作者贡献:叶圣洁负责总结归纳所收集到的文献, 起草并撰写文章; 胡凯莉负责论文选题、指导、审阅文章和提供行政、技术上的支持。
利益冲突:论文不涉及相关知识产权, 不存在利益冲突。
1981年, Trams等[1]首次在电子显微镜下观察到来自不同正常细胞系和肿瘤细胞系的培养物产生的具有5'-核苷酸酶活性的小泡, 其平均直径为500~1 000 nm, 并且通常包含直径约40 nm的第二个囊泡群。随后Pan等[2]首次报道了在绵羊网织红细胞成熟过程中, 外泌体是通过质膜向内出芽形成胞内内质体, 并成为细胞表面蛋白质交换的手段。1987年Johnstone正式将其命名为“exosome”, 即外泌体。
进一步研究表明, 外泌体是一种起源于内吞的小膜泡, 大小为40~100 nm。外泌体的纳米膜型结构(图 1)是由脂质双层构成的, 由表面黏附蛋白、特定配体(四跨膜蛋白、整合素、CD11b和CD18受体)和主要组织相容性复合体(major histocompatibility complex, MHC)附着, 包含微小核糖核酸(micro ribonucleic acid, miRNA)、信使核糖核酸(messenger ribonucleic acid, mRNA)、脱氧核糖核酸片段和蛋白质。此外, 外泌体还富含脂类, 包括胆固醇、神经酰胺和磷酸甘油酸, 以及饱和脂肪酸链。外泌体可以从供体细胞传递到受体细胞, 并且可以在新的位置发挥功能[3-5]。
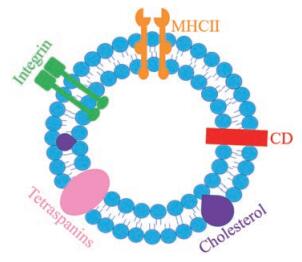
|
Figure 1 Structure diagram of exosomes. CD: Cluster of differentiation; MHC II: Major histocompatibility |
多种细胞在正常及病理状态下均可分泌外泌体。在免疫细胞、间充质干细胞、成纤维细胞、神经元、内皮细胞和上皮细胞等细胞中都能观察到外泌体的产生[6], 且外泌体天然存在于体液中, 包括血液、唾液、尿液、脑脊液、乳汁、羊水、腹水和胆汁中[7-14]。目前外泌体被视为特异性分泌的膜泡, 有介导细胞间通讯、免疫系统功能、发育和分化、神经元功能、细胞信号、肿瘤侵袭和再生等方面的作用[15-22]。
2 外泌体作为脑靶向释药载体的优势由于血脑屏障的限制, 将药物递送入脑内一直是治疗脑部疾病时面临的重大挑战。血脑屏障由脑微血管内皮细胞、星形胶质细胞、周细胞、内皮基底膜及邻近神经元组成。
脑内皮细胞具有紧密连接和黏附连接的复杂排列, 在调节细胞旁通透性中起着关键作用[23]。血脑屏障是一种动态界面, 限制和控制物质在外周血管循环和中枢神经系统(central nervous system, CNS)之间的通过, 防止神经毒性血浆成分、血细胞和病原体进入大脑[24], 从而保护CNS免受有害物质或过度免疫反应的影响[25-27]。然而这种复杂的界面在保护大脑免受可能存在于全身循环中的有害化学物质或毒素伤害的同时, 也导致治疗药物无法跨越血脑屏障, 约98%的小分子药物和几乎所有的大分子生物药物, 包括重组蛋白、单克隆抗体或基因药物, 不能穿过血脑屏障[28, 29]。
纳米载体的小尺寸造成的“纳米效应”, 使其可以通过正常的内吞作用内化到大多数细胞(包括脑毛细血管内皮细胞)中[30]。因而被广泛用于多种脑部疾病治疗的研究。常见的纳米载体包括纳米粒、脂质体和胶束等, 药物可以通过溶解、包埋、吸附、封装或共价连接到纳米材料上。无机材料(如二氧化硅、碳、金属或金属氧化物)的纳米粒尺寸稳定, 其表面具有独特的介孔结构, 巨大的比表面积和孔容、选择性的表面功能, 易于进行功能化以促进血脑屏障的渗透[31, 32]。但其不可降解而产生的潜在毒性问题极大限制了其在脑部疾病治疗中的应用[33]。脂质体是由单层或多层磷脂双层包裹的水性内核的纳米囊泡, 有良好的生物相容性, 能够包载亲水性药物, 可以作为完整的形式穿过血脑屏障, 因此被广泛应用于脑部药物递送[34, 35]。但其稳定性差, 以及可用表面基团数量少和空间位阻等问题限制了其进一步应用[31]。胶束是由两亲性嵌段共聚物制成的, 在水溶液中聚集形成具有疏水核和亲水表面的稳定球状纳米结构[36]。由于胶束有可能通过疏水作用和氢键溶解疏水核心区不易溶于水的亲脂性化合物, 并且有能力与某些靶向配体结合, 因此胶束也被广泛用于脑部给药载体研究[37]。然而, 由于胶束的特殊结构, 其对于亲水性的药物递送受到限制。
近年来, 有大量研究显示外泌体在CNS的稳态、病理和随后的恢复中起着关键作用, 与上述脑靶向递释载体相比, 外泌体具有免疫原性低、半衰期长和传递率高等特点, 可以与其他生物工程方法相结合以增强其生物分布, 尤其是其天然的绕过血脑屏障的能力[38-40], 能提高药物的脑内递送效率。Alvarez-Erviti等[41]用自衍生树突状细胞外泌体降低了免疫原性, 加载神经元特异性狂犬病毒衍生肽(rabies-virus-derived peptide, RVG)实现了靶向大脑神经元、小胶质细胞和少突胶质细胞。Rupert等[42]研究证实外泌体的zeta负电位赋予其体内的长循环时间。在Qu等[43]研究中发现, 以外泌体为载药系统包载多巴胺通过静脉注射进入大脑比注射游离的多巴胺效果增强了15倍, 证实了外泌体的高传递率。多种来源的外泌体都已被证实具有跨越血脑屏障的能力。在脑胶质瘤荷瘤小鼠和人胶质母细胞瘤患者的血液循环中检测到肿瘤来源的外泌体, 证实外泌体具有穿越血脑屏障的能力[44]。树突状细胞的外泌体能够将治疗用的小干扰核糖核酸(small interfering ribonucleic acid, siRNA)通过血脑屏障传递到小鼠大脑[39]。来自小鼠淋巴瘤细胞系的外泌体可通过鼻内给药将姜黄素通过血脑屏障传递给脑内小胶质细胞, 以减轻实验性自身免疫性脑脊髓炎中的脑内炎症和自身免疫反应[45]。
3 外泌体脑靶向的机制外泌体可以通过介导脑微血管内皮细胞内吞作用穿过血脑屏障[46]。而内吞作用可以通过许多不同的途径实现。通过一系列表面黏附蛋白、特定载体配体(四跨膜蛋白、整合素、CD11b和CD18受体)和MHC附着, 外泌体被有效传递给靶细胞[47, 48]。各种黏附蛋白如细胞间黏附分子-1 (intercellular cell adhesion molecule-1, ICAM-1)及从人脑微血管内皮D3细胞(human microvascular endothelial cells/D3, HCMEC/D3)中分离的四跨膜蛋白CD9、CD63和CD81在星形胶质细胞和皮层神经元之间的通讯中起着非常重要的作用[41], 也是外泌体跨越血脑屏障的重要途径。
在体外模型中证实Mϕ衍生的外泌体从其亲本细胞中继承了淋巴细胞功能相关抗原-1 (lymphocyte function associated antigen-1, LFA-1)和ICAM-1共同介导了HCMEC/D3对Mϕ外泌体的摄取[49]。Yang等[50]在斑马鱼体内证实外泌体能够通过介导与脑微血管内皮细胞CD63结合内化而透过血脑屏障, 这种介导是能量依赖性内化过程。ICAM-1和树突状细胞表面标志物DEC205被认为是小鼠巨噬细胞源性外泌体内化入HCMEC/D3细胞的受体[51]。另有研究显示, 基于交联和综合蛋白质组学鉴定, CD46是HCMEC/D3摄取脑转移癌细胞外泌体的主要受体[52]。
4 外泌体在脑部疾病治疗中的应用基于以上外泌体的优点和能够通过血脑屏障的特性, 其已被广泛用于CNS退行性疾病、脑肿瘤和脑血管疾病等多种脑部疾病的治疗(图 2)。
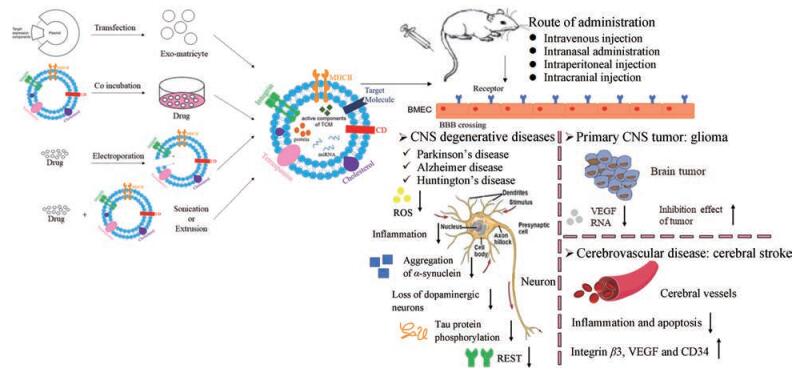
|
Figure 2 The application of exosomes in the treatment of brain diseases. BMEC: Brain microvascular endothelial cells; CNS: Central nervous system; ROS: Reactive oxygen species; REST: Repressor element 1 silencing transcription factor; VEGF: Vascular endothelial growth factor |
CNS退行性疾病是指一组由慢性进行性的中枢神经组织退行性变性而产生的疾病总称。病理上可见脑或脊髓发生神经元退行性变性、丢失, 随着时间的推移而恶化, 出现功能障碍。主要疾病包括帕金森病(Parkinson's disease, PD)、阿尔茨海默病(Alzheimer disease, AD)和亨廷顿病(Huntington's disease, HD)等。
神经元与胶质细胞的通讯是CNS功能性突触传递和生理学的基础[53, 54]。外泌体在体内的定位, 尤其是在神经元室中的定位尚不清楚。通过建立细胞型特异性外显子报告小鼠并进行随后的共聚焦和免疫电镜分析, 显示了神经元CaMKII-Cre诱导的细胞外定位的CD63-绿色荧光蛋白(green fluorescent protein, GFP)+小泡, 提供了第1个从大脑神经元分泌CD63+小泡的原位证据。脑切片免疫电镜分析和培养神经元共聚焦分析均发现, Cre诱导的细胞内CD63-GFP+点状结构主要定位于核周胞体和树突, 而非轴突[55]。
PD是一种常见的运动障碍, 其典型运动特征与路易体和黑质多巴胺能神经元的丧失有关。由于病灶位于脑内, 药物选择有限。目前左旋多巴是主要的治疗药物, 但左旋多巴诱发的运动障碍在PD患者中普遍存在, 因此需要开发更有效的治疗药物, 提高PD患者的生活质量[56, 57]。多巴胺能黑质神经元的丢失和含有路易小体的α-突触核蛋白的形成被认为是导致PD的原因[58, 59]。用饱和溶液孵育法能有效地将多巴胺装入外泌体, 通过三维成像及流式细胞术, 观察到所制备的外泌体在bEnd.3细胞的细胞质中大量聚集。通过静脉注射负载多巴胺的外泌体, 近红外荧光成像显示这些外泌体能成功地将多巴胺传递到大脑的纹状体和黑质[43]。Izco等[60]运用电穿孔将短发夹核糖核酸(short hairpin RNA, shRNA)加载到RVG外泌体中, 通过静脉注射至小鼠体内的包载shRNA的RVG外泌体, 减少了PD小鼠大脑中α-突触核蛋白的聚集和多巴胺能神经元的丢失。PD与脑内炎症、小胶质细胞活化和分泌性神经毒性活动有关, 包括活性氧增多[61-64]。PD患者大脑中的样本检测结果显示, 氧化还原酶、过氧化氢酶和超氧化物歧化酶以及其他抗氧化剂的水平降低, 导致PD患者脑内的氧化应激和神经变性[65]。Haney等[66]将过氧化氢酶通过室温孵育、皂甙渗透、冻融循环、超声或挤压4种不同的方法体外装载到外泌体中。共聚焦成像显示, 与PC12神经细胞孵育3 h后外泌体有效地被神经元细胞摄取; 这些外泌体经鼻腔给药后, PD小鼠脑内检测到大量的载过氧化氢酶的外泌体。实验结果显示, 空白外泌体载体在大脑中没有神经毒性作用, 负载过氧化氢酶的外泌体在PD体内外模型中具有明显的神经保护作用[65, 66]。Kojima等[67]运用6-羟基多巴胺诱导PD模型, 通过质粒转染的方式, 将含过氧化氢酶mRNA的外泌体通过静脉注射至C57BL/6J小鼠体内, 结果显示, 外泌体减少了活性氧引发的神经炎症, 且能减轻脂多糖导致的神经元毒性。
以健忘症为主要临床表现的AD, 研究标准主要是通过正电子发射断层扫描和脑脊液分析来研究淀粉样蛋白和Tau蛋白的生物标记物状态。目前的流行病学资料大多依赖于生物标志物未确诊的AD病例, 强调载脂蛋白E和年龄是主要的危险因素。罕见的常染色体显性突变也占早发性AD的一小部分。然而, 目前对AD发病机制的认识还很有限, 由于缺乏可靠的非AD性神经退行性疾病生物标记物, 早期诊断和治疗仍是当前AD治疗的挑战[68, 69]。AD与淀粉样β蛋白沉积形成的细胞外炎性斑块和磷酸化Tau蛋白形成的神经元纤维缠结有关[70, 71]。Wang等[72]研究发现, 外泌体能提高姜黄素的溶解度和生物利用度, 并通过LFA-1和ICAM-1增加药物在血脑屏障的渗透性。通过共孵育的方式得到包载姜黄素的巨噬细胞外泌体, 与HCMEC/D3细胞共孵育后, 相较于几乎没有被摄取的游离姜黄素, 包载姜黄素的外泌体展现出较强的荧光, 进一步实验发现外泌体的内化是通过LFA-1与ICAM-1相互作用介导的。腹腔注射包载姜黄素的外泌体通过激活AKT/GSK-3β途径抑制Tau蛋白磷酸化, 减轻AD症状。通过冰浴超声孵育得到载槲皮素外泌体, 具有较高的生物相容性和安全性, 能提高槲皮素的脑靶向性, 显著提高槲皮素的生物利用度。体内实验结果证明, 载槲皮素外泌体通过静脉注射至SD大鼠体内, 能抑制细胞周期蛋白依赖性激酶5介导的Tau磷酸化和减少不溶性神经纤维缠结的形成, 更好地缓解AD的症状[73]。
HD是一种常染色体显性遗传性神经退行性疾病, 由编码亨廷顿蛋白的基因中CAG重复序列的异常扩增引起。HD主要由基因异常扩增的父母传播, 每个后代有50%的机会在突变基因中遗传可变重复长度的不稳定CAG扩增, 即所谓的预期。考虑到HD发病年龄和临床预后的不可预测性, 以及与潜在的不同遗传变异前和变异条件相关的许多可能的遗传和临床情况, 疾病的诊断和治疗仍面临着挑战[74, 75]。尽管HD神经退行性病变的确切机制尚不清楚, 但其中一个机制涉及转录调节因子的改变, 如抑制元件1沉默转录因子(repressor element 1 silencing transcription factor, REST)[76]。突变的亨廷顿蛋白不再沉默REST的活性, 这种缺失导致REST与抑制元件1神经元限制性沉默元件的结合增加, 产生转录功能障碍[77], 因此降低突变的亨廷顿蛋白和REST水平为治疗HD提供了有效的策略[78]。在Lee等[79]研究中, 通过质粒转染的方式, 从miRNA-124过度表达的细胞系中得到了含miRNA-124的外泌体。当含miRNA-124的外泌体被注射到大脑纹状体时, 显著降低了大脑中REST的表达, 提高了对HD的治疗效果。
4.2 外泌体在脑部肿瘤治疗中的应用穿透血脑屏障并向肿瘤输送治疗剂量的抗癌药物是脑部肿瘤治疗中的一个重大挑战[80]。化疗是癌症治疗的金标准, 但对脑部肿瘤的疗效有限, 因为药物不能有效地通过血脑屏障到达大脑[80-82]。抗癌药物如紫杉醇、多柔比星、甲氨蝶呤和长春新碱等都不能跨越血脑屏障[80, 83, 84]。而外泌体具有低免疫原性、高生物降解性和低毒性, 且对所载药物有很强的保护作用, 更能够跨越血脑屏障[85-88]。在斑马鱼脑室注射DiD标记的U-78MG细胞建立原发性脑癌模型, 共孵育得到载多柔比星的外泌体经主静脉给药, 能够显著抑制斑马鱼脑肿瘤模型中血管内皮生长因子的核糖核酸(ribonucleic acid, RNA)[51]。
神经胶质瘤是最常见的原发性CNS肿瘤, 其死亡率和致残率高, 预后差[89]。Jia等[90]根据电穿孔和点击化学的原理, 制备了神经肽-1靶向肽修饰的、超顺磁性氧化铁纳米粒和姜黄素负载的外泌体, 用于胶质瘤细胞和原位异种移植瘤的靶向成像和治疗。结果表明, 制备的外泌体通过静脉注射能顺利穿过血脑屏障, 并准确识别胶质瘤; 且磁流热疗联合姜黄素外泌体应用有协同作用, 与未联合磁流热疗组相比, 使得对胶质瘤抑制效果显著提高。Zhu等[91]构建了体外胶质母细胞瘤模型和体内皮下及原位异种移植模型, 负载紫杉醇的胚胎干细胞外泌体通过环(精氨酸-甘氨酸-天门冬氨酸-D-酪氨酸-赖氨酸)肽[cyclo(arginine-glycine-aspartate-D-tyrosine-lysine) peptide, c (RGDyK)]修饰来增强肿瘤靶向性, 尾静脉给药后, 修饰c (RGDyK)靶头与未修饰的外泌体相比, 显著抑制了肿瘤的生长, 提高了紫杉醇治疗小鼠胶质母细胞瘤的疗效。在原位磷酸酶和张力素同源物缺陷的胶质瘤小鼠模型中, 通过质粒转染的方式, 得到含有mRNA的外泌体, 比较了将内源性转录的RNA封装成外泌体和将外源核苷酸导入预先分离的外泌体的效率, 结果显示前者是后者的100多倍。将进一步修饰了胶质瘤靶向肽的外泌体静脉注射到原位移植的人U87胶质瘤免疫缺陷小鼠体内, 结果显示外泌体恢复了磷酸酶和张力素同源物的表达, 从而达到对肿瘤生长的抑制作用, 提高了小鼠存活率[92]。
外泌体可以帮助药物很好地跨越血脑屏障, 与脑内退行性病变相比, 在脑部肿瘤治疗的应用中, 外泌体上通常会修饰一个肿瘤靶向分子, 例如c (RGDyK)就是一个常用的肿瘤靶向分子, 能更好靶向脑肿瘤细胞, 提高药物的传递效率和抗肿瘤效果。
4.3 外泌体在脑血管疾病治疗中的应用脑卒中是一种急性脑血管疾病, 是由于脑部血管突然破裂或因血管阻塞导致血液不能流入大脑而引起脑组织损伤的一组疾病。由脑动脉血栓栓塞引起的缺血性脑卒中占所有脑卒中的80%以上[93, 94]。目前唯一批准的治疗这类脑卒中的方法是通过应用重组组织纤溶酶原激活剂[95]。然而, 这种方法受到治疗窗口窄(< 4.5 h)的限制[96]。由于外泌体具有免疫原性低、生物降解性好、毒性低和能跨越血脑屏障等优点, 可以作为一个安全有效的递药系统, 应用于缺血性脑卒中的治疗。Tian等[97]在构建的短暂性大脑中动脉闭塞小鼠模型中, 验证了c (RGDyK)修饰的骨髓间充质干细胞外泌体可作为姜黄素治疗大脑缺血的有效载体, 静脉注射外泌体有效地抑制了C57BL/6小鼠缺血性脑损伤区域的炎症和细胞凋亡。Zhang等[98]设计了c (RGDyK)偶联到骨髓间充质干细胞外泌体, 并被加载有胆固醇修饰的miRNA-210, 通过静脉注射修饰后的载miRNA-210的外泌体, 结果显示C57BL/6小鼠大脑中整合素β3、血管内皮生长因子和CD34显著升高, 说明血管生成得到改善。
4.4 外泌体在其他脑部疾病治疗中的应用创伤性脑损伤(traumatic brain injury, TBI)是世界范围内最常见的死亡和神经营养不良相关损伤的发病原因之一[99]。国际上每年有超过5 000万人被新诊断为TBI[100]。TBI的产生涉及一系列复杂的病理过程, 主要可分为两个阶段。原发性脑损伤主要由外部冲击引起, 并导致急性病理改变, 包括脑挫伤、脑出血和轴突剪切。继发性病理改变, 包括氧化应激、钙超载、线粒体损伤、谷氨酸兴奋性毒性和神经炎症等, 导致神经功能进一步受损[101, 102]。Li等[103]通过模拟体外脑损伤的微环境, 发现富含miRNA-21-5p的神经元外泌体可以通过靶向Rab11a抑制神经元自噬活性, 从而减轻创伤诱导的、自噬介导的神经损伤。
Liu等[104]通过转染的方式得到负载阿片受体Mu (opioid receptor mu, MOR) siRNA的狂犬病病毒糖蛋白外泌体, MOR是吗啡、芬太尼和美沙酮等临床阿片类镇痛药的主要靶点, 也导致了阿片类药物的成瘾性。制备的外泌体静脉注射至小鼠体内, 显著降低了MOR mRNA和蛋白水平, 且外泌体传递的MOR siRNA通过下调MOR表达水平强烈抑制吗啡成瘾的复发。
5 总结与展望无机纳米粒存在尚未解决的潜在毒性问题, 胶束的结构限制其对于水溶性药物的递送, 脂质体存在稳定性差, 以及脂质体可用表面基团数量少和空间位阻而难以将配体结合到脂质体表面等问题。而外泌体免疫原性低、天然稳定性好、半衰期长、传递率高和能穿越血脑屏障等特点, 使其成为安全有效脑部递药载体的研究热点, 广泛应用于CNS退行性疾病、脑肿瘤和脑血管疾病等的治疗中。外泌体包载的药物可以是蛋白、核酸类药物, 也可以是化疗药物或中药单体, 如姜黄素、槲皮素和紫杉醇等。根据药物性质的不同可以通过质粒转染、饱和溶液孵育法、电穿孔、超声或挤压等方法将药物载入外泌体, 通过静脉注射、经鼻给药、腹腔注射及颅内注射等方式给药。
与细胞膜一样, 外泌体具有脂质双分子结构, 能有效地装载疏水性和亲水性药物, 特别适合于核酸类药物的包载。基于核酸治疗的一个主要限制是其细胞和组织的递送率低。多年来, 多种体内基因传递技术尝试改善核酸的递送, 包括病毒载体和合成纳米载体[105, 106]。然而, 这些策略仍面临着毒性、免疫原性、质量控制和高成本, 及其无法通过特殊的生理屏障(如血脑屏障)等问题。外泌体的优点有望改善核酸药物的递送[41, 107, 108]。外泌体具有生物相容性, 表达跨膜和膜锚定蛋白, 这些蛋白质的存在可延长血液循环, 促进组织定向输送, 并促进细胞摄取包裹的外泌体内容物[107, 109]。Yang等[92]研究发现, 通过质粒转染的方式将内源性转录的RNA封装成外泌体的效率是外源核苷酸导入预先分离的外泌体的效率的100多倍。
虽然用电穿孔将miRNA和shRNA质粒插入外泌体后, 在抑制癌基因靶点方面比合成的纳米载体显示出更大的治疗效果, 但将大核酸包载入外泌体仍存在技术上的挑战性, 可能仅限于特定细胞类型的外泌体[110]。尽管已经提出了生物修饰细胞源以促进RNA在外泌体中的包封策略[67, 111], 但在不进行基因修饰的情况下, 诱导从多个有核细胞源释放大量载有所需核苷酸转录的外泌体还未实现。其次, 尽管外泌体具有穿透血脑屏障的能力, 但也有研究表明, 静脉注射的外泌体主要分布在肝脏或脾脏, 脑内或肿瘤部位的分布仍然较低。用肿瘤靶向配体直接修饰外泌体表面已被证明能有效地提高外泌体的肿瘤递送率[90, 112]。c (RGDyK)是一种已知的肿瘤化疗靶向配体[97, 98], 与αvβ3整合素受体具有高度的亲和力, c (RGDyK)修饰的纳米载体能更有效地将化疗药物导入肿瘤细胞, 并能显著提高抗肿瘤作用。此外, 用c (RGDyK)修饰的外泌体也具有更强的跨血脑屏障能力[97]。因此, c (RGDyK)可作为一个可靠的靶向修饰分子提高外泌体的靶向性。
外泌体可以携带母细胞膜上细胞特异性蛋白质, 如少突胶质细胞外泌体的髓鞘蛋白, 具有独特的归巢选择性[41]。因此, 外泌体母细胞的选择也是影响其靶向性的重要因素。
综上所述, 外泌体作为药物载体在脑部疾病治疗领域的进一步应用, 仍有赖于解决大核酸的包载、外泌体母细胞的选择及脑靶向效率的提高等问题, 这些问题仍有待进一步的研究探讨。
| [1] |
Trams EG, Lauter CJ, Salem N, et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles[J]. Biochim Biophys Acta, 1981, 645: 63-70. DOI:10.1016/0005-2736(81)90512-5 |
| [2] |
Pan BT, Teng K, Wu C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes[J]. J Cell Biol, 1985, 101: 942-948. DOI:10.1083/jcb.101.3.942 |
| [3] |
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes:current perspectives and future challenges[J]. Acta Pharm Sin B, 2016, 6: 287-296. DOI:10.1016/j.apsb.2016.02.001 |
| [4] |
Balaj L, Lessard R, Dai LX. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences[J]. Nat Commun, 2011, 2: 180. DOI:10.1038/ncomms1180 |
| [5] |
Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nat Cell Biol, 2007, 9: 654-659. DOI:10.1038/ncb1596 |
| [6] |
Kalluri R. The biology and function of exosomes in cancer[J]. J Clin Invest, 2016, 126: 1208-1215. DOI:10.1172/JCI81135 |
| [7] |
Caby MP, Danielle L, Claude VS, et al. Exosomal-like vesicles are present in human blood plasma[J]. Int Immunol, 2005, 17: 879-887. DOI:10.1093/intimm/dxh267 |
| [8] |
Ogawa Y, Miura Y, Harazono A, et al. Proteomic analysis of two types of exosomes in human whole saliva[J]. Biol Pharm Bull, 2011, 34: 13-23. DOI:10.1248/bpb.34.13 |
| [9] |
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine[J]. Proc Natl Acad Sci U S A, 2004, 101: 13368-13373. DOI:10.1073/pnas.0403453101 |
| [10] |
Vella LJ, Sharples RA, Lawson VA, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing[J]. J Pathol, 2007, 211: 582-590. DOI:10.1002/path.2145 |
| [11] |
Admyre C, Johansson SM, Qazi KRA, et al. Exosomes with immune modulatory features are present in human breast milk[J]. J Immunol, 2007, 179: 1969-1978. DOI:10.4049/jimmunol.179.3.1969 |
| [12] |
Asea A, Claudel JP, Punit K, et al. Heat shock protein-containing exosomes in mid-trimester amniotic fluids[J]. J Reprod Immunol, 2008, 79: 12-17. DOI:10.1016/j.jri.2008.06.001 |
| [13] |
Andre F, Schartz EC, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes[J]. Lancet, 2002, 360: 295-305. DOI:10.1016/S0140-6736(02)09552-1 |
| [14] |
Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia[J]. Am J Physiol Gastrointest Liver Physiol, 2010, 299: G990-G999. DOI:10.1152/ajpgi.00093.2010 |
| [15] |
Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication[J]. Curr Opin Cell Biol, 2009, 21: 575-581. DOI:10.1016/j.ceb.2009.03.007 |
| [16] |
Borges FT, Melo SA, Özdemir BA, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis[J]. J Am Soc Nephrol, 2013, 24: 385-392. DOI:10.1681/ASN.2012101031 |
| [17] |
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles[J]. Annu Rev Cell Dev Biol, 2014, 30: 255-289. DOI:10.1146/annurev-cellbio-101512-122326 |
| [18] |
Gross JC, Chaudhary V, Bartscherer K, et al. Active Wnt proteins are secreted on exosomes[J]. Nat Cell Biol, 2012, 14: 1036-1045. DOI:10.1038/ncb2574 |
| [19] |
Akers JC, Gonda D, Kim Y, et al. Biogenesis of extracellular vesicles (EV):exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies[J]. J Neurooncol, 2013, 113: 1-11. DOI:10.1007/s11060-013-1084-8 |
| [20] |
Wendler F, Rabassedas NB, Marro XF. Cancer becomes wasteful:emerging roles of exosomes (dagger) in cell-fate determination[J]. J Extracell Vesicles, 2013. DOI:10.3402/jev.v2i0.22390 |
| [21] |
Zhang HG, Grizzle WE. Exosomes:a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions[J]. Am J Pathol, 2014, 184: 28-41. DOI:10.1016/j.ajpath.2013.09.027 |
| [22] |
Costa SB, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver[J]. Nat Cell Biol, 2015, 17: 816-826. DOI:10.1038/ncb3169 |
| [23] |
Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier:development, composition and regulation[J]. Vascul Pharmacol, 2002, 38: 323-337. DOI:10.1016/S1537-1891(02)00200-8 |
| [24] |
Montagne A, Zhao Z, Zlokovic BV. Alzheimer's disease:a matter of blood-brain barrier dysfunction?[J]. J Exp Med, 2017, 214: 3151-3169. DOI:10.1084/jem.20171406 |
| [25] |
Andreone BJ, Lacoste B, Gu C. Neuronal and vascular interactions[J]. Annu Rev Neurosci, 2015, 38: 25-46. DOI:10.1146/annurev-neuro-071714-033835 |
| [26] |
Banks WA. From blood-brain barrier to blood-brain interface:new opportunities for CNS drug delivery[J]. Nat Rev Drug Discov, 2016, 15: 275-292. DOI:10.1038/nrd.2015.21 |
| [27] |
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier[J]. Nat Med, 2013, 19: 1584-1596. DOI:10.1038/nm.3407 |
| [28] |
Pardridge WM. Drug transport across the blood-brain barrier[J]. J Cereb Blood Flow Metab, 2012, 32: 1959-1972. DOI:10.1038/jcbfm.2012.126 |
| [29] |
Pardridge WM. The blood-brain barrier:bottleneck in brain drug development[J]. NeuroRx, 2005, 2: 3-14. DOI:10.1602/neurorx.2.1.3 |
| [30] |
Vilella A, Tosi G, Grabrucker AM, et al. Insight on the fate of CNS-targeted nanoparticles. Part I:Rab5-dependent cell-specific uptake and distribution[J]. J Control Release, 2014, 174: 195-201. DOI:10.1016/j.jconrel.2013.11.023 |
| [31] |
Li X, Tsibouklis J, Weng T, et al. Nano carriers for drug transport across the blood-brain barrier[J]. J Drug Target, 2017, 25: 17-28. DOI:10.1080/1061186X.2016.1184272 |
| [32] |
Zhou Y, Quan G, Wu Q, et al. Mesoporous silica nanoparticles for drug and gene delivery[J]. Acta Pharm Sin B, 2018, 8: 165-177. DOI:10.1016/j.apsb.2018.01.007 |
| [33] |
Yang Z, Zhang Y, Yang Y, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease[J]. Nanomedicine, 2010, 6: 427-441. DOI:10.1016/j.nano.2009.11.007 |
| [34] |
Wen CJ, Zhang LW, Al-Suwayeh SA, et al. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging[J]. Int J Nanomedicine, 2012, 7: 1599-1611. |
| [35] |
Dai T, Jiang K, Lu W. Liposomes and lipid disks traverse the BBB and BBTB as intact forms as revealed by two-step Förster resonance energy transfer imaging[J]. Acta Pharm Sin B, 2018, 8: 261-271. DOI:10.1016/j.apsb.2018.01.004 |
| [36] |
Zhang P, Hu L, Yin Q, et al. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy[J]. Mol Pharm, 2012, 9: 1590-1598. DOI:10.1021/mp200600t |
| [37] |
Jiao X, Yu Y, Meng J, et al. Dual-targeting and microenvironment-responsive micelles as a gene delivery system to improve the sensitivity of glioma to radiotherapy[J]. Acta Pharm Sin B, 2019, 9: 381-396. DOI:10.1016/j.apsb.2018.12.001 |
| [38] |
Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke[J]. Front Cell Neurosci, 2014, 8: 377. |
| [39] |
Morad G, Carman CV, Hagedorn EJ, et al. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis[J]. ACS Nano, 2019, 13: 13853-13865. DOI:10.1021/acsnano.9b04397 |
| [40] |
Matsumoto J, Stewart T, Banks WA, et al. The transport mechanism of extracellular vesicles at the blood-brain barrier[J]. Curr Pharm Des, 2017, 23: 6206-6214. |
| [41] |
Alvarez-Erviti L, Seow YQ, Yin HF, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes[J]. Nat Biotechnol, 2011, 29: 341-345. DOI:10.1038/nbt.1807 |
| [42] |
Rupert DLM, Claudio V, Lässer C, et al. Methods for the physical characterization and quantification of extracellular vesicles in biological samples[J]. Biochim Biophys Acta Gen Subj, 2017, 1861: 3164-3179. DOI:10.1016/j.bbagen.2016.07.028 |
| [43] |
Qu MK, Lin Q, Huang LY, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease[J]. J Control Release, 2018, 287: 156-166. DOI:10.1016/j.jconrel.2018.08.035 |
| [44] |
Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers[J]. Nat Cell Biol, 2008, 10: 1470-1476. DOI:10.1038/ncb1800 |
| [45] |
Zhuang XY, Xiang XY, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain[J]. Mol Ther, 2011, 19: 1769-1779. DOI:10.1038/mt.2011.164 |
| [46] |
Chen CC, Liu LN, Ma FX, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro[J]. Cell Mol Bioeng, 2016, 9: 509-529. DOI:10.1007/s12195-016-0458-3 |
| [47] |
Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses[J]. Nat Rev Immunol, 2009, 9: 581-593. DOI:10.1038/nri2567 |
| [48] |
Thery C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids[J]. Curr Protoc Cell Biol, 2006. DOI:10.1002/0471143030.cb0322s30 |
| [49] |
Haqqani AS, Delaney CE, Tremblay TL, et al. Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells[J]. Fluids Barriers CNS, 2013, 10: 4. DOI:10.1186/2045-8118-10-4 |
| [50] |
Yang TZ, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio[J]. Pharm Res, 2015, 32: 2003-2014. DOI:10.1007/s11095-014-1593-y |
| [51] |
Yuan D, Zhao YL, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain[J]. Biomaterials, 2017, 142: 1-12. DOI:10.1016/j.biomaterials.2017.07.011 |
| [52] |
Kuroda H, Tachikawa M, Yagi Y, et al. Cluster of differentiation 46 is the major receptor in human blood-brain barrier endothelial cells for uptake of exosomes derived from brain-metastatic melanoma cells (SK-Mel-28)[J]. Mol Pharm, 2019, 16: 292-304. DOI:10.1021/acs.molpharmaceut.8b00985 |
| [53] |
Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions[J]. Physiol Rev, 2008, 88: 983-1008. DOI:10.1152/physrev.00036.2007 |
| [54] |
Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function[J]. Science, 2018, 362: 181-185. DOI:10.1126/science.aat0473 |
| [55] |
Men YQ, Yelick J, Jin SJ, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS[J]. Nat Commun, 2019, 10: 4136. DOI:10.1038/s41467-019-11534-w |
| [56] |
Kalia LV, Lang AE. Parkinson's disease[J]. Lancet, 2015, 386: 896-912. DOI:10.1016/S0140-6736(14)61393-3 |
| [57] |
Radhakrishnan DM, Goyal V. Parkinson's disease:a review[J]. Neurol India, 2018, 66: S26-S35. DOI:10.4103/0028-3886.226451 |
| [58] |
Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease[J]. Science, 2003, 302: 819-822. DOI:10.1126/science.1087753 |
| [59] |
Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein:new targets for drug discovery[J]. Neuron, 2006, 52: 33-38. DOI:10.1016/j.neuron.2006.09.026 |
| [60] |
Izco M, Blesa J, Schleef M, et al. Systemic exosomal delivery of shRNA minicircles prevents Parkinsonian pathology[J]. Mol Ther, 2019, 27: 2111-2122. DOI:10.1016/j.ymthe.2019.08.010 |
| [61] |
McGeer PL, Itagaki S, Boyes BE, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains[J]. Neurology, 1988, 38: 1285-1291. DOI:10.1212/WNL.38.8.1285 |
| [62] |
Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro[J]. Nature, 1995, 378: 776-779. DOI:10.1038/378776a0 |
| [63] |
Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson's disease[J]. Prog Neurobiol, 1996, 48: 1-19. DOI:10.1016/0301-0082(95)00029-1 |
| [64] |
Wu DC, Teismann P, Tieu K, et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine model of Parkinson's disease[J]. Proc Natl Acad Sci U S A, 2003, 100: 6145-6150. DOI:10.1073/pnas.0937239100 |
| [65] |
Abraham S, Soundararajan CC, Vivekanandhan S, et al. Erythrocyte antioxidant enzymes in Parkinson's disease[J]. Indian J Med Res, 2005, 121: 111-115. |
| [66] |
Haney MJ, Klyachko NL, Zhao YL, et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy[J]. J Control Release, 2015, 207: 18-30. DOI:10.1016/j.jconrel.2015.03.033 |
| [67] |
Kojima R, Bojar D, Rizzi G, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment[J]. Nat Commun, 2018, 9: 1305. DOI:10.1038/s41467-018-03733-8 |
| [68] |
Villain N, Dubois B. Alzheimer's disease including focal presentations[J]. Semin Neurol, 2019, 39: 213-226. DOI:10.1055/s-0039-1681041 |
| [69] |
Sun BL, Li WW, Zhu C, et al. Clinical research on Alzheimer's disease:progress and perspectives[J]. Neurosci Bull, 2018, 34: 1111-1118. DOI:10.1007/s12264-018-0249-z |
| [70] |
Holtzman DM, Goate A, Kelly J, et al. Mapping the road forward in Alzheimer's disease[J]. Sci Transl Med, 2011, 3: 114ps48. |
| [71] |
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease:progress and problems on the road to therapeutics[J]. Science, 2002, 297: 353-356. DOI:10.1126/science.1072994 |
| [72] |
Wang H, Sui HJ, Zheng Y, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway[J]. Nanoscale, 2019, 11: 7481-7496. DOI:10.1039/C9NR01255A |
| [73] |
Qi Y, Guo L, Jiang YB, et al. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles[J]. Drug Deliv, 2020, 27: 745-755. DOI:10.1080/10717544.2020.1762262 |
| [74] |
Pandey M, Rajamma U. Huntington's disease:the coming of age[J]. J Genet, 2018, 97: 649-664. DOI:10.1007/s12041-018-0957-1 |
| [75] |
Migliore S, Jankovic J, Squitieri F. Genetic counseling in Huntington's disease:potential new challenges on Horizon?[J]. Front Neurol, 2019, 10: 453. DOI:10.3389/fneur.2019.00453 |
| [76] |
Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease[J]. Trends Genet, 2003, 19: 233-238. DOI:10.1016/S0168-9525(03)00074-X |
| [77] |
Zuccato C, Tartari M, Crotti A, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes[J]. Nat Genet, 2003, 35: 76-83. DOI:10.1038/ng1219 |
| [78] |
Orozco DR, Sánchez AA, Hernández HJM, et al. The interaction between RE1-silencing transcription factor (REST) and heat shock protein 90 as new therapeutic target against Huntington's disease[J]. PLoS One, 2019, 14: e0220393. DOI:10.1371/journal.pone.0220393 |
| [79] |
Lee ST, Wooseok I, Jae JB, et al. Exosome-based delivery of miR-124 in a Huntington's disease model[J]. J Mov Disord, 2017, 10: 45-52. DOI:10.14802/jmd.16054 |
| [80] |
Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer[J]. Clin Cancer Res, 2010, 16: 5664-5678. DOI:10.1158/1078-0432.CCR-10-1564 |
| [81] |
Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier[J]. Adv Drug Deliv Rev, 2012, 64: 640-665. DOI:10.1016/j.addr.2011.11.010 |
| [82] |
Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients:a prospective study[J]. Neuro Oncol, 2015, 17: 289-295. DOI:10.1093/neuonc/nou141 |
| [83] |
Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy:a reality check[J]. J Clin Oncol, 2007, 25: 2295-2305. DOI:10.1200/JCO.2006.09.9861 |
| [84] |
Sathornsumetee S, Rich JN. New approaches to primary brain tumor treatment[J]. Anticancer Drugs, 2006, 17: 1003-1016. DOI:10.1097/01.cad.0000231473.00030.1f |
| [85] |
El AS, Lakhal S, Mäger I, et al. Exosomes for targeted siRNA delivery across biological barriers[J]. Adv Drug Deliv Rev, 2013, 65: 391-397. DOI:10.1016/j.addr.2012.08.008 |
| [86] |
Vader P, Emma AM, Gerard P, et al. Extracellular vesicles for drug delivery[J]. Adv Drug Deliv Rev, 2016, 106: 148-156. DOI:10.1016/j.addr.2016.02.006 |
| [87] |
Ingato D, Lee JU, Sim SJ, et al. Good things come in small packages:overcoming challenges to harness extracellular vesicles for therapeutic delivery[J]. J Control Release, 2016, 241: 174-185. DOI:10.1016/j.jconrel.2016.09.016 |
| [88] |
Kooijmans SAA, Schiffelers RM, Zarovni N, et al. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles:new nanotools for cancer treatment[J]. Pharmacol Res, 2016, 111: 487-500. DOI:10.1016/j.phrs.2016.07.006 |
| [89] |
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas:a clinical review[J]. JAMA, 2013, 310: 1842-1850. DOI:10.1001/jama.2013.280319 |
| [90] |
Jia G, Han Y, An YL, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo[J]. Biomaterials, 2018, 178: 302-316. DOI:10.1016/j.biomaterials.2018.06.029 |
| [91] |
Zhu QW, Ling XZ, Yang YL, et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy[J]. Adv Sci (Weinh), 2019, 6: 1801899. DOI:10.1002/advs.201801899 |
| [92] |
Yang ZG, Shi JF, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation[J]. Nat Biomed Eng, 2020, 4: 69-83. DOI:10.1038/s41551-019-0485-1 |
| [93] |
Kelly PJ, Kavanagh E, Murphy S. Stroke:new developments and their application in clinical practice[J]. Semin Neurol, 2016, 36: 317-323. DOI:10.1055/s-0036-1586261 |
| [94] |
Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update:a report from the American Heart Association[J]. Circulation, 2016, 133: e38-360. DOI:10.1161/CIR.0000000000000350 |
| [95] |
Rother J, Ford GA, Thijs VN. Thrombolytics in acute ischaemic stroke:historical perspective and future opportunities[J]. Cerebrovasc Dis, 2013, 35: 313-319. DOI:10.1159/000348705 |
| [96] |
Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy[J]. Lancet Neurol, 2015, 14: 758-767. DOI:10.1016/S1474-4422(15)00054-X |
| [97] |
Tian T, Zhang HX, He CP, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy[J]. Biomaterials, 2018, 150: 137-149. DOI:10.1016/j.biomaterials.2017.10.012 |
| [98] |
Zhang HX, Wu J, Wu JH, et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice[J]. J Nanobiotechnol, 2019, 17: 29. DOI:10.1186/s12951-019-0461-7 |
| [99] |
Johnson WD, Griswold DP. Traumatic brain injury:a global challenge[J]. Lancet Neurol, 2017, 16: 949-950. DOI:10.1016/S1474-4422(17)30362-9 |
| [100] |
Gao GY, Jiang JY. Chinese Head Trauma Data Bank:effect of gender on the outcome of patients with severe traumatic brain injury[J]. J Neurotrauma, 2012. DOI:10.1089/neu.2011.2134 |
| [101] |
Levin H, Smith D. Traumatic brain injury:networks and neuropathology[J]. Lancet Neurol, 2013, 12: 15-16. DOI:10.1016/S1474-4422(12)70300-9 |
| [102] |
Shlosberg D, Benifla M, Kaufer D, et al. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury[J]. Nat Rev Neurol, 2010, 6: 393-403. DOI:10.1038/nrneurol.2010.74 |
| [103] |
Li D, Huang S, Zhu JL, et al. Exosomes from miR-21-5p-increased neurons play a role in neuroprotection by suppressing Rab11a-mediated neuronal autophagy in vitro after traumatic brain injury[J]. Med Sci Monit, 2019, 25: 1871-1885. DOI:10.12659/MSM.915727 |
| [104] |
Liu YC, Li DM, Liu ZY, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse[J]. Sci Rep, 2015, 5: 17543. DOI:10.1038/srep17543 |
| [105] |
Fraietta JA, Nobles CL, Sammons MA, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells[J]. Nature, 2018, 558: 307-312. DOI:10.1038/s41586-018-0178-z |
| [106] |
Borducchi EN, Cabral C, Stephenson KE, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys[J]. Nature, 2016, 540: 284-287. DOI:10.1038/nature20583 |
| [107] |
Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer[J]. Nature, 2017, 546: 498-503. DOI:10.1038/nature22341 |
| [108] |
El AS, Mäger I, Breakefield XO, et al. Extracellular vesicles:biology and emerging therapeutic opportunities[J]. Nat Rev Drug Discov, 2013, 12: 347-357. DOI:10.1038/nrd3978 |
| [109] |
Squadrito ML, Cianciaruso C, Hansen SK, et al. EVIR:chimeric receptors that enhance dendritic cell cross-dressing with tumor antigens[J]. Nat Methods, 2018, 15: 183-186. DOI:10.1038/nmeth.4579 |
| [110] |
Usman WM, Pham TC, Kwok YY, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles[J]. Nat Commun, 2018, 9: 2359. DOI:10.1038/s41467-018-04791-8 |
| [111] |
Wang QY, Yu JJ, Kadungure T, et al. ARMMs as a versatile platform for intracellular delivery of macromolecules[J]. Nat Commun, 2018, 9: 960. DOI:10.1038/s41467-018-03390-x |
| [112] |
Wang J, Li W, Lu ZC, et al. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis[J]. Nanoscale, 2017, 9: 15598-15605. DOI:10.1039/C7NR04425A |
 2020, Vol. 55
2020, Vol. 55


