作者贡献: 于恒彩负责文献搜索、分析及整理, 手稿撰写及修改; 侯少聪负责文献搜索策略制定, 文献分析, 手稿修改; 崔冰负责思路指导, 图片制作指导, 手稿修改; 李平平负责主题制定, 思路指导, 手稿修改。
利益冲突:所有作者之间无利益冲突, 所有作者与这项研究没有任何潜在的利益冲突。
2. 山东第一医科大学附属省立医院药学部, 山东 济南 250021
2. Department of Pharmacy, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, China
糖脂代谢紊乱是肥胖、2型糖尿病和非酒精性脂肪肝(non-alcoholic fatty liver disease, NAFLD)等疾病的主要临床表现, 并可诱发心脑血管等疾病[1]。长期以来, 胆汁酸(bile acids, BAs)被认为仅仅溶解胆囊中胆固醇、促进肠道脂质乳化和吸收[2], 但是自从1999年报道BAs是法尼醇X受体(farnesoid X receptor, FXR)的自然配体[3-5]以来, BAs作为信号分子日益被人们所关注。研究报道, 胆汁酸主要通过核受体FXR和膜受体G蛋白偶联胆汁酸受体5 (transmembrane G protein-coupled receptor 5, TGR5)发挥调节糖脂代谢的作用[6-9]。FXR和TGR5在多种组织中均有表达, 但是FXR高表达于肝脏、回肠和结肠, TGR5高表达于肠内分泌细胞、胆囊和胆管[8]。本文主要就BAs通过肝肠FXR与肠道TGR5调节糖脂代谢的作用及机制进行系统综述, 为后续基础及临床研究提供参考。
1 胆汁酸的代谢 1.1 胆汁酸合成BAs是胆汁的重要成分, 为双亲性类固醇分子, 由肝脏胆固醇专一合成。BAs合成有两条主要途径:经典途径和替代途径。经典途径始于细胞质和内质网中胆固醇的甾体环的修饰(包括羟化、异构化、还原和脱羟基), 然后是线粒体中甾体侧链氧化和过氧化物酶体中侧链的氧化裂解。替代途径始于甾体侧链的氧化, 然后是甾体环的修饰和甾体侧链的氧化裂解[10]。经典途径主要合成胆酸(cholic acid, CA)和鹅去氧胆酸(chenodeoxycholic acids, CDCA), 细胞色素P450家族7亚家族A成员1 (cytochrome P450 family 7 subfamily A member 1, CYP7A1)是经典途径的起始酶, 也是限速酶, 决定BAs合成量的多少; 细胞色素P450家族8亚家族B成员1 (cytochrome P450 family 8 subfamily B member 1, CYP8B1)是CA合成的必需酶, 决定CA和CDCA产物的比例[11]。替代途径起始于细胞色素P450家族27亚家族A成员1 (cytochrome P450 family 27 subfamily A member 1, CYP27A1), 该酶将胆固醇转化为27-羟基胆固醇, 然后经过细胞色素P450家族7亚家族B成员1 (cytochrome P450 family 7 subfamily B member 1, CYP7B1)羟化和一系列的酶促反应, 主要合成CDCA。在正常生理情况下, 人体替代途径合成的胆汁酸量不到胆汁酸总量的10%[12], 而啮齿动物替代途径对胆汁酸合成的贡献与经典途径大致相同[10]。
BAs按其来源分为初级胆汁酸和次级胆汁酸, 人类的初级胆汁酸为CA和CDCA, 鼠类的为CA、CDCA、熊去氧胆酸(ursodeoxycholic acid, UDCA)和鼠胆酸(muricholic acids, MCAs), 后者包括αMCA和βMCA, 其中βMCA占主要部分。在人类结肠中, 初级结合型胆汁酸首先被胆汁酸盐水解酶(bile acid salt hydrolysis enzyme, BSH)水解成游离型胆汁酸, 经过差向异构生成UDCA, 经过7α-脱羟基酶生成次级胆汁酸脱氧胆酸(deoxycholic acid, DCA)和石胆酸(lithocholic acid, LCA), 在鼠类中, 则可以生成猪胆酸(hyocholic acid, HCA)、鼠脱氧胆酸(murideoxycholic acid, MDCA)、ωMCA、猪去氧胆酸(hyodeoxycholic acid, HDCA)、DCA和LCA。此外, BAs按其是否与甘氨酸或牛磺酸结合分为结合型胆汁酸和游离型胆汁酸。肝脏中初级胆汁酸一旦合成即与甘氨酸和牛磺酸结合, 排泄入胆汁, 需要时输送到十二指肠, 在人类主要与甘氨酸结合, 在鼠类主要与牛磺酸结合[13-15](图 1)。
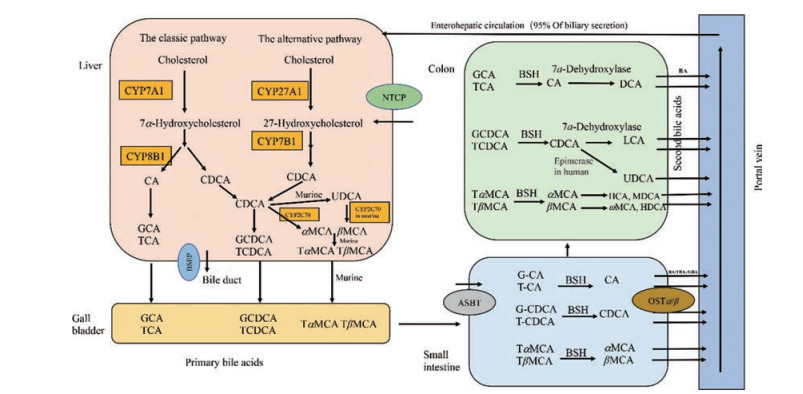
|
Figure 1 Synthesis and circulation of bile acids. The conversion of cholesterol into bile acids in the liver involves the classic (CA and CDCA) and alternative (CDCA) pathway. The initial products of the two pathways are the primary bile acids (CA and CDCA in humans and CA, CDCA, UDCA, αMCA and βMCA in rodents). Primary bile acids are conjugated to either taurine or glycine, secreted into bile and stored in the gallbladder to be discharged into the intestinal lumen upon ingestion of a meal. In the colon, primary bile acids are converted into secondary bile acids (DCA, LCA, UDCA in humans and DCA, LCA, HCA, MDCA, ωMCA, HDCA in murine). Bile acids were reabsorbed into the portal vein from the terminal ileum and colon, followed by hepatic uptake from the portal blood and resecretion into bile, as is enterohepatic circulation. CA: Cholic acid; CDCA: Chenodeoxycholic acid; UDCA: Ursodeoxycholic acid; αMCA: α-Muricholic acid; βMCA: β-Muricholic acid; DCA: Deoxycholic acid; LCA: Lithocholic acid; HCA: Hyocholic acid; MDCA: Murideoxycholic acid; ωMCA: ω-Muricholic acid; HDCA: Hyodeoxycholic acid; BSH: Bile acid salt hydrolysis enzyme; BSEP: Bile salt export protein; NTCP: Sodium taurocholate co-transporting polypeptide; ASBT: Apical sodium dependent bile acid transporter; OSTα/β: Organic solute transporter α/β; BA: Bile acid; T: Taurine-conjugated species; G: Glycine-conjugated species |
胆汁酸从肝细胞合成后, 通过三磷酸腺苷(adenosine triphosphate, ATP)依赖的胆汁酸盐输出蛋白(bile salt export pump, BSEP)[16]分泌入胆管。随后, 胆汁酸与胆固醇、卵磷脂、钾、钠和钙等形成微胶粒, 储存在胆囊中, 进食刺激胆囊收缩排空胆汁进入十二指肠。在小肠中(主要是回肠末端), BAs依赖胆汁酸转运蛋白(apical sodium dependent bile acid transporter, ASBT)被高效重吸收入肠上皮细胞。在肠上皮细胞基底侧, 胆汁酸通过有机转运体(organic solute transporter α/β, OSTα/β)进入门静脉[17], 经过门脉循环回到肝脏, 最终经肝细胞膜上钠牛磺胆酸共转运多肽(sodium taurocholate co-transporting polypeptide, NTCP, SLC10A1)转运入肝细胞[18]。游离胆汁酸在小肠和大肠被动扩散重吸收, 初级结合胆汁酸在回肠末端高效主动重吸收(依赖ASBT), 经过门脉循环入肝转运进肝细胞然后再分泌入胆汁, 这一过程称为肠肝循环[16]。人体1个胆汁酸池平均约有2 g胆汁酸, 每天循环10次, 肝肠每24 h可转运20 g胆汁酸。经过肠肝循环, 95%的胆汁酸被重新利用, 只有5%的胆汁酸随粪便排出, 人类每天的排泄量约为0.6 g, 这部分需要肝脏重新从胆固醇合成, 以维持胆汁酸池的恒定[19](图 1)。
2 胆汁酸代谢的调节胆汁酸量主要受胆汁酸自身的调节, 机体通过多种途径限制循环中胆汁酸的过多积聚。BAs主要通过肝脏FXR-小异源二聚体伴侣(small heterodimer partner, SHP)和回肠FXR-成纤维细胞生长因子(fibroblast growth factor, FGF) 15/19信号通路反馈性抑制肝脏CYP7A1表达, 减少BAs含量[20]。在肝脏, BAs激活FXR, 后者诱导靶基因Shp表达, SHP与肝受体类似物1 (liver receptor homolog-1, LRH-1)结合, 抑制Cyp7a1基因表达, 减少BAs生成[21-23]。除了肝脏局部负反馈作用, BAs还通过肠-肝途径远程负反馈调节肝脏BAs合成。在回肠末端, BAs也可激活FXR, 后者诱导靶基因Fgf15 (人类为Fgf19)的表达。FGF15/19通过门脉系统到达肝脏, 与FGF受体4 (FGF receptor 4, FGFR4)/β-Klotho异质二聚体复合物结合, 启动c-Jun N端激酶(c-Jun N-terminal kinase, JNK) 1/2和细胞外信号调节激酶(extracellular signal-regulated kinase, ERK) 1/2级联反应, 后者抑制CYP7A1表达, 抑制BAs生成(图 2)。
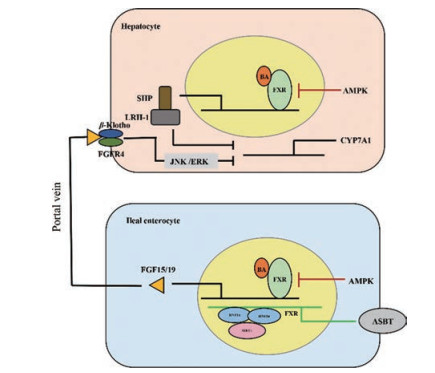
|
Figure 2 The self-regulation of bile acids metabolism. Bile acid synthesis is regulated by negative feedback pathway (FXR-SHP in the liver and FXR-FGF15/19 in the ileum). Furthermore, AMPK and SIRT1 regulate BAs amount by liver and intestinal FXR. FXR: Farnesoid X receptor; FGF15/19: Fibroblast growth factor 15/19; SHP: Small heterodimer partner; LRH-1: Liver receptor homolog-1; FGFR4: Fibroblast growth factor receptor 4; JNK: c-Jun N-terminal kinase; ERK: Extracellular signal-regulated kinase; AMPK: Adenosine monophosphate activated protein kinase; SIRT1: Sirtuin 1 |
此外, 腺苷酸活化的蛋白激酶(adenosine monophosphate activated protein kinase, AMPK)和去乙酰化酶(sirtuin 1, SIRT1)通过调节肝脏和肠道FXR从而调节胆汁酸量。在体外培养的人和小鼠肝细胞和肠上皮细胞中, 激活AMPK抑制了FXR的转录活性, 并阻止了FXR共激活因子对FXR靶基因启动子的招募; 在小鼠肝内胆汁淤积模型中, 二甲双胍(AMPK激动剂)诱导FXR磷酸化, 抑制FXR的转录活性, 扰乱胆汁酸稳态, 加重胆汁淤积状态下的肝损伤[24]。SIRT1是最保守的哺乳动物氧化烟酰胺腺嘌呤二核苷酸依赖性蛋白去乙酰化酶, 是许多组织中重要的代谢传感器。动物实验表明, SIRT1可以使肝细胞核因子1α二聚化辅因子(dimerization cofactor of hepatocyte nuclear factor 1α, Dcoh2)去乙酰化, 促进其与肝细胞核因子1α (hepatocyte nuclear factor 1α, HNF-1α)的相互作用, 并诱导HNF-1α与DNA结合; 肠道上皮细胞SIRT1敲除(SIRT1ΔIE)小鼠通过HNF-1α降低肠道FXR表达, 从而降低BAs转运基因Asbt的表达和回肠BAs的重吸收, 进而通过肠肝循环到达肝脏的BAs减少, 肝脏胆汁酸的生物合成代偿性地增加, 肝脏胆汁酸的积聚减少, 并保护动物免受高胆汁酸饮食的肝脏损害; 提示, 肠道SIRT1作为关键的营养传感器, 通过调节HNF-1α-FXR-ASBT信号通路, 调节小鼠回肠胆汁酸吸收和维持全身胆汁酸稳态[25] (图 2)。
3 胆汁酸通过肝脏FXR影响糖脂代谢FXR属于核受体超家族, 高表达于肝脏和肠道组织, BAs是其天然配体。与结合型胆汁酸相比, 游离型胆汁酸激活FXR能力更强, 激活强度顺序为CDCA > DCA > LCA > CA[26]。最近研究发现, 牛磺酸结合α鼠胆酸(tauro-alpha-muricholic acid, TαMCA)、牛磺酸结合β鼠胆酸(tauro-beta-muricholic acid, TβMCA)和UDCA对FXR具有明显的拮抗作用[27]。各类胆汁酸作用于FXR后, 通过不同的代谢通路调节糖脂代谢。
3.1 胆汁酸通过肝脏FXR减少肝脏脂质CA可激动肝脏FXR, 后者诱导靶基因Shp表达增加, SHP抑制转录因子固醇调节因子结合蛋白1c (sterol regulatory element-binding protein 1c, SREBP-1c)及其下游肝脏脂质合成基因表达, 减少肝脏脂质合成[28]。人体内CDCA也可激动FXR, 诱导脂肪酸代谢主要调节子核受体过氧化物酶体增殖物激活受体α (peroxisome proliferator-activated receptor alpha, PPARα)表达, 增加脂肪酸氧化[29]。因此, 激活肝脏FXR可以导致脂类分解, 脂质合成减少, 脂肪酸氧化增加, 肝脏脂质水平降低[30]。
3.2 胆汁酸通过肝脏FXR减少肝脏葡萄糖除上述通路, CA通过FXR诱导SHP表达后, SHP还可与肝细胞核因子4α (hepatocyte nuclear factor-4 alpha, HNF4α)和叉头盒蛋白O1 (forkhead box protein O1, FOXO1)相互作用, 抑制HNF4α靶基因如磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxykinase, Pepck)和果糖二磷酸酶-1 (fructose 1, 6-bis phosphatase, Fbp1), 以及FOXO1靶基因如葡萄糖6磷酸酶(glucose-6-phosphatase, G6p)启动子活性, 减少肝脏糖异生[31]。
4 胆汁酸通过抑制肠道FXR影响糖脂代谢 4.1 肠道FXR-SHP/FGF15/19代谢通路高脂喂养(high fat diet, HFD)后, 与野生型小鼠相比, 肠道特异性Fxr敲除(FxrΔIE)小鼠显示出较低的肥胖和胰岛素抵抗水平, 同时肠道脂肪酸β-氧化相关基因的表达水平也显著增加。与对照组相比, 给予四甲基哌啶(tempol)的小鼠肠道菌群组成改变, TβMCA增加, 肠道FXR信号传导减弱, 通过肠肝循环到达肝脏的SHP和FGF15减少, CYP7A1表达增加; 相应的, 肝脏胆固醇减少, 肥胖和胰岛素抵抗改善[32]。研究显示, 口服二甲双胍可改变肠道菌群, 增加牛磺酸结合熊去氧胆酸(tauro-ursodeoxycholic acid, TUDCA)水平, 抑制肠道FXR信号通路, 从而改善高血糖等代谢紊乱[33]。另有研究表明, 给予HFD仓鼠抗生素处理, 消除其肠道菌群后, 其肝脏CYP7B1上调, 通过替代途径合成的CDCA增加, 进而肠道TβMCA增加, 肠道FXR-SHP/FGF19信号通路被抑制, 肝脏脂质合成相关基因Srebp1c、脂肪酸合酶(fatty acid synthase, Fas)、硬脂酸CoA去饱和酶1 (stearoyl coenzyme A desaturase-1, Scd-1)、乙酰辅酶A羧化酶(acetyl CoA carboxylase, Acc)水平下降, 肝脏脂质合成降低, 整体糖耐量、脂肪肝改善[34] (图 3)。

|
Figure 3 Bile acids regulate glucose and lipid metabolism through intestinal FXR and TGR5. Bile acids activate or inhibit the intestinal FXR, further regulate the expression of FGF15/19, ceramide, GLP-1, NPC1L1, and affect glucolipid metabolism. Moreover, bile acids can activate the pathways of TGR5-GLP-1 in the intestine and TGR5-DiO2 in the WAT and BAT, and improve the glucose and lipid metabolism. TβMCA: Tauro-beta-muricholic acid; TUDCA: Tauro-ursodeoxycholic acid; Gly-MCA: Glycine-β-muricholic acid; TGR5: Transmembrane G protein-coupled receptor 5; TLCA: Taurolithocholic acid; SREBF2: Sterol regulatory element-binding factor 2; NPC1L1: NPC1-like intracellular cholesterol transporter; FFA: Free fatty acids; SCFA: Short-chain fatty acids; FFAR2: SCFA receptor 2; IP3: Inositol tri-phosphate; ATP: Adenosine triphosphate; GLP-1: Glucagon-like peptide-1; cAMP: Cyclic adenosine monophosphate; CREBP: cAMP response element‐ binding protein; PC1: Prohormone convertases subtilisin/kexin type 1; NFAT: Nuclear factor of activated T cells; PC1/3: Prohormone convertases 1/3; mTORC1: Mechanistic target of rapamycin complex 1; G6PC: Glucose-6-phsophatase catalytic; PCK: Phosphoenolpyruvate carboxykinase; PGC-1α: Peroxisome proliferator-activated receptor γ coactivator-1α; CYP7A1: Cytochrome P450 family 7 subfamily A member 1; TC: Total cholestetol; GS: Glycogen synthase; SREBP-1c: Sterol regulatory element-binding protein 1c; FA: Fatty acid; PC: Pyruvate carboxylase; WAT: White adipose tissue; BAT: Brown adipose tissue; DIO2: Deiodinase 2 |
神经酰胺(ceramide)是鞘脂家族的成员, 由神经鞘氨醇长链碱基与脂肪酸组成。动物实验表明, 血清和组织中神经酰胺水平的增高, 可诱发胰岛素抵抗, 损害糖耐量, 进而发展成糖尿病或NAFLD等疾病[35]。肠道Fxr敲除的小鼠血浆神经酰胺水平降低, 可抵抗高脂饮食诱导的代谢性疾病[36]。
高脂喂养后, 与野生型小鼠相比, FxrΔIE小鼠肝脏重量、肝脏/体重比例及肝甘油三酯含量显著降低。当给予肥胖小鼠抗氧化剂tempol或抗生素后, 其肠道BSH活性减弱, TβMCA水平增加, 肠道FXR活性被抑制, 肠道和血中神经酰胺水平降低。降低的神经酰胺一方面通过SREBP-1c抑制肝脏脂质从头合成[37]; 另一方面增强了白色脂肪组织(white adipose tissue, WAT)棕色化和棕色脂肪组织(brown adipose tissue, BAT)产热能力[38]。肠道FXR特异性抑制剂甘氨酸结合鼠胆酸(glycine-β-muricholic acid, Gly-MCA)同样可改善小鼠肥胖和糖脂代谢[38]。研究显示, CYP8B1敲低的小鼠CA和DCA减少, FXR-ceramide-SREBP-1c通路信号减弱, 肝脏脂质合成减弱, 血脂紊乱改善, 减少CA可能是改善血脂紊乱和NAFLD的一个潜在靶点[39] (图 3)。
咖啡酸苯乙酯(caffeic acid phenethyl ester, CAPE)可预防HFD小鼠体重增加, 降低空腹血糖和血胰岛素水平, 改善2型糖尿病[40], 但是对FxrΔIE小鼠的上述有益作用均消失; 其机制与tempol类似, CAPE通过BSH-TβMCA-FXR通路减少肠道和血中神经酰胺, 进而降低内质网(endoplasmic reticulum, ER)应激、内质网-线粒体偶联和Ca2+超载, 抑制肝脏线粒体乙酰辅酶A和丙酮酸羧化酶(pyruvate carboxylase, PC)活性, 减少肝糖异生, 这一机制即为FXR-ceramide-PC通路[41] (图 3)。
4.3 肠道FXR-糖酵解-GLP-1代谢通路胰高血糖素样肽1 (glucagon-like peptide-1, GLP-1)是一种多功能肽类激素, 主要由肠黏膜上皮L细胞合成和分泌, 可促进餐后胰岛素的分泌, 抑制食欲, 促进胰岛β细胞增殖等, 从而改善肥胖和胰岛素抵抗[42]。GLP-1是前胰高血糖素原基因(preproglucagon gene, Gcg)编码的胰高血糖素原肽(proglucagon peptide)的产物, 由胰高血糖素原肽经激素原转化酶1/3 (prohormone convertases 1/3, PC 1/3)[42]加工修饰而成, 其分泌受激素、神经、营养刺激和肠道菌群等多种因素的影响[43]。Trabelsi等[44]表明L细胞也表达FXR, Fxr敲除导致葡萄糖刺激的GLP-1表达和分泌增加, 小鼠糖代谢改善。其机制是FXR被激活后, 糖酵解减弱, 一方面通过降低L细胞转录因子碳水化合物反应元件结合蛋白(carbohydrate responsive element-binding protein, ChREBP)表达, 减少胰高血糖素原转录和GLP-1生成; 另一方面通过抑制细胞内ATP产生, 减少GLP-1分泌[44] (图 3)。
4.4 肠道FXR-FFAR2-GLP1代谢通路菊粉型果聚糖(inulin-type fructans, ITF)可增加微生物发酵产生的短链脂肪酸(short-chain fatty acids, SCFA, 如乙酸盐、丙酸盐和丁酸盐)并改善能量稳态[45, 46]。研究报道, 小鼠HFD喂养同时给予ITF, 与野生型小鼠相比, Fxr-/-小鼠血清GLP-1水平显著升高, 其机制是Fxr敲除后结肠短链脂肪酸受体2 (SCFA receptor 2, Ffar2)基因上调, 其下游的Gαq-Ca2+/三磷酸肌醇(inositol tri-phosphate, IP3)被激活, 引起SCFA刺激的GLP-1分泌增加[47]。CYP8b1-/-敲除小鼠CA及衍生物完全缺乏, 肠道脂肪吸收减少, 到达回肠L细胞的游离脂肪酸(free fatty acids, FFA)增加, 导致GLP-1分泌增加[48]。这一过程可能与肠道菌群改变致回肠TβMCA增加, 抑制FXR活性, 继而激活FFAR2-GLP1通路有关。在体外, 给予STC-1细胞FXR激动剂GW4064, FXR与环磷酸腺苷(cyclic adenosine monophosphate, cAMP)反应元件结合蛋白(cAMP response element‐binding protein, CREBP)结合后抑制CREBP转录活性, 降低蛋白原转化酶1型(proprotein convertase subtilisin/kexin type 1)水平, 最终导致GLP-1分泌减少[49] (图 3)。
4.5 肠道FXR-SCFA-脂质从头合成代谢通路研究报道, HFD小鼠口服肠道FXR抑制剂Gly-MCA显著降低厚壁门菌/拟杆菌(Firmicutes /Bacteroidetes, F/B)比例, 从而降低SCFA (肝脏脂质合成原料)产生, 减少肝脏脂质从头合成, 改善肥胖等代谢综合征, 而在FxrΔIE小鼠中, 上述代谢改善的作用消失[50] (图 3)。
5 胆汁酸通过激动肠道FXR影响糖脂代谢 5.1 肠道FXR-FGF15/19通路Fang等[51]的研究表明, 给予HFD小鼠肠道特异性FXR激动剂非沙明(fexaramine, FEX)后, 肠道Fgf15基因表达上调, BAT能量消耗增加, WAT棕色化, 如解偶联蛋白(uncoupling protein-1, Ucp-1)基因表达上调, 肝糖异生基因如葡萄糖-6-磷酸酶(glucose-6-phsophatase catalytic, G6pc)、磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxykinase, Pck)和脂质合成基因如Srebp1c、Fas和Scd-1表达下调, 肥胖和炎症等代谢综合征改善。另有研究表明, 静脉给予小鼠FGF15可激活ERK, 继而抑制糖原合成酶激酶3 (glycogen synthase kinase 3, GSK3), 激活肝脏糖原合成酶(glycogen synthase, GS), 促进肝糖原合成[52]。FGF15也可使肝脏转录因子CREB去磷酸化而失活, 继而抑制过氧化物酶体增殖物激活受体γ辅激活因子1α (peroxisome proliferator-activated receptor γ coactivator-1α, PGC-1α)表达及下游糖异生相关靶基因表达, 减少肝糖产生[53] (图 3)。
5.2 肠道FXR-TGR5-GLP1通路与野生型小鼠相比, Fxr-/-或者Tgr5-/-小鼠葡萄糖诱导的血浆GLP-1水平下降约40%[54]。Tgr5-/-小鼠中FXR表达正常, 当给予FXR和TGR5双激动剂INT-767后, GLP-1分泌显著增加; Fxr-/-小鼠中Tgr5表达则显著下降, INT-767刺激后GLP-1分泌未见显著增加, 说明FXR和TGR5都参与GLP-1分泌, 并且FXR起着更关键的作用[54]。研究表明, Tgr5基因启动子上含有FXR反应元件(FXR-responsive element, FXRE), FXR被INT-767激活后可与FXRE结合, 诱导Tgr5基因表达, L细胞内Ca2+水平增加, cAMP活性增加, 进而刺激GLP-1分泌, 改善HFD小鼠的肝脏糖脂代谢[54]。在基因诱导的db/db小鼠中, FEX也可激活肠道FXR, 导致肠道菌群重整, 胆汁酸成分比例改变, LCA和牛磺酸结合石胆酸(taurolithocholic acid, TLCA)增加, TGR5被激活, GLP-1分泌增加, 肝糖和胰岛素敏感性得到改善[55]。可见, FXR和TGR5在促进L细胞分泌GLP-1调节糖脂代谢方面可能具有交叉作用(图 3)。
5.3 肠道FXR-cAMP-GLP1通路胃旁路手术(Roux-en-Y gastric bypass, RYGB)通过切断胃形成一个小的垂直胃袋(30 mL), 然后将胃袋重新吻合到空肠, 重塑消化道; 再通过空肠吻合术恢复肠的连续性。新形成的消化道绕过胃的大部分、十二指肠和近端空肠, 导致食物摄入量和营养吸收减少。RYGB之后, 胆汁和胰腺分泌物通过前肠排出, 并通过新创建的空肠空肠造口汇合入空肠中远端[56]。临床研究表明, RYGB术后15个月肥胖患者体重指数显著下降, 空腹血糖降低, 伴随空腹血浆总胆汁酸和GLP-1水平的增加[57], 但其具体机制尚不清楚。在肥胖小鼠上, 胆汁酸分流手术(bile diversion to the ileum, GB-IL)和RYGB具有相似的代谢改善作用。研究表明, GB-IL可降低HFD喂养的Tgr5-/-小鼠体重, 改善葡萄糖耐量, 而FxrΔIE小鼠或GLP-1R-/-小鼠则无上述改善作用, 说明GB-IL可改变肠道胆汁酸生物利用度, 激活FXR-GLP-1轴, 从而改善糖耐量和体重[58], 提示小鼠术后代谢的改善依赖于肠道FXR介导的胆汁酸信号通路(图 3)。
5.4 肠道FXR-FGF19/15-SHP-SREBF2-NPC1L1通路NPC样细胞内胆固醇转运蛋白(NPC1-like intracellular cholesterol transporter, NPC1L1)高表达于空肠和回肠[59], 是肠腔细胞表达的跨膜转运蛋白, 负责饮食和胆汁中胆固醇的限速转运, 同时也是降脂药物依折麦布的靶点[60-62]。正常小鼠先禁食再给予饮食后, 其肠道NPC1L1表达降低, Shp-/-或Fgf15-/-小鼠并无此变化。给小鼠注射FGF19使SHP (Thr-55)磷酸化, 进而抑制固醇调节因子结合因子2 (sterol regulatory element-binding factor 2, SREBF2)活性, 减少肠道NPC1L1表达, 最终肠道胆固醇吸收减少, 高胆固醇血症减轻[63]。研究表明, 摄食大麦可以显著降低HFD喂养小鼠血浆总胆固醇和低密度脂蛋白胆固醇(low density lipoprotein cholesterol, LDLC)浓度, 增加粪便胆固醇排泄量, 其机制是摄食大麦诱导肠道FXR高表达, 继而后者抑制肠道NPC1L1表达, 减少饮食中胆固醇的摄取[64] (图 3)。
6 抑制或激动肠道FXR对糖脂代谢的差异性分析前文提到抑制或激动肠道FXR均可通过FXR-FGF15/19通路改善HFD喂养肥胖小鼠的糖脂代谢。具体情况是, 一方面, 灌胃给予HFD诱导的肥胖小鼠tempol或二甲双胍后, 其肠道菌群改变, BSH活性降低, 肠道胆汁酸组成改变, TβMCA或TUDCA增加, 抑制肠道FXR, 肠道和循环FGF15含量降低, 反馈性引起肝脏CYP7A1表达或活性增加, 继而其底物胆固醇含量减少, 肥胖和胰岛素抵抗改善[32, 33]; 或者灌胃给予HFD仓鼠抗生素, 其肝脏CYP7B1表达水平上调, CDCA合成增加, 肠道GCA减少, TβMCA增加, 抑制肠道FXR-FGF15/19, 肝脏脂质合成减少, 皮下脂肪组织(subcutaneous white adipose tissue, sWAT)产热增加[34], 也可改善肥胖及胰岛素敏感性。另一方面, 灌胃给予HFD小鼠FEX, 回肠FXR被激活, 回肠和血中FGF15水平增加, 进而肝脏CYP7A1表达水平下降, CYP7B1表达水平上升, 肝脏CDCA合成增多, 血循环TCA减少, LCA增加, BAT能量消耗增加, WAT棕色化, 肝脏糖脂减少[51]。经分析造成这种差异的原因可能有以下3个: ①影响胆汁酸组成变化的发生部位不同(肠道与肝脏), 激活分子顺序不同(BA-FXR-FGF15与FXR-FGF15-BA), 即BAs既可以通过FXR调节糖脂代谢, 也可以被FXR调节其合成。给予tempol、二甲双胍或抗生素, 首先影响肠道菌群, 进而影响肠道胆汁酸组成, 从而抑制FXR-FGF15, 改善糖脂代谢[32-34]; 给予FEX, 首先激动FXR-FGF15, 进而影响肝脏胆汁酸合成, 增加LCA水平, 通过FGF15或LCA影响糖脂代谢[51]; ②肠道菌群因素, 研究表明多种肠道微生物来源的酶参与胆汁酸的修饰, 包括去结合、转运和7α/β-去羟基化[65]。口服抗生素和FEX均可上调肝脏CYP7B1, CDCA合成增加[34, 51], 但是口服抗生素使肠道TβMCA增加[34], 口服FEX使肠道LCA增加[51], 肠道菌群差异可能起着重要作用; ③多因素参与调节, Sun等[33]提到TUDCA可以促进GLP-1分泌, Fang等[51]提到FGF15只是一个因素, LCA-TGR5或者其他未知信号通路对FEX引起的糖脂改善作用不能排除。
同样, 前文提到抑制或激动肠道FXR均可促进GLP-1增加, 分析差异其原因可能有以下3个: ①肠道激动剂不同, 小鼠口服非特异性FXR激动剂GW4064, 通过降低糖酵解, 一方面降低转录因子ChREBP表达, 减少GLP-1产生, 同时降低ATP产生, 减少GLP-1分泌[44]; 口服肠道特异性FXR激动剂FEX, 激活肠道FXR, 肠道菌群改变, 肠道胆汁酸组成改变, LCA和TLCA增加, 进而通过激活TGR5促进GLP-1分泌[55]。给予小鼠口服FXR和TGR5双激动剂INT-767, 激活的FXR诱导TGR5基因表达, cAMP增加, GLP-1分泌增加[54]。非特异性FXR激动剂通过直接激动FXR, 减少GLP-1的合成和分泌, 肠道FXR特异性激动剂, FXR和TGR5双激动剂通过间接激动TGR5, 增加GLP-1分泌; ②体内外实验差别, 体外给予STC-1细胞GW4064, FXR激活, CREBP转录活性被抑制, PC水平降低, GLP-1分泌减少[49]; 给予小鼠口服FEX或者手术分流胆汁酸至回肠, 激动肠道FXR, 肠道菌群和胆汁酸改变, GLP-1分泌增加[55, 58]。体外实验是单一刺激对单一细胞内的变化, 体内实验有多因素参与调节, 因此引起结果可能不同; ③实验条件不同, HFD喂养同时给予ITF, Fxr敲除引起结肠Ffar2基因上调, 下游Ca2+/ IP3激活, SCFA刺激的GLP-1分泌增加, 特定条件ITF可能引起FXR-FFAR2-Ca2+/ IP3- GLP-1这条通路激活[47]; 给予小鼠口服INT-767或FEX, FXR的激活直接或间接激活TGR5, 增加GLP-1分泌[54, 55]。
7 胆汁酸通过TGR5影响糖脂代谢TGR5为膜结合G蛋白偶联BA受体, 存在于许多器官和组织, 高表达于小肠、胃、肝脏、肺、胎盘和脾脏[66, 67]。胆汁酸是目前已知的TGR5的唯一内源性配体, 次级胆汁酸比初级胆汁酸具有更高的TGR5亲和力, BAs激活TGR5能力顺序为TLCA > LCA > DCA > CDCA > CA[68]。
7.1 胆汁酸通过激动TGR5-cAMP-DIO2影响糖脂代谢Tgr5-/-小鼠表现出严重代谢综合征, 如肥胖、胰岛素抵抗和糖脂代谢紊乱等[69]。研究报道, CA喂养可以逆转HFD小鼠的肥胖, 其机制不仅仅依赖FXR, 还通过TGR5激活BAT的产热和甲状腺素信号通路进而加强能量消耗[70]。在BAT中, TGR5激活启动cAMP信号通路, 诱导甲状腺激素脱碘酶2 (deiodinase 2, Dio2)基因表达, 后者促使甲状腺素(T4)转化为三碘甲状腺氨酸(T3)增多, 能量消耗增加。除了Dio2, 参与产热的基因Pgc1α、Pgc1β、解偶联蛋白1 (uncoupling protein-1, Ucp1)、Ucp3和直链酰基辅酶A氧化酶1 (straight-chain acyl-CoA oxidase 1)均显著上调, 而Dio2-/-的小鼠并无此变化[70]。小鼠胆汁酸分流模型(bile diversion, BD)将胆囊吻合到远端空肠, 是用来测定BA调节糖脂代谢的模型。肥胖小鼠进行BD手术后代谢表型得到改善, 能量消耗增加, 伴随附睾和腹股沟WAT的Tgr5、Dio2和产热基因Ucp1、PR结构域(PR domain-containing 16, Prdm16)、Pgc-1α、Pgc-1β和血小板衍生生长因子受体α (alpha-type platelet-derived growth factor receptor, Pdgfrα)表达增加, 肠道菌群F/B比例降低[71]。可见, BA可通过激动TGR5-cAMP-DIO2信号通路控制能量代谢(图 3)。
7.2 胆汁酸通过激动肠道TGR5-cAMP-GLP1影响糖脂代谢在HFD诱导的肥胖小鼠中, 给予TGR5特异性激动剂INT-777 (CDCA衍生物), 可增加GLP-1分泌, 改善肝脂肪变性和肥胖, 改进胰岛素敏感性, 而Tgr5-/-小鼠GLP-1水平无显著增加[72]。在STC-1细胞中, 胆汁酸处理能够激活TGR5, 启动cAMP信号通路, Ca2+内流增加, 刺激肠道L细胞GLP-1分泌[72]。研究表明, 与Tgr5-/-小鼠相比, 野生型小鼠给予胆汁酸结合树脂(bile acid binding resins, BABRs)考来替泊后血清GLP-1水平增加, 糖耐量改善, 同时肠道PC1/3基因表达增加[73]。BABRs使肠道BAs组成改变, 如TGR5激动剂TCA显著增加, 肠道TGR5活性增加, 经过T细胞核因子(nuclear factor of activated T cells, NFAT)介导, NFTA与肠道PC1/3启动子区域结合, 促进PC1/3的表达, 继而促进GLP-1的释放, 葡萄糖耐量显著改善; cAMP-PKA-Ca2+/钙调蛋白-钙调磷酸酶-NFAT-PC1/3信号通路可能参与了TGR5激动剂引起的GLP-1释放[73] (图 3)。
7.3 胆汁酸通过激动肠道TGR5-mTORC1-GLP-1影响糖脂代谢雷帕霉素靶蛋白(mechanistic target of rapamycin, mTOR)是一种高度保守的丝氨酸/苏氨酸激酶, 其下游靶蛋白包括核糖体蛋白S6激酶(S6 kinases, S6Ks)、S6和真核翻译起始因子4E结合蛋白1 (4E binding protein 1, 4EBP1)[74-76]。mTOR信号通路具有促进物质代谢、参与细胞凋亡和自噬的作用, 其异常激活与糖尿病、肥胖和癌症相关[76, 77]。mTOR包括两种复合体, 分别为mTOR复合体1 (mTOR complex 1, mTORC1)和mTORC2, 其中mTORC1发挥着更为重要的作用, 主要负责营养感知[75]。动物实验和细胞实验表明, 肠道mTORC1参与调节L细胞GLP-1的合成[78]。小鼠和人RYGB术后, 循环BAs增加, 回肠TGR5和mTORC1信号通路激活, GLP-1产生和分泌增加[79]。体外实验提示, DCA使STC-1细胞内TGR5-mTORC1信号显著增强, mTOR、S6K和S6磷酸化增加, GLP-1合成和分泌增加; 敲低TGR5或mTORC1后, DCA诱导的GLP-1合成增加作用消失[79] (图 3)。
8 胆汁酸通过其他机制影响糖脂代谢 8.1 改变肝脏胰岛素敏感性野生型小鼠颈静脉灌注DCA后, 血浆BAs水平急性升高, 升高的血浆BAs通过阻断胰岛素抑制肝糖产生的作用, 损坏肝胰岛素敏感性; Tgr5-/-小鼠上述现象同样存在; 但是门静脉灌注DCA并不损坏肝胰岛素敏感性, 说明循环中急性升高的BAs可不依赖TGR5信号通路间接损害肝胰岛素敏感性[80]。临床研究表明, TUDCA改善肥胖患者肝脏和肌肉的胰岛素敏感性, 增加肌肉胰岛素信号传导[81]。
8.2 促进脂肪细胞和肝细胞GLUT4表达葡萄糖转运蛋白4 (glucose transporter 4, GLUT4)专一表达于胰岛素靶组织, 如BAT、WAT、骨骼肌和心肌, 在维持全身葡萄糖稳态方面具有重要作用[82, 83]。体外实验表明, FXR和FXR激动剂CDCA可诱导3T3-L1和HepG2细胞GLUT4转录, 其机制是FXR与GLUT4启动子区域FXRE结合诱导GLUT4表达, 参与调节葡萄糖稳态[84]。
8.3 促进胰岛β细胞分泌胰岛素TCDCA激活小鼠胰岛β细胞FXR, 抑制ATP敏感性K+通道(ATP-sensitive potassium channel, KATP)的亚单位磺脲类受体1 (sulphonylurea receptor 1, SUR1)活性, K+外流减少, 细胞质Ca2+浓度增加, 胰岛素分泌增加, TCDCA并不能改变Sur1-/-或Fxr-/-小鼠胰岛β细胞的胰岛素分泌[85]。
8.4 调节炎症调节因子NF-κB和IKKb研究表明, CDCA能够纠正棕榈酸盐处理的3T3-L1细胞和HFD小鼠脂肪组织的脂肪因子分泌紊乱, 其机制是CDCA通过抑制炎症调节因子如核转录因子(nuclear factor-κB, NF-κB)和抑制性κB激酶(inhibitory kappa B kinase, IKKb)磷酸化抑制其活化, 进而抑制了促炎脂肪因子如肿瘤坏死因子α (tumor necrosis factor-α, TNF-α)、白介素-6 (interleukin-6, IL-6)和单核细胞趋化蛋白-1 (monocyte chemotactic protein-1, MCP-1)等的分泌, 增加了抗炎脂肪因子如脂联素(adiponectin)和瘦素(leptin)的分泌, 从而缓解了胰岛素抵抗[86]。
8.5 减少细胞内氧化应激研究报道, 棕榈酸盐诱导HepG2细胞内活性氧(reactive oxygen species, ROS)水平增加, 显著抑制胰岛素诱导的HepG2细胞AKT磷酸化; UDCA联合胰岛素处理可以降低细胞内ROS水平, UDCA处理部分恢复被棕榈酸盐抑制的PI3K/AKT磷酸化[87]。UDCA可以改善果糖所致大鼠的糖脂代谢紊乱, 降低尿酸水平, 改善胰岛素抵抗, 减少血管组织的氧化应激[88]。
8.6 抑制内质网应激, 恢复自噬内质网应激(endoplasmic reticulum stress, ER stress)是肥胖、胰岛素抵抗和2型糖尿病的一个关键连接。TUDCA可以缓解肥胖和2型糖尿病小鼠的内质网应激, 改善高血糖和胰岛素抵抗, 增强胰岛素在肝脏、肌肉和脂肪组织的作用[89]。UDCA可抑制db/db小鼠内质网应激, 恢复自噬, 改善糖尿病肾病的生化指标[90]。
8.7 缓解肠道炎症, 改善肠道屏障, 减少肠道脂质吸收转运TUDCA可以缓解HFD小鼠肝脂肪变性、炎症反应、肥胖和胰岛素抵抗; TUDCA还可缓解肠道炎症反应, 改善肠道屏障功能, 降低肠道脂质转运相关基因水平, 如脂肪酸移位酶分化族36 (cluster of differentiation 36, Cd36)、脂肪酸结合蛋白(fatty acid-binding protein, FABP)、脂肪酸转运蛋白4 (fatty acid transport protein 4, FATP4)和脂肪酸受体3 (fatty acid receptor 3, FAR3), 继而缓解高脂诱导的NAFLD[91]。最近有研究表明, 正常饮食条件下, 与野生型小鼠相比, Cyp8b1-/-小鼠合成及分泌入近端小肠肠腔的12α-OH BAs减少, 继而乳化脂质的能力减弱, 近端小肠肠腔甘油三酯水解和水解产物吸收减少, 导致运送到远端小肠的甘油三酯及水解产物FFA和2-单酰基甘油(2-monoacylglycerol, 2-MAG)增多; 在远端小肠肠腔2-MAG通过激动GPR119诱导GLP-1的分泌, 后者通过减缓胃排空和食物摄入, 降低体重及改善血糖[92]。
8.8 减少肠道BAs重吸收, 改善肥胖和肝脂肪变性与野生型小鼠相比, Asbt-/-小鼠可以抵抗短期HFD诱导的肝脂肪变性。给予HFD诱导的肥胖小鼠口服ASBT抑制剂(ASBTi), 其回肠BAs重吸收减少, 粪便BAs排泄增加, 回肠Fgf15基因表达减少, 肝脏亲水性胆汁酸(FXR拮抗剂)减少, FXR激动型BAs增加, 脂质合成基因如Srebp1表达减少, 糖耐量恢复, 肝脏甘油三酯和总胆固醇含量降低, NAFLD改善[93]。与野生型小鼠相比, Ntcp-/-小鼠HFD喂养后其血浆BAs水平增加, 肥胖和肝脂肪变性改善, 血浆胆固醇水平降低, 这些变化与肠道脂质吸收减少、粪便脂质排泄增加、BAT产热增加和能量消耗增加有关[94]。胆汁酸重吸收转运蛋白ASBT和NTCP有望成为肥胖和NAFLD的一个治疗靶点[95]。
8.9 调节外周T细胞平衡, 维持肠道免疫稳态体外研究表明, CA、CDCA、DCA、LCA和ωMCA在或高于其临界胶束浓度(约2.5~10 mmol·L-1)时对野生型T细胞具有细胞毒性, 可以抑制多药耐药蛋白1 (multidrug resistance protein 1, MDR1, 一种膜相关的、ATP依赖的外排泵, 用于将化疗药物转运出肿瘤细胞)介导的异型物质外排, 胆汁酸螯合剂消胆胺可以恢复Mdr1缺陷T细胞小鼠的肠道稳态[96]。体内外实验共同表明, 3-羟基脱氧胆酸(3β-hydroxydeoxycholic acid, isoDCA)通过降低树突状细胞的免疫刺激特性, 增加对转录因子叉头蛋白3 (forkhead box P3, FOXP3)的诱导, 进而增加外周调节性T (regulatory T, Treg)细胞的增殖, 维持结肠免疫稳态[97]。
9 结语BAs作为信号分子调节糖脂代谢引起人们广泛关注, 本文综述了BAs的合成、循环和调节, 以及其激动肝脏FXR、抑制或激动肠道FXR和TGR5对糖脂代谢的影响, 进一步从SHP、FGF15/19、神经酰胺和GLP-1等相关信号通路阐述了BAs调节糖脂代谢的分子机制, 为后续基础及临床研究提供了一定的参考价值。不同胆汁酸作用的部位不同, 激活的受体不同, 激动的下游信号通路不同, 引起的效果可能不同, 最终结果可能是特定环境因素下几条不同信号通路竞争的结果。有关BAs调节糖脂代谢的分子机制仍需进一步的研究。
| [1] |
Grundy SM. Metabolic syndrome update[J]. Trends Cardiovasc Med, 2016, 26: 364-373. DOI:10.1016/j.tcm.2015.10.004 |
| [2] |
Dumaswala R, Berkowitz D, Heubi JE. Adaptive response of the enterohepatic circulation of bile acids to extrahepatic cholestasis[J]. Hepatology, 1996, 23: 623-629. DOI:10.1002/hep.510230330 |
| [3] |
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR[J]. Mol Cell, 1999, 3: 543-553. |
| [4] |
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor[J]. Science, 1999, 284: 1365-1368. DOI:10.1126/science.284.5418.1365 |
| [5] |
Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids[J]. Science, 1999, 284: 1362-1365. DOI:10.1126/science.284.5418.1362 |
| [6] |
Jadhav K, Xu Y, Xu Y, et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR[J]. Mol Metab, 2018, 9: 131-140. DOI:10.1016/j.molmet.2018.01.005 |
| [7] |
Molinaro A, Wahlström A, Marschall HU. Role of bile acids in metabolic control[J]. Trends Endocrinol Metab, 2018, 29: 31-41. DOI:10.1016/j.tem.2017.11.002 |
| [8] |
Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development[J]. Nat Rev Gastroenterol Hepatol, 2014, 11: 55-67. DOI:10.1038/nrgastro.2013.151 |
| [9] |
Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap-bile acids in metabolic control[J]. Nat Rev Endocrinol, 2014, 10: 488-498. DOI:10.1038/nrendo.2014.60 |
| [10] |
Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology[J]. Gene Expr, 2018, 18: 71-87. DOI:10.3727/105221618X15156018385515 |
| [11] |
Li-Hawkins J, Gåfvels M, Olin M, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice[J]. J Clin Invest, 2002, 110: 1191-1200. DOI:10.1172/JCI0216309 |
| [12] |
Hardwick JP. Cytochrome P450 function and pharmacological roles in inflammation and cancer. Preface[J]. Adv Pharmacol, 2015, 74: xv-xxxi. DOI:10.1016/S1054-3589(15)00047-2 |
| [13] |
Falany CN, Fortinberry H, Leiter EH, et al. Cloning, expression, and chromosomal localization of mouse liver bile acid CoA: amino acid N-acyltransferase[J]. J Lipid Res, 1997, 38: 1139-1148. |
| [14] |
Falany CN, Johnson MR, Barnes S, et al. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA: amino acid N-acyltransferase[J]. J Biol Chem, 1994, 269: 19375-19379. |
| [15] |
Vessey DA. The biochemical basis for the conjugation of bile acids with either glycine or taurine[J]. Biochem J, 1978, 174: 621-626. DOI:10.1042/bj1740621 |
| [16] |
Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications[J]. Pharm Res, 2007, 24: 1803-1823. DOI:10.1007/s11095-007-9289-1 |
| [17] |
Dawson PA, Hubbert ML, Rao A. Getting the mOST from OST: role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism[J]. Biochim Biophys Acta, 2010, 1801: 994-1004. DOI:10.1016/j.bbalip.2010.06.002 |
| [18] |
Doring B, Lutteke T, Geyer J, et al. The SLC10 carrier family: transport functions and molecular structure[J]. Curr Top Membr, 2012, 70: 105-168. |
| [19] |
Brufau G, Groen AK, Kuipers F. Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion[J]. Arterioscler Thromb Vasc Biol, 2011, 31: 1726-1733. DOI:10.1161/ATVBAHA.108.181206 |
| [20] |
Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis[J]. Cell, 2000, 102: 731-744. DOI:10.1016/S0092-8674(00)00062-3 |
| [21] |
Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: key regulator of whole-body energy metabolism[J]. Trends Endocrinol Metab, 2011, 22: 458-466. |
| [22] |
Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis[J]. Mol Cell, 2000, 6: 517-526. DOI:10.1016/S1097-2765(00)00051-4 |
| [23] |
Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors[J]. Mol Cell, 2000, 6: 507-515. DOI:10.1016/S1097-2765(00)00050-2 |
| [24] |
Lien F, Berthier A, Bouchaert E, et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk[J]. J Clin Invest, 2014, 124: 1037-1051. DOI:10.1172/JCI68815 |
| [25] |
Kazgan N, Metukuri MR, Purushotham A, et al. Intestine-specific deletion of SIRT1 in mice impairs DCoH2-HNF-1alpha-FXR signaling and alters systemic bile acid homeostasis[J]. Gastroenterology, 2014, 146: 1006-1016. DOI:10.1053/j.gastro.2013.12.029 |
| [26] |
Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis[J]. Nat Rev Gastroenterol Hepatol, 2018, 15: 111-128. DOI:10.1038/nrgastro.2017.119 |
| [27] |
Sanz Y, DiMarzio M, Rusconi B, et al. Identification of a mouse Lactobacillus johnsonii strain with deconjugase activity against the FXR antagonist T-β-MCA[J]. PLoS One, 2017, 12: e0183564. DOI:10.1371/journal.pone.0183564 |
| [28] |
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c[J]. J Clin Invest, 2004, 113: 1408-1418. DOI:10.1172/JCI21025 |
| [29] |
Pineda Torra I, Claudel T, Duval C, et al. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor[J]. Mol Endocrinol, 2003, 17: 259-272. DOI:10.1210/me.2002-0120 |
| [30] |
Ferrebee CB, Dawson PA. Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids[J]. Acta Pharm Sin B, 2015, 5: 129-134. DOI:10.1016/j.apsb.2015.01.001 |
| [31] |
Yamagata K, Daitoku H, Shimamoto Y, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and foxo1[J]. J Biol Chem, 2004, 279: 23158-23165. DOI:10.1074/jbc.M314322200 |
| [32] |
Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity[J]. Nat Commun, 2013, 4: 2384. DOI:10.1038/ncomms3384 |
| [33] |
Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin[J]. Nat Med, 2018, 24: 1919-1929. DOI:10.1038/s41591-018-0222-4 |
| [34] |
Sun L, Pang Y, Wang X, et al. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters[J]. Acta Pharm Sin B, 2019, 9: 702-710. DOI:10.1016/j.apsb.2019.02.004 |
| [35] |
Turpin SM, Nicholls HT, Willmes DM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance[J]. Cell Metab, 2014, 20: 678-686. DOI:10.1016/j.cmet.2014.08.002 |
| [36] |
Gonzalez FJ, Jiang C, Patterson AD. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease[J]. Gastroenterology, 2016, 151: 845-859. DOI:10.1053/j.gastro.2016.08.057 |
| [37] |
Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease[J]. J Clin Invest, 2015, 125: 386-402. DOI:10.1172/JCI76738 |
| [38] |
Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction[J]. Nat Commun, 2015, 6: 10166. DOI:10.1038/ncomms10166 |
| [39] |
Pathak P, Chiang JYL. Sterol 12alpha-hydroxylase aggravates dyslipidemia by activating the ceramide/mTORC1/SREBP-1c pathway via FGF21 and FGF15[J]. Gene Expr, 2019, 19: 161-173. DOI:10.3727/105221619X15529371970455 |
| [40] |
Bezerra RM, Veiga LF, Caetano AC, et al. Caffeic acid phenethyl ester reduces the activation of the nuclear factor kappaB pathway by high-fat diet-induced obesity in mice[J]. Metabolism, 2012, 61: 1606-1614. DOI:10.1016/j.metabol.2012.04.006 |
| [41] |
Xie C, Jiang C, Shi J, et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice[J]. Diabetes, 2017, 66: 613-626. DOI:10.2337/db16-0663 |
| [42] |
González-García I, Milbank E, Diéguez C, et al. Glucagon, GLP-1 and thermogenesis[J]. Int J Mol Sci, 2019, 20: 3445. DOI:10.3390/ijms20143445 |
| [43] |
Tian L, Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion[J]. J Diabetes, 2016, 8: 753-765. DOI:10.1111/1753-0407.12439 |
| [44] |
Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells[J]. Nat Commun, 2015, 6: 7629. DOI:10.1038/ncomms8629 |
| [45] |
Cani PD, Delzenne NM. The gut microbiome as therapeutic target[J]. Pharmacol Ther, 2011, 130: 202-212. DOI:10.1016/j.pharmthera.2011.01.012 |
| [46] |
Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits[J]. Br J Nutr, 2010, 104 Suppl 2: S1-S63. |
| [47] |
Ducastel S, Touche V, Trabelsi MS, et al. The nuclear receptor FXR inhibits glucagon-like peptide-1 secretion in response to microbiota-derived short-chain fatty acids[J]. Sci Rep, 2020, 10: 174. DOI:10.1038/s41598-019-56743-x |
| [48] |
Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1[J]. Diabetes, 2015, 64: 1168-1179. DOI:10.2337/db14-0716 |
| [49] |
Li P, Zhu L, Yang X, et al. Farnesoid X receptor interacts with cAMP response element binding protein to modulate glucagon-like peptide-1 (7-36) amide secretion by intestinal L cell[J]. J Cell Physiol, 2019, 234: 12839-12846. DOI:10.1002/jcp.27940 |
| [50] |
Zhang L, Xie C, Nichols RG, et al. Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism[J]. mSystems, 2016, 1: e00070-16. |
| [51] |
Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance[J]. Nat Med, 2015, 21: 159-165. DOI:10.1038/nm.3760 |
| [52] |
Kir S, Beddow SA, Samuel VT, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis[J]. Science, 2011, 331: 1621-1624. DOI:10.1126/science.1198363 |
| [53] |
Potthoff Matthew J, Boney-Montoya J, Choi M, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway[J]. Cell Metab, 2011, 13: 729-738. DOI:10.1016/j.cmet.2011.03.019 |
| [54] |
Pathak P, Liu H, Boehme S, et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism[J]. J Biol Chem, 2017, 292: 11055-11069. DOI:10.1074/jbc.M117.784322 |
| [55] |
Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism[J]. Hepatology, 2018, 68: 1574-1588. DOI:10.1002/hep.29857 |
| [56] |
Flynn CR, Albaugh VL, Abumrad NN. Metabolic effects of bile acids: potential role in bariatric surgery[J]. Cell Mol Gastroenterol Hepatol, 2019, 8: 235-246. DOI:10.1016/j.jcmgh.2019.04.014 |
| [57] |
Werling M, Vincent RP, Cross GF, et al. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery[J]. Scand J Gastroenterol, 2013, 48: 1257-1264. DOI:10.3109/00365521.2013.833647 |
| [58] |
Albaugh VL, Banan B, Antoun J, et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery[J]. Gastroenterology, 2019, 156: 1041-1051. DOI:10.1053/j.gastro.2018.11.017 |
| [59] |
Xie C, Zhou ZS, Li N, et al. Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine[J]. J Lipid Res, 2012, 53: 2092-2101. DOI:10.1194/jlr.M027359 |
| [60] |
Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids[J]. J Lipid Res, 2015, 56: 1085-1099. DOI:10.1194/jlr.R054114 |
| [61] |
Degirolamo C, Sabba C, Moschetta A. Intestinal nuclear receptors in HDL cholesterol metabolism[J]. J Lipid Res, 2015, 56: 1262-1270. DOI:10.1194/jlr.R052704 |
| [62] |
Wang DQ. Regulation of intestinal cholesterol absorption[J]. Annu Rev Physiol, 2007, 69: 221-248. DOI:10.1146/annurev.physiol.69.031905.160725 |
| [63] |
Kim YC, Byun S, Seok S, et al. Small heterodimer partner and fibroblast growth factor 19 inhibit expression of NPC1L1 in mouse intestine and cholesterol absorption[J]. Gastroenterology, 2019, 156: 1052-1065. DOI:10.1053/j.gastro.2018.11.061 |
| [64] |
Hoang MH, Houng SJ, Jun HJ, et al. Barley intake induces bile acid excretion by reduced expression of intestinal ASBT and NPC1L1 in C57BL/6J mice[J]. J Agric Food Chem, 2011, 59: 6798-6805. DOI:10.1021/jf200681n |
| [65] |
Gu Y, Wang X, Li J, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment[J]. Nat Commun, 2017, 8: 1785. DOI:10.1038/s41467-017-01682-2 |
| [66] |
Tiwari A, Maiti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders[J]. Drug Discov Today, 2009, 14: 523-530. DOI:10.1016/j.drudis.2009.02.005 |
| [67] |
Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells[J]. Hepatology, 2007, 45: 695-704. DOI:10.1002/hep.21458 |
| [68] |
Guo C, Chen WD, Wang YD. TGR5, not only a metabolic regulator[J]. Front Physiol, 2016, 7: 646. |
| [69] |
Velazquez-Villegas LA, Perino A, Lemos V, et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue[J]. Nat Commun, 2018, 9: 245. DOI:10.1038/s41467-017-02068-0 |
| [70] |
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation[J]. Nature, 2006, 439: 484-489. DOI:10.1038/nature04330 |
| [71] |
Pierre JF, Martinez KB, Ye H, et al. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice[J]. Am J Physiol Gastrointest Liver Physiol, 2016, 311: G286-G304. DOI:10.1152/ajpgi.00202.2016 |
| [72] |
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis[J]. Cell Metab, 2009, 10: 167-177. DOI:10.1016/j.cmet.2009.08.001 |
| [73] |
Morimoto K, Watanabe M, Sugizaki T, et al. Intestinal bile acid composition modulates prohormone convertase 1/3 (PC1/3) expression and consequent GLP-1 production in male mice[J]. Endocrinology, 2016, 157: 1071-1081. DOI:10.1210/en.2015-1551 |
| [74] |
Dennis PB, Jaeschke A, Saitoh M, et al. Mammalian TOR: a homeostatic ATP sensor[J]. Science, 2001, 294: 1102-1105. DOI:10.1126/science.1063518 |
| [75] |
Yang Q, Guan KL. Expanding mTOR signaling[J]. Cell Res, 2007, 17: 666-681. DOI:10.1038/cr.2007.64 |
| [76] |
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease[J]. Cell, 2017, 168: 960-976. DOI:10.1016/j.cell.2017.02.004 |
| [77] |
Laplante M, Sabatini DM. mTOR signaling in growth control and disease[J]. Cell, 2012, 149: 274-293. DOI:10.1016/j.cell.2012.03.017 |
| [78] |
Xu G, Li Z, Ding L, et al. Intestinal mTOR regulates GLP-1 production in mouse L cells[J]. Diabetologia, 2015, 58: 1887-1897. DOI:10.1007/s00125-015-3632-6 |
| [79] |
Zhai H, Li Z, Peng M, et al. Takeda G protein-coupled receptor 5-mechanistic target of rapamycin complex 1 signaling contri-butes to the increment of glucagon-like peptide-1 production after Roux-en-Y gastric bypass[J]. EBioMedicine, 2018, 32: 201-214. DOI:10.1016/j.ebiom.2018.05.026 |
| [80] |
Syring KE, Cyphert TJ, Beck TC, et al. Systemic bile acids induce insulin resistance in a TGR5-independent manner[J]. Am J Physiol Endocrinol Metab, 2019, 316: E782-E793. DOI:10.1152/ajpendo.00362.2018 |
| [81] |
Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women[J]. Diabetes, 2010, 59: 1899-1905. DOI:10.2337/db10-0308 |
| [82] |
Kahn BB. Lilly lecture 1995. Glucose transport: pivotal step in insulin action[J]. Diabetes, 1996, 45: 1644-1654. DOI:10.2337/diab.45.11.1644 |
| [83] |
Stephens JM, Pilch PF. The metabolic regulation and vesicular transport of GLUT4, the major insulin-responsive glucose transporter[J]. Endocr Rev, 1995, 16: 529-546. |
| [84] |
Shen H, Zhang Y, Ding H, et al. Farnesoid X receptor induces GLUT4 expression through FXR response element in the GLUT4 promoter[J]. Cell Physiol Biochem, 2008, 22: 1-14. |
| [85] |
Dufer M, Horth K, Wagner R, et al. Bile acids acutely stimulate insulin secretion of mouse beta-cells via farnesoid X receptor activation and K(ATP) channel inhibition[J]. Diabetes, 2012, 61: 1479-1489. DOI:10.2337/db11-0815 |
| [86] |
Shihabudeen MS, Roy D, James J, et al. Chenodeoxycholic acid, an endogenous FXR ligand alters adipokines and reverses insulin resistance[J]. Mol Cell Endocrinol, 2015, 414: 19-28. DOI:10.1016/j.mce.2015.07.012 |
| [87] |
Yokoyama K, Tatsumi Y, Hayashi K, et al. Effects of ursodeoxycholic acid and insulin on palmitate-induced ROS production and down-regulation of PI3K/Akt signaling activity[J]. Biol Pharm Bull, 2017, 40: 2001-2004. DOI:10.1248/bpb.b17-00423 |
| [88] |
Mahmoud AA, Elshazly SM. Ursodeoxycholic acid ameliorates fructose-induced metabolic syndrome in rats[J]. PLoS One, 2014, 9: e106993. DOI:10.1371/journal.pone.0106993 |
| [89] |
Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes[J]. Science, 2006, 313: 1137-1140. DOI:10.1126/science.1128294 |
| [90] |
Cao AL, Wang L, Chen X, et al. Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy[J]. Lab Invest, 2016, 96: 610-622. DOI:10.1038/labinvest.2016.44 |
| [91] |
Wang W, Zhao J, Gui W, et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease[J]. Br J Pharmacol, 2018, 175: 469-484. DOI:10.1111/bph.14095 |
| [92] |
Higuchi S, Ahmad TR, Argueta DA, et al. Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice[J]. Gut, 2020. DOI:10.1136/gutjnl-2019-319693 |
| [93] |
Rao A, Kosters A, Mells JE, et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice[J]. Sci Transl Med, 2016, 8: 357ra122. DOI:10.1126/scitranslmed.aaf4823 |
| [94] |
Donkers JM, Kooijman S, Slijepcevic D, et al. NTCP deficiency in mice protects against obesity and hepatosteatosis[J]. JCI Insight, 2019, 5: e127197. |
| [95] |
Slijepcevic D, van de Graaf SF. Bile acid uptake transporters as targets for therapy[J]. Dig Dis, 2017, 35: 251-258. DOI:10.1159/000450983 |
| [96] |
Cao W, Kayama H, Chen ML, et al. The xenobiotic transporter Mdr1 enforces T cell homeostasis in the presence of intestinal bile acids[J]. Immunity, 2017, 47: 1182-1196. DOI:10.1016/j.immuni.2017.11.012 |
| [97] |
Campbell C, McKenney PT, Konstantinovsky D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells[J]. Nature, 2020, 581: 475-479. DOI:10.1038/s41586-020-2193-0 |
 2020, Vol. 55
2020, Vol. 55


