作者贡献:高丽娜负责资料的调研和初稿的撰写; 乔宏志负责全文写作构思的设计及制剂学内容的审校和修改; 胡立宏负责强心苷内容的修改和终审。
利益冲突:作者声明无利益冲突。
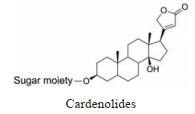
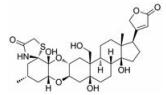
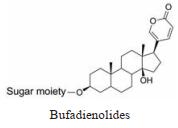
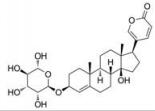
强心苷是一类对心脏具有显著生理活性的甾体苷类化合物, 在临床上已有200多年的应用历史。1775年英国医生威廉·威瑟林(William Withering)首先发现洋地黄可以治疗浮肿, 尤其对猩红热和咽喉炎引起的水肿疗效明显[1]。后被证实两类疾病导致的水肿实为链球菌引起的心瓣膜损伤所致。威瑟林医生的发现使洋地黄类药物在欧洲迅速推广。1930年, 史密斯(Sydney Smith)成功分离出地高辛, 并证明其在充血性心力衰竭和心房性心律不齐中的治疗作用。直到今日地高辛仍是治疗心力衰竭的有效药物[2]。随后, 一系列强心苷化合物被发现(表 1), 其中有不少品种如洋地黄毒苷(digitoxin)、毛花丙甙(lanatoside C)、醋洋地黄毒苷(acetyldigitoxin)和去乙酰毛花甙丙(deslanoside)获批上市。但后来的临床结果证明, 这些强心苷药物普遍存在治疗窗口窄、毒副作用大的问题, 有效剂量与中毒剂量非常接近, 且个体差异较大, 若服用不当, 极易发生中毒反应, 这直接导致多个强心苷药物被FDA撤市。20世纪末, 一项包括6 800例心衰患者为期3年的大型临床试验表明, 地高辛虽能降低患者因病情恶化而住院的风险, 但并未降低患者的全因死亡率[3]。另一项临床研究显示地高辛的使用还会增加房颤患者的死亡风险[4, 5]。这使得强心苷在治疗心衰领域中的地位不断下降。同时, 伴随一系列新型抗心衰药物, 如β受体阻滞剂、血管紧张素转化酶抑制剂的出现, 心衰的临床用药发生明显改变[6, 7], 强心苷药物逐渐退居二线[8]。
| Table 1 Typical cardiac glycosides |
但强心苷在抑制肿瘤方面的表现为其迎来新的转机。20世纪60年代, 临床医生曾尝试用强心苷治疗恶性肿瘤, 但因其作用机制不明和毒副作用大而终止[9, 10]。20世纪80年代前后, 研究者发现强心苷可以明显抑制肿瘤细胞增殖, 具有广谱抗肿瘤活性[11], 并对人源肿瘤表现出一定的选择性[12, 13]。在一项针对145名接受过强心苷治疗和290名没有接受强心苷治疗的癌症患者所作的回顾性分析中, 发现接受强心苷治疗的患者5年存活率明显高于未治疗组(风险比为0.62)[14], 表明强心苷在提高肿瘤患者生存率方面具有潜在优势。此外, 强心苷还显示具有阻止肿瘤转移的作用, 在给予乳腺癌小鼠3周的哇巴因(ouabain)治疗后, 成簇的循环肿瘤细胞减少98.8%, 表明肿瘤转移能力被抑制[15]。
2 强心苷抗肿瘤作用机制目前发现的强心苷抗肿瘤机制比较多样, 即使同一种化合物也存在多途径调控作用, 详细机制可参考相关报道[11, 12], 其中Na+/K+-ATP酶是强心苷抗肿瘤作用的靶点之一[16], 哇巴因可通过抑制Na+/K+-ATP酶活性, 诱导细胞凋亡, 同时激活下游Ras/Raf/MAPK信号级联, 抑制ERK的异常激活, 进而解除凋亡抑制[17]。夹竹桃苷(oleandrin)可以阻断NF-κB和激活蛋白-1的激活, 降低NF-κB的转录活性, 诱导肿瘤细胞凋亡[18]。近期研究发现, 蟾毒灵可下调肿瘤细胞内细胞周期A蛋白、Bcl-2、Bcl-XL和上调p21、Bax蛋白表达, 活化caspase-9, 促进肿瘤细胞凋亡并停滞在G0/G1期[19]。此外, 地高辛等强心苷还被报道可诱导免疫原性细胞死亡(immunogenic cell death, ICD)[14], 激活肿瘤特异性细胞毒性T细胞攻击肿瘤细胞, 提高机体抗肿瘤免疫反应[20, 21]。
3 强心苷抗肿瘤制剂的研究大量研究表明强心苷具有广阔的抗肿瘤应用前景, 但其水溶性差、治疗窗口窄, 以及固有的心脏、神经和胃肠道毒性问题严重阻碍了强心苷在抗肿瘤领域的临床应用[22]。通过创新制剂改变药物的体内动力学与组织分布, 从而发挥减毒增效作用, 是解决强心苷成药性难题的有效策略。近年来, 针对强心苷的制剂开发有较多报道, 选用的剂型和制剂技术也比较多样, 如脂质体、胶束、微球、包合物和贴片等(图 1, 表 2)[23-53]。这些工作很好地克服了强心苷的弊端, 推动了其临床转化进程。

|
Figure 1 Schematic illustration of the novel preparations to deliver the cardiac glycosides for reduced toxicity and enhanced antitumor efficiency |
| Table 2 Novel preparations of cardiac glycoside for cancer therapy. PEG: Polyethylene glycol; cRGD: Cyclic (arginine-glycine-aspartic acid peptide); TPGS: d-alpha-Tocopheryl polyethylene glycol 1 000 succinate; PLL: Poly-L-lysine; Oct: Octreotide; p(OEGMA): Poly(oligo(ethylene glycol) monomethyl ether methacrylate; G3-C12: Peptide G3-C12 (the sequence ANTPCGPYTHDCPVKR); PCL: Poly(e-caprolactone); FA: Folic acid; β-CD: β-Cyclodextrin.*Tumor models: human cervical carcinoma: HeLa; human lung carcinoma: A549, Lewis; gastric carcinoma: SGC7901, BGC803; human liver hepatocellular carcinoma: Hep G2; leukemia: HL-60, K562; colon carcinoma: SW1116, HCT-8, HCT116, SW620, SW480, CD-26; esophageal cancer: EC9706; prostatic carcinoma: PC-3, DU145; human breast cancer: MDA-MB-231, MCF-7, SKBR-3; human colon adenocarcinoma cells: Caco-2; glioma: U251, U87; human umbilical vein endothelial cells: HUVEC; mouse macrophage: RAW 264.7; melanoma: B16; mouse hepatoma cells: H22; mouse fibroblast: L929 |
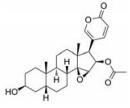
脂质体是将活性药物包封于磷脂双分子层之间(疏水性药物)或内部(亲水性药物)形成的纳米级球状载体制剂[54], 具有良好的生物相容性, 在强心苷的制剂设计中研究较多。蟾毒灵(bufalin, BF或BUF)是从传统中药蟾酥中提取得到的强心苷类化合物, 具有明显的抗肿瘤活性, 但溶解度差, 且有心脏和神经毒性。为此, 有研究者将BF制成了聚乙二醇化的脂质体(BF/PEG-LP)[26, 28], 显著扩大了BF的治疗窗口, 半数致死量(LD50)从0.156 mg·kg-1提高到3.03 mg·kg-1, 心脏部位药物浓度降低30%, AUC0-∞提高了4倍, 半衰期延长1倍, 显著降低了心脏毒性并提高了抗肿瘤效果。为进一步增加BF靶向性, 提高抗肿瘤效果, 有研究者制备了叶酸和转铁蛋白修饰的靶向脂质体[29], 利用肿瘤细胞高表达叶酸受体和转铁蛋白受体的性质, 提高药物在肿瘤部位的浓度。结果显示, 肿瘤细胞对靶向脂质体的摄取提高了6倍, 表现出更强的抗肿瘤效果。脂质体作为目前上市及申报数量最多的一类纳米制剂, 因其研发流程较为成熟、原料易得、制备工艺简单且易于放大而深受制剂研发者青睐。对已上市脂质体品种的临床回溯显示, 药物制成脂质体后避免了有机溶媒的使用, 改变了药物的体内分布规律, 可明显改善患者的顺应性, 扩大治疗窗口。因此, 对于存在溶解性和器官毒性问题的强心苷, 脂质体可作为首选开发剂型之一。在研发过程中脂质体常出现的载药量、稳定性和泄露等问题需要根据药物特点采取合适的处方和工艺技术予以解决。
3.2 胶束胶束制备方法简单, 载药能力较高, 在强心苷药物的制剂研发中也有较多应用。Jing等[32]开发了一种基于两亲性脂化肽树状大分子的递送系统, 在水中可以自组装形成发散型胶束, 利用共沉淀法制备的蟾毒灵胶束可以显著增加药物的溶解度(142.9 μg·mL-1 vs 42.4 μg·mL-1), 提高药物的细胞摄取能力。Wang等[33]以两亲性温敏性聚合物F127为基础制备了温敏和氧化还原响应的胶束, 用以装载BF。当胶束到达肿瘤部位时, 高浓度的谷胱甘肽(~10 mmol·L-1)会触发载体中的二硫键断裂, 迅速释放BF发挥抗肿瘤作用。还有学者采用壳聚糖(CS)和普郎尼克123 (P123)制备ATB-CP1胶束, 用于递送乙酰绒被素B (acetylthevetin B, ATB)[30]。CS可以通过氢键增强胶束纳米粒和肺癌细胞之间的生物黏附力, 结果显示ATB-CP1在肿瘤部位的荧光强度是游离ATB的15.31倍, 说明该递送系统具有优异的靶向递送能力, 在药效实验中ATB-CP1表现出更强的抗肿瘤活性。
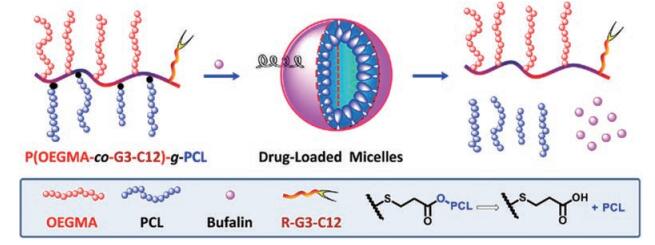
3.3 聚合物纳米粒聚合物纳米粒是以高分子材料为载体制成的载药胶体微粒, 可根据递送需求连接靶向配体设计为环境响应型制剂[55]。目前用于载强心苷的纳米粒主要有PLGA纳米粒、仿生膜-聚合物纳米粒和交联聚合物纳米粒等。这些材料一般具有良好的可调性, 可以根据药物缺陷设计专属的载体系统。Liu等[41]采用甲基丙烯酸寡聚乙二醇酯(OEGMA)、聚(ε-己内酯) (PCL)与肿瘤靶向肽反应合成刷状聚合物单体P(OEGMA-co-G3-C12)-g-PCL, 与BUF自组装形成BUF-NP-(G3-C12) (图 2), 该纳米粒借助靶向肽提高药物的肿瘤富集和抗肿瘤效果。
|
Figure 2 Schematic illustration for the fabrication of castration-resistant prostate cancer targeting micellar nanoparticles via self-assembly of the amphiphilic brush-type polymers, P(OEGMA-co-G3-C12)-g-PCL. The micelles consist of PCL as hydrophobic core and hydrophilic corona of pOEGMA and G3-C12. Hydrophobic anticancer drug, bufalin, was physically encapsulated into the hydrophobic cores of the micellar nanoparticles. p(OEGMA): Poly(oligo(ethylene glycol) monomethyl ether methacrylate; G3-C12: Peptide G3-C12 (the sequence ANTPCGPYTHDCPVKR); PCL: Poly(e-caprolactone). (Adapted from Ref. 41 with permission. Copyright © 2016 Elsevier) |
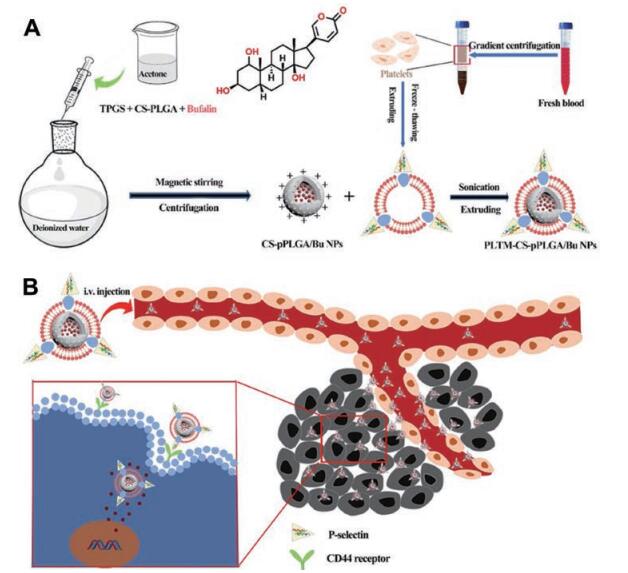
仿生膜与聚合物联用可提高载体的功能性[56]。Wang等[40]采用CS-PLGA共聚物载BF制备成纳米粒(CS-pPLGA/Bu NPs), 再与血小板膜(PLTM)通过挤出法组装形成新型纳米粒(PLTM-CS-pPLGA/Bu NPs) (图 3)。相对于无PLTM的纳米粒, H22肿瘤细胞对PLTM-CS-pPLGA/Bu NPs的摄取提高了7.4倍, 体内分布实验显示载药系统在肿瘤部位的荧光强度分别是肝和脾的1.15倍和3.12倍, 且24 h后仍具有明显的荧光强度, 展现出优越的肿瘤靶向能力。这主要归功于血小板膜上的P-selectin蛋白可以选择性靶向肿瘤细胞高表达的CD44受体, 提高了肿瘤靶向性。
|
Figure 3 Illustration of the preparation route to PLTM-CS-pPLGA/Bu NPs (A); in vivo targeted bufalin delivery to a tumor site mediated by binding of P-selectin on the surface of the PLTM to CD44 receptors of the tumor cells (B). TPGS: Vitamin E polyethylene glycol succinate (pore-forming); CS: Chitosan oligosaccharide; PLGA: Poly(lactic-co-glycolic acid); PLTM: Platelet membrane; Bu: Bufalin. (Adapted from Ref. 40 with permission. Copyright © 2019 BioMed Central Ltd.) |
聚合物纳米粒设计灵活, 可根据药物性质及预期目标选择合适的天然或合成材料作为载体, 进行功能性修饰也较方便, 是目前新型纳米制剂研究的热点。由于该类剂型高度依赖聚合物材料, 对材料的“药用载体”属性有很高的要求, 既要适合载药, 又要足够稳定地将药物送达患处, 同时不得对机体产生毒性、免疫原性和溶血等不良反应。事实上, 真正能同时满足上述条件的材料非常有限, 很多功能材料目前尚处于实验室研究阶段。已获批上市的载体多集中在PLGA聚酯材料或白蛋白天然材料上, 因此开发满足上述需要的药用载体对该类制剂的研发有重要意义。另外, 加强对已上市纳米制剂的临床评价, 追踪聚合物的体内代谢过程及对机体在更广范围的影响也非常必要。
3.4 前体药物将小分子抗癌药物与大分子聚合物偶联制备前体药物是克服小分子化合物成药性问题的有效策略之一。杠柳麻甙(periplocymarin, PPM)是从杠柳麻植物中提取的一种强心苷化合物, 对人源肿瘤细胞PC3、A549和A2780等表现出良好的抑制作用[57]。研究者采用聚乙二醇亚油酸盐衍生物(MPEG2000-LA)与PPM-维生素E共沉淀, 自组装形成新型氧化还原前药纳米粒(MPSSV-NPs)[44]。该前药可将药物半衰期从0.33 h延长到30.04 h, AUC扩大了21倍, 同时在给药1 h后显著降低了药物在心脏组织中分布并增加了在肿瘤部位药物积累量。Liu等[42]通过酯化反应偶联PEG和BUF得到前药PEGS-BUF, 提高了BUF的水溶性(从32.76 μg·mL-1到690 μg·mL-1)。体外释放实验显示, 在pH 7.4条件下前药24 h的释放量约为10%, 而在酯酶存在时释放约为80%, 表现出灵敏的触发释药机制。前体药物曾是细胞毒类药物重点研发的剂型之一, 但是很多品种在研发阶段遭叫停, 很大程度上是由于药物触发释放环节出现问题。类似情况也发生在小分子前药中, 如很多酶敏感键在体外可顺利断裂, 但体内往往不如预期, 另外药物经共价修饰后的活性也可能发生改变, 最终导致体内活性降低。前体药物目前研究较为成功的是抗体偶联药物, 也可作为强心苷制剂研发的选择之一。
3.5 微球微球是药物溶解或分散于高分子材料中所形成的微小球体或类球体, 其粒径范围一般在1~250 μm, 可以通过口服和注射等方式给药。与普通剂型相比, 长效缓释微球制剂可以减少用药次数、改善患者的顺应性和提高药物的疗效[58, 59]。有研究者制备了蟾毒化合物的脂质微球制剂[47], 显示具有高包封率(87%~93%)和高载药量(21%~50%)。微球用5%葡萄糖稀释后在4 ± 2 ℃可以稳定保存18个月以上。也有研究员用乳化聚合法制备华蟾素精明胶微球[60], 用于肝窦前动脉介入治疗。载药明胶微球在栓塞的同时, 还可缓慢释放华蟾素精, 达到减毒增效目的。微球在长效和原位制剂开发中有较好的优势和应用。将微球埋置于肿瘤部位, 通过缓慢释药抑制肿瘤生长或清除术后微小残留病灶、减少复发是该类剂型的主攻领域之一。由于强心苷主要表现为心脏毒性, 因此控制药物的入血量是有效手段之一。相较于静脉注射制剂的突释风险, 肿瘤原位微球可以在空间上降低药物与心脏的接触几率, 同时利用其缓控释优势降低药物入血速率和入血量, 更有利于控制强心苷对心脏的不良反应。目前微球制剂主要存在的问题是药物的突释现象, 因此提高微球的控释能力是该类剂型研发的关键, 尤其是对于强心苷这类药物显得尤为重要[58]。优化微球材料的分子量和制备工艺或采用联合制剂技术可以提高药物的控释水平, 开发出符合预期释药曲线的制剂。有人将微球与水凝胶联合制备微球凝胶制剂, 显示出较单一剂型更好的控释效果[61]。
3.6 其他制剂形式包合物是一种超微型药物载体, 包合难溶性药物后可有效提高药物的溶解度和渗透性。Zou等[49]采用叶酸修饰的β-环糊精包合BF制备FA/BF/β-CD, 使BF的溶解度提高了23倍。摄取实验显示, 肿瘤细胞对包合物的摄取显著提高, 肿瘤细胞毒性比BF增加了15%, 说明BF经包合后水溶性和靶向性均得到提高。另有研究者针对BF的心脏毒性问题, 采用丙二醇等材料将BF制成透皮贴剂[53], 药代动力学结果显示, 贴剂的最高入血浓度仅为静脉注射的26%, 半衰期延长3倍多, 可维持有效治疗浓度至少10 h以上。12 h的皮肤刺激实验也未见大鼠皮肤有明显红肿, 表现出良好的生物安全性。
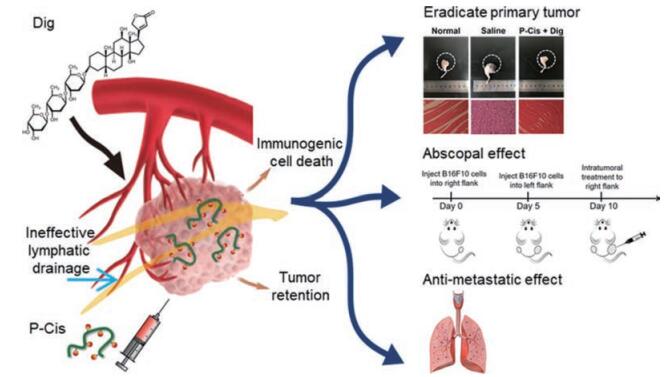
4 联合治疗策略联合治疗策略是近年来临床抗肿瘤治疗普遍采用的方法, 通过几种药物或治疗手段的联合应用, 可以减少细胞毒药物的用量, 避免耐药出现, 发挥协同治疗效果[62-67]。Calderón-Montaño等[65]发现地高辛、洋地黄毒苷和哇巴因可强效抑制肺癌细胞糖酵解过程, 与化疗药顺铂联用时表现出显著的协同作用。近年来发现, 强心苷是一种高效ICD诱导剂, 可以诱导激活机体抗肿瘤免疫反应, 当与其他化疗药[68]或免疫制剂[69]联用时可以有效解除肿瘤免疫抑制, 显著提高抗肿瘤效果。Xiang等[68]报道了联用地高辛和顺铂前药(P-Cis)的治疗效果(图 4)。结果显示, 使用地高辛后可以明显促进钙网蛋白暴露、树突状细胞的成熟和抗原呈递, 进一步招募细胞毒性T淋巴细胞发挥全身抗肿瘤免疫反应。与顺铂联用不仅完全杀死了原位的肿瘤细胞, 且对远端肿瘤也表现出显著抑制, 在肺组织中也未发现明显的转移结节。
|
Figure 4 Schematic illustration of in vivo behaviors of intratumoral injected P-Cis, including long tumor retention due to macromolecular size and dysfunctional lymphatic drainage, endocytosis into the intracellular lysosome, stimulus-responsive cisplatin release, and ultimate cytotoxicity. Dig: Digoxin; P-Cis: Cisplatin (IV) prodrug linked to the N-(2-hydroxypropyl) methacrylamide copolymer. (Adapted from Ref. 68 with permission. Copyright © 2020 American Chemical Society) |
强心苷自发现至今可谓历经波折, 从最初在强心方面的广泛应用到近年来在抗肿瘤领域崭露头角, 均体现出该类化合物的独特优势。但无论其在应用领域如何变化, 阻碍强心苷临床应用的都是其不尽人意的溶解性、明显的毒副作用和治疗窗口窄等问题, 归根结底是强心苷的成药性问题。多年来, 针对其固有弊端, 药物化学家开展了大量的结构改造工作, 并有效改善了溶解性能, 但毒副作用问题始终未得到彻底解决。现代制剂技术和治疗策略是解决这一问题的有效方法, 近年来在该领域的研究已证明, 通过制备适宜的制剂、设计精准的治疗方案, 将极大地降低强心苷的毒副作用, 尤其是对心脏的毒性, 同时提高肿瘤治疗效果。研究表明, 强心苷的毒性往往与其入血剂量有关, 因此避免药物渗漏和控制入血速率是强心苷制剂的关键技术指标。作者认为, 采用肿瘤原位给药和多种控释技术的联合可以提高肿瘤部位药物浓度, 降低药物入血量, 实现强心苷的精准可控释放, 是解决其上述问题的有效方案。此外, 强心苷对细胞的亲和力过强, 敏感性过高, 是阻碍其成药的因素之一。通过结构改造, 适度降低其细胞敏感度将有利于提高用药的“容错率”, 减少不良反应的发生。采用联合治疗策略减少强心苷的剂量、加强用药环节的血药浓度检测和临床观察等措施, 都有利于降低该药物的用药风险, 使其在发挥抗肿瘤独特优势的同时, 让临床用药更安全、患者更放心。
| [1] |
Withering W. CLASS XXIII[M]//A Botanical Arrangement of All the Vegetables Naturally Growing in Great Britain: With Descriptions of the Genera and Species, According to the System of the Celebrated Linnaeus. Cambridge: Cambridge University Press, 2015: 622-635.
|
| [2] |
Ziff OJ, Kotecha D. Digoxin:the good and the bad[J]. Trends Cardiovasc Med, 2016, 26: 585-595. DOI:10.1016/j.tcm.2016.03.011 |
| [3] |
Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure[J]. N Engl J Med, 1997, 336: 525-533. DOI:10.1056/NEJM199702203360801 |
| [4] |
DCRI-led study concludes people with atrial fibrillation should not take the drug[EB/OL]. Squibb BM, Pfizer, 2017[2020-05-09]. https://dcri.org/acc-2017-aristotle-digoxin/.
|
| [5] |
Whitbeck MG, Charnigo RJ, Khairy P, et al. Increased mortality among patients taking digoxin-analysis from the AFFIRM study[J]. Eur Heart J, 2013, 34: 1481-1488. DOI:10.1093/eurheartj/ehs348 |
| [6] |
Consensus recommendations for the management of chronic heart failure. On behalf of the membership of the advisory council to improve outcomes nationwide in heart failure[J]. Am J Cardiol, 1999, 83: 1a-38a. DOI:10.1016/S0002-9149(98)00850-9 |
| [7] |
Jin CC, Zhang XW. Research progress of natural medicines and bioactive compounds against heart failure[J]. Acta Pharm Sin (药学学报), 2020, 55: 1147-1156. |
| [8] |
Metra M, Teerlink JR. Heart failure[J]. Lancet, 2017, 390: 1981-1995. DOI:10.1016/S0140-6736(17)31071-1 |
| [9] |
Shiratori O. Growth inhibitory effect of cardiac glycosides and aglycones on neoplastic cells:in vitro and in vivo studies[J]. Gan, 1967, 58: 521-528. |
| [10] |
Hartwell JL, Abbott BJ. Antineoplastic principles in plants:recent developments in the field[J]. Adv Pharmacol Chemother, 1969, 7: 117-209. |
| [11] |
Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides[J]. Nat Rev Drug Discov, 2008, 7: 926-935. DOI:10.1038/nrd2682 |
| [12] |
Newman RA, Yang P, Pawlus AD, et al. Cardiac glycosides as novel cancer therapeutic agents[J]. Mol Interv, 2008, 8: 36-49. DOI:10.1124/mi.8.1.8 |
| [13] |
Yang P, Menter DG, Cartwright C, et al. Oleandrin-mediated inhibition of human tumor cell proliferation:importance of Na, K-ATPase alpha subunits as drug targets[J]. Mol Cancer Ther, 2009, 8: 2319-2328. DOI:10.1158/1535-7163.MCT-08-1085 |
| [14] |
Menger L, Vacchelli E, Adjemian S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death[J]. Sci Transl Med, 2012, 4: 143r. |
| [15] |
Gkountela S, Castro-Giner F, Szczerba BM, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding[J]. Cell, 2019, 176: 98-112. DOI:10.1016/j.cell.2018.11.046 |
| [16] |
Chen JQ, Contreras RG, Wang R, et al. Sodium/potasium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides:a new paradigm for development of anti-breast cancer drugs?[J]. Breast Cancer Res, 2006, 96: 1-15. DOI:10.1007/s10549-005-9053-3 |
| [17] |
Kulikov A, Eva A, Kirch U, et al. Ouabain activates signaling pathways associated with cell death in human neuroblastoma[J]. Biochim Biophys Acta, 2007, 1768: 1691-1702. DOI:10.1016/j.bbamem.2007.04.012 |
| [18] |
Sreenivasan Y, Sarkar A, Manna SK. Oleandrin suppresses activation of nuclear transcription factor-kappa B and activator protein-1 and potentiates apoptosis induced by ceramide[J]. Biochem Pharmacol, 2003, 66: 2223-2239. DOI:10.1016/j.bcp.2003.07.010 |
| [19] |
Nasu K, Nishida M, Ueda T, et al. Bufalin induces apoptosis and the G0/G1 cell cycle arrest of endometriotic stromal cells:a promising agent for the treatment of endometriosis[J]. Mol Hum Reprod, 2005, 11: 817-823. DOI:10.1093/molehr/gah249 |
| [20] |
Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy[J]. Nat Rev Cancer, 2012, 12: 860-875. DOI:10.1038/nrc3380 |
| [21] |
Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy[J]. Annu Rev Immunol, 2013, 31: 51-72. DOI:10.1146/annurev-immunol-032712-100008 |
| [22] |
Demiryürek AT, Demiryürek S. Cardiotoxicity of digitalis glycosides:roles of autonomic pathways, autacoids and ion channels[J]. Auton Autacoid Pharmacol, 2005, 25: 35-52. DOI:10.1111/j.1474-8673.2004.00334.x |
| [23] |
Hu K, Zhu L, Liang H, et al. Improved antitumor efficacy and reduced toxicity of liposomes containing bufadienolides[J]. Arch Pharm Res, 2011, 34: 1487-1494. DOI:10.1007/s12272-011-0910-9 |
| [24] |
Li Y, Zhao H, Duan LR, et al. Preparation, characterization and evaluation of bufalin liposomes coated with citrus pectin[J]. Colloids Surf A Physicochem Eng Asp, 2014, 444: 54-62. DOI:10.1016/j.colsurfa.2013.12.006 |
| [25] |
Li Y, Yuan J, Yang Q, et al. Immunoliposome co-delivery of bufalin and anti-CD40 antibody adjuvant induces synergetic therapeutic efficacy against melanoma[J]. Int J Nanomedicine, 2014, 9: 5683-5700. DOI:10.2147/IJN.S73651 |
| [26] |
Yuan J, Zhou X, Cao W, et al. Improved antitumor efficacy and pharmacokinetics of bufalin via PEGylated liposomes[J]. Nanoscale Res Lett, 2017, 12: 585. DOI:10.1186/s11671-017-2346-8 |
| [27] |
Gomes ER, Novais MVM, Silva IT, et al. Long-circulating and fusogenic liposomes loaded with a glucoevatromonoside derivative induce potent antitumor response[J]. Biomed Pharmacother, 2018, 108: 1152-1161. DOI:10.1016/j.biopha.2018.09.109 |
| [28] |
Yuan J, Zeng C, Cao W, et al. Bufalin-loaded PEGylated liposomes:antitumor efficacy, acute toxicity, and tissue distribution[J]. Nanoscale Res Lett, 2019, 14: 223. DOI:10.1186/s11671-019-3057-0 |
| [29] |
Chen Q, Liu J. Transferrin and folic acid co-modified bufalin-loaded nanoliposomes:preparation, characterization, and application in anticancer activity[J]. Int J Nanomedicine, 2018, 13: 6009-6018. DOI:10.2147/IJN.S176012 |
| [30] |
Zhu JJ, Zhang XX, Miao YQ, et al. Delivery of acetylthevetin B, an antitumor cardiac glycoside, using polymeric micelles for enhanced therapeutic efficacy against lung cancer cells[J]. Acta Pharmacol Sin, 2017, 38: 290-300. DOI:10.1038/aps.2016.113 |
| [31] |
Yuan Z, Yuan Y, Han L, et al. Bufalin-loaded vitamin E succinate-grafted-chitosan oligosaccharide/RGD conjugated TPGS mixed micelles demonstrated improved antitumor activity against drug-resistant colon cancer[J]. Int J Nanomedicine, 2018, 13: 7533-7548. DOI:10.2147/IJN.S170692 |
| [32] |
Jing J, Tupally K, Kokil G, et al. Development of a hybrid peptide dendrimer micellar carrier system and its application in the reformulation of a hydrophobic therapeutic agent derived from traditional Chinese medicine[J]. RSC Adv, 2019, 9: 2458-2463. DOI:10.1039/C8RA09606F |
| [33] |
Wang H, Williams GR, Wu J, et al. Pluronic F127-based micelles for tumor-targeted bufalin delivery[J]. Int J Pharm, 2019, 559: 289-298. DOI:10.1016/j.ijpharm.2019.01.049 |
| [34] |
Liu Y, Wang P, Sun C, et al. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin[J]. Int J Pharm, 2011, 419: 260-265. DOI:10.1016/j.ijpharm.2011.07.019 |
| [35] |
Liu Y, Wang P, Sun C, et al. Wheat germ agglutinin-grafted lipid nanoparticles:preparation and in vitro evaluation of the association with Caco-2 monolayers[J]. Int J Pharm, 2010, 397: 155-163. DOI:10.1016/j.ijpharm.2010.06.030 |
| [36] |
Tam YY, Chen S, Zaifman J, et al. Small molecule ligands for enhanced intracellular delivery of lipid nanoparticle formulations of siRNA[J]. Nanomedicine, 2013, 9: 665-674. DOI:10.1016/j.nano.2012.11.006 |
| [37] |
Yin P, Wang Y, Qiu Y, et al. Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles:preparation, cellular uptake, tissue distribution, and anticancer activity[J]. Int J Nanomedicine, 2012, 7: 3961-3969. DOI:10.2147/IJN.S32063 |
| [38] |
Hu Q, Liang B, Sun Y, et al. Preparation of bufalin-loaded pluronic polyetherimide nanoparticles, cellular uptake, distribution, and effect on colorectal cancer[J]. Int J Nanomedicine, 2014, 9: 4035-4041. DOI:10.2147/IJN.S64708 |
| [39] |
Tian X, Yin H, Zhang S, et al. Bufalin loaded biotinylated chitosan nanoparticles:an efficient drug delivery system for targeted chemotherapy against breast carcinoma[J]. Eur J Pharm Biopharm, 2014, 87: 445-453. DOI:10.1016/j.ejpb.2014.05.010 |
| [40] |
Wang H, Wu J, Williams GR, et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery[J]. J Nanobiotechnol, 2019, 17: 60. DOI:10.1186/s12951-019-0494-y |
| [41] |
Liu T, Huang Q. Biodegradable brush-type copolymer modified with targeting peptide as a nanoscopic platform for targeting drug delivery to treat castration-resistant prostate cancer[J]. Int J Pharm, 2016, 511: 1002-1011. DOI:10.1016/j.ijpharm.2016.08.017 |
| [42] |
Liu T, Jia T, Yuan Y, et al. Development of octreotide-conjugated polymeric prodrug of bufalin for targeted delivery to somatostatin receptor 2 overexpressing breast cancer in vitro and in vivo[J]. Int J Nanomedicine, 2016, 11: 2235-2250. DOI:10.2217/nnm-2016-0234 |
| [43] |
Liu T, Yuan X, Jia T, et al. Polymeric prodrug of bufalin for increasing solubility and stability:synthesis and anticancer study in vitro and in vivo[J]. Int J Pharm, 2016, 506: 382-393. DOI:10.1016/j.ijpharm.2016.04.041 |
| [44] |
Zhang H, Xu W, Omari-Siaw E, et al. Redox-responsive PEGylated self-assembled prodrug-nanoparticles formed by single disulfide bond bridge periplocymarin-vitamin E conjugate for liver cancer chemotherapy[J]. Drug Deliv, 2017, 24: 1170-1178. DOI:10.1080/10717544.2017.1365393 |
| [45] |
Chai XP, Sun GL, Fang YF, et al. Tumor-targeting efficacy of a BF211 prodrug through hydrolysis by fibroblast activation protein-α[J]. Acta Pharmacol Sin, 2018, 39: 415-424. DOI:10.1038/aps.2017.121 |
| [46] |
Shi XJ, Qiu YY, Yu H, et al. Increasing the anticancer performance of bufalin (BUF) by introducing an endosome-escaping polymer and tumor-targeting peptide in the design of a polymeric prodrug[J]. Colloids Surf B Biointerfaces, 2018, 166: 224-234. DOI:10.1016/j.colsurfb.2018.03.024 |
| [47] |
Weng Y, Pan C, Meng J, et al. Formulation, preparation, and stability of intravenous bufadienolides-loaded lipid microspheres[J]. Eur J Lipid Sci Technol, 2012, 114: 1154-1164. DOI:10.1002/ejlt.201200105 |
| [48] |
Qu G, Wang C, Ning L, et al. Inhibitory effect of bufadienolides lipid microsphere injection on nude mice bearing human cancer cells[J]. J Mod Oncol (现代肿瘤医学), 2017, 25: 2187-2194. |
| [49] |
Zou A, Zhao X, Handge UA, et al. Folate receptor targeted bufalin/β-cyclodextrin supramolecular inclusion complex for enhanced solubility and anti-tumor efficiency of bufalin[J]. Mater Sci Eng C Mater Biol Appl, 2017, 78: 609-618. DOI:10.1016/j.msec.2017.04.094 |
| [50] |
Chan CO, Jing J, Xiao W, et al. Enhanced intestinal permeability of bufalin by a novel bufalin-peptide-dendrimer inclusion through Caco-2 cell monolayer[J]. Molecules, 2017, 22: 2088. DOI:10.3390/molecules22122088 |
| [51] |
Li W, Lin X, Yang Z, et al. A bufadienolide-loaded submicron emulsion for oral administration:stability, antitumor efficacy and toxicity[J]. Int J Pharm, 2015, 479: 52-62. DOI:10.1016/j.ijpharm.2014.12.054 |
| [52] |
Liu Y, Chen ZQ, Zhang X, et al. An improved formulation screening and optimization method applied to the development of a self-microemulsifying drug delivery system[J]. Chem Pharm Bull, 2010, 58: 16-22. DOI:10.1248/cpb.58.16 |
| [53] |
Yang Z, Teng Y, Wang H, et al. Enhancement of skin permeation of bufalin by limonene via reservoir type transdermal patch:formulation design and biopharmaceutical evaluation[J]. Int J Pharm, 2013, 447: 231-240. DOI:10.1016/j.ijpharm.2013.02.048 |
| [54] |
Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery[J]. Chem Rev, 2015, 115: 10938-10966. DOI:10.1021/acs.chemrev.5b00046 |
| [55] |
Hu C, Gao HL. Advances in tumor microenvironment responsive and regulatory drug delivery system[J]. Acta Pharm Sin (药学学报), 2020. DOI:10.16438/j.0513-4870.2019-0798 |
| [56] |
Li R, He Y, Zhang S, et al. Cell membrane-based nanoparticles:a new biomimetic platform for tumor diagnosis and treatment[J]. Acta Pharm Sin B, 2018, 8: 14-22. DOI:10.1016/j.apsb.2017.11.009 |
| [57] |
Bloise E, Braca A, De Tommasi N, et al. Pro-apoptotic and cytostatic activity of naturally occurring cardenolides[J]. Cancer Chemother Pharmacol, 2009, 64: 793-802. DOI:10.1007/s00280-009-0929-5 |
| [58] |
Park K, Skidmore S, Hadar J, et al. Injectable, long-acting PLGA formulations:analyzing PLGA and understanding microparticle formation[J]. J Control Release, 2019, 304: 125-134. DOI:10.1016/j.jconrel.2019.05.003 |
| [59] |
Ramazani F, Chen W, van Nostrum CF, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres:state-of-the-art and challenges[J]. Int J Pharm, 2016, 499: 358-367. DOI:10.1016/j.ijpharm.2016.01.020 |
| [60] |
Fan J, Huang MK, Wu FL, et al. An experimental study on hepatic arterial chemoembolization with cinobufagin microspheres[J]. Chin J Cancer, 1995, 14: 434-437. |
| [61] |
Kim DY, Kwon DY, Kwon JS, et al. Synergistic anti-tumor activity through combinational intratumoral injection of an in-situ injectable drug depot[J]. Biomaterials, 2016, 85: 232-245. DOI:10.1016/j.biomaterials.2016.02.001 |
| [62] |
Kulikov AV, Slobodkina EA, Alekseev AV, et al. Contrasting effects of cardiac glycosides on cisplatin- and etoposide-induced cell death[J]. Biol Chem, 2016, 397: 661-670. DOI:10.1515/hsz-2016-0101 |
| [63] |
Felth J, Rickardson L, Rosén J, et al. Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs[J]. J Nat Prod, 2009, 72: 1969-1974. DOI:10.1021/np900210m |
| [64] |
Einbond LS, Wu HA, Sandu C, et al. Digitoxin enhances the growth inhibitory effects of thapsigargin and simvastatin on ER negative human breast cancer cells[J]. Fitoterapia, 2016, 109: 146-154. DOI:10.1016/j.fitote.2015.12.005 |
| [65] |
Calderón-Montaño JM, Burgos Morón E, López Lázaro M. The cardiac glycosides digitoxin, digoxin and ouabain induce a potent inhibition of glycolysis in lung cancer cells[J]. Webmed Central, 2013, 7: WMC004323. |
| [66] |
Kaushik V, Azad N, Yakisich JS, et al. Antitumor effects of naturally occurring cardiac glycosides convallatoxin and peruvoside on human ER+ and triple-negative breast cancers[J]. Cell Death Discov, 2017, 3: 17009. DOI:10.1038/cddiscovery.2017.9 |
| [67] |
Pereira DG, Salgado MAR, Rocha SC, et al. Involvement of Src signaling in the synergistic effect between cisplatin and digoxin on cancer cell viability[J]. J Cell Biochem, 2018, 119: 3352-3362. DOI:10.1002/jcb.26499 |
| [68] |
Xiang Y, Chen L, Li L, et al. Restoration and enhancement of immunogenic cell death of cisplatin by coadministration with digoxin and conjugation to HPMA copolymer[J]. ACS Appl Mater Interfaces, 2020, 12: 1606-1616. DOI:10.1021/acsami.9b19323 |
| [69] |
Haux J. Digitoxin has specific properties for potential use to treat cancer and inflammatory diseases[J]. Res Rev Health Care Open Acc J, 2018. DOI:10.32474/RRHOAJ.MS.ID.000137 |
 2020, Vol. 55
2020, Vol. 55