2. 南开大学药学院, 药物化学生物学国家重点实验室, 天津 300350
2. State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin 300350, China
扎冲十三味丸出自《蒙医经典》由诃子、制草乌、石菖蒲、木香、人工麝香、珊瑚(制)、丁香、肉豆蔻、沉香、禹粮土、磁石(煅)、甘草等十三味药材组成, 主要用于治疗脑缺血等心脑血管疾病等, 是蒙医临床常用的经典方剂之一[1]。但目前扎冲十三味丸研究主要集中于整方层面的药效学评价, 鲜见其物质基础及作用机制的相关报道。且扎冲十三味丸的质量控制标准中缺少统一的含量测定项(中华人民共和国卫生部药品标准蒙药分册·1998年版), 研究者们大多以扎冲十三味丸中某种药材的一种或几种代表性成分的含量为标准进行扎冲十三味丸的质量评价[2, 3]。这些都严重影响了其推广应用。因此, 有必要对扎冲十三味丸的物质基础、作用机制及质量控制做进一步研究, 且作为一种复方药物, 其质量也应该可以参照中药质量标志物的方法[4, 5]进行控制, 通过现代研究方法将其与药效结合获得潜在的质量标志物[6]。
炎症反应是引起扎冲十三味丸主治证脑缺血病理变化的主要原因之一, 有文献报道扎冲十三味丸可调节大鼠脑缺血后各种炎症因子白介素-8 (interleukin-8, IL-8)、细胞间黏附分子(intercellular cell adhesion molecule-1, ICAM-1)、肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)等的表达[7, 8], 但其具体作用物质基础及机制未见报道。此次研究拟以NF-κB、NO释放抑制作用为切入点, 筛选扎冲十三味丸中抗炎质量标志物, 以期初步明确其抗炎方面药效物质基础及作用机制, 并为完善扎冲十三味丸质量控制标准提供实验依据。
材料与方法实验细胞 人胚肾细胞系(human embryonic kidney293T, HEK293T, 美国Type Culture Collection Rockville MD公司); 小鼠单核巨噬细胞白血病细胞(mouse leukemia cells of monocyte macrophage RAW264.7, 江苏凯基生物技术股份有限公司)。
实验仪器 Waters ACQUITY UPLC I-Class液相色谱仪、Waters XEVO-G2- Q-TOF质谱仪(美国Waters公司); TECAN Spark 10M多功能微孔板检测仪(瑞士Tecan公司); 共聚焦成像仪Leica TCS SP8 (德国Leica公司); 5200型化学发光成像仪(上海天能科技有限公司)。
实验试剂 双荧光素酶报告基因试剂盒(美国Promega公司, 货号: e1960); NO含量检测试剂盒(上海碧云天生物技术有限公司, 货号: S00210); TNF-α、polyethyleneimine转染试剂(美国Peprotech公司, 货号: 04152、B600070);脂多糖(lipopolysaccharide, LPS, 美国Sigma公司, 货号: L2880), 青霉素(10 000 U)和10 mg·mL-1链霉素(双抗, 美国Hyclone公司, 货号: SV30010); DMEM高糖培基(high glucose of Dulbecco's modified Eagle medium)、RPMI1640培基(Roswell Park Memorial Institute 1640, RPMI1640, 以色列Bioind公司, 货号分别为: 01-052-1A、01-100-1A)、胎牛血清(美国Gibco公司, 货号: 10099-141);兔抗人AKT多克隆抗体、兔抗人phosphorylated protein kinase B (Ser473)单克隆抗体(P-AKT473)、Alexa Fluor®594-共轭荧光二抗(英国Abcam公司, 货号分别为: ab235958、ab81283、ab150088)、HRP标记的羊抗兔IgG (horseradish peroxidase labeled goat anti-rabbit immunoglobulin G)、兔抗人NF-κB p65单克隆抗体(美国Cell Signaling Technology公司, 货号分别为: 7274、8242S); 质粒pGL4.32及Renilla由实验室自行构建和保存。
扎冲十三味丸(内蒙古蒙药股份有限公司, 批号: 1908003);没食子酸(上海麦克林生化技术有限公司, 批号: C10371394, 质量分数99%); 鞣花酸(大连美仑生物科技有限公司, 批号: M0104AS, 质量分数≥98%); 甘草素(成都普思生物科技有限公司, 批号: PS010083, 质量分数≥98%); 甘草次酸(北京百灵威科技有限公司, 批号: LTB0Q07, 质量分数≥98%); 木香烃内酯(上海阿拉丁生化科技股份有限公司, 批号: J1519044, 质量分数≥98%); 去氢木香内酯(上海吉至生化科技有限公司, 批号: D2722E769, 质量分数≥98%); 地塞米松(dexamethasone, Dex, 德国Ehrenstorfer Quality公司, 批号G145673, 质量分数99.1%)
细胞培养 HEK293T培养于含10%特级胎牛血清及1%双抗的DMEM高糖培基中, 置于37 ℃、5% CO2培养箱中培养。RAW264.7培养于含10%特级胎牛血清及1%双抗的RPMI 1640培基中, 置于37 ℃、5% CO2培养箱中培养。
供试品的制备 取扎冲十三味丸粉末, 以70%甲醇为溶剂, 料液比1:20超声提取1 h, 离心, 取上清, 利用旋转蒸发仪浓缩除去溶剂中的甲醇, 然后置于冷冻干燥机中干燥即得扎冲十三味丸总提物。扎冲十三味丸总提物一部分用水复溶, 依次用石油醚、二氯甲烷、乙酸乙酯、正丁醇萃取, 萃取后剩余部分为水层, 除去各有机溶剂后即得相应萃取层的产物。
基于双荧光素酶报告基因系统的NF-κB拮抗活性检测 将HEK293T细胞接种于96孔板进行培养, 待细胞融合度达到60%时, 以脂质体2000为载体按说明书将荧光素酶报告基因质粒pGL4.32及荧光素酶内参质粒Renilla瞬时共转染20 h, 然后将细胞分组:空白组(control group, Con)、模型组(model group, Mod)、阳性药地塞米松组(Dex, 20 μmol·L-1)、总提物高低剂量组(total extraction, TE; 1 μg·mL-1、10 μg·mL-1)、各有机溶剂萃取层高低剂量组(0.5 μg·mL-1、5 μg·mL-1), 除空白组外, 其余组均加TNF-α (20 ng·mL-1)刺激同时给药6 h。各组NF-κB抑制活性以相对荧光强度比值(relative luciferase activity ratio, RLU ratio)表示, 具体操作详见双荧光素酶报告基因试剂盒说明书。
抑制LPS诱导巨噬细胞释放NO的活性检测 将RAW264.7细胞接种于96孔板, 待细胞融合度达60%时将细胞进行分组:空白组(Con)、模型组(Mod)、阳性药地塞米松组(Dex, 20 μmol·L-1)、总提物高低剂量组(TE, 0.1 μg·mL-1、1 μg·mL-1)、各有机溶剂萃取层高低剂量组(12.5 μg·mL-1、25 μg·mL-1), 空白组除外, 其余各组均加LPS (100 ng·mL-1)刺激同时给药24 h, 然后按照检测剂盒说明书测定各组NO含量(C)。若模型组NO含量平均值为M, 则各组NO抑制率计算公式为抑制率= (M-C)/M×100%。
UPLC/Q-TOF条件 液相色谱条件:色谱柱Waters ACQUITY UPLC®BEHC18色谱柱(2.1 mm×100 mm, 1.7 μm); 柱温45 ℃; 流速0.4 mL·min-1; 进样量2 μL; 流动相组成: A (0.1%甲酸-水) B (乙腈); 二元洗脱梯度程序: 0~4 min, 2%~15% B; 4~5 min, 15%~17% B; 5~8 min。17%~19% B; 8~10 min, 19%~28% B; 10~15 min, 28%~100% B (适用于NF-κB双荧光素酶报告基因系统), 0~12 min, 2%~41% B; 12~22 min, 41%~57% B; 22~30 min, 57%~100% B (适用于抑制LPS诱导的巨噬细胞释放NO的活性成分筛选)。
质谱条件:离子源为软电离模式电喷雾离子源(ESI), 正、负离子模式, 毛细管电压:正离子模式为3.0 kV, 负离子模式为2.5 kV; 离子源温度110 ℃; 锥孔电压:正离子模式为30 V, 负离子模式为45 V; 雾化气高纯氮气的流速为600 L·h-1, 雾化器温度设定为350 ℃; 离子质量扫描范围100~1 500 Da, 扫描频率0.1 s, 扫描间隔延时0.02 s; 校正液为亮氨酸脑啡肽, 内参设定为([M+H]+ = 555.293 1、[M+H]+ = 553.277 5);数据采集工作站为MassLynx4.1。
UPLC分段样品的制备 取有效部位样品配制成10 mg·mL-1的甲醇溶液, 13 000 r·min-1离心15 min取上清用于UPLC分析, 流出液按10:1分流, 小部分用于质谱检测, 另一部分每间隔30 s (用于NF-κB拮抗筛选)或1 min (用于NO抑制作用筛选)收集至96孔深孔板中, 真空干燥, 分别加原溶剂15倍量的基础培养基充分振荡使其完全溶解用于活性筛选。通过质谱精确分子量及二级碎片裂解规律对活性时间段对应的化合物进行鉴定。并选择代表性化合物进行抗炎活性验证。
扎冲十三味丸抗炎活性成分靶点通路预测分析 将活性单体化合物结构式以mol.2格式导入PharmMapper (www.lilab-ecust.cn/pharmmapper)数据库进行靶标预测。利用string9.1 (http://www.string-db.org/)进行靶点相互作用分析, 并选择Kyoto Encyclopedia of Genes and Genomes (https://www.genome.jp/kegg/pathway.htm)数据库注释的炎症相关通路。
AKT磷酸化验证 将RAW264.7细胞接种于6孔板中, 待细胞融合度达到80%时进行分组:空白组Con、模型组Mod、阳性药地塞米松组(Dex, 20 μmol·L-1)、木香烃内酯高中低剂量组(CT, 0.25、0.5、1 μmol·L-1), 给药6 h后除空白组外每孔加入LPS (100 ng·mL-1)刺激30 min。30 min后弃上清, PBS润洗两遍后提蛋白, 以Western blot方法评价活性化合物对AKT磷酸化的影响。
扎冲十三味丸活性成分对NF-κB核转移的影响 将RAW264.7细胞接种于共聚焦小皿中, 待细胞融合度达到60%时进行分组:空白组Con、模型组LPS、木香烃内酯组(CT, 1 μmol·L-1)给药6 h后除空白组外每孔加入LPS (100 ng·mL-1)刺激30 min。采用细胞免疫荧光方法检测活性化合物对NF-κB核转移情况的影响[9]。
统计学方法 采用Graphpadprism7.00进行统计分析, 实验结果以mean ± SEM表示, 组间比较采用单因素方差分析(one-way ANOVA), 以P < 0.05为差异有统计学差异。
结果 1 扎冲十三味丸总提物及各有机溶剂萃取层抗炎活性的评价结果显示, 扎冲十三味丸总提物对NF-κB及NO释放有较强抑制作用(图 1)。其中具有NF-κB抑制活性成分集中在乙酸乙酯层(图 1A), 而具有NO释放抑制活性成分集中在石油醚、二氯甲烷及乙酸乙酯层(图 1B)。
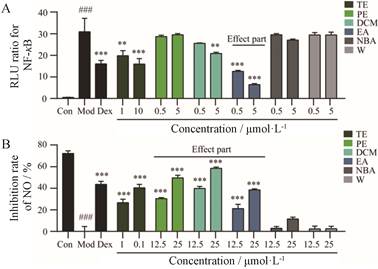
|
Figure 1 The anti-inflammatory activity of total extraction and each extraction layer of Zhachong shisanwei pills (ZC-13). A: The antagonistic activity of total extraction and each extraction layer of ZC-13 on NF-κB; B: Inhibitory effect of total extraction and each extraction layer of ZC-13 on LPS-induced macrophage release of NO. TE: Total extraction of ZC-13; PE: Petroleum ether; DCM: Dichloromethane; EA: Ethyl acetate; NBA: n-Butyl alcohol; W: Water. A: n = 5, B: n = 3, x ± s. ###P < 0.001vs control; **P < 0.01, ***P < 0.001vs model |
为了进一步明确扎冲十三味丸中具体抗炎活性成分, 实验中将拮抗NF-κB的有效部位和抑制NO释放的有效部位分别进行谱效筛选。结果显示乙酸乙酯层中3个时间段对应的馏分(1~1.5、4.5~6、11.5~12.5 min)对NF-κB显示出较好的拮抗活性(图 2), 经过质谱解析, 确定了其中4个单体化合物, 分别为没食子酸、鞣花酸、芹糖甘草苷及甘草酸(表 1[10-12])。
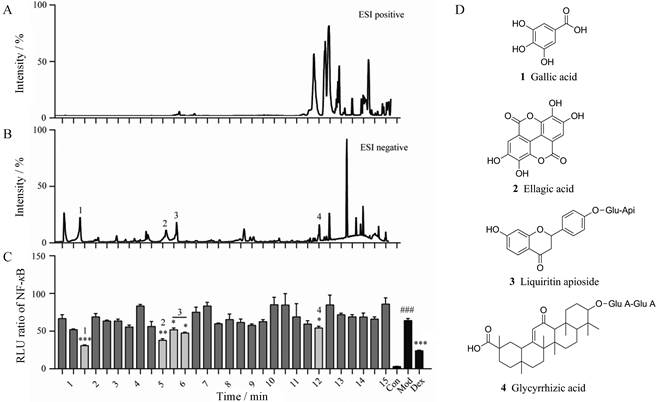
|
Figure 2 Screening of NF-κB antagonistic active ingredients from ZC-13 based UPLC/Q-TOF-MS/MS. A: UPLC/Q-TOF base peak intensity (BPI) in the ESI positive; B: UPLC/Q-TOF BPI in the ESI negative; C: Screening of the antagonistic activity of NF-κB; D: Structure of NF-κB inhibiting active components. Glu-Api: Glucose-apiose; Glu A: Glucuronide acid.n = 4, x ± s. ###P < 0.001vs control; *P < 0.05, **P < 0.01, ***P < 0.001 vs model |
| Table 1 Mass spectrometry analysis and identification of NF- κB antagonistic components |
石油醚、二氯甲烷及乙酸乙酯合并层中3个时间段对应的馏分(1~2、9~11、18~20 min)对NO释放显示出较好的抑制作用(图 3), 经过质谱解析, 确认了其中4个单体化合物, 分别为没食子酸、甘草素、木香烃内酯、去氢木香内酯(表 2[10, 11, 13])。
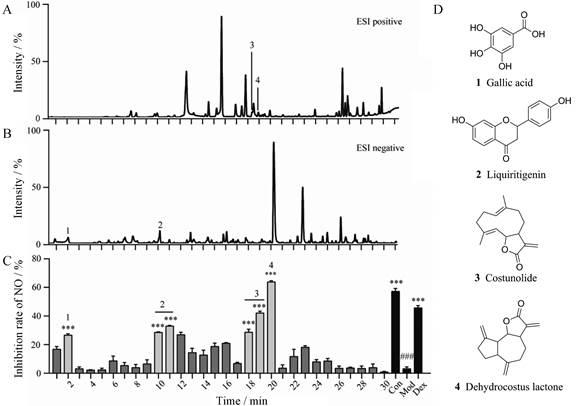
|
Figure 3 Screening of the antagonistic activities of NO release from ZC-13 based UPLC/Q-TOF-MS/MS. A: UPLC/Q-TOF BPI in the ESI positive; B: UPLC/Q-TOF BPI in the ESI negative; C: Screening of the antagonistic activity of NO release; D: Structure of NO inhibiting active components. n = 3, x ± s. ###P < 0.001vs control; ***P < 0.001 vs model |
| Table 2 Mass spectrometry analysis and identification of NO-releasing inhibitors |
为了确定筛选结果的准确性, 分别对芹糖甘草苷、甘草酸、没食子酸、鞣花酸拮抗NF-κB活性进行验证, 其中芹糖甘草苷和甘草酸皆为糖苷类物质, 一般口服后会转化为更易入血的苷元来发挥作用, 考虑到扎冲十三味丸主要是通过口服发挥作用, 因此实验中选用它们相对应的苷元甘草素和甘草次酸进行单体验证(图 4A), 同时对没食子酸、甘草素、木香烃内酯、去氢木香内酯NO释放抑制作用(图 4B)进行验证。结果显示上述化合物均具有一定拮抗NF-κB活性及NO释放抑制作用。
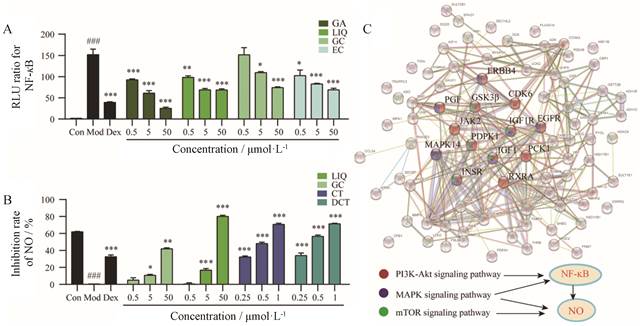
|
Figure 4 Verification of anti-inflammatory active ingredients from ZC-13 and network pharmacological prediction of the action mechanism of active ingredients. A: Confirmation the antagonistic activity on NF- κB of gallic acid (GC), ellagic acid (EC), liquiritigenin (LIQ), glycyrrhetic acid (GA); B: Confirmation the suppress release effect on NO of gallic acid, liquiritigenin, costunolide (CT), dehydrocostus lactone (DCT); C: Network pharmacological prediction of the action mechanism of active ingredients. ERBB4: Receptor tyrosine-protein kinase ErbB-4; GSK3β: Glycogen synthase kinase-3β; CDK6: Cyclin-dependent kinase 6; PGF: Placenta growth factor; JAK2: Janus kinase 2; IGF1R: Insulin-like growth factor 1 receptor; IGF1: Insulin-like growth factor 1; PDPK1:3-Phosphoinositide-dependent protein kinase 1; MAPK14: Mitogen-activated protein kinase 14; PCK1: Recombinant phosphoenolpyruvate carboxy kinase 1; INSR: Insulin receptor; RXRA: Retinoid X receptor alpha. A: n = 4, B: n= 3, x ± s. ###P < 0.001vs control; *P < 0.05, **P < 0.01, ***P < 0.001 vs model |
对上述活性成分作用靶点及通路进行预测结果表明, 上述活性成分可通过作用于MAPK14、PDPK1、GSK3β、胎盘生长因子(placenta growth factor, PGF)、受体酪氨酸受体激酶ErbB-4 (receptor tyrosine-protein kinase, ErbB-4 ERBB4)、周期蛋白依赖激酶6 (cyclin-dependent kinase, 6 CDK6)、Janus激酶2 (Janus kinase 2, JAK2)、表皮生长因子受体(epidermal growth factor receptor, EGFR)、胰岛素样生长因子1受体(insulin-like growth factor 1 receptor, IGF1R)、胰岛素样生长因子1 (insulin-like growth factor 1, IGF1)、磷酸烯醇式丙酮酸羧激酶1 (recombinant phosphoenolpyruvate carboxy kinase 1, PCK1)、维甲类X受体α (retinoid X receptor alpha, RXRA)、胰岛素受体(insulin receptor, INSR)等靶点, 进而调控T细胞受体、Toll样受体、瞬时感受器电位离子通道炎症介导调节通路、PI3K-AKT、MAPK、mTOR等信号通路, 其中PI3K-AKT、MAPK及mTOR信号通路与NF-κB、NO关系密切[14, 15] (图 4C)。
4 炎症通路的验证为了验证预测结果的准确性, 实验中通过检测木香烃内酯对AKT磷酸化及NF-κB核转移的影响, 以评价其对PI3K-AKT-NF-κB通路的影响。实验结果显示, 木香烃内酯可以抑制AKT磷酸化(图 5A), 并抑制NF-κB核转移(图 5B)。提示抑制PI3K-AKT-NF-κB通路是其抗炎作用机制之一。

|
Figure 5 Confirmation the anti-inflammatory pathway of costunolide (CT). A: Effect of costunolide on AKT phosphorylation induced by LPS in macrophages; B: Inhibition of costunolide on NF-κB nuclear transfer stimulated by LPS in macrophages. n = 4, x ± s. ##P < 0.01 vs control; *P < 0.05, ***P < 0.001 vs model |
NF-κB是炎症反应的关键环节之一[16], 其在脑缺血的发生发展过程中也有重要作用, 有文献[17]报道脑缺血过程中NF-κB显著激活, 而NF-κB抑制剂可降低脑缺血再灌注后炎症小体的表达以及脑细胞的死亡。NO同样是炎症关键环节之一, 在机体炎症情况下, 诱导型一氧化氮合酶被激活, 产生大量NO, 过量的NO可引起脂质过氧化, 并作为炎症因子刺激上游炎症相关蛋白的表达, 进一步加重炎症反应[18]。抑制NF-κB的激活和NO的释放可减轻炎症反应对细胞的损伤。扎冲十三味丸是临床常用治疗脑缺血蒙药之一, 此次研究结果显示, 其可以显著拮抗NF-κB, 并抑制NO释放, 提示抗炎作用可能是其治疗脑缺血关键机制之一。
药物物质基础不清是影响蒙药推广应用的关键因素之一, 通过谱效筛选可知扎冲十三味丸中没食子酸、鞣花酸、芹糖甘草苷及甘草酸是拮抗NF-κB活性的主要成分, 而抑制NO释放的活性成分为没食子酸、甘草素、木香烃内酯及去氢木香内酯。其中没食子酸、鞣花酸、甘草酸、木香烃内酯及去氢木香内酯[19-25]等化合物拮抗NF-κB及抑制NO释放的活性已有报道, 芹糖甘草苷是首次发现可拮抗NF-κB。也有文献[26-28]报道肉豆蔻醚、麝香酮、α-细辛醚等等扎冲十三味丸中成分也有抗炎作用, 但此次研究并未观察到其显著作用, 可能与其在复方含量较低有关。
作用机制不明是影响蒙药推广应用的另一关键因素, 实验中网络药理学的方法预测了扎冲十三味丸抗炎关键药效成分抗炎作用机制, 结果表明其可以通过木香烃内酯等成分多靶点的调控MAPK、PI3K-AKT、mTOR等信号通路发挥抗炎作用。初步揭示了扎冲十三味丸抗炎作用机制。
综上所述, 该实验初步阐明了扎冲十三味丸可以通过没食子酸、鞣花酸、芹糖甘草苷、甘草酸、甘草素、木香烃内酯、去氢木香内酯等成分多靶点的调控MAPK、PI3K-AKT、mTOR等信号通路发挥抗炎作用以治疗脑缺血。没食子酸、鞣花酸、芹糖甘草苷、甘草酸、甘草素、木香烃内酯、去氢木香内酯等化合物作为潜在质量标志物可以作为提升扎冲十三味丸质量标准参考。但也应看到, 扎冲十三味丸组成成分较多, 化学成分复杂,除抗炎作用外, 还有舒筋活血, 镇静安神等其他方面作用。该复方其他功效的物质基础及作用机制还需进一步探究。
| [1] |
Yang CJ, Gu YL, Liu X, et al. Research progress of traditional Mongolian medicine zhachong-thirteen[J]. J North Pharm (北方药学), 2018, 15: 154-155. |
| [2] |
Li W, Wu XQ, Yuan WH, et al. Determination of costunolide and dehydrocostus lactone in Mongolia medicine zhachong shisanwei pills by UHPLC[J]. Chin J Ethnomed Ethnopharm (中国民族民间医药), 2017, 26: 24-25. |
| [3] |
Su NH. Experimental research progress of mongolian medicine garidi-13 flavor pills[J]. Electron J Clin Med Lit (临床医药文献电子杂志), 2017, 4: 12267. |
| [4] |
Liu CX, Chen SL, Xiao XH, et al. A new concept on quality marker of Chinese materia medica:quality control for Chinese medicinal products[J]. Chin Tradit Herb Drugs (中草药), 2016, 47: 1443-1457. |
| [5] |
Zhang TJ, Bai G, Liu CX. The concept, core theory and research methods of Chinese medicine quality markers[J]. Acta Pharm Sin (药学学报), 2019, 54: 187-196. |
| [6] |
Li XJ, Huang YY, Yang Z, et al. Research strategy for quality-biomarkers of traditional Chinese medicine based on effect benchmarks[J]. Acta Pharm Sin (药学学报), 2019, 54: 204-210. |
| [7] |
Tian CY, Zhang HN, He JB, et al. Effect of garidi-13 on the expression of NSE, TNF-α and IL-8 at different phase after focal cerebral ischemia in rats[J]. Pharm Clin Chin Mater Med (中药药理与临床), 2014, 30: 158-161. |
| [8] |
Tao C, Lin L, Song BH, et al. Effect of Mongolian medicine garidi-13 pills on IL-1β, TNF-α protein and nerve cell apoptosis of cerebral ischemia hippocampus tissue of rats[J]. Chin J Gerontol (中国老年学杂志), 2015, 35: 152-154. |
| [9] |
Han MH, Lee WS, Nagappan A, et al. Flavonoids isolated from flowers of Lonicera japonica Thunb. inhibit inflammatory responses in BV2 microglial cells by suppressing TNF-α and IL-β through PI3K/Akt/NF-κB signaling pathways[J]. Phytother Res, 2016, 30: 1824-1832. DOI:10.1002/ptr.5688 |
| [10] |
Pfundstein B, Desouky SKE, Hull WE, et al. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida):characterization, quantitation and determination of antioxidant capacities[J]. Phytochemistry, 2010, 71: 1132-1148. DOI:10.1016/j.phytochem.2010.03.018 |
| [11] |
Zhao YM, Liu SX, Zhang CX, et al. Analysis on chemical constituents from Glycyrrhizae Radix et Rhizoma by HPLC-Q-TOF-MS[J]. Chin Tradit Herb Drugs (中草药), 2016, 47: 2061-2068. |
| [12] |
Shakeri A, Masullo M, D'Urso G, et al. In depth chemical investigation of Glycyrrhiza triphylla Fisch roots guided by a preliminary HPLC-ESIMSn profiling[J]. Food Chem, 2018, 248: 128-136. DOI:10.1016/j.foodchem.2017.12.031 |
| [13] |
Hu F, Feng S, Wu Y, et al. Quantitative analysis of costunolide and dehydrocostus lactone in rat plasma by ultraperformance liquid chromatography-electrospray ionization-mass spectrometry[J]. Biomed Chromatogr, 2011, 25: 547-554. DOI:10.1002/bmc.1482 |
| [14] |
Kim H, Banerjee N, Barnes RC, et al. Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo[J]. Mol Carcinog, 2017, 56: 197-207. DOI:10.1002/mc.22484 |
| [15] |
Sun SC. The non-canonical NF-κB pathway in immunity and inflammation[J]. Nat Rev Immunol, 2017, 17: 545-558. DOI:10.1038/nri.2017.52 |
| [16] |
Afonina IS, Zhong Z, Karin M, et al. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome[J]. Nat Immunol, 2017, 18: 861-869. DOI:10.1038/ni.3772 |
| [17] |
Fann YW, Lim YA, Cheng YL, et al. Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke[J]. Mol Neurobiol, 2018, 55: 1082-1096. DOI:10.1007/s12035-017-0394-9 |
| [18] |
Li H, Fö rstermann U. Nitric oxide in the pathogenesis of vascular disease[J]. J Pathol, 2000, 190: 244-254. DOI:10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8 |
| [19] |
Bensaad LA, Kim KH, Quah CC, et al. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A & B isolated from Punica granatum[J]. BMC Complement Altern Med, 2017, 17: 47. DOI:10.1186/s12906-017-1555-0 |
| [20] |
Zhu L, Gu P, Shen H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis[J]. Int Immunopharmacol, 2019, 67: 129-137. DOI:10.1016/j.intimp.2018.11.049 |
| [21] |
Ebrahimi R, Sepand MR, Seyednejad SA, et al. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IκBα/NF-κB in rats[J]. DARU J Pharm Sci, 2019, 27: 721-733. DOI:10.1007/s40199-019-00309-9 |
| [22] |
Tanaka M, Kishimoto Y, Sasaki M, et al. Terminalia bellirica (Gaertn.) Roxb. extract and gallic acid attenuate LPS-induced inflammation and oxidative stress via MAPK/NF-κB and Akt/AMPK/Nrf2 pathways[J]. Oxid Med Cell Longev, 2018, 2018: 9364364. |
| [23] |
He Y, Moqbel SAA, Xu L, et al. Costunolide inhibits matrix metalloproteinases expression and osteoarthritis via the NF κB and Wnt/βcatenin signaling pathways[J]. Mol Med Rep, 2019, 20: 312-322. |
| [24] |
Wang CY, Kao TC, Lo WH, et al. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions[J]. J Agric Food Chem, 2011, 59: 7726-7733. DOI:10.1021/jf2013265 |
| [25] |
Nie Y, Wang Z, Chai G, et al. Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-κB signaling pathway mediated by p38 MAPK and Akt[J]. Molecules, 2019, 24: 1510. DOI:10.3390/molecules24081510 |
| [26] |
Lee JY, Park W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid[J]. Molecules, 2011, 16: 7132-7142. DOI:10.3390/molecules16087132 |
| [27] |
Du Y, Gu X, Meng H, et al. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome[J]. Am J Transl Res, 2018, 10: 4235-4246. |
| [28] |
Sutariya B, Saraf M. α-Asarone reduce proteinuria by restoring antioxidant enzymes activities and regulating necrosis factor κB signaling pathway in doxorubicin-induced nephrotic syndrome[J]. Biomed Pharmacother, 2018, 98: 318-324. DOI:10.1016/j.biopha.2017.12.051 |
 2020, Vol. 55
2020, Vol. 55


