2. 广东药科大学, 广东 广州 510006
2. Guangdong Pharmaceutical University, Guangzhou 510006, China
化疗药物多为细胞毒类药物, 其在杀伤肿瘤细胞的同时, 也破坏了肠道免疫功能而影响肠道菌群的比例及结构稳定。接受常规化疗药物治疗的患者, 肠黏膜炎发病率为40%, 而接受高剂量化疗药物的患者肠黏膜炎发病率几乎为100%[1]。因此, 化疗性肠黏膜炎(chemotherapy-induced intestinal mucositis, CIM)是肿瘤患者化疗过程中主要的剂量限制性不良反应, 也是化疗被迫停止的一个重要原因。CIM不仅限制化疗药物的临床应用, 也严重影响患者的生活和生存质量。目前, 尚无预防或治疗CIM的有效手段。
国内外的临床研究显示, 化疗药物可导致肠道菌群的种类和丰度明显减少, 菌种比例在化疗期间有明显变化, 总体呈现菌群失调趋势。动物实验研究结果与临床观察基本一致, 提示化疗期间肠道微生态的改变更有利于致病菌的生长, 甚至发生易位而改变菌群结构, 导致菌群比例失衡[2]。体外研究发现, 肠道上皮是化疗药物介导的细胞毒性损伤的主要目标, 单次大剂量以5-氟尿嘧啶(5-fluorouracil, 5-FU)腹腔注射及小剂量多次给药均可引起肠上皮紧密连接蛋白表达下降, 破坏肠道机械屏障, 造成肠黏膜通透性增高而影响肠道微生态平衡。此外, 以5-FU为代表的化疗药物可快速引起肠道内的杯状细胞空泡化, 导致杯状细胞储存的黏蛋白迅速耗尽, 引起肠道黏液层变化, 降低肠道屏障功能[3]。而以伊立替康(irinotecan, CPT-11)为代表的细胞毒类化疗药物可诱导双链DNA从肠黏膜上皮内大规模释放, 导致肠黏膜炎和晚发性腹泻[4]。通过调节肠道菌群的平衡来改善CIM已成为目前肠道菌群研究的热点之一。
隐丹参酮(cryptotanshinone, CTS)是丹参的主要脂溶性提取物, 属于二萜醌类化合物, 其骨架上大多具有三元或四元环的邻醌或对醌结构, 被报道具有抗肿瘤、抗氧化、抗炎、抗菌等活性[5]。然而隐丹参酮是否可以调控粪便菌群, 以改善CIM病理变化, 尚无相关报道。本研究考察了CTS对CIM小鼠的防治作用, 同时探讨了CTS对粪便菌群、炎性因子及血脂含量的调控及其可能的相关性。
材料与方法实验动物 雄性Balb/c小鼠, 6~8周龄, 体重20~22 g, SPF级, 购于北京维通利华实验动物技术有限公司。许可证号SCXK (京) 2016-0006。自由进食、饮水。饲养环境:温度22~27 ℃, 相对湿度60%~65%, 光照12 h。动物福利和实验过程均遵循中国医学科学院药物研究所动物伦理委员会的规定。
药品及试剂 隐丹参酮(成都曼思特生物科技有限公司); 5-FU (CAS: 51-21-8)和伊立替康(CAS: 100286-90-6) (MCE公司); 白介素6 (interleukin 6, IL-6)检测试剂盒、髓过氧化物酶(myeloperoxidase, MPO)检测试剂盒、二胺氧化酶(diamine oxidase, DAO)检测试剂盒和白介素11 (interleukin 11, IL-11)检测试剂盒(武汉伊莱瑞特生物科技股份有限公司); 总甘油三酯(triglyceride, TG)和总胆固醇(total cholesterol, TC)检测试剂盒(南京建成生物工程研究所); 脂肪酶(lipase)检测试剂盒(Cayman公司)。
化疗性肠黏膜炎小鼠模型的建立 参考Ribeiro等[4]报道的CIM小鼠模型制备的基础上改进。腹腔注射(ip) 5-FU (25 mg·kg-1)及伊立替康(20 mg·kg-1), 连续注射4天造模。
实验分组及样品采集 选取雄性Balb/c小鼠32只, 按体重随机分为正常对照组、模型组、伪无菌造模组和隐丹参酮给药组, 每组8只。正常对照组:灌胃(ig) 0.9%生理盐水, 每日1次, 连续8天; 模型组:第1~2天灌胃0.9%生理盐水, 第3~6天连续造模4天, 第7~8天灌胃0.9%生理盐水; 伪无菌造模组:每3天灌胃2次混合抗生素(100 mg·kg-1, 甲硝唑、万古霉素、青霉素比例为1:1:1), 2次灌胃时间间隔12 h, 诱导小鼠肠道伪无菌状态, 采集伪无菌造模组新鲜粪便检验肠道菌群干扰模型是否成功。伪无菌模型成功后腹腔注射5-FU (25 mg·kg-1)及伊立替康(20 mg·kg-1), 连续注射4天造模。隐丹参酮给药组:造模前1天, 腹腔注射CTS (20 mg·kg-1), 每日1次, 连续8天。
体重及排便量检测 每日记录各组小鼠体重, 观察各组小鼠的生理状态、粪便颜色、性状等, 称重并统计白天给药后3 h内各组小鼠的排便量。
疾病活动指数(disease activity index, DAI) 评分取小鼠体重下降分数(无: 0分; 1%~5%, 1分; 6%~10%, 2分; 11%~15%, 3分; > 15%, 4分)、小鼠腹泻等级分数(正常, 0分; 软便, 1分; 大便稀湿不成形且轻度肛周着色, 2分; 重度肛周着色, 3分)和小鼠白天3 h排便量(> 0.6 g, 0分; 0.4~0.6 g, 1分; 0.2~0.4 g, 2分; 0~0.2 g, 3分)三者的平均值。在实验的第3~8天分别对各组小鼠进行疾病活动指数评分。
血清及肠黏膜组织炎性因子、总甘油三酯、总胆固醇含量测定 在给药结束后次日各组小鼠眼球取血, 4 ℃、5 000 r·min-1离心15 min, 取上清制备血清样品。肛门至回盲部的结肠沿肠系膜边缘纵行剖开, 刮取肠黏膜转入无菌冻存管中, 液氮速冻, 于生理盐水中制备组织匀浆样品。酶联免疫吸附法(ELISA)测定血清及组织匀浆中IL-6、IL-11、DAO、MPO及血清脂肪酶活性。TG酶法及TCH酶法测定血清中TG和TC的含量。
组织病理学评价 取肛门至回盲部的结肠, 用生理盐水冲洗肠道内容物后, 放入4%多聚甲醛固定。结肠组织按照常规包埋、切片, H & E (hematoxylin and eosin)染色。镜下观察结肠组织形态及是否出现以下病理变化: ①黏膜层不同数量的单个核细胞浸润及与嗜中性粒细胞混合浸润; ②黏膜厚度变薄、腺体扩张伴有杯状细胞减少, 隐窝及腺体远离基底膜; ③隐窝消失, 以中性粒细胞为主; ④上皮破坏, 基底浆细胞增多, 淋巴细胞聚集。病理学评分标准如下:具有以上1种病理学表现的评分1分; 具有以上2种病理学表现的评分2分; 具有以上3种病理学表现的评分3分; 具有以上病理学表现多于3种的评分4分。
粪便菌群多样性检测 提取小鼠粪便DNA, 利用NanoDrop及1%琼脂糖凝胶电泳进行总DNA质检。以稀释后的基因组DNA为模板, 使用KAPA HIiFi hotstart ReadyMix PCR kit高保真酶进行PCR实验, 2%琼脂糖凝胶电泳检测并用AxyPrep DNA凝胶试剂盒(Axygen)切胶回收PCR产物。NanoDrop及1%琼脂糖凝胶电泳进行文库质检, 进行Illumina测序。测序引物为16S rRNA基因的V3~V4高变区的特异引物338F (5'-ACTCCTACGGGAGGCAGCAG-3')和806R (5'-GGACTACHVGGGTWTCTAAT-3')。根据Illumina MiSeq平台标准操作规程将纯化后的扩增片段构建末端测序(paired-end, PE)文库, 区分样本后于Majorbio I-Sanger云平台进行分类单位(operational taxonomic units, OTU)聚类分析和物种分类学分析, 包括α多样性分析、β多样性分析和线性判别分析(linear discriminant analysis size effect, LEfSe)物种差异及spearman相关性分析等。
数据统计与分析 采用GraphPad Prism8.0软件进行数据分析, 数据结果以
与正常组小鼠比较, 模型组小鼠体重明显下降, 出现腹泻及明显肛门着色。伪无菌造模组小鼠体重虽然比正常组明显下降, 但疾病活动指数显著降低。隐丹参酮给药组小鼠肛周着色程度降低, 体重及肠道内粪便量显著增加。DAI评分显示:伪无菌造模组及给药组小鼠的DAI评分显著低于模型组(P < 0.001) (图 1)。

|
Figure 1 Effects of cryptotanshinone (CTS) on perianal coloration, body weight, and disease activity index (DAI) in chemotherapy induced colitis (CIM) mice (NC: Normal control group; M: Model group; GM: Pseudo-sterile model; CTS: CTS treated group).A: Effect of CTS on perianal coloration in CIM mice; B: Effect of CTS on body weight of CIM mice; C: Effect of CTS on DAI in CIM mice. n = 8, |
镜下观察小鼠结肠组织, 正常对照组小鼠结肠黏膜结构完整, 组织排列紧密整齐, 未见异常。模型组小鼠肠黏膜萎缩/变性, 隐窝腺体远离基底膜甚至消失, 杯状细胞减少, 基底浆细胞增多, 黏膜层嗜中性粒细胞浸润; 伪无菌造模组及CTS给药组的小鼠结肠黏膜病变及炎性细胞浸润与模型组相比有显著的改善(图 2)。

|
Figure 2 Effect of CTS on histopathologic changes in CIM mice.A: Representative H & E (hematoxylin and eosin)-stained pictures of colon sections; B: Histopathological scores in each group. n = 5, |
与正常对照组比较, 模型组小鼠血清中DAO、MPO、IL-6及肿瘤坏死因子α (tumor necrosis factor alpha, TNFα)显著升高, 肠黏膜组织中IL-11和DAO显著升高。伪无菌造模组小鼠中血清炎性因子含量与正常对照组无显著差异。隐丹参酮显著降低血清中DAO、MPO、IL-6及肠黏膜组织中DAO含量, 但对血清TNFα含量及肠黏膜组织中IL-11含量无显著影响(表 1、2)。
|
Table 1 Effects of CTS on inflammatory factors in the serum of CIM mice. DAO: Diamine oxidase; MPO: Myeloperoxidase; IL-6: Interleukin 6; TNFα: Tumor necrosis factor alpha; IL-11: Interleukin 11. n = 5, |
|
Table 2 Effects of CTS on inflammatory factors in colon tissue of CIM mice. n = 5, |
与正常对照组比较, 模型组小鼠血清中TC含量及脂肪酶活性显著升高, TG含量及TG与TC的比率显著降低。伪无菌造模组小鼠中TG、TC、TG/TC及脂肪酶活性与正常对照组无明显差异。隐丹参酮可有效逆转模型组小鼠血清中TG、TC、TG/TC及脂肪酶含量的变化(图 3)。

|
Figure 3 Effects of CTS on TG, TC, TG/TC, and lipase activity in the serum of CIM mice.A: Effect of CTS on TG in serum; B: Effect of CTS on TC in serum; C: Effect of CTS on the ratio of TG to TC in serum; D: Effect of CTS on lipase activity in serum. n = 5, |
伪无菌小鼠使用混合抗生素干扰后6天, 收集小鼠新鲜粪便并进行粪便菌群测序, 包括α多样性分析、主坐标分析(principal co-ordinates analysis, PCoA)和基于β多样性距离的相似性分析(analysis of similarities, ANOSIM)。结果显示, 与正常对照组及模型组相比, 伪无菌组小鼠的粪便样本中的物种多样性和丰度都显著低于正常对照组及模型组(图 4A、B), 证明伪无菌小鼠模型制备成功。α多样性分析显示, CIM模型组粪便菌群物种丰富度较正常对照组显著降低, 而隐丹参酮可显著恢复CIM小鼠菌群物种丰富度。PCoA主坐标分析及ANOSIM分析结果显示, 各组小鼠的粪便菌群构成存在明显差异(图 4C、D)。在门水平:和正常对照组相比, 模型组厚壁菌门(Firmicutes, 48.53% vs 36.83%)及变形菌门(Proteobacteria, 20.08% vs 0.96%)相对丰度增加, 拟杆菌门(Bacteriodetes, 31.84% vs 80.1%)及放线菌门菌群(Actinobacteria, 0.49% vs 1.06%)相对丰度减少。在属水平:和正常对照组相比, 模型组乳杆菌属(Lactobacillus, 26.08% vs 5.32%)和拟杆菌属(Bacteroides, 12.61% vs 6.67%)相对丰度增加; norank_f_Muribaculaceae (11.3% vs 34.46%)、拟普雷沃菌属(Alloprevotella, 3.75% vs 9.84%)和普雷沃氏菌属(Prevotellaceae_UCG_001, 0.61% vs 6.99%)相对丰度降低。隐丹参酮可有效调控CIM小鼠肠道菌群恢复至接近正常, 并增加八叠球菌属(Sarcina)、普雷沃氏菌科(Prevotellaceae_NK3B31_group)和断链真杆菌(Eubacterium_fissicatena_group)相对丰度(图 4E、F)。

|
Figure 4 Effect of CTS on the species composition of fecal microbiota in CIM mice.A: Difference tests on α diversity in sobs index among NC, M, and GM group; B: PCoA analysis for β diversity among NC, M, and GM group; C: α diversity analysis among NC, M, and CTS group; D: PCoA analysis among NC, M, and CTS group; E: Species composition of fecal microbiota in phylum level among NC, M, and CTS group; F: Species composition of fecal microbiota in genus level among NC, M, and CTS group. n = 5, |
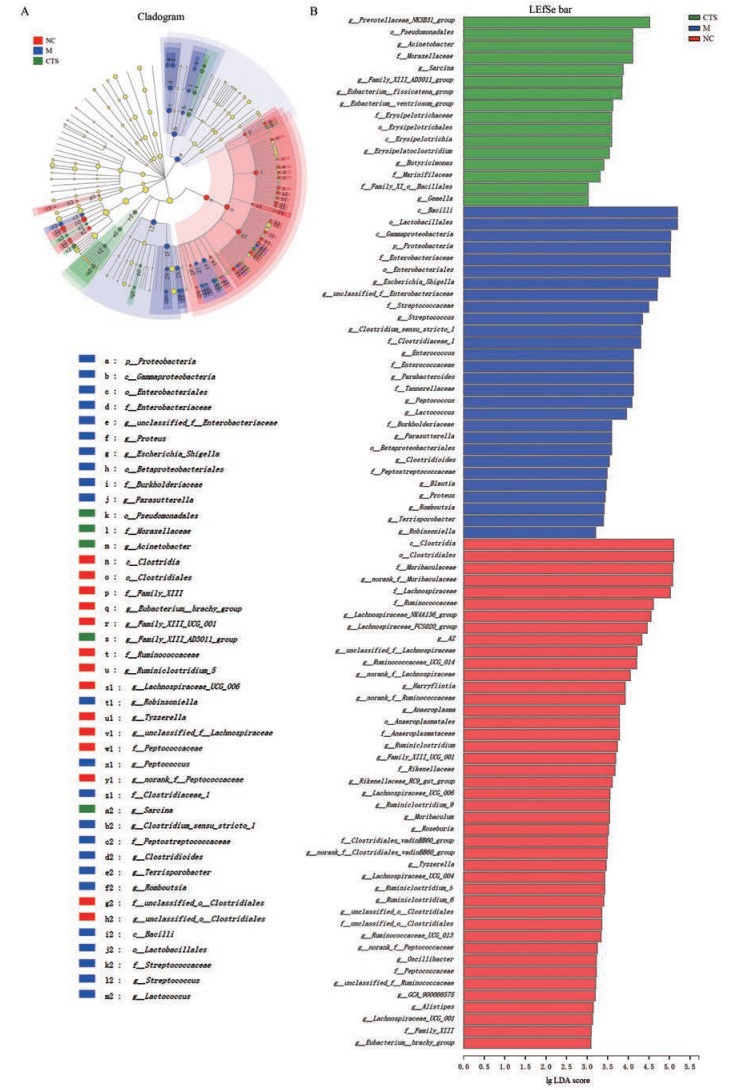
LEfSe及LDA (linear discriminant analysis)分析结果显示:正常对照组、模型组及隐丹参酮处理组的小鼠菌群物种构成具有显著差异。模型组瘤胃菌科(g_UBA1819)、布劳特氏菌属(g_Blautia)、肠球菌属(g_ Enterococcus)、埃希氏杆菌属(g_Escherichia_Shigell)、梭菌属(g_Clostridium_sensu_stricto_1)、链球菌属(g_ Streptococcus)、乳球菌属(g_Lactococcus)、毛螺旋菌属(g_Parasutterella)和布劳特氏菌属(g_Blautia)显著富集, 而隐丹参酮处理组中不动杆菌属(g_Acinetobacte)、丁酸弧菌属(g_Butyricimonas)、克里斯滕森菌(g_ Clostridium_innocuum_groupg_Christensenellaceae_R_ 7_group)和断链真杆菌(g_Eubacterium_fissicatena_ group)显著富集(图 5)。
|
Figure 5 Effect of CTS on species differences in fecal microbiota in CIM mice.A: Cladogram of LEfSe (linear discriminate analysis size effect) among groups; B: LEfSe bar of LDA (linear discriminant analysis) among groups |
Spearman相关性分析显示结果显示, 肠道菌群丰度与CIM小鼠血清中IL-6、IL-11、MPO、DAO含量相关性具有显著性差异。其中, 与DAO、MPO、IL-6含量均有显著相关性的肠道菌包括:梭菌属(g_Clostridium_ sensu_stricto_1, 正相关)、嗜木聚糖真杆菌(g_Eubacterium_xylanophilum_group, 负相关)、瘤胃梭菌属(g_Ruminiclostridium, 负相关)和副萨特氏菌属(g_Parasutterella, 正相关)。与MPO、IL-6、IL-11含量均有显著相关性的肠道菌包括: g_norank_f_Muribaculaceae (负相关)和理研菌科-RC9 (g_Rikenellaceae_RC9_gut_group, 负相关)。志贺氏菌(g_Escherichia-Shigella)则与MPO水平呈显著正相关(图 6)。

|
Figure 6 Correlation analysis between fecal microbiota and inflammatory factors in CIM mice.A: Spearman correlation heatmap for the relationship between fecal microbiota and inflammatory factors. n = 5, |
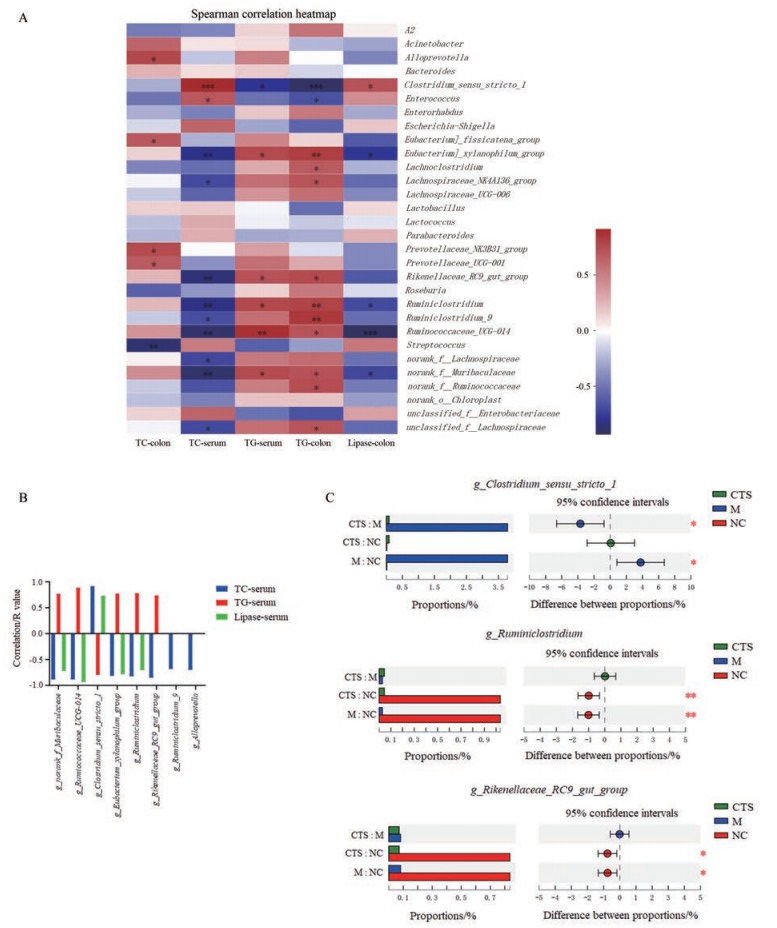
Spearman相关性分析显示结果显示, 肠道菌群丰度与CIM小鼠血清及结肠黏膜组织中TC、TG含量及结肠黏膜组织中脂肪酶含量具有显著相关性(图 7A)。其中, 与TC、TG及脂肪酶均有显著相关性的肠道菌包括: g_norank_f_Muribaculacea、g_Ruminococcaceae_ UCG-014、g_Clostridium_sensu_stricto_1、g_Eubacterium_ xylanophilum_group和g_Ruminiclostridium (图 7B)。模型组中g_norank_f_Muribaculaceae、g_Ruminococcaceae_UCG-014、g_Clostridium_sensu_stricto_1、g_ Ruminiclostridium和g_Rikenellaceae_RC9_gut_group (与TG呈正相关, 而与TC及脂肪酶呈负相关)丰度显著降低; g_Clostridium_sensu_stricto_1 (与TG呈负相关, 而与TC及脂肪酶呈正相关)丰度显著提高。隐丹参酮处理组g_norank_f_Muribaculaceae和g_Ruminococcaceae_UCG-014丰度显著增加; g_Clostridium_ sensu_stricto_1丰度显著降低(图 7B、C)。
|
Figure 7 Correlation analysis between fecal microbiota, TG, TC, and lipase activity in CIM mice.A: Spearman correlation heatmap for the relationship between fecal microbiota, TG, TC, and lipase activity in CIM mice; B: Significant correlation of fecal microbiota, TG, TC, and lipase activity; C: CTS significantly increased the abundance of g_norank_f_Muribaculaceaeand and g_Ruminococcaceae_UCG-014, but decreased that of g_Clostridium_sensu_stricto_1 in CIM mice. n = 5, |
正常状态下, 肠道微生物与宿主间存在着复杂而微妙的动态平衡, 这一平衡一旦被打破, 就会造成肠道菌群结构紊乱, 影响宿主的健康[6, 7]。肠道微生物在肠道内将多糖降解成单糖和短链脂肪酸, 提高能量利用效率, 而短链脂肪酸是结肠和小肠上皮细胞的主要供能物质, 能够较强地维护肠道形态及功能, 并对肠道疾病具有一定的治疗作用[8, 9]。化疗药物可导致免疫紊乱和肠上皮屏障的破坏, 使得肠道菌群的种类和组成比例发生显著变化[10]。
与人类肠道菌群类似, 正常小鼠肠道优势菌群为拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes)、变形杆菌门(Proteobacteria)和放线菌门(Actinobacteria)[11]。化疗性肠黏膜炎小鼠中模型组厚壁菌门及变形菌门较正常对照组显著增加, 拟杆菌门及放线菌门菌群较正常对照组显著减少。拟杆菌门在肠道中占绝对的优势, 具有酵解碳水化合物, 参与代谢多糖、胆汁酸和类固醇, 维持肠道正常生理等诸多功能。拟杆菌可募集IL-6, 在结肠诱导上皮内淋巴细胞, 促进屏障的完整性[12]。拟杆菌以生成乙酸和丙酸为主, 而厚壁菌以生成丁酸为主[13, 14]。有研究报道, 活动期溃疡性结肠炎组患者的梭菌属(Fusobacterium)、乳杆菌属(Lactobacillus)、双歧杆菌属(Bifidobacterium)和拟杆菌属(Bacteroides)菌落含量下降; 溃疡性结肠炎模型小鼠中拟杆菌门(Bacteroidetes)和柔壁菌门(Tenericures)显著减少; 大肠杆菌志贺属(Escherichia·Shigella)显著增多, 同时拟杆菌属(Bacteroidales_S24-7_group)、普雷沃氏菌属(Prevotellaceae UCG-001)、葡萄球菌属(Staphylococcus)、理研菌属(Alistipes)和酸杆菌属(Odoribacter)显著减少[15, 16]。然而, 除了大肠杆菌志贺属显著增多, 化疗性肠黏膜炎小鼠中乳杆菌属和拟杆菌属也明显增加; 而norank_f_Muribaculaceae、拟普雷沃菌属(Pseudoprevostella)和普雷沃氏菌属(Prevotella)降低。与对照组比较, 活动期溃疡性结肠炎患者血清中DAO和MPO升高, 而TC和TG水平被报道明显降低[17, 18]。本文研究结果显示化疗性肠黏膜炎小鼠血清中TC、DAO、MPO和脂肪酶活性显著升高, 而TG显著降低。因此, 化疗性肠黏膜炎与溃疡性结肠炎模型中肠道菌群的变化具有各自的特点, 发病机制及治疗方式也并不相同。已有报道证实, 随着动物体内TC水平的升高, 肠上皮细胞分裂速度加快, 导致肠道组织扩张, 肠道也随之延长, 这些变化显著加快了结肠肿瘤形成的速度[19]。因此, 与溃疡性结肠炎比较, 化疗性肠黏膜炎可能更易诱发结肠癌。
隐丹参酮可有效改善小鼠化疗性黏膜炎, 使CIM小鼠肠道菌群构成恢复至接近正常状态, 且诱导不动杆菌属、丁酸弧菌属、克里斯滕森菌和断链真杆菌显著富集。值得注意的是, 隐丹参酮有效降低IL-6、MPO、DAO及TC含量的同时, 可显著增加TG含量并降低脂肪酶活性。环境因子相关性分析显示, g_norank_f_Muribaculaceae、瘤胃球菌(g_Ruminococcaceae_UCG-014)和梭菌属(g_Clostridium_sensu_stricto_1)与脂质及炎性因子表达均有显著相关性。此外, 本研究报道了伪无菌小鼠中CIM显著缓解, 炎性因子及TG/TC比例也与正常对照组无显著差异, 证明肠道菌群在小鼠CIM发生发展中具有关键作用。已有研究证实炎症介质与脂质代谢密切相关, 炎性过程中产生的IL-6、IL11及TNFα等, 可能通过影响3-羟基-3-甲基戊二酰辅酶A还原酶(3-hydroxy-3-methylglutaryl coenzyme A reductase, HMG-CoA)及胆固醇羟化酶(cholesterol hydroxylase)的表达, 调节肝细胞载脂蛋白的合成与分泌, 降低肝脏TC的分解代谢, 提高TC含量[20]。在CIM小鼠中, 作者确实发现IL-6的表达与TC的含量呈正相关, 而与TG含量呈负相关。据报道, TNFα可以诱导炎症脂肪组织降解, 促进TG从极低密度脂蛋白中释放, 并增加肝脂肪酸合成及减弱脂肪酸氧化而提高TG含量。虽然CIM小鼠中TNFα含量显著增加, 但TG含量却显著降低。脂肪酶隶属于羧基酯水解酶类, 能够逐步将TG水解成甘油和脂肪酸, 在微生物中有广泛的分布[21]。由于隐丹参酮可以有效降低CIM小鼠中脂肪酶活性而对TNFα含量无明显抑制作用, 其很有可能是通过抑制脂肪酶活性和抑制TG水解而提高血清中TG含量。结合相关性分析结果, 隐丹参酮防治小鼠CIM的作用很有可能是通过降低粪便菌群梭菌属(g_Clostridium_sensu_stricto_1)丰度、提高g_norank_f_Muribaculaceae及瘤胃球菌属(g_Ruminococcaceae_UCG-014)丰度, 调控炎性因子及脂质代谢实现的。
综上所述, 本研究证实了肠道菌群在化疗性肠黏膜炎发生发展中的关键作用, 报道隐丹参酮可能通过调控肠道菌群, 有效改善小鼠化疗性肠黏膜炎, 其作用机制除了抑制CIM小鼠炎性因子表达及脂肪酶活性, 还可能与脂质代谢调控密切相关。
作者贡献:王霖和杜冠华负责实验设计; 王睿、魏广义和王霖负责动物实验及相关指标检测和数据处理; 王霖和王睿负责论文的撰写; 杜冠华和王淑美负责论文的指导及审阅。
利益冲突:无相关利益冲突。
| [1] |
Ichim TE, Kesari S, Shafer K. Protection from chemotherapy- and antibiotic-mediated dysbiosis of the gut microbiota by a probiotic with digestive enzymes supplement[J]. Oncotarget, 2018, 9: 30919-30935. DOI:10.18632/oncotarget.25778 |
| [2] |
Alexander JL, Wilson ID, Teare J, et al. Gut microbiota modulation of chemotherapy efficacy and toxicity[J]. Nat Rev Gastroenterol Hepatol, 2017, 14: 356-365. DOI:10.1038/nrgastro.2017.20 |
| [3] |
Kim SH, Chun HJ, Choi HS, et al. Ursodeoxycholic acid attenuates 5-fluorouracil-induced mucositis in a rat model[J]. Oncol Lett, 2018, 16: 2585-2590. |
| [4] |
Ribeiro RA, Wanderley CW, Wong DV, et al. Irinotecan- and 5-fluorouracil-induced intestinal mucositis:insights into pathogenesis and therapeutic perspectives[J]. Cancer Chemother Pharmacol, 2016, 78: 881-893. DOI:10.1007/s00280-016-3139-y |
| [5] |
Xia C, Bai X, Hou X, et al. Cryptotanshinone reverses cisplatin resistance of human lung carcinoma A549 cells through down-regulating Nrf2 pathway[J]. Cell Physiol Biochem, 2015, 37: 816-824. DOI:10.1159/000430398 |
| [6] |
Blander JM, Longman RS, Iliev ID, et al. Regulation of inflammation by microbiota interactions with the host[J]. Nat Immunol, 2017, 18: 851-860. DOI:10.1038/ni.3780 |
| [7] |
Gao J, Xu K, Liu H, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism[J]. Front Cell Infect Microbiol, 2018, 8: 13. DOI:10.3389/fcimb.2018.00013 |
| [8] |
Ibrahim A, Hugerth LW, Hases L, et al. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity[J]. Int J Cancer, 2019, 144: 3086-3098. DOI:10.1002/ijc.32037 |
| [9] |
Liang H, Dai Z, Liu N, et al. Dietary L-tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets[J]. Front Microbiol, 2018, 9: 1736. DOI:10.3389/fmicb.2018.01736 |
| [10] |
Florez AB, Sierra M, Ruas-Madiedo P, et al. Susceptibility of lactic acid bacteria, bifidobacteria and other bacteria of intestinal origin to chemotherapeutic agents[J]. Int J Antimicrob Agents, 2016, 48: 547-550. DOI:10.1016/j.ijantimicag.2016.07.011 |
| [11] |
Lin Z, Zu XP, Xie HS, et al. Research progress in mechanism of intestinal microorganisms in human diseases[J]. Acta Pharm Sin (药学学报), 2016, 51: 843-852. |
| [12] |
Kuhn KA, Schulz HM, Regner EH, et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity[J]. Mucosal Immunol, 2018, 11: 357-368. DOI:10.1038/mi.2017.55 |
| [13] |
Sivaprakasam S, Bhutia YD, Yang S, et al. Short-chain fatty acid transporters:role in colonic homeostasis[J]. Compr Physiol, 2017, 8: 299-314. |
| [14] |
Zeng H, Umar S, Rust B, et al. Secondary bile acids and short chain fatty acids in the colon:a focus on colonic microbiome, cell proliferation, inflammation, and cancer[J]. Int J Mol Sci, 2019, 20: E1214. DOI:10.3390/ijms20051214 |
| [15] |
Ohkusa T, Koido S. Intestinal microbiota and ulcerative colitis[J]. J Infect Chemother, 2015, 21: 761-768. DOI:10.1016/j.jiac.2015.07.010 |
| [16] |
Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis:mechanisms and clinical application of probiotics and fecal microbiota transplantation[J]. World J Gastroenterol, 2018, 24: 5-14. DOI:10.3748/wjg.v24.i1.5 |
| [17] |
Koutroumpakis E, Ramos-Rivers C, Regueiro M, et al. Association between long-term lipid profiles and disease severity in a large cohort of patients with inflammatory bowel disease[J]. Dig Dis Sci, 2016, 61: 865-871. DOI:10.1007/s10620-015-3932-1 |
| [18] |
Saresella M, Mendozzi L, Rossi V, et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients:a pilot study[J]. Front Immunol, 2017, 8: 1391. DOI:10.3389/fimmu.2017.01391 |
| [19] |
Wang B, Rong X, Palladino END, et al. Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis[J]. Cell Stem Cell, 2018, 22: 206-220.e4. DOI:10.1016/j.stem.2017.12.017 |
| [20] |
Lubrano V, Gabriele M, Puntoni MR, et al. Relationship among IL-6, LDL cholesterol and lipid peroxidation[J]. Cell Mol Biol Lett, 2015, 20: 310-322. |
| [21] |
Wang C, Niimi M, Kitajima S, et al. Sex hormones affect endothelial lipase-mediated lipid metabolism and atherosclerosis[J]. Lipids Health Dis, 2019, 18: 226. DOI:10.1186/s12944-019-1175-4 |
 2020, Vol. 55
2020, Vol. 55


