作者贡献:侯旭东是本文的第一作者, 负责文献中相关数据的调研和整理, 文章主题内容的撰写。胡情负责相关文献的调研与整理。马丽娟负责部分文献资料的搜集整理和归纳汇总。于浩楠负责前沿技术相关文献调研与整理, 负责部分内容的撰写。葛广波为本文的通讯作者, 负责文章整体结构的设计、内容的撰写与稿件修改。侯洁为本文的并列通讯作者, 负责文章部分内容的撰写、全文的审核与校订。
利益冲突:本文的研究内容无任何利益冲突。
2. 上海中医药大学交叉科学研究院, 上海 201203
2. Institute of Interdisciplinary Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
近20年来, 由于现代生活方式的改变和高脂、高糖饮食的增加, 肥胖的发病率急剧升高。目前, 肥胖已被世界卫生组织(WHO)认定是一个全球性的问题, 在2016年, 全球的肥胖或超重人口占比将近50%[1-4], 新近研究表明肥胖是由于人体内能量吸收和能量消耗的长期失衡所导致的, 还发现肥胖也是高血压、高脂血症、动脉硬化、2型糖尿病、冠心病以及多种类型癌症等代谢性疾病的重要诱因[5-10]。因此, 研发高效且安全的减肥药物对于肥胖、高血脂症等代谢性疾病的防治尤为重要。人体有多种酶参与脂质的吸收与代谢, 比如舌脂肪酶、胃脂肪酶、胰脂肪酶(pancreatic lipase, PL), 其中部分关键酶可作为肥胖的潜在治疗靶点[8]。其中最为重要且研究最深入的肥胖治疗靶点是PL (又称三酰基甘油酰基水解酶)。国内外大量研究显示抑制PL的活性可减少消化器官中膳食脂肪的消化和吸收, 进而显著改善肥胖和高脂血症等代谢性疾病的症状[11-15]。
PL是由哺乳动物胰腺分泌后释放到胃肠体系, 其与肝脏分泌的胆汁酸盐协作, 将脂肪分解为脂肪酸和甘油。PL负责水解约50%~70%的膳食脂肪, 是调控哺乳动物脂类物质吸收的关键酶。近年来, 越来越多的研究表明, 抑制PL可减少消化器官中膳食脂肪的分解和吸收, 进而改善肥胖和高脂血症等代谢性疾病的症状。因此, 研发PL抑制剂已成为肥胖防治新药研发领域的热点[16, 17]。目前临床上常用的肥胖防治药物奥利司他是一种源于毒三素链霉菌代谢产物利普斯他汀的衍生物, 是一种强效的PL抑制剂, 其在1999年被美国食品与药品管理局批准为抗肥胖药物[18-21]。虽然奥利司他显现出了极佳的脂肪酶抑制效果, 且对临床肥胖治疗有很好的作用, 能够阻止大约人体内30%膳食脂肪的吸收[22], 但奥利司他有着不可忽视的胃肠道不良反应, 比如大便失禁、胃肠道胀气、腹部痉挛、腹泻和油性斑点等[23-26]。因此, 研发强效且安全的新型PL抑制剂并将其用于肥胖等代谢性疾病的防治显得尤为重要。近年来, 从中草药中(尤其是药食同源的中草药)发现有活性的天然产物已成为新药研发的热点。中药作为我国的民族瑰宝, 其有效性和安全性在千百年临床实践中已得到反复验证和深入的了解。近年来, 国内外大量研究发现茶叶、桑白皮等多种中药提取物及其化学成分可通过抑制PL达到调节脂质代谢、治疗肥胖的效果。本文结合近年来PL抑制剂研发领域的国内外研究进展, 着重汇总了具有PL抑制效应的中药提取物及其化学成分, 并对其抑制效应和抑制机制等进行了系统归纳和总结。
2 中药提取物及其化学成分对PL的抑制作用 2.1 中药提取物对PL的抑制作用许多中草药都显示出了良好的降脂活性。近年来, 越来越多的研究开展了中药提取物对PL的抑制作用, 以及中药提取物对高脂模型动物的治疗作用研究。表 1总结了多种中药提取物对PL的抑制效果。从表中可以看出, 姜黄、桑枝、桑白皮、桑叶、一枝黄花、岩白菜、穿山龙、苍术、茶叶等中药提取物对PL有较为强烈的抑制作用, 尤其是桑树的3个部分对PL均有较强的抑制作用, 这提示了桑树在PL抑制剂以及肥胖治疗方面有比较好的应用价值。值得注意的是, 多数研究选择了方便获取并且与人类胰脂肪酶(human pancreatic lipase)相似性较高的猪胰脂肪酶(porcine pancreatic lipase)作为酶源。而在酶抑制作用的检测方面, 使用较多的是以4-甲基伞形酮油酸酯(4-methylumbelliferyl oleate, 4-MUO)为底物, 其可被PL水解产生具有荧光属性的产物4-甲基伞形酮(4-methylumbelliferon, 4-MU)。荧光检测由于具有灵敏度高、特异性强、检测准确的优势, 常被用于酶抑制剂的高通量筛选和评价[27-32]。此外, PNPB (p-nitrophenyl butyrate)、PNPP (p-nitrophenyl phosphate)等荧光底物也常被用于PL抑制剂的高效筛选。
| Table 1 The inhibitory effects of Chinese herbs against PL. PL: Pancreatic lipase. 4-MUO: 4-Methylumbelliferyl oleate; PPL: Porcine pancreatic lipase; GT: Glyceryl tributyrate; PNPP: p-Nitrophenylpalmitate; PNPB: p-Nitrophenyl butyrate; DNPB: 2, 4-Dinitrophenyl butyrate; HPL: Human pancreatic lipase |
桑白皮(Cortex Mori)是一种著名的“药食同源”类中草药, 已经被广泛应用于高血压、高脂血症、糖尿病等代谢性疾病的治疗[52-54]。桑白皮的主要成分包括黄酮类成分(桑根酮、桑黄酮等)、香豆素类以及多糖[55-59]。Hou等[60]发现桑白皮中的主要成分桑根酮C (1)和桑根酮D (2)具有很强的PL抑制活性, 能够以混合性抑制方式减缓PL催化的4-MUO水解反应, 抑制常数(Ki)分别为1.07和0.43 μmol·L-1 (图 1, 1~6)。桑根酮C和桑根酮D作为桑白皮的主要化学成分, 其含量分别达到781.4和576.5 μg·g-1, 中药口服后其在胃肠体系的生理浓度能够超过其Ki, 提示桑白皮口服后其化学成分能够有效地抑制胃肠体系中的PL。此外, 作者借助分子对接模拟, 进一步证实了桑根酮D能够与PL活性中心的催化残基Ser-152发生强烈的相互作用。Ha等[61]鉴定了桑白皮中33种天然成分的化学结构, 并且以奥利司他作为阳性对照, 初步测定了天然成分对PL介导的p-NPB水解反应的抑制作用, 其中, 9种桑白皮化学成分显示出较为强效的抑制作用, IC50值范围为0.09~0.92 μmol·L-1 (图 1, 7~15), 其中化合物7~13均属于香豆酮类化合物的衍生物(含有法尼基、香叶基、异戊二烯基等), 这提示香豆酮类化合物能够作为与PL相关代谢疾病治疗的药物先导物。
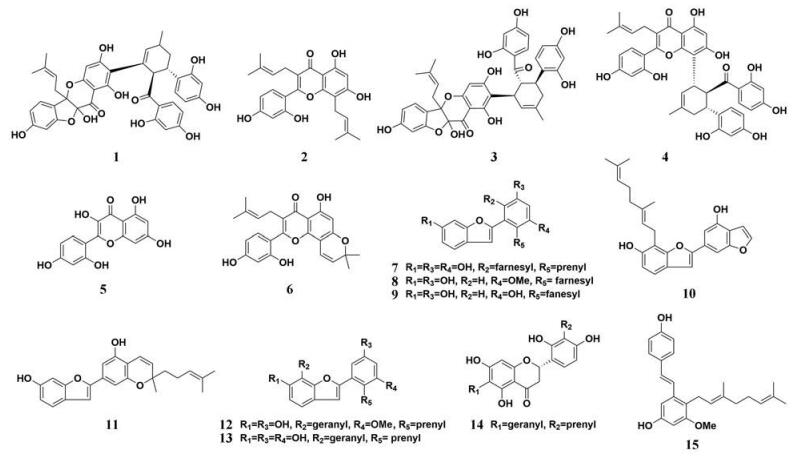
|
Figure 1 Chemical structures of natural PL inhibitors from Cortex Mori |
桑叶(Folium Mori)作为“药食同源”类中草药, 能够用于多种代谢疾病(糖尿病、高血压、抗氧化等)的治疗[62-64], 主要成分为黄酮类(槲皮素、异槲皮苷等)、生物碱类(葫芦巴碱)、三萜类、多酚类和多糖类[65-67]。Jeong等[68]研究了桑叶中20种化学成分(黄酮类、苯并呋喃类、芪类和查尔酮类)对PL的抑制作用, 8种天然化合物(图 2, 16~23)显示较为强效的抑制能力(IC50为6.2~64.8μmol·L-1), 其中, 查尔酮类化合物——桑叶查尔酮A (23)显示出最强的抑制效果, IC50达到6.2 μmol·L-1。与其他类别天然产物相比, 含有异戊二烯基团的化合物显示出了相对强效的PL抑制作用, 提示异戊二烯基团在抑制PL中发挥了重要作用。上述研究提示桑树作为一种较为常见的药用植物, 其多个药用部位均对PL有较强的抑制作用, 提示其在肥胖防治领域具有重要的研究价值和应用前景。
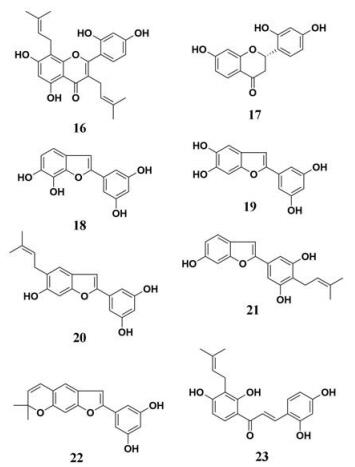
|
Figure 2 Chemical structures of natural PL inhibitors from Folium Mori |
银杏叶(Folium Ginkgo)是银杏科植物银杏Ginkgo biloba L.的干燥叶, 在很多国家都有较长的使用历史, 其在东西方均被作为饮食补充剂或者植物药使用[69-71], 银杏叶提取物的主要成分包含萜类、双黄酮类和黄烷醇类等[72, 73]。同时, 银杏叶提取物还被用于多种代谢性疾病的治疗, 如高脂血症、心血管疾病和动脉硬化等。Liu等[74]对银杏叶中的4种双黄酮成分(图 3, 24~27)进行了PL抑制效应评价, 发现其中3种双黄酮类成分对PL有强烈的抑制作用(IC50 < 10 μmol·L-1), 且异银杏双黄酮(24)对PL的抑制常数Ki达到了0.81 μmol·L-1, 分子对接结果显示, 异银杏双黄酮能够与PL活性中心的Ser-152形成氢键, 进而发生强烈的相互作用。而Bustanji等[36]报道了银杏叶中的7种三萜内酯类化合物对PL的抑制作用, 其中, 银杏内酯A (28)显示出PL抑制活性, IC50值为56.07 μmol·L-1。
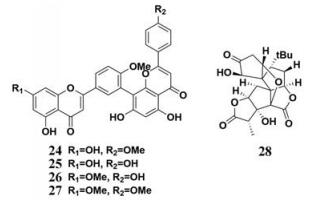
|
Figure 3 Chemical structures of natural PL inhibitors from Folium Ginkgo |
穿山龙(Rhizoma Dioscoreae Nipponicae)主要成分包括甾体皂苷类(薯蓣皂苷、纤细皂苷和水溶性皂苷), 具有镇咳、祛痰、平喘的作用[75, 76]。Kwon等[44]以4-MUO为底物测定了穿山龙提取物对PL的抑制活性, 同时对穿山龙主要化学成分的PL抑制活性做了进一步的研究(图 4, 29~32), 发现薯蓣次皂苷A显示出较为强效的PL抑制作用, IC50值为2.49 μmol·L-1。此外, 作者还以高脂小鼠为模型, 以穿山龙提取物及其化学成分为实验组, 通过测定小鼠体重及其脂肪相关指标, 在动物整体层面对穿山龙的抗肥胖作用进行了活性验证, 结果显示穿山龙提取物能够有效缓解高脂鼠体重和脂肪组织重量的快速增长。
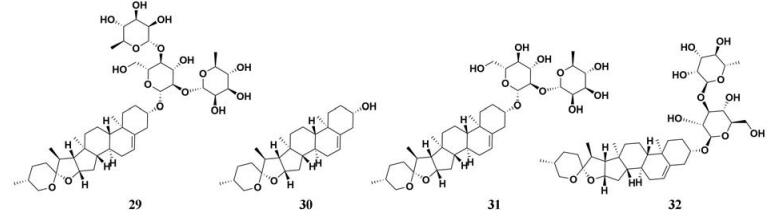
|
Figure 4 Chemical structures of natural PL inhibitors from Rhizoma Dioscoreae Nipponicae |
苍术(Rhizoma Atractylodis)的化学成分包括挥发油、倍半萜类、酚类和有机酸类。苍术的药理作用很多, 包括抗炎、抗氧、抗菌、降血糖、保肝等[77, 78]。Jiao等[45]报道了苍术提取物及其化学成分对PL的抑制作用(图 5, 33~38), 其中苍术素的抑制效果较好。作者还采用小鼠为动物模型, 比较了苍术提取物及其化学成分的抗肥胖作用, 研究发现与阳性对照组(奥利司他组)相比, 苍术对小鼠体重的变化并不明显, 提示苍术提取物及其化学成分整体层面的肥胖防治效果并不突出。
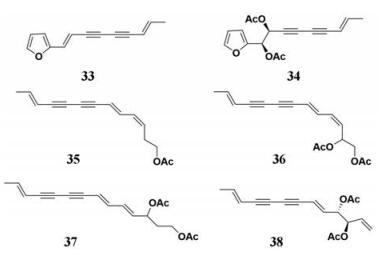
|
Figure 5 Chemical structures of natural PL inhibitors from Rhizoma Atractylodis |
娑罗子(Semen Aesculi)作为七叶树的干燥成熟种子, 主要含有皂苷类、香豆素类、黄酮类和有机酸等成分[79, 80]。Kimura等[81]研究了七叶树种子(娑罗子)的化学成分及其衍生物对PL的抑制作用, 结果显示娑罗子中的七叶皂苷化合物对PL有中等强度的抑制作用(IC50 < 70 μmol·L-1, 图 6, 39~42), 而其衍生物的PL抑制性较弱, 七叶皂苷IIb (42)显示出最强的PL的抑制作用, IC50值为12.71 μmol·L-1。上述研究表明具有当归酰基的七叶皂苷Ib (41)和七叶皂苷IIb的PL抑制活性优于具有顺酯酰基团的七叶皂苷Ia (39)和七叶皂苷IIa (40)。
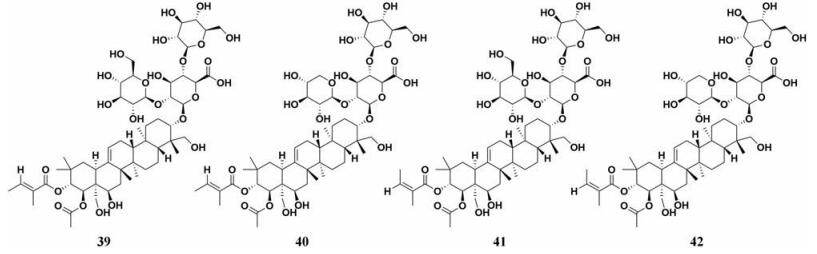
|
Figure 6 Chemical structures of natural PL inhibitors from Semen Aesculi |
荷叶(Folium Nelumbinis)作为一种中国传统中草药主要用于暑热烦渴、暑湿泄泻、脾虚泄泻等。荷叶中含有多种化学成分, 包括:黄酮类、生物碱、有机酸和挥发油等, 其中最为主要的有效成分是生物碱和黄酮类化合物。而且, 荷叶作为第二批“药食同源”的中草药, 在使用以及用药研究方面有广泛的应用, 其中的生物碱和黄酮成分具有调血脂活性, 因此其减肥降脂的作用也受到越来越多的关注。Tao等[82]使用其新型的筛选方法从药用植物提取物中筛选抑制剂, 并且使用高效液相色谱-质谱法快速鉴定了活性化合物(图 7, 43~44), 其中槲皮素-3-O-β-D-吡喃阿拉伯糖基-(1→2)-β-D-半乳糖(43)表现出中等的抑制作用, IC50为66.86 μmol·L-1。Tang等[83]开发验证了用于检测脂肪酶抑制剂的新型TLC生物自动测定法, 测定了荷叶中的主要成分槲皮素(45)和荷叶碱(46)对PL的抑制作用(图 7, 45、46), 均展现出了强效的抑制能力, IC50分别为17.8和16.2 μmol·L-1。荷叶作为一种常用的中草药, 易得安全, 为开发新型的PL抑制剂提供了依据, 并且为肥胖和高脂血症的治疗提供了更好的药物制备材料。
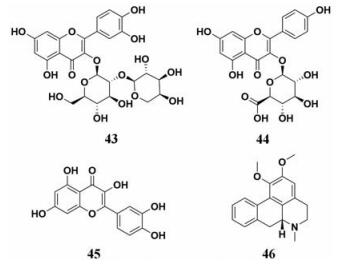
|
Figure 7 Chemical structures of natural PL inhibitors from Folium Nelumbinis |
茶叶是一种日常生活中极其常见的中草药, 现代药理学研究表明茶叶能够提高中枢神经系统的兴奋度, 具有利尿、抑菌和治疗哮喘的功效。茶叶种类繁多, 常见的有乌龙茶、黑茶、绿茶等, 其主要成分基本相似, 主要包含三萜皂苷、维生素、甾醇、黄酮类、没食子酸酯和黄酮醇等。Nakai等[46]报道了乌龙茶里多酚类化合物对PL的抑制作用, 发现茶叶中的多种化学成分对PL均显示出了强效的抑制效果(图 8, 47~61), 其中没食子儿茶素-3, 5-di-O-没食子酸酯(53)的抑制作用最强, IC50值为0.10 μmol·L-1。对于乌龙茶提取物, 乌龙茶提取物中的聚合多酚抑制效果(IC50= 0.28 μg·mL-1)较乌龙茶提取物的抑制效果(IC50= 0.91 μg·mL-1)更优, 表现出极强的PL抑制作用。上述研究提示乌龙茶对于高脂血症和肥胖的防治可能有较好的效果。此外, Yuda等[47]对黑茶提取物及其化学成分的PL抑制作用评价, 发现没食子酸酯类化合物具有很强的PL抑制活性(图 9, 62~67)。上述研究提示可以没食子酸酯类化合物为先导化合物, 设计合成活性更优且安全性好的PL抑制剂。由于茶叶提取物及其化学成分拥有非常强的PL抑制活性, 加之其来源广泛, 茶叶及其制品在高脂血症及肥胖的防治领域会有更广泛的应用前景。
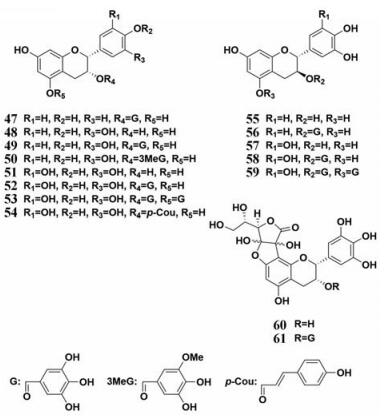
|
Figure 8 Chemical structures of natural PL inhibitors from Oolong Tea |
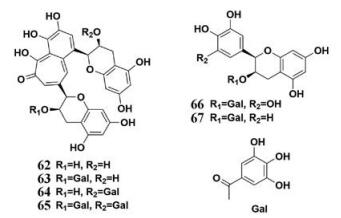
|
Figure 9 Chemical structures of natural PL inhibitors from Black Tea |
连翘(Fructus Forsythiae)广泛存在于中国、日本和韩国, 其果实具有抗炎、抗病毒、解热、抗肝损伤和其他治疗作用。连翘叶经常作为茶叶来使用, Chen等[84]分离鉴定了连翘叶中的10种天然成分, 并且准确筛选了10种成分对PL的作用能力, 其中, 2种活性成分表现出一定的PL抑制作用(图 10, 68、69), IC50值分别为2.9和52.4 μmol·L-1, 同时, 通过抑制动力学分析, 揭示了3种活性成分对PL的抑制类型和作用机制。这提示连翘茶叶与乌龙茶叶、黑茶叶以及绿茶叶一样, 能够抑制PL的生物活性, 借此达到降脂减肥的目的。
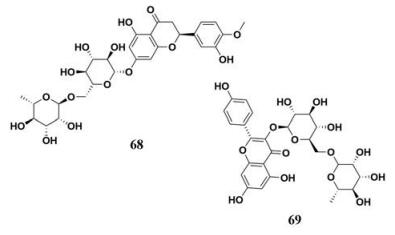
|
Figure 10 Chemical structures of natural PL inhibitors from Fructus Forsythiae |
补骨脂(Fructus Psoraleae)是一种在亚洲国家广泛使用的可食用中药, 有较好地降血脂作用, 并且对于多种疾病有一定治疗作用。补骨脂含有多种化学成分, 包括黄酮类、查尔酮类等, 补骨脂乙素能够有效地扩张哺乳动物离体心脏的冠状血管, 并且能够对抗脑垂体后叶素对冠脉的收缩作用, 此外, 还能够强效抑制脂质积聚, 降低血清脂质和肝脏甘油三酯水平[85]。Hou等[86]收集了补骨脂中的11种主要化学成分(图 11, 70~76), 以4-甲基伞形酮油酸酯作为探针底物检测补骨脂中的化学成分对PL的抑制效果, 结果显示, 其中的查尔酮类化合物(包括补骨脂乙素、补骨脂查尔酮)显示出较为强效的抑制效果, IC50分别为3.30和5.73 μmol·L-1。进一步分子对接研究显示补骨脂中的查尔酮类化合物与PL催化空腔的氨基酸残基主要通过疏水作用相结合, 同时C-4酚羟基还可与PL催化空腔的部分氨基酸残基形成氢键作用, 提示该类化合物主要通过疏水和氢键作用与PL结合。
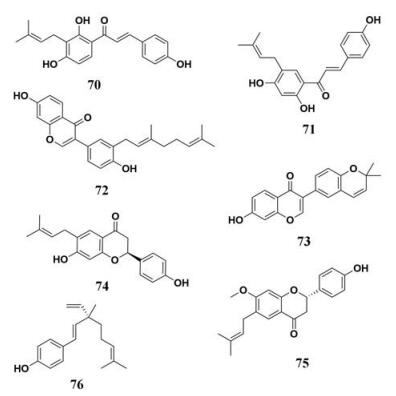
|
Figure 11 Chemical structures of natural PL inhibitors from Fructus Psoraleae |
贯叶连翘(Herba Hyperici Perforati)在全球范围内被广泛使用, 是一种“药食同源”的中草药, 能够作为膳食补充剂和中药方剂制备的关键材料。同时, 全球范围内已经上市了多种贯叶连翘产品, 其治疗效果得到了充分肯定。众多的药理学研究表明, 贯叶连翘提取物及其主要成分(包括蒽酮类、黄酮类、双黄酮类和单宁类)有良好的抗抑郁、抗炎、抗肿瘤和多种酶抑制活性等作用, 而且贯叶连翘能够通过降低体重、脂肪细胞体积和脂肪含量达到抗肥胖作用[87-89]。Hou等[34]贯叶连翘中的化学成分进行了PL抑制活性测定(图 12, 77~91), 发现两种萘骈二蒽酮类化合物(金丝桃素、伪金丝桃素)和1个双黄酮类化合物(I3, II8-双芹菜苷元)对PL表现出了较为强效的抑制作用, IC50值均小于1 μmol·L-1。其中, I3, II8-双芹菜苷元(C-8/C-3连接)的抑制能力优于已报道的其他双黄酮类化合物(如C-8/C-3′连接的异银杏素)。抑制动力学和分子反应动力学研究显示上述3种天然成分是PL的混合性抑制剂, 其可与PL结合在两个不同的配体结合位点。
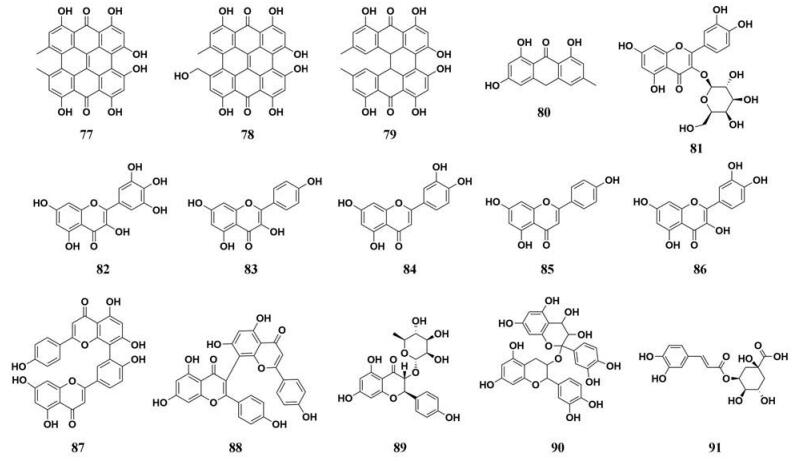
|
Figure 12 Chemical structures of natural PL inhibitors from Herba Hyperici Perforati |
甘草(Radix Et Rhizoma Glycyrrhizae, Licorice)作为一种医用药物已经被全球广泛应用, 甘草中含有多种的天然成分, 比如类黄酮、皂苷类和香豆素类等。甘草被称为“药草之先”, 具有丰富的医药价值, 能够治疗动脉疾病、心绞痛, 并且其提取物能够用于治疗发炎和消化性溃疡。药理学研究表明, 甘草及其生物活性成分具有抗炎、抗病毒、抗氧化等功效, 此外, 有研究报道甘草黄酮油能够通过调节与肝脏中脂肪酸合成和氧化相关的限速酶活性来抑制腹部脂肪积聚, 进而达到降脂减肥的目的[90]。Birari等[91]从甘草中分离并鉴定了12中黄酮类化合物, 测定了12种天然成分对PL的抑制活性, 结果显示, 异甘草素等4种化合物表现出较为强效的PL抑制能力(图 13, 92~95), IC50值在7.3~37.6 μmol·L-1之间, 分子对接结果也表明, 甘草中的PL抑制剂能够与PL活性位点的关键氨基酸残基发生相互作用。动物体内实验结果显示, 异甘草素(92)能够控制大鼠体重的快速增长, 并且降低血清中甘油三酯和总胆固醇的水平。以上结果进一步阐明了甘草在减肥降脂方面发挥着巨大作用, 对于临床上治疗肥胖和药物的研发提供了实验依据。
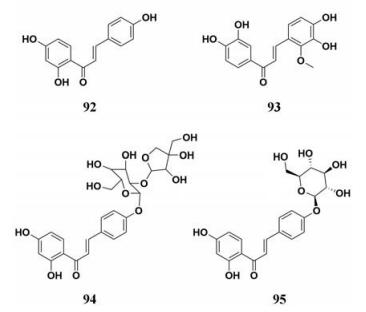
|
Figure 13 Chemical structures of natural PL inhibitors from Radix Et Rhizoma Glycyrrhizae |
多种中药中天然成分对于PL的抑制作用被广泛地研究, 天然成分的化学结构及其抑制能力如图 14、15和表 2所示。
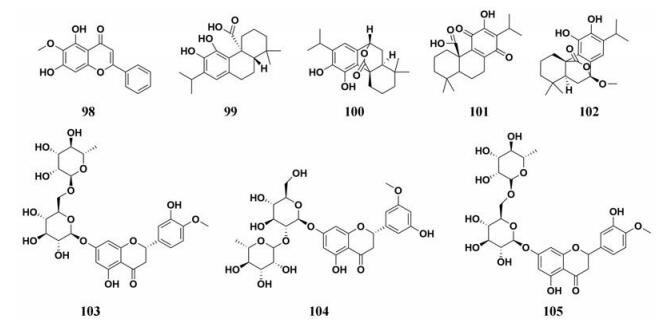
|
Figure 14 Chemical structures of natural PL inhibitors from Radix Scutellariae, Herba Salvia Japonica, Rubrum Citri Exocarpium, and Spica Prunellae |
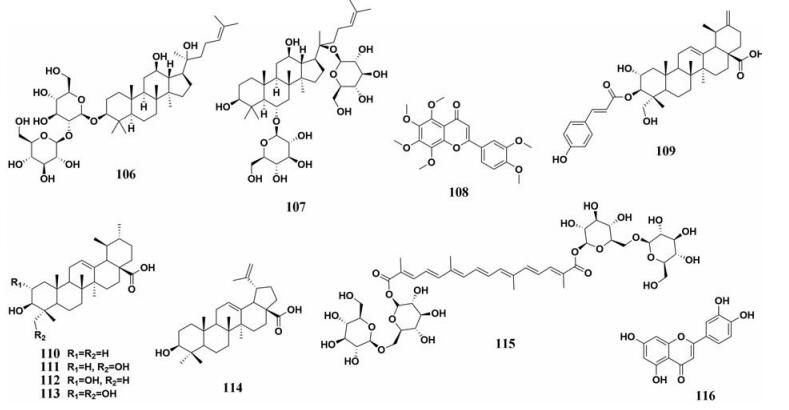
|
Figure 15 Chemical structures of natural PL inhibitors from Radix Et Rhizoma Ginseng, Pericarpium Citri Reticulatae, Radix Actinidia arguta, Fructus Gardeniae, and Folium Tecoma stans |
| Table 2 The inhibitory effects of herbal constituents against PL. β-NPM: β-Naphthyl myristate; PNPL: p-Nitrophenyl laurate |
随着全球范围内肥胖发病率的急剧升高, 研发安全、有效的肥胖防治药物已成为国内外新药研发的热点之一。中草药(尤其是药食两用的中草药)在长期的临床实践中显示了良好的安全性, 因此其在肥胖等代谢性疾病防治中的应用有着得天独厚的优势。近20年来, 围绕着PL这一肥胖防治的关键靶点, 国内外学者已从中草药中(尤其是药食同源的中草药)中发现了多种PL的天然抑制剂。虽然天然PL抑制剂的抑制能力较弱, 但由于PL主要分布在胃肠体系中, 中草药口服后其活性成分可与PL直接结合进而发挥抑制作用。考虑到中草药口服后其胃肠暴露量远高于其他组织, 其活性成分的胃肠暴露量往往高于其抑制PL的IC50[60, 74, 86, 101]。因此, 从中草药中发现天然的PL抑制剂进而将其开发成减肥降脂药物/产品具有较高的可行性和应用前景。中草药化学成分极其复杂, 如何快速准确地从中草药中发现强效的PL抑制剂是减肥降脂药物/产品研发的关键。近年来, 随着现代分离分析技术的快速发展, 采用中药化学指纹图谱和抑制效应谱相结合(谱效结合)的策略从中草药中快速发现有PL抑制活性的天然产物已成为现实[81, 84, 102, 103]。同时, 基于磁性纳米粒子多样的表面结构、较强的酶负载能力等优势, 已经开发出更为优良的固定化酶技术, 利用磁珠固定化PL和LC-MS/MS相结合, 筛选和鉴定中药提取物中的有效抑制成分, 此技术不仅能够在短时间内快速富集、鉴定中药提取物中的天然活性成分, 而且能够提升筛选过程的稳定性和精确性[104-106]。此外, 还有学者利用基于亲和超滤质谱技术等现代分析技术快速发现可与PL强效结合的小分子, 进而结合酶抑制实验发现中草药中的PL抑制剂[107-109]。值得注意的是, 目前业界发现与评价PL抑制剂多采用廉价易得的猪胰脂酶(PPL)为酶源, 其与人胰脂酶(HPL)对抑制剂的响应差异及结合/抑制动力学上的差异还有待深入研究。因此, 未来PL抑制剂的研发需要进一步验证其对HPL的抑制效果。对于发现的可强效抑制HPL的天然产物, 药物化学家可将其作为先导化合物并利用高效化学衍生获取结构多样的系列衍生物, 进而开展更为细致的构效关系研究和结构-成药性/安全性关系研究, 最终有望设计研发出安全、强效且具有良好成药性的新型HPL抑制剂, 最终达到治疗肥胖及其相关代谢疾病的目的[110]。因此对于有强效抑制作用的中草药, 还可在中医药理论的指导下, 通过配伍配比优化和现代体内外药理学研究, 研发出可用于肥胖、糖尿病等代谢性疾病防治的中药复方或中药新药。此外, 临床专家还可通过开展中西药联用的临床研究评价具有PL抑制活性的中草药与已上市的肥胖防治药物(如奥利司他)的联合应用, 以期寻找更为安全、有效的防治肥胖等代谢性疾病的新疗法。
| [1] |
Lunagariya NA, Patel NK, Jagtap SC, et al. Inhibitors of pancreatic lipase:state of the art and clinical perspectives[J]. EXCLI J, 2014, 13: 897-921. |
| [2] |
Malik VS, Willett WC, Hu FB. Global obesity:trends, risk factors and policy implications[J]. Nat Rev Endocrinol, 2013, 9: 13-27. DOI:10.1038/nrendo.2012.199 |
| [3] |
Ahima RS. Digging deeper into obesity[J]. J Clin Invest, 2011, 121: 2076-2079. DOI:10.1172/JCI58719 |
| [4] |
Bray GA. Soft drink consumption and obesity:it is all about fructose[J]. Curr Opin Lipidol, 2010, 21: 51-57. DOI:10.1097/MOL.0b013e3283346ca2 |
| [5] |
Aronne LJ. Classification of obesity and assessment of obesity-related health risks[J]. Obesity, 2012, 10: 105S-115S. |
| [6] |
Kopelman PG. Obesity as a medical problem[J]. Nature, 2000, 404: 635-643. DOI:10.1038/35007508 |
| [7] |
Antonopoulos AS, Oikonomou EK, Antoniades C, et al. From the BMI paradox to the obesity paradox:the obesity-mortality association in coronary heart disease[J]. Obes Rev, 2016, 17: 989-1000. DOI:10.1111/obr.12440 |
| [8] |
Shi Y, Burn P. Lipid metabolic enzymes:emerging drug targets for the treatment of obesity[J]. Nat Rev Drug Discov, 2004, 3: 695-710. DOI:10.1038/nrd1469 |
| [9] |
Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001[J]. JAMA, 2003, 289: 76-79. DOI:10.1001/jama.289.1.76 |
| [10] |
Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States:comparative risk assessment of dietary, lifestyle, and metabolic risk factors[J]. PLoS Med, 2009, 6: e1000058. DOI:10.1371/journal.pmed.1000058 |
| [11] |
Kushner RF. Weight loss strategies for treatment of obesity[J]. Prog Cardiovasc Dis, 2014, 56: 465-472. DOI:10.1016/j.pcad.2013.09.005 |
| [12] |
Rubio MA, Gargallo M, Isabel MA, et al. Drugs in the treatment of obesity:sibutramine, orlistat and rimonabant[J]. Public Health Nutr, 2007, 10: 1200-1205. DOI:10.1017/S1368980007000717 |
| [13] |
Al DLG, Milagro FI, Boque N, et al. Natural inhibitors of pancreatic lipase as new players in obesity treatment[J]. Planta Med, 2011, 77: 773-785. DOI:10.1055/s-0030-1270924 |
| [14] |
Jeong JY, Jo YH, Kim SB, et al. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions[J]. Bioorg Med Chem Lett, 2015, 25: 2269-2274. DOI:10.1016/j.bmcl.2015.04.045 |
| [15] |
Mukherjee M. Human digestive and metabolic lipases-a brief review[J]. J Mol Catal B Enzym, 2003, 22: 369-376. DOI:10.1016/S1381-1177(03)00052-3 |
| [16] |
Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources:unexplored potential[J]. Drug Discov Today, 2007, 12: 879-889. DOI:10.1016/j.drudis.2007.07.024 |
| [17] |
Mcclendon KS, Riche DM, Uwaifo GI. Orlistat:current status in clinical therapeutics[J]. Expert Opin Drug Saf, 2009, 8: 727-744. DOI:10.1517/14740330903321485 |
| [18] |
Ballinger A, Peikin SR. Orlistat:its current status as an anti-obesity drug[J]. Eur J Pharmacol, 2002, 440: 109-117. DOI:10.1016/S0014-2999(02)01422-X |
| [19] |
Leung WY, Thomas GN, Chan JC, et al. Weight management and current options in pharmacotherapy:orlistat and sibutramine[J]. Clin Ther, 2003, 25: 58-80. DOI:10.1016/S0149-2918(03)90009-9 |
| [20] |
Hochuli E, Kupfer E, Maurer R, et al. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. II. Chemistry and structure elucidation[J]. J Antibiot, 1987, 40: 1286-1091. |
| [21] |
Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity[J]. Pharmacotherapy, 2000, 20: 270-279. DOI:10.1592/phco.20.4.270.34882 |
| [22] |
Guerciolini R. Mode of action of orlistat[J]. Int J Obes, 1997, 21: S12-S23. |
| [23] |
Chaput JP, St-Pierre S, Tremblay A. Currently available drugs for the treatment of obesity:sibutramine and orlistat[J]. Mini-Rev Med Chem, 2007, 7: 3-10. DOI:10.2174/138955707779317849 |
| [24] |
Cheung BMY, Cheung TT, Samaranayake NR. Safety of antiobesity drugs[J]. Ther Adv Drug Saf, 2013, 4: 171-181. DOI:10.1177/2042098613489721 |
| [25] |
Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group[J]. Lancet, 1998, 352: 167-172. DOI:10.1016/S0140-6736(97)11509-4 |
| [26] |
Guarino AH. Treatment of intractable constipation with orlistat:a report of three cases[J]. Pain Med, 2010, 6: 327-328. DOI:10.1111/j.1526-4637.2005.00053.x |
| [27] |
Weng ZM, Ge GB, Dou TY, et al. Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2[J]. Bioorg Chem, 2018, 77: 320-329. DOI:10.1016/j.bioorg.2018.01.011 |
| [28] |
Lv X, Wang XX, Hou J, et al. Comparison of the inhibitory effects of tolcapone and entacapone against human UDP-glucuronosyltransferases[J]. Toxicol Appl Pharmacol, 2016, 301: 42-49. DOI:10.1016/j.taap.2016.04.009 |
| [29] |
Xin H, Qi XY, Wu JJ, et al. Assessment of the inhibition potential of licochalcone A against human UDP-glucuronosyltransferases[J]. Food Chem Toxicol, 2016, 90: 112-122. DOI:10.1016/j.fct.2016.02.007 |
| [30] |
Zou LW, Li YG, Wang P, et al. Design, synthesis, and structure-activity relationship study of glycyrrhetinic acid derivatives as potent and selective inhibitors against human carboxylesterase 2[J]. Eur J Med Chem, 2016, 112: 280-288. DOI:10.1016/j.ejmech.2016.02.020 |
| [31] |
Shah S, Javaid K, Zafar H, et al. Synthesis, and in vitro and in silico α-glucosidase inhibitory studies of 5-chloro-2-aryl benzo[d]thiazoles[J]. Bioorg Chem, 2018, 78: 269-279.
|
| [32] |
Cheng X, Lv X, Qu H, et al. Comparison of the inhibition potentials of icotinib and erlotinib against human UDP-glucuronosyltransferase 1A1[J]. Acta Pharm Sinica B, 2017, 7: 657-664. DOI:10.1016/j.apsb.2017.07.004 |
| [33] |
Sun XL, Zhang KB, Ji XH, et al. Screening of pancreatic lipase and α-glucosidase inhibitors from Chinese dietary herbs[J]. China J Chin Mater Med (中国中药杂志), 2012, 37: 1319-1323. |
| [34] |
Hou XD, Guan XQ, Cao YF, et al. Inhibition of pancreatic lipase by the constituents in St. John's Wort:in vitro and in silico investigations[J]. Int J Biol Macromol, 2020, 145: 620-633. DOI:10.1016/j.ijbiomac.2019.12.231 |
| [35] |
Adisakwattana S, Intrawangso J, Hemrid A, et al. Extracts of edible plants inhibit pancreatic lipase, cholesterol esterase and cholesterol micellization, and bind bile acids[J]. Food Technol Biotechnol, 2012, 50: 11-16. |
| [36] |
Bustanji Y, Al-Masri IM, Mohammad M, et al. Pancreatic lipase inhibition activity of trilactone terpenes of Ginkgo biloba[J]. J Enzym Inhib Med Chem, 2011, 26: 453-459. DOI:10.3109/14756366.2010.525509 |
| [37] |
Shimura S, Tsuzuki W, Kobayashi S, et al. Inhibitory effect on lipase activity of extracts from medicinal herbs[J]. Biosci Biotechnol Biochem, 1992, 56: 1478-1479. DOI:10.1271/bbb.56.1478 |
| [38] |
Uchholz TB, Chen C, Zhang XY, et al. Pancreatic lipase and α-amylase inhibitory activities of plants used in traditional Chinese medicine (TCM)[J]. Pharmazie, 2016, 71: 420-424. |
| [39] |
Kim YS, Lee Y, Kim J, et al. Inhibitory activities of Cudrania tricuspidata leaves on pancreatic lipase in vitro and lipolysis in vivo[J]. Evid Based Complement Alternat Med, 2012, 2012: 878365. |
| [40] |
Teixeira LS, Lima AS, Boleti AP, et al. Effects of Passiflora nitida Kunth leaf extract on digestive enzymes and high caloric diet in rats[J]. J Nat Med, 2014, 68: 316-325. DOI:10.1007/s11418-013-0800-1 |
| [41] |
Kaewpiboon C, Lirdprapamongkol K, Srisomsap C, et al. Studies of the in vitro cytotoxic, antioxidant, lipase inhibitory and antimicrobial activities of selected Thai medicinal plants[J]. BMC Complement Altern Med, 2012, 12: 217. DOI:10.1186/1472-6882-12-S1-P217 |
| [42] |
Ivanov SA, Nomura K, Malfanov IL, et al. Isolation of a novel catechin from Bergenia rhizomes that has pronounced lipase-inhibiting and antioxidative properties[J]. Fitoterapia, 2011, 82: 212-218. DOI:10.1016/j.fitote.2010.09.013 |
| [43] |
Kim HY, Kang MH. Screening of Korean medicinal plants for lipase inhibitory activity[J]. Phytother Res, 2010, 19: 359-361. |
| [44] |
Kwon CS, Sohn HY, Kim SH, et al. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents[J]. Biosci Biotechnol Biochem, 2003, 67: 1451-1456. DOI:10.1271/bbb.67.1451 |
| [45] |
Jiao P, Tseng-Crank J, Corneliusen B, et al. Lipase inhibition and antiobesity effect of Atractylodes lancea[J]. Planta Med, 2014, 80: 577-582. DOI:10.1055/s-0034-1368354 |
| [46] |
Nakai M, Fukui Y, Asami S, et al. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro[J]. J Agric Food Chem, 2005, 53: 4593-4598. DOI:10.1021/jf047814+ |
| [47] |
Yuda N, Tanaka M, Suzuki M, et al. Polyphenols extracted from black tea (Camellia sinensis) residue by hot-compressed water and their inhibitory effect on pancreatic lipase in vitro[J]. J Food Sci, 2012, 77: H254-H261. DOI:10.1111/j.1750-3841.2012.02967.x |
| [48] |
Ninomiya K, Matsuda H, Shimoda H, et al. Carnosic acid, a new class of lipid absorption inhibitor from sage[J]. Bioorg Med Chem Lett, 2004, 14: 1943-1946. DOI:10.1016/j.bmcl.2004.01.091 |
| [49] |
Zhang J, Kang MJ, Kim MJ, et al. Pancreatic lipase inhibitory activity of Taraxacum officinale in vitro and in vivo[J]. Nutr Res Pract, 2008, 2: 200-203. DOI:10.4162/nrp.2008.2.4.200 |
| [50] |
Buchholz T, Melzig MF. Medicinal plants traditionally used for treatment of obesity and diabetes mellitus-screening for pancreatic lipase and α-amylase inhibition[J]. Phytother Res, 2016, 30: 260-266. DOI:10.1002/ptr.5525 |
| [51] |
Zheng CD, Duan YQ, Gao JM, et al. Screening for antilipase properties of 37 traditional Chinese medicinal herbs[J]. J Chin Med Assoc, 2010, 73: 319-324. DOI:10.1016/S1726-4901(10)70068-X |
| [52] |
Wei B, Yang W, Yan ZX, et al. Prenylflavonoids sanggenon C and kuwanon G from mulberry (Morus alba L.) as potent broad-spectrum bacterial β-glucuronidase inhibitors:biological evaluation and molecular docking studies[J]. J Funct Food, 2018, 48: 210-219. DOI:10.1016/j.jff.2018.07.013 |
| [53] |
Ma LL, Yuan YY, Zhao M, et al. Mori Cortex extract ameliorates nonalcoholic fatty liver disease (NAFLD) and insulin resistance in high-fat-diet/streptozotocin-induced type 2 diabetes in rats[J]. Chin J Nat Med, 2018, 16: 411-417. |
| [54] |
Liu YJ, Li SY, Hou J, et al. Identification and characterization of naturally occurring inhibitors against human carboxylesterase 2 in white mulberry root-bark[J]. Fitoterapia, 2016, 115: 57-63. DOI:10.1016/j.fitote.2016.09.022 |
| [55] |
Lian J, Chen J, Yuan Y, et al. Cortex Mori Radicis extract attenuates myocardial damages in diabetic rats by regulating ERS[J]. Biomed Pharmacother, 2017, 90: 777-785. DOI:10.1016/j.biopha.2017.03.097 |
| [56] |
Qi SZ, Li N, Tuo ZD, et al. Effects of Morus root bark extract and active constituents on blood lipids in hyperlipidemia rats[J]. J Ethnopharmacol, 2016, 180: 54-59. DOI:10.1016/j.jep.2016.01.024 |
| [57] |
Seo CS, Lim HS, Jeong SJ, et al. HPLC-PDA analysis and anti-inflammatory effects of Mori Cortex Radicis[J]. Nat Prod Commun, 2013, 8: 1443-1446. |
| [58] |
Kim HJ, Lee HJ, Jeong SJ, et al. Cortex Mori Radicis extract exerts antiasthmatic effects via enhancement of CD4(+)CD25(+)Foxp3(+) regulatory T cells and inhibition of Th2 cytokines in a mouse asthma model[J]. J Ethnopharmacol, 2011, 138: 40-46. DOI:10.1016/j.jep.2011.08.021 |
| [59] |
Zhang Y, Feng TT, Zhao Z, et al. Study on the chemical constituents of Mori Cortex[J]. J Chin Med Mate (中药材), 2013, 36: 1101-1103. |
| [60] |
Hou XD, Ge GB, Weng ZM, et al. Natural constituents from Cortex Mori Radicis as new pancreatic lipase inhibitors[J]. Bioorg Chem, 2018, 80: 577-584. DOI:10.1016/j.bioorg.2018.07.011 |
| [61] |
Ha MT, Tran MH, Ah KJ, et al. Potential pancreatic lipase inhibitory activity of phenolic constituents from the root bark of Morus alba l[J]. Bioorg Med Chem Lett, 2016, 26: 2788-2794. DOI:10.1016/j.bmcl.2016.04.066 |
| [62] |
Ji T, Zhang LL, Huang XC, et al. The action mechanisms of Morus alba leaves extract for the treatment of diabetes based on plasma metabolomics[J]. Acta Pharm Sin (药学学报), 2015, 50: 830-835. |
| [63] |
Choi J, Kang HJ, Kim SZ, et al. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells[J]. Arch Pharm Res, 2013, 36: 912-917. DOI:10.1007/s12272-013-0090-x |
| [64] |
Wilson RD, Islam MS. Effects of white mulberry (Morus alba) leaf tea investigated in a type 2 diabetes model of rats[J]. Acta Pol Pharm, 2015, 72: 153-160. |
| [65] |
Yang Y, Wang HQ, Chen RY. Flavonoids from the leaves of Morus alba L[J]. Acta Pharm Sin (药学学报), 2010, 45: 77-81. |
| [66] |
Dugo P, Donato P, Cacciola F, et al. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system[J]. J Sep Sci, 2015, 32: 3627-3634. |
| [67] |
Doi K, Kojima T, Makino M, et al. Studies on the constituents of the leaves of Morus alba L.[J]. Chem Pharm Bull, 2001, 49: 151-153. DOI:10.1248/cpb.49.151 |
| [68] |
Jeong JY, Jo YH, Kim SB, et al. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions[J]. Bioorg Med Chem Lett, 2015, 25: 2269-2274. DOI:10.1016/j.bmcl.2015.04.045 |
| [69] |
Chang JY, Chang MN. Medicinal uses of Ginkgo biloba[J]. Todays Ther Trends, 1997, 15: 63-74. |
| [70] |
Isah T. Rethinking Ginkgo biloba L.:medicinal uses and conservation[J]. Pharmacogn Rev, 2015, 9: 140-148. DOI:10.4103/0973-7847.162137 |
| [71] |
Cassileth B. Ginkgo (Ginkgo biloba)[J]. Oncology, 2011, 25: 971. |
| [72] |
Czigle S, Tóth J, Jedlinszki N, et al. Ginkgo biloba food supplements on the European market-adulteration patterns revealed by quality control of selected samples[J]. Planta Med, 2018, 84: 475-482. DOI:10.1055/a-0581-5203 |
| [73] |
Bustanji Y, Al-Masri IM, Mohammad M, et al. Pancreatic lipase inhibition activity of trilactone terpenes of Ginkgo biloba[J]. J Enzym Inhib Med Chem, 2011, 26: 453-459. DOI:10.3109/14756366.2010.525509 |
| [74] |
Liu PK, Weng ZM, Ge GB, et al. Biflavones from Ginkgo biloba as novel pancreatic lipase inhibitors:inhibition potentials and mechanism[J]. Int J Biol Macromol, 2018, 118: 2216-2223. DOI:10.1016/j.ijbiomac.2018.07.085 |
| [75] |
Shan HL, Shan RP, Fu XC. Hypouricemic effect of ethanol extracts from Dioscoreae Nipponicae Rhizoma[J]. J Zhejiang Univ Med Sci (浙江大学学报医学版), 2015, 44: 49-53. |
| [76] |
Wang Y, Luo J, Song H, et al. New ionic liquid-based preparative method for diosgenin from Rhizoma Dioscoreae Nipponicae[J]. Pharmacogn Mag, 2013, 9: 250-254. DOI:10.4103/0973-1296.113282 |
| [77] |
Chen YM, Chen J, Chou GX. Research review on chemical constituent and pharmacological action of Rhizoma Atractylodis[J]. Acta Univ Tradit Med Sin Pharmacol Shanghai (上海中医药大学学报), 2006, 20: 95-98. |
| [78] |
Liu F, Liu YJ, Tian CM. Effect of Rhizoma Atractylodis extract in protecting gastric mucosa and modulating gastrointestinal immune function in a rat model of spleen deficiency[J]. J South Med Univ (南方医科大学学报), 2015, 35: 343-347. |
| [79] |
Shi ZH, Ye LC, Guan XY, et al. HPLC fingerprint of Aesculi Semen and its application in identification of medicinal materials[J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2018, 24: 52-56. |
| [80] |
Yang M, Pei XH. Pharmacological study on Semen Aesculi active ingredient and clinical application progress[J]. World J Tradit Chin Med (世界中医药), 2017, 12: 3138-3141. |
| [81] |
Kimura H, Ogawa S, Jisaka M, et al. Identification of novel saponins from edible seeds of Japanese horse chestnut (Aesculus turbinata Blume) after treatment with wooden ashes and their nutraceutical activity[J]. J Pharm Biomed Anal, 2006, 41: 1657-1665. DOI:10.1016/j.jpba.2006.02.031 |
| [82] |
Tao Y, Zhang YF, Wang Y, et al. Hollow fiber based affinity selection combined with high performance liquid chromatography-mass spectroscopy for rapid screening lipase inhibitors from lotus leaf[J]. Anal Chim Acta, 2013, 785: 75-81. DOI:10.1016/j.aca.2013.04.058 |
| [83] |
Tang J, Zhou J, Tang Q, et al. A new TLC bioautographic assay for qualitative and quantitative estimation of lipase inhibitors[J]. Phytochem Anal, 2016, 27: 5-12. DOI:10.1002/pca.2581 |
| [84] |
Chen T, Li Y, Zhang L. Nine different chemical species and action mechanisms of pancreatic lipase ligands screened out from Forsythia suspensa leaves all at one time[J]. Molecules, 2017, 22: 795. DOI:10.3390/molecules22050795 |
| [85] |
Li ZJ, Abulizi A, Zhao GL, et al. Bakuchiol contributes to the hepatotoxicity of Psoralea corylifolia in rats[J]. Phytother Res, 2017, 31: 1265-1272. DOI:10.1002/ptr.5851 |
| [86] |
Hou XD, Song LL, Cao YF, et al. Pancreatic lipase inhibitory constituents from Fructus Psoraleae[J]. Chin J Nat Med, 2020, 18: 369-378. |
| [87] |
Garcia-de la Cruz L, Galvan-Goiz Y, Caballero-Caballero S, et al. Hypericum silenoides Juss. and Hypericum philonotis Cham. & Schlecht. extracts:in-vivo hypolipidaemic and weight-reducing effects in obese rats[J]. J Pharm Pharmacol, 2013, 65: 591-603. DOI:10.1111/jphp.12015 |
| [88] |
You MK, Rhuy J, Jeong KS, et al. Effect of St. John's Wort (Hypericum perforatum) on obesity, lipid metabolism and uterine epithelial proliferation in ovariectomized rats[J]. Nutr Res Pract, 2014, 8: 292-296. DOI:10.4162/nrp.2014.8.3.292 |
| [89] |
Ma J, Ji TF, Tian J, et al. A new 2, 3-dioxoflavonoid from the aerial part of Hypericum perforatum[J]. Acta Pharm Sin (药学学报), 2019, 54: 2286-2288. |
| [90] |
Nakagawa K, Kishida H, Arai N, et al. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level inobese diabetic KK-A(y) mice[J]. Biol Pharm Bull, 2004, 27: 1775-1778. |
| [91] |
Birari RB, Gupta S, Mohan CG, et al. Antiobesity and lipid lowering effects of glycyrrhiza chalcones:experimental and computational studies[J]. Phytomedicine, 2011, 18: 795-801. DOI:10.1016/j.phymed.2011.01.002 |
| [92] |
Wan LH, Jiang XL, Liu YM, et al. Screening of lipase inhibitors from Scutellaria baicalensis extract using lipase immobilized on magnetic nanoparticles and study on the inhibitory mechanism[J]. Anal Bioanal Chem, 2016, 408: 2275-2283. DOI:10.1007/s00216-016-9320-7 |
| [93] |
Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors[J]. Planta Med, 2015, 81: 771-783. DOI:10.1055/s-0035-1546173 |
| [94] |
Kawaguchi K, Mizuno T, Aida K, et al. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas[J]. Biosci Biotechnol Biochem, 1997, 61: 102-104. DOI:10.1271/bbb.61.102 |
| [95] |
Chen TG, Li YL, Wei YR, et al. Screening, identification and activity evaluation of pancreatic lipase inhibition in Prunella vulgaris[J]. China J Chin Mater Med (中国中药杂志), 2018, 43: 4665-4671. |
| [96] |
Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors[J]. Planta Med, 2015, 81: 771-783. DOI:10.1055/s-0035-1546173 |
| [97] |
Zeng SL, Li SZ, Lai CJS, et al. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae Pericarpium from different harvest time[J]. Phytomedicine, 2018, 43: 103-109. DOI:10.1016/j.phymed.2018.04.008 |
| [98] |
Jang DS, Lee GY, Kim J, et al. A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta[J]. Arch Pharm Res, 2008, 31: 666-670. DOI:10.1007/s12272-001-1210-9 |
| [99] |
Lee IA, Lee JH, Baek NI, et al. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite Crocetin[J]. Biol Pharm Bull, 2005, 28: 2106-2110. DOI:10.1248/bpb.28.2106 |
| [100] |
Ramirez G, Zamilpa A, Zavala M, et al. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity[J]. J Ethnopharmacol, 2016, 185: 1-8. DOI:10.1016/j.jep.2016.03.014 |
| [101] |
Yu LS, Bi HC, Wu BJ, et al. 2018 DMPK research progress in China[J]. Acta Pharm Sin (药学学报), 2019, 54: 963-970. |
| [102] |
Zhang L, Shi J, Tang J, et al. Direct coupling of thin-layer chromatography-bioautography with electrostatic field induced spray ionization-mass spectrometry for separation and identification of lipase inhibitors in lotus leaves[J]. Anal Chim Acta, 2017, 967: 52-58. DOI:10.1016/j.aca.2017.03.008 |
| [103] |
Castillosantaella TD, Maldonadovalderrama J, Cabrerizovilchez MA, et al. Natural inhibitors of lipase:examining lipolysis in a single droplet[J]. J Agric Food Chem, 2015, 63: 10333. DOI:10.1021/acs.jafc.5b04550 |
| [104] |
Chen TG, Wei YR, Zhang LW. Screening and identification of pancreatic lipase inhibitors in Polygonum cuspidatum with enzyme-immobilized magnetic nanoparticles and LC-MS/MS[J]. Nat Prod Res Dev (天然产物研究与开发), 2017, 29: 198-205. |
| [105] |
Wan LH, Jiang XL, Liu YM, et al. Screening of lipase inhibitors from Scutellaria baicalensis extract using lipase immobilized on magnetic nanoparticles and study on the inhibitory mechanism[J]. Anal Bioanal Chem, 2016, 408: 2275-2283. DOI:10.1007/s00216-016-9320-7 |
| [106] |
Chen Z, Tao Y, Wang Y, et al. Rapid screening of lipase inhibitors from Fructus aurantii using lipase-immobilized magnetic beads coupled with high-performance liquid chromatography-mass spectrometry[J]. Chem J Chin Univ (高等学校化学学报), 2012, 33: 2692-2696. |
| [107] |
Xiao S, Yu R, Ai N, et al. Rapid screening natural-origin lipase inhibitors from hypolipidemic decoctions by ultrafiltration combined with liquid chromatography-mass spectrometry[J]. J Pharm Biomed Anal, 2015, 104: 67-74. DOI:10.1016/j.jpba.2014.11.022 |
| [108] |
Tao Y, Cai H, Li W, et al. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale[J]. Anal Bioanal Chem, 2015, 407: 6081-6093. DOI:10.1007/s00216-015-8781-4 |
| [109] |
Zhu L, Zou X, Liu RT, et al. Recent advances in enzyme inhibitor screening based on mass spectrometry[J]. Acta Pharm Sin (药学学报), 2019, 54: 818-827. |
| [110] |
Wang TY, Wang LN, Zhao LH, et al. Design, synthesis and inhibitory activity of indoleamine 2, 3-dioxygenase 1 inhibitors[J]. Acta Pharm Sin (药学学报), 2019, 54: 2039-2048. |
 2020, Vol. 55
2020, Vol. 55


