作者贡献:汪溪是本实验研究的执行人; 汪溪和罗祥完成数据分析和论文初稿的写作; 汪溪、罗祥、梁磊、赵锦、孙艳辉、徐红帅和蔡希强参与实验设计和实验结果分析; 栗原博、李怡芳和何蓉蓉是项目的构思者及负责人, 负责指导实验设计、数据分析和论文写作与修改。
利益冲突:所有作者均声明不存在利益冲突。
2. 暨南大学, 广东省中药药效物质基础和创新药物研究重点实验室, 广东 广州 510632;
3. 暨南大学药学院, 中药及天然药物研究所, 广东 广州 510632;
4. 锦州中心医院, 辽宁 锦州 121017;
5. 锦州市传染病医院, 辽宁 锦州 121017
2. Guangdong Province Key Laboratory of Pharmacodynamic Constituents of Traditional Chinese Medicine and New Drugs Research, Jinan University, Guangzhou 510632, China;
3. Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, China;
4. Jinzhou Central Hospital, Jinzhou 121017, China;
5. Jinzhou Infectious Disease Hospital, Jinzhou 121017, China
急性肝损伤是肝实质细胞严重受损的结果, 通常是由药物滥用、大量饮酒、病毒感染等原因引起的肝功能的急速下降, 严重时甚至危及生命[1, 2]。急性肝损伤世界发病率呈逐年上升趋势, 目前临床治疗肝损伤的有效药物较少且使用有局限性, 亟待开发出药效显著、毒副作用小的药物。近年来研究表明, 中医药对急性肝损伤具有显著的预防和治疗作用, 多环节的综合作用、稳定持久的作用效果、对机体不良反应小等优点吸引了国内外研究者的广泛关注[3]。扶正养肝合剂(Fuzheng Yanggan Fomula, FYF)由当归、五味子、枸杞、虫草菌丝4味药材组成, 均是卫生部批准的药食同源中药, 安全性有保障。FYF具有抗炎、抗氧化和抗肝损伤等作用, 同时具有补气养血、扶正固本、增强机体免疫能力的功效。现代研究表明, 五味子素A可预防d-半乳糖胺诱导的急性肝损伤, 增加肝细胞清除自由基能力和抗氧化活性[4]; 枸杞多糖可有效减轻四氯化碳(carbon tetrachloride, CCl4)诱导的急性肝毒性, 降低肝细胞一氧化氮代谢和过氧化脂质水平, 保护小鼠肝脏免受氧化应激和坏死性炎症的影响[5]; 当归多糖通过增加谷胱甘肽(glutathione, GSH)水平来抑制肝细胞凋亡并减轻乙酰氨基酚(acetaminophen, APAP)诱导的肝脏损伤[6]; 虫草菌丝能显著缓解二甲基亚硝胺诱导的过氧化损伤, 同时减少胶原纤维形成, 阻止大鼠肝硬化[7]。此外, 本课题组前期研究成果显示, FYF能抑制肝脏细胞脂质过氧化并缓解APAP诱导的小鼠药物性肝损伤[8]。然而, 目前有关由4种中药组成的FYF对肝损伤的保护作用及抗炎机制尚不明确。Nod样受体蛋白3 (Nod-like receptor protein 3, NLRP3)炎症小体是介导机体免疫炎症和细胞死亡的关键调控因子, 越来越多的证据表明, NLRP3炎症小体的活化在肝损伤以及肝纤维化过程中发挥重要作用[9, 10]。因此, 本研究采用痤疮丙酸杆菌(Propionibacterium acnes, P. acnes)和脂多糖(lipopolysaccharide, LPS)建立小鼠急性肝损伤模型, 探讨FYF对急性肝损伤小鼠肝脏的保护作用, 初步探究其作用机制, 为肝损伤疾病的治疗和预防提供理论依据和新的药物作用靶点。
材料与方法试剂 FYF [辽宁润生康泰生物医药科技有限公司, 批号Z20170002, 企业内部质量控制标准。FYF是临床经验方并已申请专利(CN201811199635.3), 该药物由当归30~50份、五味子25~45份、枸杞30~50份和虫草菌丝5~10份制成, 在临床广泛应用于免疫性肝损伤和药物性肝损伤的预防和治疗。该合剂成分清楚, 疗效确切, 质量可控]; LPS和β-actin抗体(美国Sigma公司); P. acnes (美国组织培养库); NLRP3特异性抑制剂(a specific inhibitor of NLRP3 inflammasome, MCC950) (美国Selleck公司); NLRP3抗体、白细胞介素-1β (interleukin-1β, IL-1β)抗体、pro-IL-1β抗体、天冬氨酸特异性半胱氨酸蛋白酶-1 (cysteinyl aspartate specific proteinase-1, caspase-1)抗体、pro-caspase-1抗体和凋亡相关斑点样蛋白(apoptosis-associated speck-like protein containing a CARD, ASC)抗体(美国细胞信号技术公司); 细胞表面糖蛋白F4/80 (cell surface glycoprotein F4/80, F4/80)抗体(美国Santa Cruz公司); 蛋白上样缓冲液、羊抗兔-辣根过氧化物酶(horseradish peroxidase, HRP)标记二抗、羊抗鼠-HRP标记二抗和显影液(杭州弗德生物公司); IL-1β和肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)酶联免疫吸附测定(enzyme linked immunosorbent assay, ELISA)检测试剂盒(美国诺为生物公司); 抗氧化能力指数(oxygen radical absorbance capacity, ORAC)检测试剂盒(美国Abcam公司); 谷丙转氨酶(alanine transaminase, ALT)、谷草转氨酶(aspartate aminotransferase, AST)、GSH和丙二醛(malonaldehyde, MDA)检测试剂盒(南京建成生物工程研究所); 苏木素-伊红(hematoxylinendash-eosin, HE)染色试剂盒(碧云天生物技术有限公司); 原位细胞凋亡检测试剂盒(美国罗氏公司)。
主要仪器设备 氢离子浓度指数(hydrogen ion concentration, pH)计和高速冷冻离心机(德国赛多利斯公司); 石蜡包埋机(德国徕卡公司); QM100型多功能高通量组织研磨仪(上海净信科技有限公司); 全自动化学发光成像分析系统(上海天能科技器材公司)。
动物分组与给药 无特定病原(specific pathogen free, SPF)级雄性BALB/c小鼠(实验动物许可证号: SCXK 2016-0041)购自南方医科大学实验动物中心。小鼠适应性喂养1周后随机分为5组, 每组8只小鼠, 包括:空白对照组(control); 模型组(P. acnes/LPS); 阳性对照组(P. acnes/LPS + MCC950), 腹腔注射MCC950 (20 mg·kg-1); FYF高剂量治疗组(P. acnes/LPS + high-dose FYF, P. acnes/LPS + F-H), 灌胃高剂量FYF (240 mg·kg-1); FYF低剂量治疗组(P. acnes/LPS + low-dose FYF, P. acnes/LPS + F-L), 灌胃低剂量FYF (120 mg·kg-1)。为保证实验严谨性, 其他组也灌胃等量生理盐水。FYF连续灌胃BALB/c小鼠21天, 除空白对照组外, 其他组均在第16天尾静脉注射P. acnes (20 mg·kg-1), 连续刺激5天, 第21天一次性尾静脉注射LPS (50 μg·kg-1)造模。阳性对照组在P. acnes注射后第2天腹腔注射MCC950 (20 mg·kg-1), MCC950隔天给1次药, 连续给药3次。LPS给药1天后采用异氟烷麻醉小鼠并取材。实验过程中每天记录小鼠体重。动物福利和实验过程均遵循暨南大学动物伦理委员会的规定。
血清ALT、AST、IL-1β和TNF-α含量的测定 小鼠麻醉后眼眶取血, 静置4 h后, 4 ℃、3 000 r·min-1离心10 min, 离心后取上清。血清中ALT与AST的水平按照南京建成试剂盒说明书进行检测。血清中IL-1β与TNF-α含量按照ELISA试剂盒说明书进行检测。
小鼠肝组织MDA、GSH和ORAC含量的测定 肝脏取出后称重, 取适量肝组织匀浆后提取上清液, 用二喹啉甲酸(bicinchoninic acid, BCA)法对组织匀浆液中的蛋白进行定量。根据试剂盒说明书检测肝组织MDA、GSH和ORAC水平。
肝组织病理学和免疫学观察 肝组织用4%多聚甲醛固定并包埋于石蜡中, 4 μm切片, HE染色后, 脱水, 封片, 扫描显微镜下观察肝组织病理学变化。对于F4/80免疫组织染色, 肝脏切片经苏木素染色后, 将载玻片与F4/80抗体(1:100)于4 ℃孵育过夜后, 与HRP标记二抗于室温孵育30 min, 二氨基联苯胺四盐酸盐(diaminobenzidine tetrahydrochloride, DAB)工作液显色, 中性树脂封片后, 扫描显微镜下观察肝组织巨噬细胞浸润情况。
末端脱氧核苷酸转移酶标记(terminal deoxynucleotidyl transferase dUTP nick end labeling, TUNEL)染色法检测肝脏细胞凋亡 包埋切片同HE染色, 肝脏切片经脱蜡, 水化, 抗原修复后, 3% H2O2封闭10 min, TUNEL反应液4 ℃孵育30 min, 3%牛血清白蛋白(bovine serum albumin, BSA) 37 ℃封闭15 min, 过氧化物酶(peroxidase, POD)反应液室温孵育30 min, DAB工作液显色, 然后苏木素染核, 中性树脂封片, 扫描显微镜下观察肝细胞凋亡情况, 肝细胞核显示棕色即为TUNEL阳性细胞。
Western blot法检测肝组织中NLRP3炎症小体通路蛋白表达 肝脏组织经裂解液提取后进行蛋白定量试剂盒定量。取30 μg蛋白样品经聚丙烯酰胺凝胶电泳后, 转膜, 5%脱脂奶粉封闭, 分别与β-actin (1:3 000)、NLRP3 (1:1 000)、ASC (1:1 000)、pro-caspase-1 (1:1 000)、caspase-1 (1:2 000)、pro-IL-1β (1:1 000)和IL-1β (1:1 000)一抗4 ℃孵育过夜。洗膜后, 加入羊抗兔-HRP标记二抗或羊抗鼠-HRP标记二抗(1:3 000)室温孵育1 h, 在化学发光工作站中进行拍照, 以β-actin为内参, 应用Image J软件统计NLRP3炎症小体通路蛋白相对表达量。
统计学分析 实验数据以
如图 1所示, 与正常组相比, P. acnes/LPS组小鼠体重明显下降(P < 0.001), 而FYF高、低剂量组和MCC950组小鼠体重未见明显下降; 与正常组相比, P. acnes/LPS组小鼠肝脏指数明显增加(P < 0.001), 而FYF高、低剂量组和MCC950组小鼠肝脏指数未见明显增加; FYF高、低剂量组和MCC950组小鼠肝脏指数与P. acnes/LPS组比较均明显减小(P < 0.01)。
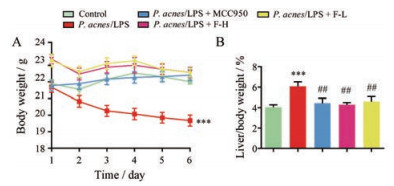
|
Figure 1 Effects of Fuzheng Yanggan Fomula (FYF) on the body weight and liver index in P. acnes/LPS mice. A: Body weight of mice; B: The percentage of liver of body weight of mice. Data are expressed as |
血清中ALT和AST水平升高是急性肝损伤肝细胞严重受损的标志。如图 2所示, 与正常组相比, P. acnes/LPS组血清ALT和AST水平显著升高(P < 0.001), 表明肝损伤模型建立成功。FYF高、低剂量组和MCC950组血清ALT、AST水平与P. acnes/LPS组比较均明显下降(P < 0.001)。
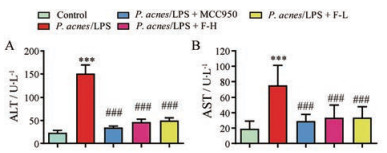
|
Figure 2 Effects of FYF on the levels of serum ALT and AST in P. acnes/LPS mice. A: The levels of serum ALT in mice; B: The levels of serum AST in mice. Data are expressed as |
如图 3所示, 与正常组相比, P. acnes/LPS组小鼠血清IL-1β和TNF-α显著增加(P < 0.001); FYF高、低剂量组和MCC950组小鼠血清IL-1β水平与P. acnes/LPS组相比均明显降低(P < 0.01)。与P. acnes/LPS组相比, 高剂量FYF治疗后, 小鼠肝脏TNF-α水平略微降低(P < 0.05), 低剂量组和MCC950组没有显著性变化。

|
Figure 3 The effects of FYF on pro-inflammatory cytokines in P. acnes/LPS-stimulated mice. The levels of IL-1β and TNF-α in the livers were determined by enzyme linked immunosorbent assay (ELISA). A: The levels of IL-1β in mice; B: The levels of TNF-α in mice. Data are expressed as |
对小鼠肝组织中GSH、MDA和ORAC的水平进行检测, 结果如图 4所示。与正常组相比, P. acnes/LPS模型组肝组织MDA水平明显升高(P < 0.001); FYF高、低剂量和MCC950组肝组织MDA水平与P. acnes/LPS组比较均明显降低(P < 0.01)。ORAC是目前抗氧化研究领域中为人们所关注的一个评价方法, AAPH (2, 2'-azobis-2-methylpropionamidine dihydrochloride)在水溶液中可释放过氧自由基, 并能将荧光素氧化, 使其荧光特性消失。当抗氧化剂水溶性维生素E (trolox)存在时, 可与荧光素竞争氧化剂, 减缓其荧光消褪的速度。因此, 根据这一特性, 可测定样品氧自由基清除活性。P. acnes/LPS组肝组织GSH和ORAC的水平显著低于正常组(P < 0.001); FYF高、低剂量和MCC950组肝组织GSH和ORAC的水平与P. acnes/LPS组相比均显著升高(P < 0.01)。
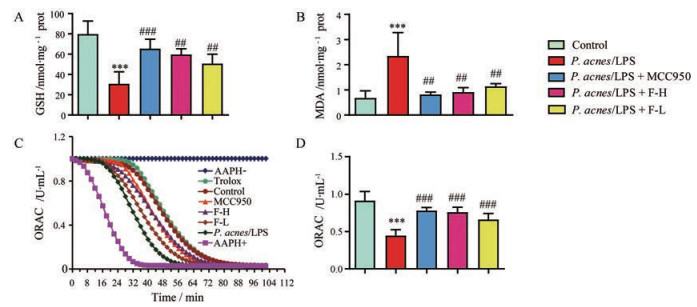
|
Figure 4 Levels of MDA, GSH, and ORAC in livers. A: The levels of GSH; B: The levels of MDA; C and D: The evaluation of direct antioxidant capacity of FYF by in vitro ORAC assay (C) and the statistical analyses (D). AAPH, 1 mmol·L-1; Trolox, 20 μmol·L-1; F-L, 10 μmol·L-1; F-H, 20 μmol·L-1; MCC950, 20 μmol·L-1. Data are expressed as |
HE染色结果显示(图 5), 正常组肝细胞形态正常, 排列整齐, 无炎症或充血。而P. acnes/LPS小鼠肝脏细胞间质大量充血, 并伴大量炎性细胞浸润, 表明其病理肝损伤程度明显增加。FYF高、低剂量组和MCC950组与P. acnes/LPS组相比, 其肝细胞炎性细胞浸润和肝细胞间质充血程度明显减少, 表明其病理肝损伤程度明显减少。

|
Figure 5 Histopathological analyses of hematoxylinendash-eosin (HE) staining. A: HE staining images of liver sections; B: Hepatic injury scores of liver sections. Scale bar: 50 μm. Data are expressed as |
HE染色结果显示FYF能缓解P. acnes/LPS对小鼠肝脏细胞的损伤, 本研究进一步采用TUNEL染色法检测FYF对各组小鼠肝脏细胞凋亡的影响。如图 6所示, 正常组几乎没有观察到凋亡的肝细胞, 而P. acnes/LPS组观察到大量的棕色颗粒, 说明凋亡的肝细胞明显增多(P < 0.001), 给予FYF高、低剂量和MCC950治疗后, 小鼠肝脏中凋亡的肝细胞明显减少(P < 0.001)。
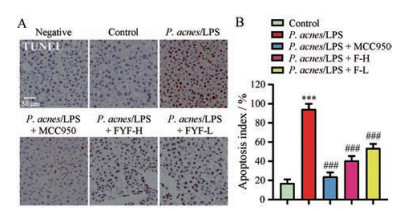
|
Figure 6 Effects of FYF on hepatocyte death in P. acnes/LPS treated mice. A: Representative photographs of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining assay of liver tissue sections; B: The apoptosis index was generated by counting the numbers of apoptosis cells in different treatments in three randomly selected regions. Scale bar: 50 μm. Data are expressed as |
F4/80是小鼠巨噬细胞表面标志物, 免疫染色结果显示(图 7):与正常组相比, P. acnes/LPS刺激的肝组织中巨噬细胞浸润显著增加(P < 0.001);而FYF高、低剂量和MCC950组肝组织巨噬细胞浸润与P. acnes/LPS组相比均明显减少(P < 0.001)。

|
Figure 7 Immunostaining of liver sections for F4/80 after P. acnes/LPS and FYF administration. A: Representative photographs of immunostaining assay for F4/80 of liver tissue sections; B: Relative intensity of F4/80-positive in liver was calculated by Image J software. Scale bar: 50 μm. Data are expressed as |
作者进一步研究了FYF缓解P. acnes/LPS诱导的急性肝损伤是否与NLRP3炎症小体活化被抑制有关。蛋白印迹实验结果显示(图 8), 与正常组相比, P. acnes/LPS组中小鼠肝脏的NLRP3、pro-IL-1β、IL-1β和caspase-1蛋白的表达显著增加(P < 0.01), 表明P. acnes/LPS确实激活NLRP3炎症小体并上调了NLRP3和IL-1β蛋白的表达。P. acnes/LPS组pro-caspase-1和ASC蛋白的表达较正常组无明显变化。与P. acnes/LPS组比较, FYF高、低剂量和MCC950均能显著抑制NLRP3炎症小体的组成成分caspase-1和IL-1β蛋白的表达下调(P < 0.05)。

|
Figure 8 FYF inhibited the activation of NLRP3 inflammasome in mice treated with P. acnes/LPS. Western blot analyses of NLRP3 (A), ASC (B), pro-caspase-1 and caspase-1 (C), and pro-IL-1β and IL-1β (D). Western blot images are the representative results of one mouse in each group of three mice, and the relative intensity analyses are the statistic results of three mice. Data are expressed as |
NLRP3炎症小体是机体介导免疫炎症和细胞死亡的关键调控因子, 主要由NLRP3、衔接蛋白ASC以及效应蛋白caspase-1和白细胞介素IL-1β组成[11]。在受到微生物、微生物毒素和细胞内危险信号的刺激后, NLRP3蛋白累积到一定量后自身寡聚化并招募下游蛋白ASC和pro-caspase-1组装, pro-caspase-1被自身蛋白酶裂解形成活化的caspase-1, 活化的caspase-1进一步将pro-IL-1β切割为成熟的IL-1β[12, 13], 炎症小体的活化在急性肝损伤以及肝纤维化过程中发挥重要作用。P. acnes/LPS肝损伤模型属于免疫肝损伤模型, P. acnes是肝损伤的致敏诱导剂, 可以导致肝脏炎性细胞浸润, 形成结节性肉芽肿。LPS是革兰阴性细菌细胞壁成分, 它能够激活单核/巨噬细胞NLRP3炎症小体信号通路, 释放大量炎症介质, 造成肝脏炎性细胞的浸润及肝细胞的损伤[14]。而经典的CCl4肝损伤模型是化学肝损伤模型, 其肝损伤机制是通过产生三氯甲基自由基(CCl3·)和过氧化三氯甲基自由基(OOCCl3·)与肝细胞膜、内质网和线粒体上的磷脂分子发生共价结合, 而引发脂质过氧化反应以损害膜的结构和功能, 导致肝细胞损伤[15]。P. acnes联合LPS是目前激活NLRP3炎症小体信号通路诱导急性肝损伤模型的常用诱导剂[16, 17]。因此, 本研究采用P. acnes/LPS方法建立小鼠急性肝损伤模型。
本研究结果显示, FYF可显著抑制P. acnes/LPS诱导的ALT和AST水平, 缓解了P. acnes/LPS造成的肝组织病变, 减少了炎性细胞浸润和出血, 并可减少P. acnes/LPS诱导的肝细胞凋亡, 抑制P. acnes/LPS诱导的巨噬细胞浸润, 这些结果均表明FYF对于P. acnes/LPS造成的急性肝损伤具有显著的治疗作用。P. acnes/LPS激活活性氧产生是导致肝损伤的主要因素[18, 19]。MDA是脂质过氧化的指标, GSH具有中和自由基和保护肝细胞免受氧化损伤的能力, 二者是用于评估机体抗氧化潜在能力的重要参数。本研究证明FYF可降低MDA含量, 增加ORAC和GSH水平, 提升肝脏的抗氧化能力, 从而产生较好的肝脏保护作用。另外, P. acnes/LPS可诱导小鼠肝脏IL-1β水平明显升高, 而FYF能显著抑制P. acnes/LPS诱导的小鼠肝脏中caspase-1和IL-1β的表达, 这提示FYF可能是通过抑制NLRP3炎症小体通路缓解了P. acnes/LPS诱导的肝损伤。综上, FYF对P. acnes/LPS诱导的急性肝损伤具有保护作用, 其作用机制与抑制NLRP3炎症小体通路激活有关。
本研究成功揭示了FYF可通过抑制NLRP3炎症小体激活并降低氧化应激来预防和治疗P. acnes/LPS诱导的肝损伤, 可为NLRP3炎症小体相关疾病提供一定的预防和治疗参考。中药、天然产物及其衍生物在新药研发中占据重要地位, 着力开发和研究具有潜在临床治疗价值的药物的作用机制具有重要意义, 将为今后研究提供一定的理论基础, 并为肝损伤治疗寻找新的突破口, 为未来治疗肝损伤带来曙光。
| [1] |
Chung RT, Stravitz RT, Fontana RJ, et al. Pathogenesis of liver injury in acute liver failure[J]. Gastroenterology, 2012, 143: e1-e7. |
| [2] |
Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults[J]. Liver Transpl, 2008, 14: S67-S79. DOI:10.1002/lt.21612 |
| [3] |
Tang X, Wei R, Deng A, et al. Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice[J]. Nutrients, 2017, 9: 1000. DOI:10.3390/nu9091000 |
| [4] |
Lu Y, Wang WJ, Song YZ, et al. The protective mechanism of schisandrin A in d-galactosamine-induced acute liver injury through activation of autophagy[J]. Pharm Biol, 2014, 52: 1302-1307. DOI:10.3109/13880209.2014.890232 |
| [5] |
Xiao J, Liong EC, Ching YP, et al. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation[J]. J Ethnopharmacol, 2012, 139: 462-470. DOI:10.1016/j.jep.2011.11.033 |
| [6] |
Cao P, Sun J, Sullivan MA, et al. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro[J]. Int J Biol Macromol, 2018, 111: 1133-1139. DOI:10.1016/j.ijbiomac.2018.01.139 |
| [7] |
Peng Y, Chen Q, Yang T, et al. Cultured mycelium Cordyceps sinensis protects liver sinusoidal endothelial cells in acute liver injured mice[J]. Mol Biol Rep, 2014, 41: 1815-1827. DOI:10.1007/s11033-014-3031-y |
| [8] |
He Y, Liu CY, He CC, et al. Protective effect of Fuzheng Yanggan Mixture on drug-induced liver injury[J]. China J Chin Mater Med (中国中药杂志), 2018, 43: 4685-4691. |
| [9] |
Peng Y, French BA, Tillman B, et al. The inflammasome in alcoholic hepatitis:its relationship with Mallory-Denk body formation[J]. Exp Mol Pathol, 2014, 97: 305-313. DOI:10.1016/j.yexmp.2014.08.006 |
| [10] |
Ouyang X, Ghani A, Mehal WZ. Inflammasome biology in fibrogenesis[J]. Biochim Biophys Acta, 2013, 1832: 979-988. DOI:10.1016/j.bbadis.2013.03.020 |
| [11] |
Subramanian N, Natarajan K, Clatworthy MR, et al. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation[J]. Cell, 2013, 153: 348-361. DOI:10.1016/j.cell.2013.02.054 |
| [12] |
Guo H, Callaway JB, Ting JP. Inflammasomes:mechanism of action, role in disease, and therapeutics[J]. Nat Med, 2015, 21: 677-687. DOI:10.1038/nm.3893 |
| [13] |
Mariathasan S, Monack DM. Inflammasome adaptors and sensors:intracellular regulators of infection and inflammation[J]. Nat Rev Immunol, 2007, 7: 31-40. DOI:10.1038/nri1997 |
| [14] |
Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria[J]. Cell, 2016, 164: 896-910. DOI:10.1016/j.cell.2015.12.057 |
| [15] |
Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism (s):a critical discussion[J]. Environ Toxicol Pharmacol, 2014, 37: 118-133. DOI:10.1016/j.etap.2013.08.015 |
| [16] |
Tsutsui H, Nishiguchi S. Importance of Kupffer cells in the development of acute liver injuries in mice[J]. Int J Mol Sci, 2014, 15: 7711-7730. DOI:10.3390/ijms15057711 |
| [17] |
Gu Q, Wu Q, Jin M, et al. Heme oxygenase-1 alleviates mouse hepatic failure through suppression of adaptive immune responses[J]. J Pharmacol Exp Ther, 2012, 340: 2-10. DOI:10.1124/jpet.111.186551 |
| [18] |
Pan CW, Pan ZZ, Hu JJ, et al. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation[J]. Eur J Pharmacol, 2016, 770: 85-91. DOI:10.1016/j.ejphar.2015.12.006 |
| [19] |
Wei Y, Chen P, de Bruyn M, et al. Carbon monoxide-releasing molecule-2(CORM-2) attenuates acute hepatic ischemia reperfusion injury in rats[J]. BMC Gastroenterol, 2010, 10: 42. DOI:10.1186/1471-230X-10-42 |
 2020, Vol. 55
2020, Vol. 55


