2. 中国科学院上海药物研究所制剂研究中心, 上海 201203;
3. 上海中医药大学科技实验中心, 上海 201203
2. Center for Pharmaceutics Research, Shanghai Institute of Materia Medica Chinese, Academy of Sciences, Shanghai 201203, China;
3. Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
肠相关淋巴组织(gut-associated lymphoid tissue, GALT)作为肠道黏膜免疫屏障系统的主要部分, 在机体抵抗病原体侵袭中发挥着关键作用[1]。GALT包括免疫诱导位点和效应位点, 其中诱导位点包括派氏结(Peyer's patches, PPs)、孤立淋巴滤泡、肠系膜淋巴结(mesenteric lymph node, MLN)等肠道淋巴组织[2]。作为GALT的重要组成部分, PPs被认为是肠道黏膜免疫反应的主要诱导部位, 药物及生物活性物质靶向递送至PPs将有利于肠道免疫相关疾病的治疗。而肠道环境与淋巴系统的复杂性使得药物靶向PPs非常具有挑战性。M细胞(microfold cells, M cells)是PPs中一种特化的上皮细胞, 虽然数量较少, 但具有摄取和转运肠腔内抗原的功能。M细胞摄取抗原后将其传递至PPs中的树突状细胞(dendritic cells, DCs)等抗原呈递细胞, 从而进一步诱导黏膜免疫应答, 实现了淋巴靶向传递的目的。因此, M细胞常被作为药物递送至PPs的主要靶标。
靶向M细胞主要通过特异性配体介导, 如凝集素配体可以特异性结合M细胞表面的α-岩藻糖残基, 或者通过聚合物、脂质等递药载体材料的应用提高M细胞对抗原的摄取能力, 此外载体粒子的粒径、电荷及疏水性等因素也会对M细胞靶向效率产生影响。因此, 本文将重点讨论靶向M细胞的主要配体、研究较多的载体材料及影响M细胞摄取的主要因素。
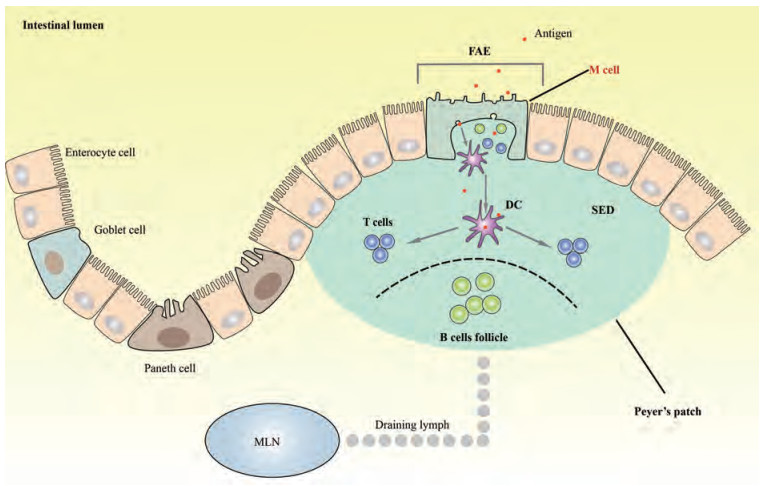
1 PPs的结构及功能PPs以瑞士解剖学家Johann Conrad Peyer的名字命名, 被称为“由黏膜淋巴结组成的高架区域”[3], 通常位于回肠末端[4], 少数在空肠[3]。PPs主要由滤泡关联上皮(follicle associated epithelium, FAE)、上皮下圆顶(sub-epithelial dome, SED)和B细胞滤泡(B cells follicle) 3个部分组成[5] (图 1)。其中FAE是M细胞的主要存在区域。M细胞是专门用于抗原摄取的一类上皮细胞, 是刺激抗原特异性黏膜免疫应答的主要门户[6], 其抗原摄取能力部分归因于FAE本身的特点。FAE没有杯状细胞(goblet cells), 隐窝中几乎没有潘氏细胞(Paneth cells), 并且FAE的肠细胞中IgA转运多聚免疫球蛋白受体的表达也很低[7, 8]。这些特点使得FAE对外源性细菌、抗原的活性具有相对较少的破坏作用, 从而促使肠腔内细菌及抗原更易于与FAE紧密结合, 有利于M细胞对其进行摄取。
|
Figure 1 The structure of intestinal Peyer's patchs and the antigens uptake of M cell. Peyer's patchs (PPs) are organized structures consisting of follicle associated epithelium (FAE), sub-epithelial dome (SED) and large B cells follicle. Antigens enter into microfold (M) cell in FAE and then are passed on to dendritic cells (DCs) in SED. DCs can directly present antigens to T cells or B cells in PPs or reach mesenteric lymph node (MLN) through drainage lymph |
被M细胞摄取的抗原会进一步转移到SED。SED含有大量的树突状细胞(dendritic cells, DCs), 特别是CD11b+ CD8-DCs和CD11b-CD8-DN DCs[9], 在抗原的处理与呈递过程中发挥关键作用。DCs通过两种途径参与免疫反应, 一种途径是M细胞将摄取的肠腔抗原传递至DCs, DCs将抗原继续呈递给T细胞或B细胞, 或者负载抗原的DCs通过淋巴引流(draining lymph)直接进入MLN; 另一途径是DCs通过M细胞延伸至肠腔并直接对抗原进行摄取[4, 10]。除此之外, SED中还分散存在着B细胞、巨噬细胞, 以及T细胞和RORγt+先天淋巴细胞等一系列免疫应答所必需的细胞[11], 更有利于引发M细胞特异性黏膜免疫反应。
2 M细胞形态及功能M细胞顶部缺乏正常的微绒毛, 具有“微褶皱”结构。此外, 它们的基底质膜深深地内陷, 形成一个大的囊状结构, 称为“M细胞囊袋(M cell pocket)”[12]。M细胞具有黏液分泌少、糖萼稀疏的特点, 且其“囊袋”结构的凹陷处被DCs和淋巴细胞占据而致使其质膜更薄, 这些结构特征促使M细胞在转胞吞作用中具有高度活性[13]。“囊袋”的形成减少了胞内距离, 加之其与局部单核吞噬细胞和淋巴细胞相邻的特点, 使腔内抗原可以被快速转运并递送至DCs以引发黏膜免疫应答[14, 15]。M细胞的溶酶体相较于其他肠上皮细胞而言更少, 并且酶活性更低。因此, M细胞摄取的抗原可以在不被处理的状态下完整地转移到DCs, 进一步进行抗原加工和呈递[16]。M细胞摄取抗原主要通过特定的受体介导的途径发生, 但已有研究发现M细胞可以吸收胶乳珠等惰性微粒, 表明它们还具有非特异性的识别吞噬能力[17, 18]。基于M细胞的特点以及其在黏膜免疫系统中发挥的重要作用, 靶向M细胞被认为是免疫激活的关键策略之一。配体与受体的特异性结合则是靶向M细胞的主要途径。
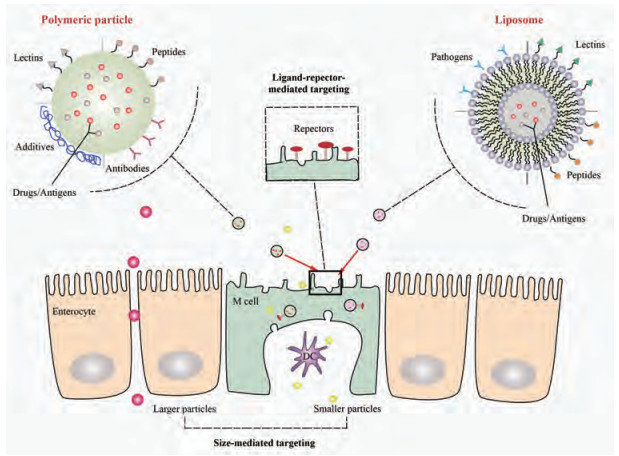
3 M细胞靶向配体M细胞在抗原转运中发挥重要作用, 在各种体内外模型中寻找M细胞的特异性靶标是构建靶向M细胞递药系统的关键策略。利用配体结合M细胞表面的特异性受体会更专一、有效地靶向M细胞(图 2)。满足靶向条件的配体主要有凝集素(lectins)、肽(peptides)、病原体(pathogens)及抗体(antibodies)。
|
Figure 2 Delivery strategies for M cell-targeting nanoparticles. Smaller particles are more easily taken up by M cell than larger particles. Polymeric nanoparticles or liposomes (loaded with drugs or antigens) modified with various M cell targeting ligands including lectins, peptides, pathogens and antibodies can specifically bind with the receptors on the apical surface of the M cell and so as to transport across M cell and deliver to dendritic cell |
M细胞表面存在可以被凝集素靶向识别的高度糖基化区域[19], 该区域中的受体可与不同的凝聚素特异性结合。其中, 凝集素1 (lectin ulex europaeus agglutinin 1, UEA-1)可以特异性结合位于M细胞顶端表面的α-L-岩藻糖残基(α-L-fucose residues)。研究发现, 与未被修饰的载体相比, 用UEA-1修饰的载体可以通过特异性识别M细胞上的α-L-岩藻糖残基以靶向小鼠M细胞并增强M细胞摄取, 从而更显著诱导免疫应答的发生。这一修饰方式对于微球[20]、脂质体[21]、纳米粒[22]等微粒体系载体而言, 均能提高其免疫应答效率。然而, 由于人体M细胞顶端表面缺乏所需的α-L-岩藻糖的表达[23], 因此该靶向策略在应用于人体方面还存在一定的不确定性。Aleuria aurantia凝集素(Aleuria aurantia lectin, AAL)是一种蘑菇衍生的凝集素, 它含有5个岩藻糖结合位点, 也可与α-L-岩藻糖特异性结合以主动靶向M细胞[24, 25]。AAL在生理条件下是一种高度带正电荷的蛋白质, 而且它的晶体结构类似于来自细菌和病毒的各种神经氨酸酶的结构, 这些神经氨酸酶可以通过PPs侵入宿主细胞[25], 因此更有利于M细胞的摄取。与UEA-1和AAL不同, 麦胚凝集素(wheat germ agglutinin lectin, WGA)则是通过与唾液酸残基(sialic acid)的相互作用来产生特异性黏附, 从而增强M细胞的摄取功能, 但缺陷在于它并不能实现对M细胞的特异性靶向[26]。
3.2 肽精氨酸-甘氨酸-天冬氨酸肽(Arg-Gly-Asp peptide, RGD肽)可以识别M细胞特异性受体β1整合素(β1 integrin)[27]。β1整合素又称为β1整联蛋白, 是M细胞顶端表面的Ⅰ型跨膜异二聚体糖蛋白受体, 可以促进细胞黏附并连接细胞内外环境[28]。β1整合素的内源配体是纤连结合蛋白(fibronectin-binding proteins, FnB), 这种蛋白可以与RGD肽相互作用[29], 所以能够通过RGD肽特异性靶向M细胞。RGD肽表面带有羧酸(-COOH)、醇(-OH)或胺(-NH2)等官能团, 可以以共价键形式键合到递药载体的表面[19]。而利用RGD肽可以将更大的载体靶向M细胞表面的β1整合素, 因为M细胞糖萼在摄取中起主要作用, 而β1整合素位于M细胞糖萼更接近的区域[30]。
M细胞表面高度表达紧密连接蛋白4 (claudin 4), claudin 4在内吞过程中发挥重要作用[31]。源自产气荚膜梭菌肠毒素(clostridium perfringens enterotoxin, CPE)的C末端30个氨基酸(CPE 30)可以与claudin 4特异性结合[32]。血凝素(hemagglutinin, HA)重组蛋白与CPE 30修饰的载体融合后可以提高载体对M细胞的黏附性, 并诱导更强的黏膜免疫反应[33]。研究表明, CKSTHPLSC (CKS 9)肽对于M细胞也显示出高亲和性。组织特异性荧光定位实验显示, CKS 9肽修饰的纳米粒在M细胞模型中可以被更高效地传递, 并且更专一地靶向PPs中的FAE[34]。CKS 9肽与猪痢疾短螺旋体膜蛋白B (membrane protein B of Brachyspira hyodysenteriae, BmpB)结合后口服给予小鼠, 与未修饰的BmpB微粒相比, 修饰后的微粒增强了M细胞靶向和转胞吞的能力, 且黏膜组织中的分泌型IgA应答与全身性IgG抗体应答均增强[35]。
3.3 病原体与抗体M细胞表面表达一系列病原体识别受体(pathogen recognition receptors, PRR), 一些肠道病原体可以与PRR结合以达到靶向M细胞的目的。细菌膜Ⅰ型菌毛中的FimH可以与M细胞顶端表面的糖蛋白2 (glycoprotein 2, GP2)受体特异性结合[36]。耶尔森氏菌侵袭蛋白可特异性靶向M细胞上的β1整合素, 通过修饰载体可介导载体靶向M细胞[37]。近年来, 利用抗体靶向M细胞已经成为开发高效黏膜疫苗的新策略。分泌型免疫球蛋白A (secretory IgA, SIgA)可以与M细胞表面的C型凝集素样受体(Dectin-1)特异性结合[38], 在黏膜疫苗的发展中具有显著应用潜力。有研究将人类免疫缺陷病毒(human immune deficiency virus, HIV)抗原P24 (P24HIV)与SIgA结合, 抗原可迅速被M细胞摄取并传递至DCs, 诱导针对P24HIV的体液和细胞免疫应答[38]。单克隆抗体NKM 16-2-4 (mAb NKM 16-2-4)可以特异性识别M细胞表面的α (1, 2)岩藻糖, 当与破伤风类毒素(tetanus toxoid, TT)疫苗结合应用时, 能够诱导更高水平的黏膜IgA免疫反应[39]。除此之外, 由于M细胞表达GP2受体, 可以利用GP2抗体增强载体靶向M细胞的能力[40]。与GP2抗体相似的还有唾液酸结合免疫球蛋白样凝集素F (Siglec-F)抗体, 研究发现Siglec-F在小鼠小肠M细胞表面表达, 通过注射其抗体可以靶向M细胞[41]。
4 M细胞靶向递药载体材料目前研究较多的M细胞靶向载体材料主要包括嵌段共聚物和脂质材料两大类。嵌段共聚物是以聚乳酸-羟基乙酸共聚物[poly (lactic-co-glycolic acid), PLGA]为代表的一类生物可降解材料, 以制备纳米粒为主要用途; 脂质材料则是以磷脂等内源性小分子材料为代表, 主要用于制备脂质体等。此外, 还有海藻糖(trehalose)和藻酸盐(alginate)等功能性添加剂, 主要用于稳定负载成分及促进配体与载体的结合。这些材料不仅是构成载体的基础材料, 而且包含与配体连接的结构基团, 使得构建的载体不仅能负载药物, 还有主动靶向M细胞进而使药物或其他活性物质在肠道淋巴中进行传递的能力。因此, M细胞靶向递药载体材料的选择是靶向载体设计尤为重要的考虑因素, 表 1[35, 42-51]将现有研究较多的、常用的载体材料及靶向策略进行了对比分析。
| Table 1 Specific ligands and vehicle materials in delivery strategies for M cell - targeting. DDS: Drug delivery system; DSPC: Disteroylphosphatidylcholine; NGPE: N-Glutaryl-phosphotidylethanolamine; PVA: Polyvinyl alcohol; BSA: Bovine serum albumin; OVA: Ovalbumin |
M细胞可以作为聚合物粒子转运的一个通道, 使生物活性成分从黏膜上皮细胞传递到淋巴组织[22]。聚合物的应用不仅可以增强载体靶向M细胞的能力, 同时又能减少胃肠道环境对生物活性成分的影响。常见的几种聚合物材料有PLGA、壳聚糖(chitosan)及藻酸盐等。
4.1.1 PLGA口服PLGA纳米粒后, M细胞会摄取纳米粒并将其从肠腔输送到上皮淋巴细胞, 然后通过淋巴系统进入血液, 避免肠细胞中的酶促降解, 更重要的是可以避免首过效应, 从而提高纳米粒中药物的生物利用度, 并减少用药剂量、降低药物的毒副作用[52]。用PLGA包载洛匹那韦后药物平均滞留时间增加, 并且抑制了P-糖蛋白对药物的外排作用及细胞色素P450引起的首过效应, 使药物的生物利用度增加了约13.9倍[53]。
为了进一步增加纳米粒靶向M细胞的特异性, 可以对载体表面进行修饰。Gupta等[42]对载有乙肝表面抗原(hepatitis B surface antigen, HBsAg)的PLGA纳米粒用UEA-1进行修饰, 发现可以诱导更强的Th1样免疫反应。Garinot等[54]将RGD肽共价连接到PLGA纳米粒表面, 通过体内研究表明纳米粒在M细胞中更为聚集。
4.1.2 壳聚糖壳聚糖具有良好的生物相容性、黏膜黏附性及吸收特性, 并且毒性低、成本低, 成为广泛使用的制备M细胞靶向递药载体材料之一[55]。已有研究证明包载抗原的壳聚糖微粒可以专一靶向PPs, 从而增加黏膜和全身免疫应答[35]。Xu等[43]利用离子凝胶法制备牛血清蛋白(bovine serum albumin, BSA)壳聚糖纳米粒, 发现其可以高度抵抗酶或酸对蛋白的降解, 并且对大鼠肠道PPs有更好的靶向能力, 包载的BSA也引起了更强的黏膜IgA应答和IgG抗体应答。壳聚糖还可以与PLGA结合应用, 结合后的微粒通过壳聚糖的黏膜黏附特性进一步加强了载体靶向M细胞的能力和转胞吞能力, 从而增强免疫应答[35]。壳聚糖还可以通过UEA-1、CPE 30和CKS9等配体的修饰来进一步提高载体靶向PPs M细胞的精准度。
4.2 脂质材料在药物递送过程中, 使用脂质材料是药物吸附、溶解和分散到基质中的常用方法之一[56]。脂质材料可以增加药物的半衰期, 降低给药频率[57]; 还可以通过与肠道表面的亲和作用从而提高药物的生物利用度[58], 并通过增强淋巴管的转运以避免首过效应[59]。据报道, 一些含有脂质分子的纳米载体如固体脂质纳米粒(solid lipid nanoparticles)和脂质体(liposomes)等, 均可以特异性地靶向M细胞, 抑制酶降解并增强M细胞摄取[60]。Ma等[47]制备了卵清白蛋白(ovalbumin, OVA) PLGA纳米粒与OVA-PLGA-脂质纳米粒, 经比较发现, 脂质纳米粒具有更高的负载能力和包封率, 体外释放曲线也显示脂质纳米粒未出现突释现象, 并且可以长时间保护负载的OVA免受胃肠环境的影响, 而纳米粒表面上的脂质层结构也促使载体表现出对M细胞更高的亲和力并有效诱导黏膜和体液免疫应答[53]。
4.3 其他功能性添加剂海藻糖是一种良好的蛋白质稳定剂, 它可以通过增加药物的包载量并减少药物在水油界面处的变性来进一步增加药物在靶向区域的释放量[61]。聚乙二醇(PEG)及其衍生物也可以起到相似的稳定作用, 且PEG可以促进各种配体与纳米粒或微粒表面的结合, 并有利于黏膜给药[62]。例如, 可将PEG包覆在PLGA纳米粒表面, 再结合RGD肽以靶向M细胞并实现更有效的免疫应答[45]。藻酸盐也是一种较为常用的添加剂, 它可以控制纳米粒的突释, 多用于缓释制剂的制备中[63]。此外, 有研究将负载抗原的纳米粒用藻酸盐包衣后口服靶向PPs区域, 藻酸盐也可以作为一种口服抗原的纳米载体材料被广泛应用[64]。
5 影响M细胞摄取的主要因素粒子与M细胞表面的“微褶皱”接触后被顶膜快速吞噬, 而该过程与粒子本身的物理性质、载体形式等因素密切相关。尽管聚合物和脂质材料的使用及它们与配体的结合已成为靶向M细胞的有效手段, 但针对影响M细胞摄取的因素进行评估与应用将对于更好地实现M细胞靶向具有积极意义。
5.1 粒径较小的粒子比较大的粒子更容易被吸收, 也容易分布到更深的部位(图 2)。50 nm的聚苯乙烯粒子相较于500 nm和1 μm的粒子而言其吸收速度最快、分布最深[65]。Awaad等[66]使用不同的荧光素标记有机二氧化硅粒子, 给药后小鼠PPs SED中100、180和365 nm粒子的荧光面积大于745和925 nm粒子。Rieux等[67]分别将200和500 nm粒子与Caco-2和Raji细胞共培养物进行孵育, 经监测后发现200 nm粒子的转胞数量比500 nm粒子高7倍。而5 μm以上粒径较大的微粒也可以被M细胞摄取, 但仍然会停留在PPs中[68]。因此, 减小粒径是增强药物靶向M细胞能力的一个有效方法。
5.2 疏水性聚合物粒子的疏水性对它们的吸收也有显著影响。研究表明, 疏水性聚合物聚苯乙烯纳米粒与M细胞的亲和力及相互作用比与其他肠上皮细胞的更强[69]。与三乙酸纤维素等亲水性聚合物纳米粒相比, 聚苯乙烯、聚甲基丙烯酸甲酯和PLGA等疏水性聚合物纳米粒可优先被M细胞吸收[70]。研究还发现, 通过使用泊洛沙姆降低纳米粒表面的疏水性, 就会减弱免疫细胞的摄取能力[71]。以上结果均证明了载体材料疏水性的考察在处方优化过程中的不可或缺性。
5.3 电荷荷正电的纳米粒可以更高效地进行M细胞转运, 这可能是由于黏液中存在更多带负电的成分。因此, 在PPs中M细胞可以优先吸引带正电荷的纳米粒[67]。然而有研究发现中性或荷负电粒子对M细胞的亲和力更强, 并且比荷正电粒子显示出更高的转胞吞能力[72]。此外, 负电荷纳米粒结合疏水性特征可以进一步促进M细胞摄取[69]。由此可见, 粒子表面的电荷对M细胞摄取的影响机制较为复杂, 虽然电荷吸引能够促进吸收, 但某些特殊情况下, 电荷的吸引作用并不能起到决定性作用。
除粒径、疏水性和电荷因素外, 物种及给药时间也会影响M细胞摄取。研究发现, 家兔的M细胞摄取能力比小鼠的高[73], 且给药时间越长, 粒子在PPs区域越容易被识别[74]。除此之外, 改变给药载体的形式也可以增加粒子的吸收[75]。因此, 在进行M细胞靶向策略研究过程中, 应该充分评估M细胞模型因种属差异导致的实验结果的偏差, 以及给药载体选择的合理性。
6 展望M细胞摄取抗原后通过其较强的细胞转运能力从而成为促进黏膜免疫反应的理想靶点, 现已有许多口服疫苗被成功研制以用于将疫苗抗原递送至M细胞来诱导更高水平的免疫应答。M细胞与炎症性肠病(inflammatory bowel disease, IBD)等免疫相关疾病也有密切联系, 研究发现, 发生在FAE中的免疫反应是引发IBD的关键步骤[76], 且M细胞数量的变化可以作为IBD的临床诊断指标[77]。因此, 在未来的研究中, 作者建议可以将M细胞的量化作为预防IBD发生的有效手段, 并构建M细胞靶向载体将药物及生物活性物质递送至PPs以期发挥对IBD的治疗作用。然而M细胞数量很少, 人类PPs FAE中的M细胞比例不到5%[78]。如果递送的药物没有特异性靶向M细胞, 则M细胞摄取率将极低。为了提高M细胞靶向精准度, 达到更好的疾病治疗效果, 应该选择合适的载体及特异性配体。在此基础上创建更合适的M细胞模型并不断发现新的M细胞特异性标志物, 依据靶向策略选择不同的微粒载药系统, 并对粒度、疏水性和电荷等载体性质加以优化和控制, 进一步提高M细胞靶向载体的药物及生物活性物质的淋巴传递效率。
| [1] |
Mabbott NA, Donaldson DS, Ohno H, et al. Microfold (M) cells:important immunosurveillance posts in the intestinal epithelium[J]. Mucosal Immunol, 2013, 6: 666-677. DOI:10.1038/mi.2013.30 |
| [2] |
Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier[J]. Nat Rev Microbiol, 2018, 16: 457-470. DOI:10.1038/s41579-018-0036-x |
| [3] |
Heel KA, Mccauley RD, Papadimitriou JM, et al. Review:Peyer's patches[J]. J Gastroenterol Hepatol, 2010, 12: 122-136. |
| [4] |
Ahluwalia B, Magnusson MK, Öhman L. Mucosal immune system of the gastrointestinal tract:maintaining balance between the good and the bad[J]. Scand J Gastroenterol, 2017, 52: 1185-1193. DOI:10.1080/00365521.2017.1349173 |
| [5] |
Reboldi A, Cyster JG. Peyer's patches:organizing B-cell responses at the intestinal frontier[J]. Immunol Rev, 2016, 271: 230-245. DOI:10.1111/imr.12400 |
| [6] |
Ohno H. Intestinal M cells[J]. J Biochem, 2016, 159: 151. DOI:10.1093/jb/mvv121 |
| [7] |
Coles M, Kioussis D, Veiga-Fernandes H. Cellular and molecular requirements in lymph node and Peyer's patch development[J]. Prog Mol Biol Transl Sci, 2010, 92: 177-205. DOI:10.1016/S1877-1173(10)92008-5 |
| [8] |
Kato T, Owen RL. Structure and function of intestinal mucosal epithelium[M]//Mucosal Immunology. San Diego: Academic Press, 2005: 131-151.
|
| [9] |
Bonnardel J, Da Silva C, Wagner C, et al. Distribution, location, and transcriptional profile of Peyer's patch conventional DC subsets at steady state and under TLR7 ligand stimulation[J]. Mucosal Immunol, 2017, 10: 1412-1430. DOI:10.1038/mi.2017.30 |
| [10] |
Reboldi A, Arnon TI, Rodda LB, et al. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer's patches[J]. Science, 2016, 352: 4822. DOI:10.1126/science.aaf4822 |
| [11] |
Ebisawa M, Hase K, Takahashi D, et al. CCR6hiCD11cint B cells promote M-cell differentiation in Peyer's patch[J]. Int Immunol, 2011, 23: 261-269. DOI:10.1093/intimm/dxq478 |
| [12] |
Kimura S. Molecular insights into the mechanisms of M-cell differentiation and transcytosis in the mucosa-associated lymphoid tissues[J]. Anat Sci Int, 2018, 93: 23-24. DOI:10.1007/s12565-017-0418-6 |
| [13] |
Li HF, Zou J, Bai RY, et al. M cell in vitro model and its application in oral delivery of macromolecular drugs[J]. Acta Pharm Sin (药学学报), 2011, 46: 1429-1435. |
| [14] |
Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens:mechanisms of interaction, consequences for the disease process[J]. Semin Immunol, 1999, 11: 193-203. DOI:10.1006/smim.1999.0175 |
| [15] |
Soares EFP, Borges OMF. Oral vaccination through Peyer's patches:update on particle uptake[J]. Curr Drug Deliv, 2018, 15: 321-330. DOI:10.2174/1567201814666170825153955 |
| [16] |
Roth-Walter F. Oral allergen immunotherapy by targeting Peyer's patches[J]. Allergy, 2019. DOI:10.1111/all.13828 |
| [17] |
Macpherson AJ, McCoy KD, Johansen FE, et al. The immune geography of IgA induction and function[J]. Mucosal Immunol, 2008, 1: 11-22. DOI:10.1038/mi.2007.6 |
| [18] |
Klisuric A, Thierry B, Delon L, et al. Identifying human and murine M cells in vitro[J]. Exp Biol Med, 2019, 224: 554-564. DOI:10.1177/1535370219838674 |
| [19] |
Managuli RS, Raut SY, Reddy MS, et al. Targeting the intestinal lymphatic system:a versatile path for enhanced oral bioavailability of drugs[J]. Expert Opin Drug Deliv, 2018, 15: 787-804. DOI:10.1080/17425247.2018.1503249 |
| [20] |
Yeboah KG, Akande J, Addo RT, et al. In vitro and ex vivo characterization of lectin-labeled Mycobacterium tuberculosis antigen-containing microspheres for enhanced oral delivery[J]. J Drug Target, 2014, 22: 34-47. DOI:10.3109/1061186X.2013.833206 |
| [21] |
Clark MA, Blair H, Liang L, et al. Targeting polymerised liposome vaccine carriers to intestinal M cells[J]. Vaccine, 2001, 20: 208-217. DOI:10.1016/S0264-410X(01)00258-4 |
| [22] |
Manocha M, Pal PC, Chitralekha KT, et al. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex europaeus-I lectin as M cell target[J]. Vaccine, 2005, 23: 5599-5617. DOI:10.1016/j.vaccine.2005.06.031 |
| [23] |
Islam MA, Firdous J, Badruddoza AM, et al. M cell targeting engineered biomaterials for effective vaccination[J]. Biomaterials, 2019, 192: 75-94. DOI:10.1016/j.biomaterials.2018.10.041 |
| [24] |
Houser J, Kozmon S, Mishra D, et al. Influence of Trp flipping on carbohydrate binding in lectins. An example on Aleuria aurantia lectin AAL[J]. PLoS One, 2017, 12: e0189375. DOI:10.1371/journal.pone.0189375 |
| [25] |
Romano PR, Mackay A, Vong M, et al. Development of recombinant Aleuria aurantia lectins with altered binding specificities to fucosy-lated glycans[J]. Biochem Biophys Res Commun, 2011, 414: 84-89. DOI:10.1016/j.bbrc.2011.09.027 |
| [26] |
Choudhry N, Bajaj-Elliott M, Mcdonald V. The terminal sialic acid of glycoconjugates on the surface of intestinal epithelial cells activates excystation of Cryptosporidium parvum[J]. Infect Immun, 2008, 76: 3735-3741. DOI:10.1128/IAI.00362-08 |
| [27] |
Humphries JD. Integrin ligands at a glance[J]. J Cell Sci, 2006, 119: 3901-3903. DOI:10.1242/jcs.03098 |
| [28] |
Ley K, Rivera-Nieves J, Sandborn WJ, et al. Integrin-based therapeutics:biological basis, clinical use and new drugs[J]. Nat Rev Drug Discov, 2016, 15: 173-183. DOI:10.1038/nrd.2015.10 |
| [29] |
Campbell ID, Humphries MJ. Integrin structure, activation, and interactions[J]. Cold Spring Harbor Perspect Biol, 2011, 3: a004994. |
| [30] |
Jepson MA, Clark MA, Hirst BH. M cell targeting by lectins:a strategy for mucosal vaccination and drug delivery[J]. Adv Drug Deliv Rev, 2004, 56: 511-525. DOI:10.1016/j.addr.2003.10.018 |
| [31] |
Ahmad T, Gogarty M, Walsh E, et al. A comparison of three Peyer's patch "M-like" cell culture models:particle uptake, bacterial interaction, and epithelial histology[J]. Eur J Pharm Biopharm, 2017, 119: 426-436. DOI:10.1016/j.ejpb.2017.07.013 |
| [32] |
Ling J, Liao H, Clark R, et al. Structural constraints for the binding of short peptides to claudin-4 revealed by surface plasmon resonance[J]. J Biol Chem, 2008, 283: 30585-30595. DOI:10.1074/jbc.M803548200 |
| [33] |
Shrestha B, Rath JP. Poly (vinyl alcohol)-coated chitosan microparticles act as an effective oral vaccine delivery system for hepatitis B vaccine in rat model[J]. IET Nanobiotechnol, 2014, 8: 201-207. DOI:10.1049/iet-nbt.2013.0035 |
| [34] |
Yoo MK, Kang SK, Choi JH, et al. Targeted delivery of chitosan nanoparticles to Peyer's patch using M cell-homing peptide selected by phage display technique[J]. Biomaterials, 2010, 31: 7738-7747. DOI:10.1016/j.biomaterials.2010.06.059 |
| [35] |
Jiang T, Singh B, Li HS, et al. Targeted oral delivery of BmpB vaccine using porous PLGA microparticles coated with M cell homing peptide-coupled chitosan[J]. Biomaterials, 2014, 35: 2365-2373. DOI:10.1016/j.biomaterials.2013.11.073 |
| [36] |
Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of Fim H (+) bacteria by M cells initiates mucosal immune response[J]. Nature, 2009, 462: 226-230. DOI:10.1038/nature08529 |
| [37] |
Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells[J]. Infect Immun, 1998, 66: 1237-1243. DOI:10.1128/IAI.66.3.1237-1243.1998 |
| [38] |
Rochereau N, Pavot V, Verrier B, et al. Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells[J]. Eur J Immunol, 2015, 45: 773-779. DOI:10.1002/eji.201444816 |
| [39] |
Nochi T, Yuki Y, Matsumura A, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses[J]. Exp Med, 2007, 204: 2789-2796. DOI:10.1084/jem.20070607 |
| [40] |
Shima H, Watanabe T, Fukuda S, et al. A novel mucosal vaccine targeting Peyer's patch M cells induces protective antigen-specific IgA responses[J]. Int Immunol, 2014, 26: 619-625. DOI:10.1093/intimm/dxu061 |
| [41] |
Gicheva N, Macauley MS, Arlian BM, et al. Siglec-F is a novel intestinal M cell marker[J]. Biochem Biophys Res Commun, 2016, 479: 1-4. DOI:10.1016/j.bbrc.2016.08.055 |
| [42] |
Gupta PN, Khatri K, Goyal AK, et al. M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B[J]. J Drug Target, 2007, 15: 701-713. DOI:10.1080/10611860701637982 |
| [43] |
Xu B, Zhang W, Chen Y, et al. Eudragit®L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery[J]. Int J Biol Macromol, 2018, 113: 534-542. DOI:10.1016/j.ijbiomac.2018.02.016 |
| [44] |
Gupta PM, Vyas SP. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization[J]. Colloids Surf B Biointerfaces, 2011, 82: 118-125. DOI:10.1016/j.colsurfb.2010.08.027 |
| [45] |
Ogra PL. Mucosal immunity:some historical perspective on host-pathogen interactions and implications for mucosal vaccines[J]. Immunol Cell Biol, 2003, 81: 23-33. DOI:10.1046/j.0818-9641.2002.01142.x |
| [46] |
Roth-Walter F, Bohle B, Schöll I, et al. Targeting antigens to murine and human M-cells with Aleuria aurantia lectin-functionalized microparticles[J]. Immunol Lett, 2005, 100: 182-188. DOI:10.1016/j.imlet.2005.03.020 |
| [47] |
Ma TT, Wang LY, Yang TY, et al. Homogeneous PLGA-lipid nanoparticle as a promising oral vaccine delivery system for ovalbumin[J]. Asian J Pharm Sci, 2014, 9: 129-136. DOI:10.1016/j.ajps.2014.03.002 |
| [48] |
Chen H, Torchilin V, Langer R. Lectin-bearing polymerized liposomes as potential oral vaccine carriers[J]. Pharm Res, 1996, 13: 1378-1383. DOI:10.1023/A:1016030202104 |
| [49] |
Manolova V, Flace A, Bauer M, et al. Nanoparticles target distinct dendritic cell populations according to their size[J]. Eur J Immunol, 2008, 38: 1404-1413. DOI:10.1002/eji.200737984 |
| [50] |
Rajapaksa TE, Stover-Hamer M, Fernandez X, et al. Claudin 4-targeted protein incorporated into PLGA nanoparticles can mediate M cell targeted delivery[J]. J Control Release, 2010, 142: 196-205. DOI:10.1016/j.jconrel.2009.10.033 |
| [51] |
Lo DD, Ling J, Eckelhoefer AH. M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses[J]. BMC Biotechnol, 2012, 12: 7-10. DOI:10.1186/1472-6750-12-7 |
| [52] |
Jain AK, Swarnakar NK, Godugu C, et al. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen[J]. Biomaterials, 2011, 32: 503-515. DOI:10.1016/j.biomaterials.2010.09.037 |
| [53] |
Joshi G, Kumar A, Sawant K. Bioavailability enhancement, Caco-2 cells uptake and intestinal transport of orally administered lopinavir loaded PLGA nanoparticles[J]. Drug Deliv, 2016, 23: 3492-3504. DOI:10.1080/10717544.2016.1199605 |
| [54] |
Garinot M, Fiévez V, Pourcelle V, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination[J]. J Control Release, 2007, 120: 195-204. DOI:10.1016/j.jconrel.2007.04.021 |
| [55] |
VDIM Lubben, Verhoef JC, Aelst VAC, et al. Chitosan microparticles for oral vaccination:preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches[J]. Biomaterials, 2001, 22: 687-694. DOI:10.1016/S0142-9612(00)00231-3 |
| [56] |
Attama AA, Umeyor CE. The use of solid lipid nanoparticles for sustained drug release[J]. Ther Deliv, 2015, 6: 669-684. DOI:10.4155/tde.15.23 |
| [57] |
Ghasemiyeh P, Samani SM. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems:applications, advantages and disadvantages[J]. Res Pharm Sci, 2018, 13: 288-303. DOI:10.4103/1735-5362.235156 |
| [58] |
Chakraborty S, Shukla D, Vuddanda PR, et al. Effective in-vivo utilization of lipid-based nanoparticles as drug carrier for carvedilol phosphate[J]. J Pharm Pharmacol, 2011, 63: 774-779. DOI:10.1111/j.2042-7158.2011.01270.x |
| [59] |
Lin CH, Chen CH, Lin ZC, et al. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers[J]. J Food Drug Anal, 2017, 25: 219-234. DOI:10.1016/j.jfda.2017.02.001 |
| [60] |
Vieira AC, Chaves LL, Pinheiro M, et al. Design and statistical modeling of mannose-decorated dapsone-containing nanoparticles as a strategy of targeting intestinal M-cells[J]. Int J Nanomed, 2016, 11: 2601-2617. |
| [61] |
Lee J, Ko JH, Mansfield KM, et al. Glucose-responsive trehalose hydrogel for insulin stabilization and delivery[J]. Macromol Biosci, 2018, 18: e1700372. DOI:10.1002/mabi.201700372 |
| [62] |
Fievez V, Plapied L, Rieux AD, et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination[J]. Eur J Pharm Biopharm, 2009, 73: 16-24. DOI:10.1016/j.ejpb.2009.04.009 |
| [63] |
Malik B, Goyal AK, Markandeywar TS, et al. Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization[J]. J Drug Target, 2012, 20: 76-84. DOI:10.3109/1061186X.2011.611516 |
| [64] |
Borges O, Tavares J, Sousa AD, et al. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles[J]. Eur J Pharmaceut Sci, 2007, 32: 278-290. DOI:10.1016/j.ejps.2007.08.005 |
| [65] |
Jani PU, Mccarthy DE, Florence AT. Nanosphere and microsphere uptake via Peyer's patches:observation of the rate of uptake in the rat after a single oral dose[J]. Int J Pharm, 1992, 86: 239-246. DOI:10.1016/0378-5173(92)90202-D |
| [66] |
Awaad A, Nakamura M, Ishimura K. Imaging of size-dependent uptake and identification of novel pathways in mouse Peyer's patches using fluorescent organosilica particles[J]. Nanomed Nanotechnol Biol Med, 2012, 8: 627-636. DOI:10.1016/j.nano.2011.08.009 |
| [67] |
Rieux AD, Ragnarsson EGE, Gullberg E, et al. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium[J]. Eur J Pharmaceut Sci, 2005, 25: 455-465. DOI:10.1016/j.ejps.2005.04.015 |
| [68] |
McClements DJ. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems:a review[J]. Adv Colloid Interface Sci, 2018, 253: 1-22. DOI:10.1016/j.cis.2018.02.002 |
| [69] |
Kettler K, Veltman K, Dik VDM, et al. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type[J]. Environ Toxicol Chem, 2014, 33: 481-492. DOI:10.1002/etc.2470 |
| [70] |
Le P, Akiyoshi T. Epidermal growth factor enhances cellular uptake of polystyrene nanoparticles by clathrin-mediated endocytosis[J]. Int J Mol Sci, 2017, 18: 1301. DOI:10.3390/ijms18061301 |
| [71] |
Lim C, Moon J, Sim T, et al. Cyclic RGD-conjugated Pluronic® blending system for active, targeted drug delivery[J]. Int J Nano- med, 2018, 13: 4627-4639. DOI:10.2147/IJN.S171794 |
| [72] |
Shakweh M, Besnard M, Nicolas V, et al. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by Peyer's patches in mice[J]. Eur J Pharm Biopharm, 2005, 61: 1-13. DOI:10.1016/j.ejpb.2005.04.006 |
| [73] |
Xie Y, Shi B, Xia F, et al. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies[J]. J Control Release, 2017, 270: 65-75. |
| [74] |
Hussain N. Regulatory aspects in the pharmaceutical development of nanoparticle drug delivery systems designed to cross the intestinal epithelium and M-cells[J]. Int J Pharm, 2016, 514: 15-23. DOI:10.1016/j.ijpharm.2016.07.053 |
| [75] |
Liu Y, Jiang Z, Hou X, et al. Functional lipid polymeric nanoparticles for oral drug delivery:rapid mucus penetration and improved cell entry and cellular transport[J]. Nanomedicine, 2019, 21: 102075. DOI:10.1016/j.nano.2019.102075 |
| [76] |
Yaron I. Oral immune therapy:targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease[J]. Clin Transl Immunol, 2016, 5: e60. DOI:10.1038/cti.2015.47 |
| [77] |
Chassaing B, Etienne-Mesmin L, Bonnet R, et al. Bile salts induce long polar fimbriae expression favouring Crohn's disease-associated adherent-invasive Escherichia coli interaction with Peyer's patches[J]. Environ Microbiol, 2013, 15: 355-371. DOI:10.1111/j.1462-2920.2012.02824.x |
| [78] |
Tahoun A, Mahajan S, Paxton E, et al. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion[J]. Cell Host Microbe, 2012, 12: 645-656. DOI:10.1016/j.chom.2012.10.009 |
 2020, Vol. 55
2020, Vol. 55


