2. 湖州师范学院生命科学学院, 浙江 湖州 313000
2. School of Life Sciences, Huzhou University, Huzhou 313000, China
迷迭香, 拉丁学名Rosmarinus officinalis L, 系唇形科迷迭香属植物, 多年生常绿亚灌木, 气味芳香、浓郁, 原产于地中海沿岸, 近年来作为经济作物在我国也大范围栽培。迷迭香及其提取物被广泛应用于食品、化妆品和饲料产业[1]。研究发现, 迷迭香具有良好的抗癌、抗炎、抑菌及调节代谢等作用, 体外研究发现迷迭香对于乳腺癌细胞具有显著的抑制作用, 体内实验证实迷迭香具有良好的降血脂和降血糖作用, 并具有抗抑郁和神经保护等效果[2-7]。迷迭香提取物成分复杂, 含鼠尾草酸(carnosic acid, CA)、鼠尾草酚、迷迭香酚、熊果酸和迷迭香酸等, 其中CA推测是迷迭香抗衰老作用的重要组分之一[8, 9]。CA分子式为C20H25O4, 相对分子质量为332.43。作者前期研究发现CA具有延缓复制性细胞衰老的功效[10], 但是其延缓衰老的体内作用和具体机制尚不明确。衰老动物模型是研究人类衰老机制和研发抗衰老药物的重要手段。目前, 衰老模型涵盖了从果蝇、线虫等无脊椎动物到小型鱼类(如非洲青鳉鱼和斑马鱼)、啮齿动物和非人灵长类动物等, 且近年来各种基因修饰小鼠在研究衰老发生、发展及干预评价中亦起到重要作用。但是由于不同的模型都有其自身的局限性, 因此各模型都有不同程度的应用[11]。D-半乳糖(D-gal)诱导的啮齿类动物亚急性衰老模型, 在评价药物抗衰老活性及相关机制的研究中得到了广泛应用, 具有氧化应激水平和炎症水平增加、学习记忆障碍和免疫力降低等多种衰老特征, 机制上与D-gal诱导的非酶糖基化相关[12]。本实验利用过氧化氢诱导的早熟性人胚肺二倍体成纤维2BS细胞衰老模型和D-gal诱导的小鼠衰老模型, 进一步验证CA干预衰老的体内外效应, 并研究其相关机制, 为迷迭香抗衰老作用的进一步开发利用提供实验依据。
材料与方法主要试剂和药品 CA (纯度98%, 上海诗丹德生物技术有限公司); 脂质过氧化产物丙二醛(MDA)检测试剂盒、总超氧化物歧化酶(T-SOD)检测试剂盒(南京建成生物工程研究所); 衰老相关β-半乳糖苷酶(SA-β-Gal)染色试剂盒(碧云天生物技术研究所); Bradford法蛋白质定量试剂盒(北京普利莱基因技术公司); 鼠抗p53抗体、鼠抗p21抗体和鼠抗β-actin抗体(美国Santa Cruz Biotechnology公司); 兔单抗p16INK4a (Cell Signaling Technology公司); 小鼠晚期糖基化终末产物(AGEs)、白介素-6 (IL-6)和肿瘤坏死因子α (TNFα) ELISA检测试剂盒(上海酶联生物科技有限公司)。
实验动物 雄性C57 BL/6J小鼠2月龄, 合格证号: SCXK (沪) 2013-0016, 购于上海西普尔-必凯实验动物有限公司, 动物实验获得浙江医院伦理委员会批准。
细胞培养 2BS细胞由北京天坛生物制品有限公司建株, 广泛用于衰老相关的体外研究[13], 一般认为代龄在30PD (population doubling, 倍增次数, 亦称作“代”)以下为年轻细胞, 55PD以上为复制性衰老细胞。使用含有10%胎牛血清的DMEM培养基, 在95%相对饱和湿度、37 ℃、5% CO2通用培养条件下培养2BS细胞。当细胞生长到约80%汇合时, 将细胞进行1:2或1:4传代。
衰老相关SA-β-Gal染色 SA-β-Gal染色按照试剂盒说明书进行, 主要步骤: 32PD 2BS细胞按1×105/孔接种于6孔板中, 每组3个平行孔, 培养24 h待细胞完全贴壁后, 采用200 μmol·L-1 H2O2处理2 h, 之后更换新鲜培养基继续培养5天; 药物处理组提前2 h加入1和5 μmol·L-1 CA, 换液后继续加入同样浓度的药物。采用磷酸盐缓冲液(PBS)洗2遍后, 再用4 ℃预冷的2%甲醛/0.2%戊二醛固定10 min, 以PBS洗3遍, 细胞和新配制的SA-β-Gal染液于37 ℃ (无CO2)温育3~16 h, 于显微镜下(100×)拍照并计算阳性(蓝绿色)细胞所占百分比。
Western blot 接种5×105 32PD 2BS细胞至60 mm培养皿, 细胞完全贴壁后, 用200 μmol·L-1 H2O2处理, 药物处理组提前2 h加入不同浓度的CA (0.2、1、5 μmol·L-1), 换液后继续加入同样浓度的药物, 作用2天。采用2 mmol·L-1 N-乙酰半胱氨酸(NAC)作为抗氧化剂阳性对照, 加药时间同CA。收集细胞时, 弃掉培养基, 用冰冷的PBS清洗细胞2遍后, 加入RIPA裂解液(含蛋白酶抑制剂Cocktail), 用细胞刮刀收集细胞, 4 ℃、12 000 r·min-1离心15 min, 吸取上清, 采用Bradford蛋白定量试剂盒测定蛋白浓度, 并制作蛋白上样缓冲液。组织样本采用液氮研磨后加入RIPA裂解液进行裂解, 余下同细胞样品处理。每个样本取20 μg总蛋白, 常规方法电泳、转膜, 采用5%脱脂牛奶室温封闭1 h, 再应用抗p53、抗p21、抗p16和抗β-actin抗体4 ℃孵育过夜(p16抗体稀释比例1:500, 其余为1:1 000), HRP标记的二抗室温孵育1 h (抗体稀释比例1:5 000), 进而应用ECL化学发光试剂盒显色, ChemiDoc MP多功能成像系统拍照保存显色图片, Image J软件分析光密度值并计算相对蛋白表达水平。
D-半乳糖衰老动物模型构建及CA干预 将40只小鼠适应培养1周后, 随机分为4组, 即对照组、模型组、CA低剂量给药组(5 mg·kg-1·d-1)和高剂量给药组(10 mg·kg-1·d-1), 每组10只。小鼠连续8周皮下注射D-gal (100 mg·kg-1·d-1)构建小鼠衰老模型, 同时灌胃给予低、高剂量CA。测定小鼠认知功能变化并获取组织和血液样本, 进行相关指标的测定。
水迷宫实验 采用Morris水迷宫法对小鼠认知功能进行评估[14]。定位航行实验:将受试小鼠按顺时针方向依次由第1、2、3、4象限入水点顺序放入水中, 记录2 min内寻找平台的时问(逃避潜伏期)。空间探索实验:定位航行实验全部结束后, 次日进行空间探索实验。撤去平台, 然后选第1象限相同的入水点将小鼠面向池壁放入水中, 测其2 min内跨越原平台位置(目标区域)的次数, 以判断小鼠记忆储存及提取再现能力。如此连续训练4天, 取第5天测试数据用于评价各组小鼠的学习记忆能力。
MDA和T-SOD测定 脂质过氧化产物MDA和T-SOD的测定按照试剂盒说明书进行。
其他因子测定 AGEs、IL-6和TNFα采用对应的ELISA试剂盒进行测定。
数据处理 数据以x±s表示, 多组间样本间比较用SPSS 17.0统计软件包进行单因素方差分析, P < 0.05表示差异有统计学意义。
结果 1 CA对H2O2诱导的2BS细胞SA-β-Gal活性的影响2BS细胞(32PD)用亚致死量(200 μmol·L-1) H2O2处理2 h后, 继续培养5天, 其SA-β-Gal染色阳性率高达95%左右, 继续传代培养后细胞几乎不增殖, 呈现显著的细胞衰老表型, 同时发现32PD的2BS细胞SA-β-Gal染色阳性率不足5%, 说明其仍可能是年轻细胞; H2O2处理前, 加入1和5 μmol·L-1CA, 处理细胞至5天, 均能显著降低SA-β-Gal染色阳性率(P < 0.01), 且两种浓度CA的效果相近。结果表明(图 1), CA能显著改善亚致死量H2O2诱导的早熟型细胞衰老。
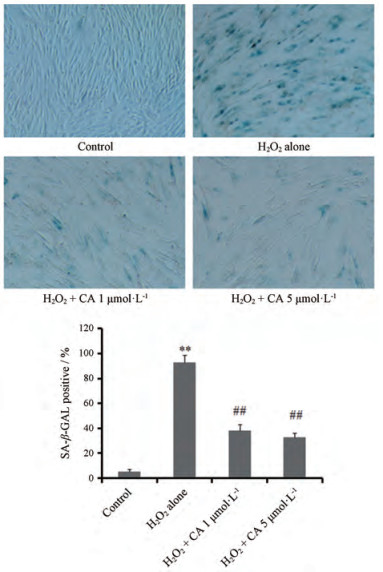
|
Figure 1 Effect of carnosic acid (CA) on H2O2 (200 μmol·L-1) induced senescence-related β-galactosidase (SA-β-Gal) activity in 32 PD (population doubling) 2BS fibroblasts. 2BS cells were pre-treated by CA at indicated concentrations 2 h before exposure to H2O2 challenge (200 μmol·L-1 for 2 h) and with its presence for SA-β-Gal staining test at 5th day (100×). n = 3, ± s. **P < 0.01 vs control group; ##P < 0.01 vs H2O2 alone group |
通过Western blot检测发现, H2O2处理的32 PD 2BS细胞中衰老相关蛋白p53、p21和p16表达水平显著高于对照组; 给予0.2、1、5 μmol·L-1 CA处理后, 这些分子的蛋白表达均有一定程度的下降, 其效果与抗氧化阳性药物2 mmol·L-1 NAC接近。图 2结果表明, CA可在细胞水平改善衰老相关分子p53、p21和p16的表达。

|
Figure 2 Inhibitive role of CA on H2O2 (200 μmol·L-1) induced increment of protein expression of senescence associated molecules p53, p21 and p16 in 32 PD 2BS cells. N-acetyl-L-cysteine (NAC) was used as a positive control for antioxidant. 2BS cells were pre-treated by CA or NAC at indicated concentrations 2 h before exposure to H2O2 challenge (200 μmol·L-1 for 2 h) and with its presence until cells were harvested at 2 days after H2O2 challenge. Relative protein expression levels were analyzed according to the optical density for each ladder which was calculated by the Image J software. n = 10, x± s. **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs H2O2 group |
给药前, 各组小鼠间的体重没有明显差异(P > 0.05);实验过程中未见小鼠死亡, 给药结束后各组小鼠体重均有增长, 但组间没有统计学差异(P > 0.05)。通过检测小鼠体内AGEs含量发现, 模型组(D-gal)小鼠的血清AGEs和脑AGEs均有显著性上升, 而不同剂量的CA均有效下调模型组的AGEs水平, 5和10 mg·kg-1·d-1剂量之间无显著差别(表 1), 说明CA对D-gal诱导的衰老小鼠的保护作用与其抑制非酶糖基化作用有关。
| Table 1 Effect of CA on the body weight and AGEs levels in D-galactose (D-gal, s.c.)-induced aging model mice. D-gal mice were treated with saline or CA (ig) at doses of 5 and 10 mg·kg-1·d-1once a day for 8 weeks. The body weight of each mouse was observed before and after experiment. At the end of experiment, serum and brain tissues were collected for the detection of AGEs levels. AGEs: Advanced glycation end products. n = 10, x± s. *P < 0.05, **P < 0.01 vs group Ⅰ; #P < 0.05 vs group Ⅱ |
Morris水迷宫实验结果见图 3, 与对照组小鼠相比, 模型组平均潜伏期显著延长(P < 0.05), 2 min内穿过目标区域次数明显减少, 表明模型组小鼠空间搜索和辨别学习能力明显减弱, 而5和10 mg·kg-1·d-1CA给药能部分改善这一状况, 其平均潜伏期较模型组显著缩短(P < 0.05), 穿过目标区域次数也明显增加, 表明CA对D-gal诱导的衰老小鼠的学习记忆有较好的改善作用。

|
Figure 3 Effects of CA on the spatial memory in Morris water maze of the D-galactose (D-gal)-induced aging model mice. D-gal mice were treated with CA (ig) at doses of 5 and 10 mg·kg-1·d-1 for 8 weeks. To all mice, the latency and numbers of crossing target area within 2 minutes were measured at eighth week before mice sacrificed. n = 10, x± s. *P < 0.05 vs control group; #P < 0.05 vs D-gal group |
采用小鼠血清和脑组织中MDA水平和总SOD活力反映氧化应激水平。与正常组比较, 衰老模型小鼠MDA含量增加, 而CA可显著抑制MDA含量的增加; 与对照组比较, 衰老模型小鼠SOD活力有所下降, CA作用后则表现出升高趋势。图 4结果表明, CA对衰老模型小鼠的保护作用与其抑制小鼠体内的脂质过氧化水平有关。

|
Figure 4 Effects of CA on T-SOD activity and MDA levels in mouse serum and cerebral samples. D-gal mice were treated with saline or CA (ig) at doses of 5 and 10 mg·kg-1·d-1 for 8 weeks. At the end of experiment, serum and brain tissues were collected for the indicated detections. T-SOD: Total superoxide dismutase; MDA: Malondialdehyde. n = 10, x± s. *P < 0.05, **P < 0.01 vs control group; ##P < 0.01 vs D-gal group |
与正常组比较, 衰老模型小鼠血清和脑组织中的IL-6和TNFα含量显著升高, 表明D-gal促进了小鼠的炎症水平。CA具有一定的抗炎作用, 表现为不同剂量的CA均能显著降低血清中的IL-6含量(P < 0.01)。在脑组织中, 高剂量(10 mg·kg-1·d-1) CA下调脑组织IL-6水平具有统计学意义(P < 0.05);与模型组比较, 各给药组对血清TNFα下调作用未见统计学差异, 而对脑组织中的TNFα增高具有明显的抑制作用(P < 0.05)。以上结果表明(表 2), CA对D-gal诱导的衰老小鼠的保护作用与其抑制衰老小鼠中的炎症水平有关。
| 表 2 Effects of CA on inflammatory cytokines in D-gal treated mice. D-gal mice were treated with saline or CA (ig) at doses of 5 and 10 mg·kg-1·d-1 for 8 weeks. At the end of experiment, serum and brain tissues were collected for the detection of IL-6 and TNFα levels. IL-6: Interleukin 6; TNFα: Tumor necrosis factor α. n = 10, x± s. *P < 0.05, **P < 0.01 vs group Ⅰ; #P < 0.05, ##P < 0.01 vs group Ⅱ |
采用Western blot检测小鼠海马中衰老相关分子p53、p21和p16的蛋白表达, 结果如图 5所示, 与对照组相比, D-gal组小鼠海马的p53、p21和p16的蛋白表达水平显著增加, 分别上调了约4.4、3.8和3.6倍, 而同时给予5和10 mg·kg-1·d-1CA后, 这些分子的蛋白表达被显著逆转, CA下调这些分子的作用在两个剂量之间无显著差别。

|
Figure 5 Effects CA on protein expression of p53, p21 and p16 in mouse hippocampus samples. D-gal mice were treated with saline or CA (ig) at doses of 5 and 10 mg·kg-1·d-1 for 8 weeks. At the end of experiment, mouse hippocampus tissues were collected, homogenized with liquid nitrogen and prepared for the Western blot analysis. Representative images were acquired from three repeated experiments. Relative protein expression levels were analyzed according to the optical density for each ladder which was calculated by the Image J software. n = 10, x± s. **P < 0.01 vs control group; ##P < 0.01 vs D-gal group |
已有研究表明, 迷迭香提取物具有多种药理活性, 如抗肿瘤、抗菌、抗氧化、消炎、抗抑郁症等作用, 但其成分复杂, 包括酚类和挥发油类化合物, 如CA、鼠尾草酚、迷迭香酸和熊果酸等, 精油中α-蒎烯、樟脑、桉叶素、莰烯和龙脑等, CA作为其一种重要的单体成分, 文献[7]报道具有良好的抗氧化和抗炎活性, 尽管推测其具有良好的延缓衰老的效应, 但缺乏具体的实验证据。本研究在前期证明CA具有良好的延缓2BS细胞复制性衰老的基础上, 进一步证实了CA具有改善氧化应激诱导的早熟型细胞衰老, 利用D-gal小鼠加速衰老模型证实了CA具有一定的体内延缓衰老的效果, 表现为改善小鼠的学习记忆能力, 抑制血清及脑组织中的脂质过氧化及炎症因子水平, 并通过抑制非酶糖基化减少AGEs的生成。在分子水平上, 证实CA可显著抑制体外早熟型衰老模型及D-gal小鼠海马中衰老相关分子p53、p21和p16的蛋白表达。
D-gal小鼠作为一种衰老加速模型, 其机制之一是通过D-gal促进非酶糖基化, 促进AGEs的生成, 进而上调机体的氧化应激和炎症水平, 在一定程度上模拟生理性衰老[15]。炎性衰老是一种衰老进程中机体内存在的增龄性慢性低度促炎性反应状态[16], 它被视为机体衰老进程速率和寿命的一个决定因素。同时, 促炎因子是衰老相关分泌表型(senescence-associated secretory phenotype, SASP)的一个重要组成部分, 是衰老细胞的关键特征。衰老细胞通过大量分泌SASP, 在诱发机体炎症的同时, 加速机体衰老, 导致多种衰老相关疾病的产生, 是目前衰老研究的热点之一[17]。作者研究发现, CA显著下调模型小鼠的外周血和大脑皮层组织中脂质过氧化和炎症因子水平, CA可能是活性氧自由基的直接清除剂, 进而通过抑制活性氧自由基介导的p38/NF-κB信号通路抑制炎症因子的生成[18]。此外, AGEs在脑组织的蓄积与脑衰老及衰老相关神经退行性疾病如阿尔茨海默病、帕金森氏病具有重要的联系, D-Gal诱导小鼠衰老模型同时作为一种脑老化模型也日益被认可[19]。作者研究发现, CA在降低血清AGEs水平的同时, 有效减少模型小鼠大脑皮层组织的AGEs生成, 这可能与其透过血脑屏障直接抑制非酶糖基化的作用相关, 也可能是通过抗氧化而间接减少AGEs的生成, 因为AGEs可诱导细胞内氧应激水平增加, 而活性氧自由基的增加反过来也可以促进非酶糖基化[20]。减少AGEs的生成和降低氧化应激水平可以直接保护神经元, 这可能是CA发挥改善学习记忆能力的可能作用机制。
总之, 本实验建立的人胚肺二倍体成纤维细胞早熟型细胞衰老模型和D-gal诱导的小鼠加速衰老模型, 为CA延缓衰老的作用提供了更为系统的实验证据, 并进一步丰富了该化合物延缓衰老的作用机制, 为CA在衰老相关领域的应用开发提供了理论依据。
| [1] |
Moran L, Giraldez FJ, Panseri S, et al. Effect of dietary carnosic acid on the fatty acid profile and flavour stability of meat from fattening lambs[J]. Food Chem, 2013, 138: 2407-2414. DOI:10.1016/j.foodchem.2012.12.033 |
| [2] |
Moore J, Yousef M, Tsiani E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols[J]. Nutrients, 2016, 8: pii:E731. DOI:10.3390/nu8110731 |
| [3] |
Naimi M, Vlavcheski F, Shamshoum H, et al. Rosemary extract as a potential anti-hyperglycemic agent:current evidence and future perspectives[J]. Nutrients, 2017, 9: pii:E968. DOI:10.3390/nu9090968 |
| [4] |
Kontogianni VG, Tomic G, Nikolic I, et al. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity[J]. Food Chem, 2013, 136: 120-129. DOI:10.1016/j.foodchem.2012.07.091 |
| [5] |
Tsai TH, Chuang LT, Lien TJ, et al. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses[J]. J Med Food, 2013, 16: 324-333. DOI:10.1089/jmf.2012.2577 |
| [6] |
Zhao YT, Sedighi R, Sang SM. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice[J]. J Agric Food Chem, 2015, 63: 4843-4852. DOI:10.1021/acs.jafc.5b01246 |
| [7] |
Andrade JM, Faustino C, Garcia C, et al. Rosmarinus officinalis L.:an update review of its phytochemistry and biological activity[J]. Future Sci OA, 2018, 4: FSO283. DOI:10.4155/fsoa-2017-0124 |
| [8] |
Shen CY, Jiang JG, Zhu W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine:pharmacological mechanisms and implications for drug discovery[J]. Br J Pharmacol, 2017, 174: 1395-1425. DOI:10.1111/bph.13631 |
| [9] |
Loussouarn M, Krieger-Liszkay A, Havaux M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms[J]. Plant Physiol, 2017, 175: 1381-1394. DOI:10.1104/pp.17.01183 |
| [10] |
Tap PH, Wen XL, Wang YZ, et al. The experimental study on effect of carnosic acid on delaying the aging process of human diploid 2BS fibroblasts[J]. Acta Nutr Sin (营养学报), 2014, 36: 273-277. |
| [11] |
Folch J, Busquets O, Ettcheto M, et al. Experimental models for aging and their potential for novel drug discovery[J]. Curr Neuropharmacol, 2018, 16: 1466-1483. DOI:10.2174/1570159X15666170707155345 |
| [12] |
Zhao FF, Zhou YZ, Gao L, et al. Advances in the study of the rat model of aging induced by D-galactose[J]. Acta Pharm Sin (药学学报), 2017, 52: 347-354. |
| [13] |
Mao GX, Wang Y, Qiu Q, et al. Salidroside protects human fibroblast cells from premature senescence induced by H2O2 partly through modulating oxidative status[J]. Mech Ageing Dev, 2010, 131: 723-731. DOI:10.1016/j.mad.2010.10.003 |
| [14] |
Wang WG, Zhou JB, Zhu ML. Morris water maze in the analysis of mouse phenotype[J]. Chin J Cell Biol (中国细胞生物学学报), 2011, 33: 8-14. |
| [15] |
Azman KF, Zakaria R. D-Galactose-induced accelerated aging model:an overview[J]. Biogerontology, 2019, 20: 763-782. DOI:10.1007/s10522-019-09837-y |
| [16] |
De Martinis M, Franceschi C, Monti D, et al. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity[J]. FEBS Lett, 2005, 579: 2035-2039. DOI:10.1016/j.febslet.2005.02.055 |
| [17] |
He S, Sharpless NE. Senescence in health and disease[J]. Cell, 2017, 169: 1000-1011. DOI:10.1016/j.cell.2017.05.015 |
| [18] |
Xia G, Wang X, Sun H, et al. Carnosic acid (CA) attenuates collagen-induced arthritis in db/db mice via inflammation suppression by regulating ROS-dependent p38 pathway[J]. Free Radic Biol Med, 2017, 108: 418-432. DOI:10.1016/j.freeradbiomed.2017.03.023 |
| [19] |
Shwe T, Pratchayasakul W, Chattipakorn N, et al. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions[J]. Exp Gerontol, 2018, 101: 13-36. DOI:10.1016/j.exger.2017.10.029 |
| [20] |
Bo-Htay C, Palee S, Apaijai N, et al. Effects of D-galactose-induced ageing on the heart and its potential interventions[J]. J Cell Mol Med, 2018, 22: 1392-1410. DOI:10.1111/jcmm.13472 |
 2020, Vol. 55
2020, Vol. 55


