细胞外基质(extracellular matrix, ECM)相互作用, 保护肿瘤细胞免受顺铂诱导的细胞凋亡影响[73], 而凋亡受阻的肿瘤细胞产生耐药性之后又会对ECM进行重组, 进一步降低细胞凋亡。此外, 肿瘤细胞分泌的胶原蛋白VI也被证实可诱导顺铂耐药性的产生, 卵巢癌中过表达胶原蛋白VI的细胞和生长在胶原蛋白VI上的细胞均表现出较高的顺铂耐药性[74]。E-钙黏蛋白(E-cadherin)调控的细胞-细胞之间的黏附接触也会导致顺铂耐药, 通过增强细胞周期蛋白依赖性激酶抑制剂p27 KIP1的活性或表达来引起细胞G1周期阻滞并相应地增强细胞凋亡抗性[75]。
1.6 肿瘤干细胞作为肿瘤细胞祖细胞的肿瘤干细胞(cancer stem cell, CSC)也表现出对顺铂化疗具有内在抗性, 而且其耐药性显著大于已分化的肿瘤细胞[76]。CSC随机分布在肿瘤内, 但主要存在于低氧、低pH值和低营养的环境中[77], 且具有自我更新和分化的能力, 在肿瘤的起始、复发和转移扩散中起重要作用[78]。CSC在肿瘤细胞中的比例约为0.1%~1%, 然而随着化疗耐药性的产生, 这一比例可增至30%[79]。药物积累减少、DNA损伤修复、细胞凋亡抑制和上游存活途径激活等促使已分化肿瘤细胞产生耐药性的机制同样适用于CSC[80], 值得注意的是, 随着化疗进行使得对化疗敏感的肿瘤细胞凋亡而对化疗耐药的CSC实现了富集[81], 由此产生了对化疗的耐药性。Levina等[82]发现顺铂化疗可导致肺癌干细胞的增殖并抑制其分化, Bertolini等[83]研究也表明具有CSC特征的CD133+非小细胞肺癌细胞可在顺铂化疗结束后存活, 在顺铂耐药的膀胱癌和卵巢癌细胞中也发现了类似的具有自我更新的CSC[84, 85]。此外, 敲除BRCA1/p53基因的小鼠一开始表现出对顺铂化疗的敏感性, 而随着CSC的不断增殖, 顺铂耐药性也随即出现[86]。另一方面, CSC可通过黏附分子和旁分泌因子与肿瘤微环境相互作用, 肿瘤微环境为CSC的自我更新和分化提供了合适的环境并促使CSC异质性和适应性, 诱导CSC耐药性的产生[87]。癌症相关成纤维细胞(carcinoma-associated fibroblasts, CAFs)产生ECM蛋白占领CSC的细胞外微环境, 促进整体环境缺氧也是CSC耐药性的产生因素[88]。
2 中药逆转顺铂耐药性策略中药在我国使用已经历经几千年, 然而未有耐药性相关报道产生。中药治疗针对多通路、多部位与多靶点, 其安全性高、毒副作用小等优点, 不易产生耐药性。中药与顺铂联用, 不仅起协同抗肿瘤作用, 而且逆转顺铂耐药性。目前以中药复方和中药活性成分的形式表现出逆转顺铂耐药性的能力均有报道。本团队欲引入超分子化学理论, 解决中药逆转顺铂耐药性问题。
2.1 中药活性成分随着提取与分离技术的逐渐成熟, 中药活性成分逐渐成为有效的抗肿瘤制剂和化疗耐药性逆转药物。灵芝孢子粉破壁提取的有效成分与顺铂联合作用于A2780DDP细胞, 呈现明显的生长抑制[89], 细胞内Bcl-2表达水平明显降低而p53表达水平升高, 对顺铂耐药性有明显的逆转作用[90]。同样, 灵芝多糖联合顺铂作用于卵巢癌耐药细胞SKOV 3/DDP, GST蛋白的表达水平下调, 改善了顺铂耐药性[91]。Chen等[92]发现香菇多糖可降低MRP的表达, 起到逆转顺铂耐药性的作用。黄芪多糖有较好的抗肿瘤、降低化疗毒副作用和提高免疫力等作用, 还可通过调节细胞自噬来增加HeLa细胞对顺铂的敏感性, 对A549/DDP耐药细胞顺铂化疗也具有增敏作用[93]。
姜黄素已被证实能使卵巢和乳腺肿瘤细胞对顺铂恢复敏感。姜黄素联合顺铂作用于A549/DDP细胞, 呈现细胞存活率与细胞增殖率降低, 且表现出对A549/DDP细胞移植瘤的生长抑制[94]。另外, 顺铂与姜黄素联用作用于卵巢癌耐药细胞株COC1/DDP, 细胞Bcl-2基因的表达降低, caspase-3基因表达增加, 整体呈现对顺铂耐药性的逆转作用[95]。中药芦荟提取物大黄素具有协同抗肿瘤作用, 也是一种治疗顺铂耐药性的逆转剂。对比大黄素联用顺铂作用于卵巢癌SKOV3与耐药细胞株SKOV3/DDP, SKOV3/DDP细胞的GSTP1 mRNA表达较SKOV3高, 而大黄素通过下调这些蛋白的表达实现了顺铂耐药性的逆转[96]。此外, 大黄素增加A549/DDP细胞内顺铂浓度, 也在一定程度显示出对顺铂耐药性的逆转[97]。Yan等[98]发现苦参碱可以抑制神经母细胞瘤SH-SY5Y细胞的增殖并诱导凋亡, 降低顺铂化疗耐药性。丹参提取物丹参酮ⅡA在与顺铂联用中, 也表现出对细胞内顺铂浓度的增加, 并调控caspase及Bcl-2家族成员促进细胞的凋亡, 实现对顺铂的增敏效果[99]。除姜黄素、大黄素和苦参碱外, 葫芦素B、冬凌草甲素、甲基莲心碱、雷公藤红素、银杏酚和人参皂苷等均在不同程度上表现出对顺铂耐药性的逆转作用。
2.2 中药复方中药复方是在中医药基本理论指导下、由多种单味药按君臣佐使配伍组成, 多成分作用于人体的多通路、多靶点, 在化疗中可提高化疗疗效并减轻毒副作用。有研究报道, 使用中药复方可逆转顺铂耐药性。Pan等[100]将多味具有抗肿瘤作用的单味药配伍成中药复方消积煎剂用于治疗上皮性卵巢癌患者, 结果表明, 此方增强了顺铂在耐药细胞系CAOV3/DDP中的敏感度, CAOV3/DDP出现了典型的凋亡现象, 且这种现象与该中药消积煎剂用量呈现正相关性。Chen等[101]则使用参芪扶正注射液逆转肺癌耐药细胞系A549/DDP顺铂耐药性, 结果显示, 顺铂杀伤A549/DDP细胞的能力与添加的参芪扶正注射液的浓度成正比, 参芪扶正注射液也能抑制MDR与肺耐药蛋白mRNA和蛋白的表达。此外, 该方对A549/DDP在裸鼠体内的成瘤能力也呈现出显著的抑制作用, 还可下调Bcl-2的表达促使细胞凋亡来逆转K562/ADM耐药性[102]。Gao等[103]同样针对A549/DDP细胞系研究, 使用十全大补汤含药血清与顺铂联用促进肿瘤细胞凋亡, 增强了A549/ DDP对顺铂的敏感性。另外, 理冲生髓饮有效组分下调MRP及其mRNA表达水平, 提高肿瘤细胞中顺铂浓度, 逆转了顺铂耐药性[104]。
2.3 中药归经有研究报道, 中药与人体都是自然界超分子的聚集体, 各种子层次的“印迹模板”, 按照一定的空间孔穴通道结构联接形成了人体和中药巨复超分子体[105]。当中药进入人体后, 中药的超分子“印迹模板”产物与人体经络脏腑内相似的“印迹模板”形成超分子而产生作用, 以此解释了中药的归经作用[106]。联用引经药与顺铂以治疗肿瘤已被证实可在肿瘤细胞内富集顺铂, 如归肺经的桔梗联用顺铂治疗裸鼠原位肺癌移植瘤, 降低了肺中P糖蛋白(P-glycoprotein)表达量并增强了顺铂在裸鼠肺组织的药物分布[107]。中药超分子理论可对顺铂体内富集进行解释, 中药与顺铂通过非分子键力结合形成类超分子结构, 进入人体后与对应的相匹配的空穴结合, 由此可能更易将顺铂带入归经部位而超分子的匹配结合又降低了顺铂的排出效应, 最终引起顺铂在体内相应脏腑的富集。而该现象产生的细胞内表现便是摄入蛋白的过表达或对外排蛋白的表达抑制。
3 总结与展望顺铂对多种癌症均有较好疗效, 然而随着顺铂投入临床使用, 在许多癌症治疗过程中产生的耐药性抑制了顺铂的疗效。顺铂的耐药机制较为复杂, 不同的癌症类型及阶段、顺铂不同转运部位、不同研究层面和不同作用通路及靶点, 其发生机制是不一样的。为了抑制顺铂的耐药性, 西医药界通过多种化药联用的方式来降低其耐药性, 但存在各种缺陷和不良反应。中药在我国已经沿用几千年, 具有辨证论治, 多成分多靶点的整体施治特点, 其耐药性问题不明显。许多研究已证实中药联用顺铂可逆转顺铂耐药性, 也从分子水平层面探索了逆转机制, 但鲜有从宿主(机体)层面、以机体的吸收、分布、代谢和排泄(ADME)为切入点, 探索顺铂耐药性机制及中药逆转机制的研究报道。与其他抗癌药物一样, 顺铂主要是注射给药, 以减少吸收环节的影响, 但顺铂在机体内仍有分布、代谢与排泄环节。目前中药逆转顺铂耐药性的实验研究大多是在耐药癌细胞株进行, 忽略了ADME对耐药性的影响, 故应该增加对癌症宿主(机体)的ADME环节进行研究。因此, 提出“中药归经性能可定点(靶向)富集顺铂, 提高顺铂在癌细胞周边及细胞内的浓度, 以逆转其耐药性”的假说。结合中药超分子与“印迹模板”等理论基础, 探索并验证此假说的可行性, 从整体视角解决顺铂临床的耐药性问题。
作者贡献:赵靖负责本文的选题、图表制作及文章初稿撰写; 李原华、张喜利负责文献的调研及整理; 刘文龙、肖小河负责指导本文的中心思想并对本文进行初稿审阅和修改。
利益冲突:文章内容不涉及相关利益冲突。
| [1] |
Siddik ZH. Cisplatin:mode of cytotoxic action and molecular basis of resistance[J]. Oncogene, 2003, 22: 7265-7279. DOI:10.1038/sj.onc.1206933 |
| [2] |
Ghosh S. Cisplatin:the first metal based anticancer drug[J]. Bioorg Chem, 2019. DOI:10.1016/j.bioorg.2019.102925 |
| [3] |
Yimit A, Adebali O, Sancar A, et al. Differential damage and repair of DNA-adducts induced by anti-cancer drug cisplatin across mouse organs[J]. Nat Commun, 2019, 10: 1-11. DOI:10.1038/s41467-018-07882-8 |
| [4] |
Hu J, Wu TM, Li HZ, et al. The synthesis, structure-toxicity relationship of cisplatin derivatives for the mechanism research of cisplatin-induced nephrotoxicity[J]. Bioorg Med Chem Lett, 2017, 27: 3591-3594. DOI:10.1016/j.bmcl.2017.04.077 |
| [5] |
Kritsch D, Hoffmann F, Steinbach D, et al. Tribbles 2 mediates cisplatin sensitivity and DNA damage response in epithelial ovarian cancer[J]. Int J Cancer, 2017, 141: 1600-1614. DOI:10.1002/ijc.30860 |
| [6] |
Sarin N, Engel F, Kalayda GV, et al. Cisplatin resistance in non-small cell lung cancer cells is associated with an abrogation of cisplatin-induced G2/M cell cycle arrest[J]. PLoS One, 2017, 12. |
| [7] |
Zhang Y, Zhang J. Research advances in resistance to platinum-based chemotherapy in lung cancer[J]. Acta Acad Med Sin (中国医学科学院学报), 2017, 39: 150. |
| [8] |
Guttmann S, Chandhok G, Groba SR, et al. Organic cation transporter 3 mediates cisplatin and copper cross-resistance in hepatoma cells[J]. Oncotarget, 2018, 9: 743. DOI:10.18632/oncotarget.23142 |
| [9] |
Chaney SG, Campbell SL, Temple B, et al. Protein interactions with platinum-DNA adducts:from structure to function[J]. J Inorg Biochem, 2004, 98: 1551-1559. DOI:10.1016/j.jinorgbio.2004.04.024 |
| [10] |
Akerfeldt MC, Tran CMN, Shen C, et al. Interactions of cisplatin and the copper transporter CTR1 in human colon cancer cells[J]. J Biol Inorg Chem, 2017, 22: 765-774. DOI:10.1007/s00775-017-1467-y |
| [11] |
Ishida S, Lee J, Thiele DJ, et al. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals[J]. Proc Natl Acad Sci U S A, 2002, 99: 14298-14302. DOI:10.1073/pnas.162491399 |
| [12] |
Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin[J]. Mol Pharmacol, 2006, 70: 1390-1394. DOI:10.1124/mol.106.022624 |
| [13] |
Lai YH, Kuo C, Kuo MT, et al. Modulating chemosensitivity of tumors to platinum-based antitumor drugs by transcriptional regulation of copper homeostasis[J]. Int J Mol Sci, 2018, 19: 1486. DOI:10.3390/ijms19051486 |
| [14] |
Arnesano F, Nardella MI, Natile G. Platinum drugs, copper transporters and copper chelators[J]. Coord Chem Rev, 2018, 374: 254-260. DOI:10.1016/j.ccr.2018.07.003 |
| [15] |
Palm ME, Weise CF, Lundin C, et al. Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro[J]. Proc Natl Acad Sci U S A, 2011, 108: 6951-6956. DOI:10.1073/pnas.1012899108 |
| [16] |
Palm-Espling ME, Andersson CD, Björn E, et al. Determinants for simultaneous binding of copper and platinum to human chaperone Atox1:hitchhiking not hijacking[J]. PLoS One, 2013, 8. |
| [17] |
Safaei R, Maktabi MH, Blair BG, et al. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin[J]. J Inorg Biochem, 2009, 103: 333-341. DOI:10.1016/j.jinorgbio.2008.11.012 |
| [18] |
Zheng JQ, Yang X, Wang Q, et al. Research progress in toxic effects of antitumor drugs based on drug transporter[J]. Acta Pharm Sin (药学学报), 2017, 52: 1496-1504. |
| [19] |
Burger H, Loos WJ, Eechoute K, et al. Drug transporters of platinum-based anticancer agents and their clinical significance[J]. Drug Resist Updates, 2011, 14: 22-34. DOI:10.1016/j.drup.2010.12.002 |
| [20] |
Sani FV, Palizban A, Mosaffa F, et al. Glucosamine reverses drug resistance in MRP2 overexpressing ovarian cancer cells[J]. Eur J Pharmacol, 2020, 868: 172883. DOI:10.1016/j.ejphar.2019.172883 |
| [21] |
Korita PV, Wakai T, Shirai Y, et al. Multidrug resistance-associated protein 2 determines the efficacy of cisplatin in patients with hepatocellular carcinoma[J]. Oncol Rep, 2010, 23: 965-972. |
| [22] |
Petruzzelli R, Polishchuk RS. Activity and trafficking of copper-transporting ATPases in tumor development and defense against platinum-based drugs[J]. Cells, 2019, 8: 1080. |
| [23] |
Nakayama K, Kanzaki A, Terada K, et al. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy[J]. Clin Cancer Res, 2004, 10: 2804-2811. |
| [24] |
Zhu S, Shanbhag V, Wang Y, et al. A role for the ATP7A copper transporter in tumorigenesis and cisplatin resistance[J]. J Cancer, 2017, 8: 1952. |
| [25] |
Samimi G, Howell SB. Modulation of the cellular pharmacology of JM118, the major metabolite of satraplatin, by copper influx and efflux transporters[J]. Cancer Chemother Pharmacol, 2006, 57: 781-788. |
| [26] |
Kawahara B, Ramadoss S, Chaudhuri G, et al. Carbon monoxide sensitizes cisplatin-resistant ovarian cancer cell lines toward cisplatin via attenuation of levels of glutathione and nuclear metallothionein[J]. J Inorg Biochem, 2019, 191: 29-39. DOI:10.1016/j.jinorgbio.2018.11.003 |
| [27] |
Zou M, Hu X, Xu B, et al. Glutathione S-transferase isozyme alpha 1 is predominantly involved in the cisplatin resistance of common types of solid cancer[J]. Oncol Rep, 2019, 41: 989-998. |
| [28] |
Kolfschoten GM, Pinedo HM, Scheffer PG, et al. Development of a panel of 15 human ovarian cancer xenografts for drug screening and determination of the role of the glutathione detoxification system[J]. Gynecol Oncol, 2000, 76: 362-368. |
| [29] |
Daubeuf S, Leroy P, Paolicchi A, et al. Enhanced resistance of HeLa cells to cisplatin by overexpression of γ-glutamyltransferase[J]. Biochem Pharmacol, 2002, 64: 207-216. |
| [30] |
Ferreira JA, Peixoto A, Neves M, et al. Mechanisms of cisplatin resistance and targeting of cancer stem cells:adding glycosylation to the equation[J]. Drug Resist Updates, 2016, 24: 34-54. |
| [31] |
Okuno S, Sato H, Kuriyama-Matsumura K, et al. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines[J]. Br J Cancer, 2003, 88: 951-956. |
| [32] |
Amable L. Cisplatin resistance and opportunities for precision medicine[J]. Pharmacol Res, 2016, 106: 27-36. |
| [33] |
Holford J, Beale PJ, Boxall FE, et al. Mechanisms of drug resistance to the platinum complex ZD0473 in ovarian cancer cell lines[J]. Eur J Cancer, 2000, 36: 1984-1990. |
| [34] |
Wong DL, Stillman MJ. Capturing platinum in cisplatin:kinetic reactions with recombinant human apo-metallothionein 1a[J]. Metallomics, 2018, 10: 713-721. DOI:10.1039/c8mt00029h |
| [35] |
Meijer C, Timmer A, De Vries EGE, et al. Role of metallothionein in cisplatin sensitivity of germ-cell tumours[J]. Int J Cancer, 2000, 85: 777-781. |
| [36] |
Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance[J]. Oncogene, 2012, 31: 1869-1883. |
| [37] |
Damia G, Broggini M. Platinum resistance in ovarian cancer:role of DNA repair[J]. Cancers, 2019, 11: 119. DOI:10.3390/cancers11010119 |
| [38] |
Sawant A, Floyd AM, Dangeti M, et al. Differential role of base excision repair proteins in mediating cisplatin cytotoxicity[J]. DNA Repair, 2017, 51: 46-59. |
| [39] |
Hamilton G, Rath B. Pharmacogenetics of platinum-based chemotherapy in non-small cell lung cancer:predictive validity of polymorphisms of ERCC1[J]. Expert Opin Drug Metab Toxicol, 2018, 14: 17-24. |
| [40] |
Ahmad A, Robinson AR, Duensing A, et al. ERCC1-XPF endonuclease facilitates DNA double-strand break repair[J]. Mol Cell Biol, 2008, 28: 5082-5092. |
| [41] |
Chang IY, Kim MH, Kim HB, et al. Small interfering RNA-induced suppression of ERCC1 enhances sensitivity of human cancer cells to cisplatin[J]. Biochem Biophys Res Commun, 2005, 327: 225-233. |
| [42] |
Rudolph C, Melau C, Nielsen JE, et al. Involvement of the DNA mismatch repair system in cisplatin sensitivity of testicular germ cell tumours[J]. Cell Oncol, 2017, 40: 341-355. |
| [43] |
Papouli E, Cejka P, Jiricny J. Dependence of the cytotoxicity of DNA-damaging agents on the mismatch repair status of human cells[J]. Cancer Res, 2004, 64: 3391-3394. |
| [44] |
Topping RP, Wilkinson JC, Scarpinato KD. Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling[J]. J Biol Chem, 2009, 284: 14029-14039. |
| [45] |
Li Z, Pearlman AH, Hsieh P. DNA mismatch repair and the DNA damage response[J]. DNA Repair, 2016, 38: 94-101. |
| [46] |
Talens F, Jalving M, Gietema JA, et al. Therapeutic targeting and patient selection for cancers with homologous recombination defects[J]. Exp Opin Drug Discov, 2017, 12: 565-581. |
| [47] |
Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers[J]. Nature, 2008, 451: 1116-1120. |
| [48] |
Shah NP. BRCA:from therapeutic target to therapeutic shield[J]. Nat Med, 2008, 14: 495-496. |
| [49] |
Dhillon KK, Swisher EM, Taniguchi T. Secondary mutations of BRCA1/2 and drug resistance[J]. Cancer Sci, 2011, 102: 663-669. |
| [50] |
Husain A, He G, Venkatraman ES, et al. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum (II)[J]. Cancer Res, 1998, 58: 1120-1123. |
| [51] |
Wang L, Wei J, Qian X, et al. ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel[J]. BMC Cancer, 2008, 8: 97. |
| [52] |
James CR, Quinn JE, Mullan PB, et al. BRCA1, a potential predictive biomarker in the treatment of breast cancer[J]. Oncologist, 2007, 12: 142-150. |
| [53] |
Rocha CRR, Silva MM, Quinet A, et al. DNA repair pathways and cisplatin resistance:an intimate relationship[J]. Clinics, 2018, 73. |
| [54] |
Brabec V, Kasparkova J. Modifications of DNA by platinum complexes:relation to resistance of tumors to platinum antitumor drugs[J]. Drug Resist Updates, 2005, 8: 131-146. |
| [55] |
Slupianek A, Schmutte C, Tombline G, et al. BCR/ABL regulates mammalian RecA homologs, resulting in drug resistance[J]. Mol Cell, 2001, 8: 795-806. |
| [56] |
Yamanaka K, Chatterjee N, Hemann MT, et al. Inhibition of mutagenic translesion synthesis:a possible strategy for improving chemotherapy?[J]. PLoS Genet, 2017, 13. |
| [57] |
Lee YS, Gregory MT, Yang W. Human Pol ζ purified with accessory subunits is active in translesion DNA synthesis and complements Pol η in cisplatin bypass[J]. Proc Natl Acad Sci U S A, 2014, 111: 2954-2959. |
| [58] |
Li X, Ren J, Chen P, et al. Co-inhibition of Pol η and ATR sensitizes cisplatin-resistant non-small cell lung cancer cells to cisplatin by impeding DNA damage repair[J]. Acta Pharmacol Sin, 2018, 39: 1359-1372. |
| [59] |
Basu A, Krishnamurthy S. Cellular responses to cisplatin-induced DNA damage[J]. J Nucl Acids, 2010. DOI:10.4061/2010/201367 |
| [60] |
Dasari S, Tchounwou PB. Cisplatin in cancer therapy:molecular mechanisms of action[J]. Eur J Pharmacol, 2014, 740: 364-378. |
| [61] |
Köberle B, Tomicic MT, Usanova S, et al. Cisplatin resistance:preclinical findings and clinical implications[J]. Biochim Biophys Acta, 2010, 1806: 172-182. |
| [62] |
Liu R, Ji P, Liu B, et al. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis[J]. Oncol Lett, 2017, 13: 1024-1030. |
| [63] |
Hientz K, Mohr A, Bhakta-Guha D, et al. The role of p53 in cancer drug resistance and targeted chemotherapy[J]. Oncotarget, 2017, 8: 8921. |
| [64] |
Sun Y, Guan Z, Liang L, et al. NF-κB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression[J]. Int J Oncol, 2016, 48: 225-234. DOI:10.3892/ijo.2015.3256 |
| [65] |
Katsman A, Umezawa K, Bonavida B. Reversal of resistance to cytotoxic cancer therapies:DHMEQ as a chemo-sensitizing and immuno-sensitizing agent[J]. Drug Resist Updates, 2007, 10: 1-12. |
| [66] |
Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of inhibitor of nuclear factor-κB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models[J]. Clin Cancer Res, 2004, 10: 7645-7654. |
| [67] |
Pierce JW, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo[J]. J Biol Chem, 1997, 272: 21096-21103. DOI:10.1074/jbc.272.34.21096 |
| [68] |
Beale PJ, Rogers P, Boxall F, et al. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma[J]. Br J Cancer, 2000, 82: 436-440. |
| [69] |
Miyamoto M, Takano M, Aoyama T, et al. Phenoxodiol increases cisplatin sensitivity in ovarian clear cancer cells through XIAP down-regulation and autophagy inhibition[J]. Anticancer Res, 2018, 38: 301-306. |
| [70] |
Michaud WA, Nichols AC, Mroz EA, et al. Bcl-2 blocks cisplatin-induced apoptosis and predicts poor outcome following chemoradiation treatment in advanced oropharyngeal squamous cell carcinoma[J]. Clin Cancer Res, 2009, 15: 1645-1654. |
| [71] |
Mansouri A, Zhang Q, Ridgway LD, et al. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation[J]. Oncol Res, 2003, 13: 399-404. |
| [72] |
Chen SH, Chang JY. New insights into mechanisms of cisplatin resistance:from tumor cell to microenvironment[J]. Int J Mol Sci, 2019, 20: 4136. |
| [73] |
Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance:a major contributor to minimal residual disease[J]. Nat Rev Cancer, 2009, 9: 665-674. |
| [74] |
Sherman-Baust CA, Weeraratna AT, Rangel LBA, et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells[J]. Cancer Cell, 2003, 3: 377-386. |
| [75] |
Zhang Y, Huang P, Liu X, et al. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis[J]. J Pharmacol Sci, 2018, 137: 305-312. |
| [76] |
Yu L, Fan Z, Fang S, et al. Cisplatin selects for stem-like cells in osteosarcoma by activating Notch signaling[J]. Oncotarget, 2016, 7: 33055. |
| [77] |
Mondal S, Bhattacharya K, Mandal C. Nutritional stress reprograms dedifferention in glioblastoma multiforme driven by PTEN/Wnt/Hedgehog axis:a stochastic model of cancer stem cells[J]. Cell Death Discov, 2018, 4: 110. |
| [78] |
Yan C, Luo L, Goto S, et al. Enhanced autophagy in colorectal cancer stem cells does not contribute to radio-resistance[J]. Oncotarget, 2016, 7: 45112. DOI:10.18632/oncotarget.8972 |
| [79] |
Iyer AK, Singh A, Ganta S, et al. Role of integrated cancer nanomedicine in overcoming drug resistance[J]. Adv Drug Deliv Rev, 2013, 65: 1784-1802. |
| [80] |
Garcia-Mayea Y, Mir C, Masson F, et al. Insights into new mechanisms and models of cancer stem cell multidrug resistance[J]. Semin Cancer Biol, 2019, 60: 166-180. |
| [81] |
Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers[J]. Life Sci, 2019, 234: 116781. |
| [82] |
Levina V, Marrangoni AM, DeMarco R, et al. Drug-selected human lung cancer stem cells:cytokine network, tumorigenic and metastatic properties[J]. PLoS One, 2008, 3. |
| [83] |
Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment[J]. Proc Natl Acad Sci U S A, 2009, 106: 16281-16286. |
| [84] |
Zhang Y, Wang Z, Yu J, et al. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer[J]. Cancer Lett, 2012, 322: 70-77. |
| [85] |
Abubaker K, Latifi A, Luwor R, et al. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden[J]. Mol Cancer, 2013, 12: 24. |
| [86] |
Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors[J]. Cancer Res, 2008, 68: 3243-3250. |
| [87] |
Yang L, Shi P, Zhao G, et al. Targeting cancer stem cell pathways for cancer therapy[J]. Signal Trans Target Ther, 2020, 5: 1-35. DOI:10.1038/s41392-019-0089-y |
| [88] |
Yu Q, Xue Y, Liu J, et al. Fibronectin promotes the malignancy of glioma stem-like cells via modulation of cell adhesion, differentiation, proliferation and chemoresistance[J]. Front Mol Neurosci, 2018, 11: 130. |
| [89] |
Wu ZZ. Antitumor mechanism of ganoderma lucidum spores[J]. J Cell Mol Immunol (细胞与分子免疫学杂志), 2006, 22: 836-837. |
| [90] |
Zhao SF. Ganoderma Lucidum Exerts Anti-tumor Effects on Ovarian Cancer Cells and Reversed Their Chemoresistance to Cisplatin (破壁灵芝孢子粉提取物抑制人卵巢上皮性癌细胞生长、转移及逆转顺铂耐药的研究)[D]. Shijiazhuang: Hebei Medical University, 2010.
|
| [91] |
Qu HG, Gao L, He D, et al. Effect of reversion of ganoderma lucidum polysaccharides on cisplatin resistant in ovarian cancer cells and its mechanism[J]. J Jilin Univ Med Ed (吉林大学学报医学版), 2011, 37: 250-254. |
| [92] |
Chen Y, Huang LH. Reversion of lentinan on A2780 ovarian cancer cell's resistance to cisplatin in vitro and mechanism[J]. Med J Wuhan Univ (武汉大学学报医学版), 2017, 38: 24-27. |
| [93] |
Liu L, Bian K. Advance in studies on molecular mechanisms of cisplatin resistance and intervention with traditional Chinese medicines[J]. China J Chin Mater Med (中国中药杂志), 2014, 39: 3216-3220. |
| [94] |
Ni YY. The Effects and Molecular Mechanisms of Curcumin on the Reverse of Cisplatin Resistance in Lung Adenocarcinoma (姜黄素逆转肺腺癌顺铂耐药作用及其分子机制的研究)[D]. Tianjing: Tianjing Medical University, 2018.
|
| [95] |
Ying HC, Zhang SL, Lu J. Drug-resistant reversing effect of curcumin on COC1/DDP and its mechanism[J]. J Mod Oncol (现代肿瘤医学), 2007, 15: 604-607. |
| [96] |
Qin CM, Hou HX, Chen DH, et al. The Reversal effect of emodin on cisplatin resistance in ovarian cancer cells and the expression of resistance-associated genes[J]. Nat Prod Res Develop (天然产物研究与开发), 2011, 23: 638-642. |
| [97] |
Wu CF, Zeng R, Zhou YL, et al. Reversal effect of aloe emodin on cisplatin resistance human lung adenocarcinoma cells[J]. Chin J Hosp Pharm (中国医院药学杂志), 2008, 28: 1061-1063. |
| [98] |
Yan XF, Xu W, Gao JZ, et al. Effect of matrine combined with cisplatin on drug sensitivity and TrkB expression in SH-SY5Y cells[J]. J Clin Pediatr (临床儿科杂志), 2012, 30: 456-459. |
| [99] |
Hou LL, Xu QJ, Hu GQ, et al. Synergistic antitumor effects of tanshinoneⅡA in combination with cisplatin via apoptosis in the prostate cancer cells[J]. Acta Pharm Sin (药学学报), 2013, 48: 675-679. |
| [100] |
Pan YZ, Yin DF, Zhang NS, et al. Effect of combined treatment with Xiaoji decoction on cisplatin-resistant ovarian cancer in vitro[J]. Chin Arch Tradit Chin Med (中华中医药学刊), 2008, 26: 78-79. DOI:10.1016/j.ygyno.2005.12.019 |
| [101] |
Chen ZQ, Zhang YZ. Reverse effect of Shenqifuzheng injection on cisplatin resistance of lung cancer cells A549/DDP[D]. Chin J Tradit Med Sci Technol (中国中医药科技), 2015, 22: 28-30.
|
| [102] |
Zhong LX, Xiong JP, Zhang XQ. Effects of Shenqifuzheng injection on multi-drug resistance of K562/ADM[J]. J Nanchang Univ (南昌大学学报), 2006, 48: 41-44. |
| [103] |
Gao Y, Yu N, Chen Q, et al. Effects of the medicated serum of Shiquandabu decoction on Wnt/20-catenin signaling pathway protein in A549/DDP cell line[J]. Chin J Exp Tradit Med Formulae (中国实验方剂学杂志), 2016, 22: 129-133. |
| [104] |
Lu P, Zhao H, Shi FQ, et al. Effects on expression of drug-resistant enzymes in transplanted tumor cells of acute lymphoblastic leukemia cell line treated by compound Zhebei granules combined with cisplatin[J]. Chin J Inf Tradit Chin Med (中国中医药信息杂志), 2019, 26: 52-56. |
| [105] |
He FY, Zhou YQ, Deng KW, et al. The special influence of supramolecular chemistry on TCM theory[J]. China J Chin Mater Med (中国中药杂志), 2014, 39: 1534-1543. |
| [106] |
He FY, He H, Deng KW, et al. Exploration of research approaches of Chinese medicine's pharmacology based on ''imprinting templates'' (medical element) of supramolecules[J]. China J Chin Mater Med (中国中药杂志), 2015, 40: 4313-4318. |
| [107] |
Li Y, Xia Q, Zhao RZ, et al. Effect of the transplanting drug platycodon grandiflorum on the distribution of cisplatin in nude mice transplanted with orthotopic lung cancer[J]. Pharmacol Clin Chin Mater Med (中药药理与临床), 2018, 34: 71-75. |
 2020, Vol. 55
2020, Vol. 55


