作者贡献:胡川负责文献的整理以及综述撰写, 高会乐负责拟定撰写思路和修订论文。
利益冲突:作者声明没有利益冲突。
化学药物治疗作为恶性肿瘤常用的治疗方式, 其治疗效果受到较低肿瘤靶向传递效率的极大限制[1, 2]。纳米递药系统具有可改善药物分子的溶解性、延长包封药物的体内循环时间、降低药物毒副作用从而提高其治疗效果等优点, 广泛地应用于肿瘤靶向药物递送研究[3-5]。肿瘤部位由于新生血管丰富及淋巴管损伤等特点而使其具有增强的渗透和滞留效应[enhanced permeability and retention (EPR) effect], 纳米递药系统可以基于此效应被动地富集于肿瘤部位[6-8]。目前已有多种基于此效应的纳米药物用于临床治疗, 如多柔比星脂质体、紫杉醇胶束等[9, 10], 但临床治疗效果改善不大。研究发现, 肿瘤部位复杂的微环境使得药物到达肿瘤部位后更多停留在肿瘤血管附近的基质, 很难到达肿瘤的深层区域, 从而在肿瘤部位呈现异质性分布, 降低了治疗效果[1, 11, 12]。因此, 设计能改善肿瘤部位异质性分布的纳米递药系统显得尤为重要。
本文主要总结近年来从肿瘤微环境的性质出发以改善纳米递药系统在肿瘤部位异质性分布的相关研究, 重点关注肿瘤微环境响应性的策略和调节肿瘤微环境以提高递药系统传递效率的相关策略。
1 基于肿瘤微环境响应性的策略 1.1 pH值响应在正常情况下, 组织外基质及血液中pH值接近7.4。由于快速增长的肿瘤中不规则的血管生成导致肿瘤部位营养和氧气的快速缺乏, 肿瘤部位存在较高的糖酵解率, 使得酸性代谢物在肿瘤间质蓄积, 肿瘤细胞外环境的pH值会降低至6.5~7.2[13]。肿瘤的微酸性常被用于响应性递药系统的刺激源。基于pH值响应性设计的主要载体类型包括含有可电离基团的多聚物[14, 15], 其在不同pH值下出现构象及溶解性改变; 以及含有酸敏感断裂基团(如腙键、亚胺键、酰腙键、缩醛/缩铜键等)的多聚物等, 其在特定pH值下发生响应性断裂。此二类载体均可实现肿瘤部位响应性释放药物、暴露靶向分子和翻转电性等以增强肿瘤部位传递效率[16-18]。本课题组设计了一种酸响应性靶向分子可断裂的递药系统[19] (图 1), 该递药系统以连接多柔比星(DOX)的树枝状聚左旋赖氨酸(DGL)为内核, 进一步修饰具有脑胶质瘤细胞靶向性的甘露糖类似物(MAN)及外层修饰酸性条件可断裂的血脑屏障靶向分子转铁蛋白(Tf), 得到具有双重靶向性的递药系统(DD-MCT)。尾静脉注射DD-MCT后, 该递药系统首先利用Tf特异性识别血脑屏障(BBB)内皮细胞的转铁蛋白受体(TfR)而被摄取入胞, 之后酸响应性的Tf可在溶酶体或内吞体的酸性条件下发生断裂, 从而使MAN修饰的递药系统与Tf-TfR复合体分离, 使递药系统更易逃离溶酶体并进一步向脑侧外排而进入脑实质中, 更好地实现脑胶质瘤细胞靶向性。结果表明, 多次尾静脉给予DD-MCT可显著延长荷脑胶质瘤小鼠的生存期。Sun等[20]报道了一种基于肿瘤微环境pH值下不稳定的化学键桥连聚乳酸与聚乙二醇共聚物(PEG-Dlinkm‐PDLLA)的策略用于响应性递送药物。当其被动靶向至肿瘤部位后, 该系统响应肿瘤微酸环境而丢弃外层PEG, 增强递药系统正电性。结果表明, 该策略可显著增强细胞摄取, 体内肿瘤抑制率可达到78.1%, 而没有酸响应性的对照组肿瘤抑制率仅为47.8%。这些递药系统尽管取得了一定进展, 但多种因素都会影响该类递药系统的响应效率, 如递药系统对于肿瘤组织与正常血管间pH值的微小变化响应的灵敏性、肿瘤部位典型的微酸环境远离血管及递药系统能否克服生理病理屏障到达肿瘤内部等。

|
Figure 1 Acid-responsive transferrin dissociation and glucose transporters (GLUT) mediated exocytosis for increased blood-brain barrier transcytosis and programmed glioma targeting delivery. Adapted from Ref. 19 with permission. Copyright©2018, John Willy and Sons. A: Preparation of acid-responsive programmed targeted delivery system of P-aminophenyl-α-D-mannopyranoside (MAN) decorated doxorubicin-loaded dendrigraft poly-L-lysine with acid-cleavable transferrin (Tf) coating outside (DD-MCT); B: Schematic illustration of DD-MCT programmed target transferrin receptor (TfR) on blood-brain barrier (BBB) and then target GLUT on glioma cells after transcytosis across BBB; C: Schematic illustration of process of the acid-responsive cleavage of Tf from DD-MCT in endothelial cell and transporter-mediated exocytosis of detached DD-MCT for increased transcytosis |
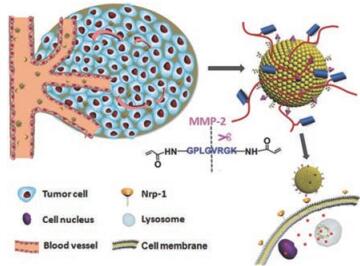
肿瘤部位上调的酶含量是另一重要的内源性刺激源。上调的酶含量对于肿瘤生长、血管生成、肿瘤细胞侵袭及转移具有重要影响。酶响应递药系统可实现肿瘤部位特异性药物释放和可控形态改变等, 从而提高肿瘤靶向传递效率及减少正常组织毒副作用。基质金属蛋白酶(MMP)属于锌依赖性内肽酶家族, 是常见肿瘤微环境高表达的酶之一, 许多基于MMP响应性的载体被用于递药系统的研究。Liu等[21]制备了一种功能化的囊泡状递药系统(ITC⊂N-G-C), 其囊泡状外部交联MMP-2可降解的高分子多肽。如图 2所示, 递药系统在血液循环中具有较好的稳定性, 当其被动靶向至肿瘤部位后, MMP-2的存在使多肽响应性断裂, 暴露出多肽封闭的细胞穿膜肽, 进而实现肿瘤部位特异性释药。本课题组设计了一个以明胶为载体, 表面修饰粒径为34.3 nm左右的小粒径纳米粒的递药系统(RGD-DOX-DGL-GNP)[22], 其中明胶为MMP-2的底物。体外实验中, RGD-DOX-DGL-GNP与MMP-2酶孵育后, 粒径可由200 nm降低至30 nm, 肿瘤球穿透实验表明, 与MMP-2孵育后具有更好的肿瘤深部穿透能力; 体内实验中, 该纳米粒取得了较好的肿瘤靶向性及最佳的抗肿瘤效果。除了MMP酶高表达外, 肿瘤微环境还高表达如透明质酸酶及豆荚蛋白酶等。本课题组设计的豆荚蛋白酶响应性聚集体系(AuNPs-A & C)[23, 24], 可以增强递药系统在肿瘤部位的滞留性从而提高治疗效果。实验结果表明, 该体系可延长荷脑胶质瘤小鼠的生存期及延缓荷4T1肿瘤小鼠的肿瘤生长率。Hu等[25]及Liu等[26]设计了基于肿瘤微环境高表达的透明质酸酶响应性粒径可降低的体系, 用于增强递药系统在肿瘤深部的穿透性, 从而达到较好的治疗乳腺癌的效果。但此类策略应当注意不同肿瘤表达酶的种类及肿瘤微环境表达该酶的浓度等, 将影响该类递药系统的响应效率。
|
Figure 2 Schematic illustrations of the mechanism of the matrix metalloproteinase (MMPs)-responsive drug‐delivery system (ITC⊂N-G-C) in the tumor microenvironment. Adapted from Ref. 21 with permission. Copyright©2015, John Willy and Sons |
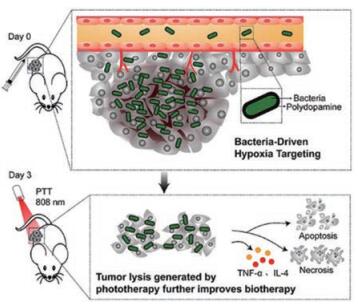
新生血管的生成是肿瘤形成的关键步骤, 但新生血管往往不能为快速增殖的肿瘤细胞提供足够的氧气, 使得肿瘤核心部位缺氧。此外, 缺氧的发生也被认为是癌症治疗中导致肿瘤复发和治疗耐受的重要原因[27, 28]。许多基于肿瘤缺氧的响应性递药系统用于肿瘤治疗。如图 3所示, Chen等[29]利用兼性厌氧菌菌株VNP20009在全身注射后对缺氧性肿瘤核心偏好的性质, 将低毒性的VNP20009作为生物治疗剂及药物载体, 其吸附光热剂聚多巴胺用于肿瘤靶向治疗(pDA-VNP)。该递药系统通过一次注射给药和激光照射, 不仅消除了原位肿瘤, 同时可避免肿瘤复发和转移的产生, 取得显著的肿瘤靶向治疗效果; 进一步为了使该治疗策略更好地应用于难以攻克的大体积肿瘤, 该课题组将一种肽类PD-1/PD-L1免疫检查点抑制剂(AUNP-12)包载于原位磷脂相变凝胶中(P-AUNP)[30]。其通过单次肿瘤周围皮下注射, 即可形成药物储库, 缓释AUNP-12可达42天, 从而持续改善肿瘤免疫抑制微环境, 恢复T细胞杀伤肿瘤能力, 进而增强药物抗大体积肿瘤疗效, 同时抑制肿瘤后期的复发与转移。在另一个研究中, 修饰有肿瘤促渗透肽iRGD的缺氧响应性脂质纳米粒展现了较好的肿瘤渗透性和缺氧响应性释药。在iRGD作用下纳米粒穿透至肿瘤深部, 随着氧分压的降低, 其脂质层表现出不稳定性, 从而释放出包载的吉西他滨, 导致胰腺癌细胞在缺氧条件下的存活率降低到35% [31]。Kulkarni等[32]构建了一种缺氧响应性自组装聚合物囊泡, 在正常氧供条件下, 该聚合物囊泡包封的药物可维持50 min不释放; 相反, 在缺氧条件下, 相同的时间间隔内90%药物可被释放。缺氧响应的智能递药系统显示出具有较大的肿瘤治疗潜力, 但同时由于典型的缺氧环境存在于肿瘤的深部, 递药系统能否克服层层生理病理屏障到达缺氧部位也会影响该类递药系统的递送效率。
|
Figure 3 Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy (pDA-VNP). Adapted from Ref. 29 with permission. Copyright©2018, American Chemical Society |
肿瘤细胞中谷胱甘肽(GSH)的浓度比血液和正常组织中分别高出100~1 000倍和100倍[33]。巨大的GSH浓度梯度差可以有效地保障氧化还原递药系统响应的敏感性, 其中二硫键容易被谷胱甘肽快速裂解, 成为最典型和最有效的响应机制。通过二硫键的使用, 递药系统可以实现响应性组分分解、纳米载体的降解及快速释药等多种优势。Lin等[34]设计了一种用透明质酸包被的还原响应性壳聚糖纳米粒。该纳米粒将壳聚糖(CS)与铜螯合剂D-青霉胺(D-pen)通过二硫键偶联(CS-ss-D-pen), DOX通过与D-pen中铜离子的络合作用进行包载。D-pen和DOX通过肿瘤细胞内高表达的GSH与二硫键发生氧化还原反应得以释放从而发挥细胞毒性的作用, 实现谷胱甘肽响应性药物释放。本课题组利用胆红素-聚乙二醇为载体原料, 包载二硫键偶联的药物二聚体(SL@BRNPs)[35], 药物的二聚作用显著增加了药物的载药量, 同时二硫键的引入使得药物二聚体具备GSH响应的能力, 其药物活性仅在富含GSH的环境被激活, 可以减少药物非特异性释放的细胞毒性。再结合肿瘤靶向促渗透肽iRGD及免疫检查点封锁抗体(anti-PD-L1), 实现较好的抗肿瘤联合治疗效果。在另一个研究中, Maggini等[36]设计了一种二硫键(S-S)连接的具有氧化还原响应性的介孔有机硅纳米粒。该响应性递药系统不仅可以在谷胱甘肽存在下释放出包载的药物分子, 同时有机载体原料的使用具有生物可降解性, 该递药系统的设计为体内靶向药物传递系统的开发提供了新思路。
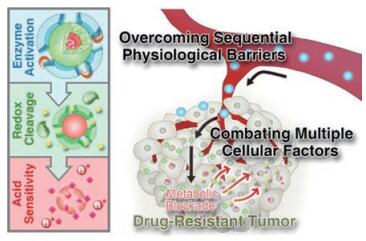
1.5 基于多种内源性刺激响应虽然上述单刺激响应性递药系统可成功地克服肿瘤部位的生理屏障, 但由于肿瘤微环境的复杂性, 可响应多种刺激的递药系统的设计将会显得更加有效。例如, 肿瘤微环境中酸性的pH值及上调的酶含量共存, 可将二者共同用于递药系统的设计。Cheng等[37]设计了一种可连续响应肿瘤微环境pH值及高表达MMP-2酶的响应递药系统。该递药系统由酸响应的3-二乙氨基丙基-异硫氰酸酯(DEAP)分子、MMP-2响应断裂的多肽序列、程序性细胞死亡配体1 (dPPA-1)的短肽拮抗剂和吲哚2-3双加氧酶的抑制剂(NLG919)组成(NLG919@DEAP- DPPA-1)。该系统在肿瘤部位微酸条件下, DEAP分子被质子化, 纳米结构发生膨胀, 同时在肿瘤部位高表达的MMP-2下, 多肽序列发生降解, 纳米结构瓦解, 实现肿瘤局部响应性释放dPPA -1及NLG919, 达到了较好的抗肿瘤效果。此外, Li等[38]同时利用上调的酶含量、酸性pH值及还原响应性设计了多响应性递药系统。如图 4所示, 该递药系统以树枝状大分子DGL为核心, 通过pH值敏感的腙键与DOX相连, 进而以还原响应的二硫键与修饰有MMP-2为底物的多肽序列的PEG连接后自组装构成该多重响应性递药系统(DNs)。该体系通过外层PEG修饰可延长其体内循环的时间。被动靶向至肿瘤部位后, 高表达的MMP-2可特异性酶切外层PEG, 释放出小粒径核心用于肿瘤间质深层穿透, 当其摄取进入肿瘤细胞内, 高浓度谷胱甘肽及溶酶体的酸性pH值可触发DOX定点释放。该策略在克服肿瘤微环境多种生理屏障及化学治疗的耐药性上取得了较好的效果。
|
Figure 4 Tumor-specific multiple stimuli-activated dendrimeric nanoassemblies (DNs). Adapted from Ref. 38 with permission. Copyright©2017, American Chemical Society |
肿瘤快速增殖导致大量新生血管的生成, 但这些新生血管呈异常性生长并具有高的渗漏性, 从而造成肿瘤部位间质压增高, 降低了药物在肿瘤部位传递的效率[39]。许多研究致力于探讨血管正常化对肿瘤部位药物递送的影响。肿瘤部位促血管生成因子与抑血管生成因子间的不一致是导致其血管异常的主要因素, 抗血管生成药物可通过调节混乱的信号传导过程, 使肿瘤新生血管暂时正常化, 改善肿瘤的灌注情况, 从而提高肿瘤部位的递送效率。Luan等[40]通过预先节律性地递送靶向肿瘤新生血管内皮细胞的纳米药物(F56-PTX-NP), 实现较好的抗新生血管生成及诱导血小板反应蛋白的分泌, 增强肿瘤微环境的氧供、血流灌注等, 从而可提高DOX在肿瘤部位的递送效率。本课题组研究了血管正常化试剂西地尼布[41]对酶响应聚集的递药系统肿瘤传递效率的影响。结果表明, 西地尼布的合用可显著提高肿瘤血管的灌注及氧供, 促进小粒径纳米粒在肿瘤部位的蓄积, 进而小粒径纳米粒发生酶响应性聚集滞留于肿瘤部位, 增强纳米粒在肿瘤部位的蓄积量并降低外排。“促渗透抑回流”相结合的方式实现了较好的抗肿瘤效果。除了血管正常化策略外, 还有一部分研究致力于通过增强肿瘤部位的血管通透性, 以增加肿瘤部位的EPR效应, 进而提高治疗药物在肿瘤部位的蓄积。Li等[42]设计了一种新型的脂多肽递药系统, 该递药系统由MMP-2响应性断裂的肽壳、聚合物包载的抗血小板抗体(R300)及化疗药物DOX组成。该系统可被动靶向至肿瘤部位并在高浓度MMP-2存在下, 响应性释放包载的药物。R300可以通过与肿瘤部位的血小板表面受体结合, 促进血小板微团聚体的形成, 随后耗竭, 从而破坏肿瘤部位血管的完整性而增强纳米药物在肿瘤部位的蓄积。一氧化氮(nitric oxide, NO)是一种具有生物活性的气体小分子调节剂, 研究表明NO参与了多种生理学过程, 如调节体内新生血管的生成、参与细胞凋亡及免疫响应等[43, 44]。近来发现, 其可增强肿瘤部位的EPR效应, 从而增加纳米粒在肿瘤部位的蓄积量。Kinoshita等[45]发现, 通过S-亚硝化的重组白蛋白二聚体(SNO-HSA-Dimer)治疗小鼠后可以增强其肿瘤部位EPR效应, 使多柔比星脂质体(Doxil)在肿瘤部位的蓄积量增加3~4倍, 增强了Doxil抗结肠癌的效果。
2.2 调节肿瘤细胞外基质肿瘤细胞外基质(ECM)为肿瘤的生长提供了结构支持, 同时其作为肿瘤部位主要的生理屏障, 可明显阻碍纳米药物在肿瘤中的渗透[46]。因此, 下调ECM的表达及降解已生成的ECM有助于提高递药系统在肿瘤部位的传递效率。作为ECM的主要组成成分, 胶原成为调节ECM的常用靶点。Kato等[47]的研究表明, 尾静脉注射胶原酶可降低肿瘤部位胶原的浓度及肿瘤间质压, 提高阳离子脂质体在肿瘤部位的分布和治疗效果。Murty等[48]将胶原酶修饰于金纳米粒(AuNCs)以提高AuNCs在肿瘤部位的分布。透明质酸是ECM的另一重要成分, Wang等[49]将透明质酸酶(HAase)通过酸敏感键修饰至具有较好生物相容性的右旋糖酐(DEX-HAase)制备成纳米辅助制剂。经尾静脉注射后, DEX-HAase表现出良好的酶稳定性、较低的免疫原性及较长的血浆半衰期。当其被动靶向至肿瘤部位后, 可在酸性条件下释放出具有活性的HAase, 从而增强氧气及后续载有光敏剂脂质体在肿瘤部位的穿透作用, 再通过联用程序化死亡配体的受体(anti-PD-L1), 实现了较好的肿瘤免疫治疗的效果。另外, 许多小分子药物可以抑制ECM的表达, 如氯沙坦可与血管紧张素I型受体作用而降低肿瘤中I型胶原蛋白的表达水平。本课题组将氯沙坦与酶响应性粒径[4]可降低的递药系统联用, 进一步增强了该递药系统在肿瘤部位的深层穿透性, 实现了较好的抗乳腺癌治疗效果。此外, 通过物理手段使ECM变性也可以一定程度提高纳米药物在肿瘤部位的分布。Raeesi等[50]利用金纳米棒的光热效应使肿瘤部位胶原变性, 可显著提高粒径为50和120 nm的纳米粒在肿瘤部位的穿透效率。
2.3 降低肿瘤组织细胞密度肿瘤部位高细胞密度是导致递药系统较差肿瘤组织穿透力的另一因素[51]。采用物理或化学手段诱导血管周围区域细胞的轻度凋亡可以有效提高药物递送效率[52]。Jang等[53]发现紫杉醇预处理导致肿瘤部位细胞密度降低, 进而增强随后给药的紫杉醇在肿瘤部位的蓄积量, 在小鼠模型上产生更强的诱导细胞凋亡的能力。Wang等[54]预先使用载紫杉醇纳米粒诱导细胞凋亡, 从而促进后续载siRNA脂质体的传递效率, 实现较好的小鼠腹膜肿瘤治疗效果。
肿瘤相关成纤维细胞(CAFs)通过与肿瘤细胞相互作用, 在肿瘤的生长及转移中起到了非常重要的作用, 同时可重塑肿瘤细胞外基质以及肿瘤微环境, 使得化疗药物难以递送至肿瘤部位。Chen等[55]利用肿瘤基质靶向的纳米载体(FH-SSL-Nav)特异性消除CAFs, 从而促进纳米药物在肿瘤部位的渗透。结果表明, 载有化疗药物的递药系统在体外肿瘤球模型及体内肿瘤组织都可渗透至更深部。在裸鼠异位HepG2肿瘤模型中, FH-SSL-Nav显著提高了载多柔比星脂质体(7pep-SSL-DOX)对肿瘤的抑制效果。
3 总结与展望综上所述, 根据肿瘤微环境的特征, 一方面, 可设计肿瘤微环境响应性的递药系统, 如pH值响应性、酶响应性、缺氧响应、氧化还原响应及多重响应性递药系统, 从而提高药物在肿瘤部位的递送效率(表 1); 另一方面, 通过调节肿瘤微环境, 如改变肿瘤部位新生血管生成、降低肿瘤部位ECM及降低肿瘤部位细胞密度等, 可改善递药系统在肿瘤部位的渗透或滞留行为, 提高递药系统在肿瘤部位递送效率。尽管许多研究报道了基于肿瘤微环境响应性的递药系统及调节肿瘤微环境相关的策略, 也有少量的内源性响应性纳米药物进入临床研究, 如具有磷酸酶A2响应性释放药物的顺铂脂质体(LiPlaCis)已进入临床II期的研究[56]及pH值响应性多柔比星的多嵌段胶束(K-912/NC-6300)已进入临床I期的研究[57], 但该类纳米药物最终用于临床抗肿瘤治疗仍存在许多挑战。首先, 肿瘤微环境与肿瘤细胞之间的相互作用仍不明确。因此, 构建实验和临床前模型来更好地描述肿瘤微环境将极大地促进现有纳米递药系统的发展; 其次, 应当对响应性纳米载体进行更彻底的临床前评估, 目前报道的多数递药系统的载体材料的体内降解性和生物相容性研究不够充分, 应采用多种手段密切监测整个递药系统在体内的药代动力学过程、体内生物分布、代谢、排泄及毒理学相关的特征; 再者, 虽然上述的肿瘤微环境特征可作为肿瘤响应性递药系统的刺激源, 但应当注意, 并非只有肿瘤具有此类特征, 如酸性pH值、酶浓度等, 同时不同的肿瘤类型、不同的肿瘤生长进程及不同个体的肿瘤生理特征可能存在较大的差异, 将限制刺激响应性纳米递药系统的临床应用; 最后, 纳米递药系统临床转化面临的另一个挑战即纳米药物从临床前研究到随后的临床转化过程中的扩大生产, 以及质量可控性等问题。对于响应性递药系统而言, 递药系统设计的复杂性, 在其扩大生产、商业化制备时将会对制药企业提出更高的要求。因此, 早期进行纳米药物设计时为其后续的临床转化进行前瞻性考虑就显得尤为重要。虽然面临上述的各类问题, 但相信在后续研究中结合生理学、材料学和化学等其他交叉学科的发展, 可进一步推进肿瘤微环境响应性的递药系统及调节肿瘤微环境的策略用于临床肿瘤治疗。
| Table 1 Tumor microenvironment stimuli-responsive nanocarrier. PEG-Dlinkm-PDLLA: Tumor-pH-labile linkage-bridged copolymers of clinically validated poly(d, l-lactide) and poly(ethylene glycol); RGD-DOX-DGL-GNP: Dendritic poly-L-lysine (DGL) and gelatin NP (GNP); AuNPs-A & C: Ala-Ala-Asn-Cys-Lys modified AuNPs (AuNPs-AK) and 2-cyano-6-aminobenzothiazole modified AuNPs (AuNPs-CABT); CS-ss-D-pen: Chitosan (CS) was conjugated with a copper chelator, D-penicillamine (D-pen) through the formation of a disulfide bond; SL@BRNPs: PEGylated bilirubin nanoparticles (BRNPs) encapsulated dimer-7-ethyl-10-hydroxycamptothecin (d-SN38) and dimer-lonidamine (d-LND); NLG919@DEAP-DPPA-1: NLG919, an inhibitor of idoleamine 2, 3-dioxygenase, DEAP, 3-diethylaminopropyl isothiocyanate, DPPA-1, D-peptide antagonist of programmed cell death-ligand |
| [1] |
Sun Q, Zhou Z, Qiu N, et al. Rational design of cancer nanomedicine:nanoproperty integration and synchronization[J]. Adv Mater, 2017, 29: 1606628. DOI:10.1002/adma.201606628 |
| [2] |
Chen H, Zhang W, Zhu G, et al. Rethinking cancer nanotheranostics[J]. Nat Rev Mater, 2017, 2: 17024. DOI:10.1038/natrevmats.2017.24 |
| [3] |
Wang H, Zhao Y, Wang H, et al. Low-molecular-weight protamine-modified PLGA nanoparticles for overcoming drug-resistant breast cancer[J]. J Control Release, 2014, 192: 47-56. DOI:10.1016/j.jconrel.2014.06.051 |
| [4] |
Cun X, Ruan S, Chen J, et al. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan[J]. Acta Biomater, 2016, 31: 186-196. DOI:10.1016/j.actbio.2015.12.002 |
| [5] |
Gao H, Yang Z, Cao S, et al. Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment[J]. Biomaterials, 2014, 35: 2374-2382. DOI:10.1016/j.biomaterials.2013.11.076 |
| [6] |
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy:mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs[J]. Cancer Res, 1986, 46: 6387-6392. |
| [7] |
Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues[J]. Microvasc Res, 1986, 31: 288-305. DOI:10.1016/0026-2862(86)90018-X |
| [8] |
Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity[J]. Adv Drug Deliv Rev, 2015, 91: 3-6. DOI:10.1016/j.addr.2015.01.002 |
| [9] |
Barenholz YC. Doxil®-the first FDA-approved nano-drug:from an idea to a product[J]. J Control Release, 2012, 160: 335-398. |
| [10] |
Fu Q. Nanoparticle albumin-bound (NAB) technology is a promising method for anti-cancer drug delivery[J]. Recent Pat Anticancer Drug Discov, 2009, 4: 262-272. DOI:10.2174/157489209789206869 |
| [11] |
Stirland DL, Nichols JW, Miura S, et al. Mind the gap:a survey of how cancer drug carriers are susceptible to the gap between research and practice[J]. J Control Release, 2013, 172: 1045-1064. DOI:10.1016/j.jconrel.2013.09.026 |
| [12] |
Bertrand N, Wu J, Xu X, et al. Cancer nanotechnology:the impact of passive and active targeting in the era of modern cancer biology[J]. Adv Drug Deliv Rev, 2014, 66: 2-25. DOI:10.1016/j.addr.2013.11.009 |
| [13] |
Stubbs M, McSheehy PMJ, Griffiths JR, et al. Causes and consequences of tumour acidity and implications for treatment[J]. Mol Med Today, 2000, 6: 15-19. DOI:10.1016/S1357-4310(99)01615-9 |
| [14] |
Crayton SH, Andrew T. pH-titratable superparamagnetic iron oxide for improved nanoparticle accumulation in acidic tumor microenvironments[J]. ACS Nano, 2011, 5: 9592-9601. DOI:10.1021/nn202863x |
| [15] |
Dreaden EC, Kong YW, Morton SW, et al. Tumor-targeted synergistic blockade of MAPK and PI3K from a layer-by-layer nanoparticle[J]. Clin Cancer Res, 2015, 21: 4410-4419. DOI:10.1158/1078-0432.CCR-15-0013 |
| [16] |
Lian L, Wei S, Zhong J, et al. Multistage nanovehicle delivery system based on stepwise size reduction and charge reversal for programmed nuclear targeting of systemically administered anticancer drugs[J]. Adv Func Mater, 2015, 25: 4101-4113. DOI:10.1002/adfm.201501248 |
| [17] |
Koren E, Apte A, Jani A, et al. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity[J]. J Control Release, 2012, 160: 264-273. DOI:10.1016/j.jconrel.2011.12.002 |
| [18] |
Yu Y, Zhang X, Qiu L. The anti-tumor efficacy of curcumin when delivered by size/charge-changing multistage polymeric micelles based on amphiphilic poly(β-amino ester) derivates[J]. Biomaterials, 2014, 35: 3467-3479. DOI:10.1016/j.biomaterials.2013.12.096 |
| [19] |
Ruan S, Qin L, Xiao W, et al. Acid-responsive transferrin dissociation and GLUT mediated exocytosis for increased blood-brain barrier transcytosis and programmed glioma targeting delivery[J]. Adv Funct Mater, 2018, 28: 1802227. DOI:10.1002/adfm.201802227 |
| [20] |
Sun CY, Liu Y, Du JZ, et al. Facile generation of tumor-pH-labile linkage-bridged block copolymers for chemotherapeutic delivery[J]. Angew Chem Int Edit, 2016, 55: 1010-1014. DOI:10.1002/anie.201509507 |
| [21] |
Liu Y, Zhang D, Qiao ZY, et al. A peptide-network weaved nanoplatform with tumor microenvironment responsiveness and deep tissue penetration capability for cancer therapy[J]. Adv Mater, 2015, 27: 5034-5042. DOI:10.1002/adma.201501502 |
| [22] |
Hu G, Zhang H, Zhang L, et al. Integrin-mediated active tumor targeting and tumor microenvironment response dendrimer-gelatin nanoparticles for drug delivery and tumor treatment[J]. Int J Pharm, 2015, 496: 1057-1068. DOI:10.1016/j.ijpharm.2015.11.025 |
| [23] |
Ruan S, He Q, Gao HL. Legumain-responsive functional gold nanoparticles for drug targeting delivery and treatment of subcutaneous xenograft tumor[J]. Acta Pharm Sin (药学学报), 2017, 52: 1756-1762. |
| [24] |
Ruan S, Hu C, Tang X, et al. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation[J]. ACS Nano, 2016, 10: 10086-10098. DOI:10.1021/acsnano.6b05070 |
| [25] |
Hu C, Cun X, Ruan S, et al. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy[J]. Biomaterials, 2018, 168: 64-75. DOI:10.1016/j.biomaterials.2018.03.046 |
| [26] |
Liu R, Xiao W, Hu C, et al. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis[J]. J Control Release, 2018, 278: 127-139. DOI:10.1016/j.jconrel.2018.04.005 |
| [27] |
Semenza GL. The hypoxic tumor microenvironment:a driving force for breast cancer progression[J]. Biochim Biophys Acta, 2016, 1863: 382-391. DOI:10.1016/j.bbamcr.2015.05.036 |
| [28] |
Brady L, Popov V, Ivan M, et al. Investigating the impact of hypoxia-induced changes in splicing on tumor microenvironment[J]. Cancer Res, 2015, 75: 3005. |
| [29] |
Chen W, Wang Y, Qin M, et al. Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy[J]. ACS Nano, 2018, 12: 5995-6005. DOI:10.1021/acsnano.8b02235 |
| [30] |
Chen W, Guo Z, Zhu Y, et al. Combination of bacterial-photothermal therapy with an anti-PD-1 peptide depot for enhanced immunity against advanced cancer[J]. Adv Funct Mater, 2020, 30: 1906623. DOI:10.1002/adfm.201906623 |
| [31] |
Kulkarni P, Haldar MK, Katti P, et al. Hypoxia responsive, tumor penetrating lipid nanoparticles for delivery of chemotherapeutics to pancreatic cancer cell spheroids[J]. Bioconjug Chem, 2016, 27: 1830-1838. DOI:10.1021/acs.bioconjchem.6b00241 |
| [32] |
Kulkarni P, Haldar MK, You S, et al. Hypoxia-responsive polymersomes for drug delivery to hypoxic pancreatic cancer cells[J]. Biomacromolecules, 2016, 17: 2507-2513. DOI:10.1021/acs.biomac.6b00350 |
| [33] |
Periannan K, Haiquan L, Govindasamy I, et al. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels[J]. Cancer Res, 2002, 62: 307-312. |
| [34] |
Lin CW, Lu KY, Wang SY, et al. CD44-specific nanoparticles for redox-triggered reactive oxygen species production and doxorubicin release[J]. Acta Biomater, 2016, 35: 280-292. DOI:10.1016/j.actbio.2016.02.005 |
| [35] |
Yang X, Hu C, Tong F, et al. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer[J]. Adv Funct Mater, 2019, 29: 1901896. DOI:10.1002/adfm.201901896 |
| [36] |
Maggini L, Cabrera I, Ruizcarretero A, et al. Breakable mesoporous silica nanoparticles for targeted drug delivery[J]. Nanoscale, 2016, 8: 7240-7247. DOI:10.1039/C5NR09112H |
| [37] |
Cheng K, Ding Y, Zhao Y, et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy[J]. Nano Lett, 2018, 18: 3250-3258. DOI:10.1021/acs.nanolett.8b01071 |
| [38] |
Li Y, Xu X, Zhang X, et al. Tumor-specific multiple stimuli-activated dendrimeric nanoassemblies with metabolic blockade surmount chemotherapy resistance[J]. ACS Nano, 2016, 11: 416-429. DOI:10.1021/acsnano.6b06161 |
| [39] |
Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers[J]. Front Oncol, 2013, 3: 211-224. |
| [40] |
Luan X, Guan YY, Lovell JF, et al. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel[J]. Biomaterials, 2016, 95: 60-73. DOI:10.1016/j.biomaterials.2016.04.008 |
| [41] |
Xiao W, Ruan S, Yu W, et al. Normalizing tumor vessels to increase the enzyme-induced retention and targeting of gold nanoparticle for breast cancer imaging and treatment[J]. Mol Pharm, 2017, 14: 3489-3498. DOI:10.1021/acs.molpharmaceut.7b00475 |
| [42] |
Li S, Zhang Y, Wang J, et al. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours[J]. Nat Biomed Eng, 2017, 1: 667-679. DOI:10.1038/s41551-017-0115-8 |
| [43] |
Kashiwagi S, Tsukada K, Xu L, et al. Perivascular nitric oxide gradients normalize tumor vasculature[J]. Nat Med, 2008, 14: 255-257. DOI:10.1038/nm1730 |
| [44] |
Carpenter AW, Schoenfisch MH. Nitric oxide release:part II. Therapeutic applications[J]. Chem Soc Rev, 2012, 41: 3742-3752. DOI:10.1039/c2cs15273h |
| [45] |
Kinoshita R, Yu I, Ikeda M, et al. S-Nitrosated human serum albumin dimer as novel nano EPR enhancer applied to macromolecular anti-tumor drugs such as micelles and liposomes[J]. J Control Release, 2015, 217: 1-9. DOI:10.1016/j.jconrel.2015.08.036 |
| [46] |
Naohide I, Padera TP, Jeroen H, et al. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function[J]. Cancer Res, 2004, 64: 4400-4404. DOI:10.1158/0008-5472.CAN-04-0752 |
| [47] |
Kato M, Hattori Y, Kubo M, et al. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex[J]. Int J Pharm, 2012, 423: 428-434. DOI:10.1016/j.ijpharm.2011.12.015 |
| [48] |
Murty S, Gilliland T, Qiao P, et al. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model[J]. Part Part Syst Charact, 2015, 31: 1307-1312. |
| [49] |
Wang H, Han X, Dong Z, et al. Hyaluronidase with pH-responsive dextran modification as an adjuvant nanomedicine for enhanced photodynamic-immunotherapy of cancer[J]. Adv Funct Mater, 2019, 29: 1902440. DOI:10.1002/adfm.201902440 |
| [50] |
Raeesi V, Chan WC. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods[J]. Nanoscale, 2016, 8: 12524-12530. DOI:10.1039/C5NR08463F |
| [51] |
Rama G, Shankar S, Tannock IF. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells[J]. Cancer Res, 2006, 66: 1033-1039. DOI:10.1158/0008-5472.CAN-05-3077 |
| [52] |
Wang J, Lu Z, Gao Y, et al. Improving delivery and efficacy of nanomedicines in solid tumors:role of tumor priming[J]. Nanomedicine, 2011, 6: 1605-1620. DOI:10.2217/nnm.11.141 |
| [53] |
Jang SH, Wientjes MG, Au JL. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment:effect of treatment schedule[J]. J Pharmacol Exp Ther, 2001, 296: 1035-1042. |
| [54] |
Wang J, Lu Z, Yeung BZ, et al. Tumor priming enhances siRNA delivery and transfection in intraperitoneal tumors[J]. J Control Release, 2014, 178: 79-85. DOI:10.1016/j.jconrel.2014.01.012 |
| [55] |
Chen B, Dai W, Mei D, et al. Comprehensively priming the tumor microenvironment by cancer-associated fibroblast-targeted liposomes for combined therapy with cancer cell-targeted chemotherapeutic drug delivery system[J]. J Control Release, 2016, 241: 68-80. DOI:10.1016/j.jconrel.2016.09.014 |
| [56] |
US National Library of Medicine. ClinicalTrials gov[EB/OL]. 2019. https://clinicaltrials.gov/ct2/show/NCT01861496?term.
|
| [57] |
Mukai H, Kogawa T, Matsubara N, et al. A first-in-human phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors[J]. Invest New Drugs, 2017, 35: 307-314. DOI:10.1007/s10637-016-0422-z |
 2020, Vol. 55
2020, Vol. 55


