肺泡毛细血管内皮屏障是肺泡与血管进行气体、营养物质以及代谢废物等交换的基础, 也是肺脏对吸入和经血液传播病原体的强有力的免疫防御防线。生理条件下, 由于毛细血管内皮以及肺泡上皮连接的紧密性, 液体和大蛋白等难以进入肺泡[1], 而在病理状态下, 体液和血液中的大蛋白容易通过受损的肺泡血管内皮和上皮屏障进入间质组织从而进入肺泡, 导致严重的肺泡水肿[2, 3], 并诱发急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)[4, 5]和急性肺损伤(acute lung injury, ALI)[6]。大量研究表明鞘氨醇-1-磷酸(sphingosine 1-phosphate, S1P)对肺泡毛细血管内皮屏障具有重要的调节作用。气道给予低浓度S1P可以介导肺泡毛细血管内皮屏障的保护作用, 降低脂多糖(lipopolysaccharide, LPS)诱导的肺水肿[7]; 激活肺部S1P的受体—鞘氨醇-1-磷酸-1型受体(sphingosine 1-phosphate receptor 1, S1P1), 亦可减轻小鼠的血管渗漏[8]。因而, 通过深入了解S1P对肺泡毛细血管内皮屏障的调节作用或为与肺泡毛细血管内皮屏障功能相关的肺部疾病治疗提供新策略、开辟新途径。
1 肺泡毛细血管内皮屏障肺泡毛细血管内皮屏障主要由肺泡上皮细胞、毛细血管内皮细胞、基底膜及细胞层之间的基质组成[9], 以促进气体以及营养物质的扩散并且防止肺泡和间质水肿的形成。在正常肺组织中, 液体和蛋白主要通过毛细血管内皮细胞之间的小间隙从血管内进入间质并通过淋巴管返流到全身循环。而在肺泡毛细血管内皮细胞间连接被破坏情况下, 毛细血管内的大蛋白和体液等容易渗出, 进而致使肺间质以及肺泡内液体积聚, 诱导相关疾病的恶化[6]。因此, 肺泡毛细血管内皮屏障稳态功能的维持具有重要的病理生理意义。
1.1 肺泡毛细血管内皮细胞肺泡毛细血管内皮细胞间连接的稳态是维持正常肺泡内稳态和损伤后肺修复的关键因素[10]。LPS等肺损伤刺激剂可介导血管内皮细胞的损伤[11]。肺泡中含有丰富的肺微血管内皮细胞, 介导分子从血管到肺间质的转运。由于毛细血管较其他血管孔径更小, 因而对流经血管内皮细胞的液体和蛋白质流通的限制更大[12]。血管内皮间连接的破坏会导致血管通透性增加, 炎症因子与炎症细胞浸润, 导致或加重相关肺部疾病, 如急性呼吸窘迫综合征和急性肺损伤等。
目前研究表明, 介导血管内皮细胞间连接主要包括黏附连接和紧密连接, 通过这2种连接, 不同细胞间的跨膜黏附蛋白通过相互作用连接从而沿细胞边界形成拉链状结构, 将不同细胞连接起来[13]。
黏附连接是血管内皮细胞间连接最常见的形式。这是一类依赖细胞肌动蛋白纤维将相邻细胞连接起来的细胞连接类型, 介导细胞间牢固黏合以抵抗机械张力。在黏附连接处, 内皮细胞黏附主要由血管内皮跨膜钙黏蛋白(vascular endothelial cadherin, VE-cadherin)介导。VE-cadherin是黏附连接的重要结构成分, 在损伤后维持血管完整性和肺修复中起着重要作用[14]。Carmeliet等[15]研究发现, VE-cadherin基因敲除不会影响动物血管丛中内皮细胞的组装, 但会影响其随后的重塑与成熟, 并在小鼠妊娠9.5天引起胚胎致死。此外, VE-cadherin的缺失还可直接诱导内皮细胞凋亡[15]。在人真皮微血管细胞系(human dermal microvascular endothelial cell line, HMEC-1)中敲除VE-cadherin胞外结构域的钙黏蛋白突变体会导致细胞屏障功能破坏[16]。VE-cadherin一方面能够与多种细胞质锚定蛋白(catenins), 如β-连环蛋白、γ-连环蛋白和p120连环蛋白等形成蛋白复合物, 以连接细胞内的骨架成分, 如纤维形肌动蛋白F-actin[17]; 另一方面, 亦可与另一个细胞表面的VE-cadherin胞外部分结合从而形成平行二聚体, 介导细胞间的黏附连接[18], 对抗机械力与刺激因子导致的细胞收缩, 保护血管内皮间连接的稳态。
紧密连接又称封闭连接, 广泛分布于各种上皮细胞与毛细血管内皮细胞之中。在紧密连接处, 相邻细胞膜之间的紧密连接点由特殊的跨膜蛋白排列形成蛋白质颗粒条索, 将相邻细胞间的缝隙封闭起来。目前已经发现数十种跨膜蛋白参与细胞间的紧密连接。封闭蛋白(claudin)和密闭蛋白(zonula occluden, ZO)是研究相对较多的2种跨膜蛋白[19], 它们在细胞膜上的定位并不相同。Claudin属于4次跨膜的膜整合蛋白, 而ZO则完全位于细胞膜内发挥作用。通过细胞间紧密连接, 细胞间缝隙被封闭, 从而有效防止细胞外物质无选择性地通过细胞间隙进入血管和组织[20]。
1.2 肺泡上皮细胞肺泡上皮细胞主要有2种类型。一型肺泡上皮细胞是其主要组成细胞, 覆盖95%的肺泡表面, 被认为是维持屏障稳态功能的关键细胞; 二型肺泡上皮细胞, 覆盖约3%~5%的肺泡表面, 在分泌表面活性剂维持肺张力以及免疫反应中发挥重要作用[21]。此外, 在一定条件如肺损伤时, 二型肺泡上皮细胞可以向一型肺泡上皮细胞转化, 从而保护肺泡上皮细胞屏障的完整性[22, 23]。肺泡上皮屏障在维持肺功能方面发挥重要作用。研究表明, 肺泡上皮细胞凋亡介导的肺泡上皮损伤是促进ARDS患者渗透性水肿加重以及随后呼吸困难的重要因素。此外, 肺泡上皮损伤后会介导肺泡上皮细胞向间质细胞转化(epithelial-mesenchymal transition, EMT), 这在疾病初期是一个代偿性的肺组织保护机制, 介导气道重塑, 但持续的肺泡上皮细胞损伤和EMT将进一步加重肺泡上皮屏障渗漏以及肺损伤[24]。除了肺泡上皮细胞损伤以及EMT导致肺泡上皮屏障的破坏外, 肺泡上皮细胞间调控细胞连接的相关蛋白(如ZO)在维持肺泡上皮屏障稳态中也发挥重要作用。肺泡上皮的细胞–细胞间连接主要是以紧密连接的形式存在[20], 该紧密连接的构成与血管内皮细胞相同。
研究表明, 除了单一的内皮或者上皮细胞间连接的稳态对整体屏障的保护作用外, 肺泡毛细血管内皮屏障多组分间的相互作用亦可调控肺泡毛细血管内皮屏障的稳态。目前研究认为肺泡上皮与血管内皮细胞存在相互作用。在生理和炎症条件下肺泡上皮细胞通过分泌前列腺素E2 (prostaglandin E2, PGE2)促进血管内皮细胞间连接的完整性[25], 而肺血管内皮细胞系(HPMEC-ST1.6R)分泌的可溶性因子也可维持肺泡上皮细胞间连接的完整性[26]。由此提示, 维持肺泡毛细血管内皮屏障稳态除了维持单一的肺泡上皮或者血管内皮细胞间连接外, 调控屏障相互作用的因子或能从整体水平调控肺泡毛细血管内皮屏障稳态功能。
2 S1PS1P最早由德国生物学家Johan Thudi-chum在19世纪末发现, 是由血浆中的鞘氨醇激酶-1 (sphingosine kinase-1, SPHK1)和鞘氨醇激酶-2 (sphingosine kinase-2, SPHK2)催化产生的一种生物活性脂类物质[27]。鞘磷脂首先被鞘磷脂酶水解为神经酰胺, 后经神经酰胺酶水解为鞘氨醇, 并激活鞘氨醇激酶, 形成S1P[28], 再经S1P磷酸酶, S1P裂解酶或溶血磷脂磷酸酶降解(图 1)。多种细胞, 包括红细胞、血小板[29]、巨噬细胞、肥大细胞及内皮细胞[30]等都与S1P的合成和分泌相关。S1P在血液中主要与高密度脂蛋白结合[31], 这有助于S1P被运输到相应的S1P受体(sphingosine 1-phosphate receptors, S1PRs)。在多种S1P合成与降解酶协调作用下, S1P在血液、淋巴液以及组织间存在独特的浓度梯度。血浆中S1P含量高达0.4~1.5 μmol·L-1, 比淋巴液中含量高25倍左右, 较组织间液中含量高约1 000倍, 这一浓度梯度与S1P的多项生理功能如调控淋巴细胞运输以及调节血管内皮屏障功能等均密切相关[32, 33]。
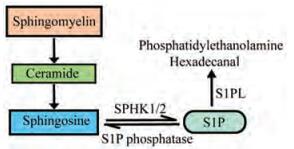
|
Figure 1 Synthesis and metabolism of sphingosine 1-phosphate (S1P). Sphingomyelin is first hydrolyzed to ceramide by sphingomyelinase, then hydrolyzed to sphingosine by ceramidase. As substrate of two kinds of sphingosine kinase (SPHK1/2), sphingosine is then phosphorylated to S1P. S1P can be degraded in two ways mainly. On the one hand, S1P can be reversibly degraded to sphingosine by S1P phosphatase, on the other hand, it can be irreversibly degraded to phosphatidylethanolamine and hexadecanal by S1P lyase (S1PL) |
S1P作为一种多效信号脂质分子, 既可作为细胞内第二信使参与细胞内信号转导, 又可由三磷酸腺苷结合盒转运体(ATP-binding cassette transporters, ABC)转运到细胞外, 结合并激活细胞膜表面的5种G蛋白偶联S1PRs (S1P receptors 1- S1P receptors 5, S1P1-S1P5)[34]。S1P在低浓度(nmol·L-1水平)即可以与此5种受体结合。其中S1P1早在1990年在人脐静脉血管内皮细胞中被分离出来, 是最早发现也是研究最多的S1P受体亚型, 在淋巴细胞运输以及屏障保护[35]方面发挥重要作用[36]。S1PRs在不同的组织和细胞类型中分布不同, 并且介导的生物学效应也不尽相同。S1P1、S1P2[37]和S1P3[38]普遍表达于体内的各种组织, 在胚胎发育阶段, S1P1在血管系统中高度表达, 对脉管系统的正常发育尤为重要; 而在成人组织中, S1P1表达于多种组织器官, 包括脑、肺、肝、心和脾[39], 并且是淋巴细胞表面的重要受体, 介导淋巴细胞的归巢。S1P1主要通过结合Gi和G12/13发挥多种生物学效应, 如血管屏障保护作用[40, 41]。S1P3主要分布于心脏成纤维细胞及心肌细胞, S1P2和S1P3主要与Gi、G12/13及Gq结合[42]。S1P4[43]主要表达于淋巴以及造血组织等; 目前S1P5[44]研究较少, 主要表达于中枢神经系统相关的通路。S1P4和S1P5主要结合Gi和G12/13。
3 S1P对肺泡毛细血管内皮屏障的调节S1P对肺泡毛细血管内皮屏障具有重要的调节作用。研究表明, 1 μmol·L-1 S1P静脉注射即可迅速降低离体灌流导致的小鼠肺重增加, 在LPS诱导的急性肺损伤小鼠模型中, S1P可显著减少肺血管渗漏和炎症反应[45]。目前关于S1P调控血管内皮屏障的作用机制研究较为深入。多种信号分子, 如神经酰胺、S1P、血管生成素以及前列腺素等均与调控血管内皮屏障密切相关[46], 其中S1P发挥重要的调控作用。Sphk1基因敲除的小鼠在肺损伤后血管渗漏加重[47, 48], 完全敲除Sphk1/2在没有组织损伤的情况下即可诱导多组织血管渗漏[49]。VE-cadherin对内皮细胞单层完整性的建立和维持具有重要作用。研究表明, S1P在nmol·L-1水平可刺激VE-cadherin和β-连环蛋白在粘附连接部位的定位, 从而促进人肺动脉内皮细胞的屏障稳定。S1P信号通路除了促进黏附连接组装, 也促进细胞紧密连接的形成。S1P通过S1P1/Gi/Akt/Rac通路, 促进密闭蛋白1 (zonula occluden 1, ZO-1)的再分布。应用siRNA技术下调ZO-1表达后, S1P的屏障保护作用也会随之减弱[50]。
大量研究表明, S1P的血管内皮屏障保护作用主要是通过S1P1介导。S1P可通过与血管内皮细胞的S1P1结合, 进而激活Gi[40, 41], 介导Rac-GTPase依赖的细胞骨架重排和黏附连接的形成[51, 52], 增强内皮细胞之间的连接, 显著降低血管通透性[51, 52]。S1P亦可通过激活肺部S1P1减轻过敏原引起的小鼠肺渗漏[8], 而干扰S1P–S1P1轴可以促进血管渗漏[53]。敲除S1p1可以破坏内皮细胞间的黏附连接, 降低VE-cadherin和血小板内皮细胞黏附分子-1 (platelet/endothelial celladhesion molecule-1, PECAM-1)的含量[14, 54]。此外, S1p1基因敲除小鼠会由于血管生成受损而表现出胚胎致死性, 这与S1p1基因在血管发育中的作用密切相关[55]。血管(而非气道)内给予体内活性手性的S1P1拮抗剂会导致小鼠皮肤和肺毛细血管完整性丧失[53]。叉头相关转录因子1 (transcription factor forkhead box F1, FOXF1)是胚胎肺发育的关键转录调节因子, 研究表明, FOXF1可以通过调节S1P1转录增强肺泡血管内皮细胞屏障功能, 逆转肺损伤。Foxf1基因敲除能够导致小鼠血管内皮屏障破坏, 而给予S1P可恢复内皮屏障功能[56]。
血管内皮细胞表面不仅大量表达S1P1, 同时也表达少量S1P3[57], 这对于S1P对血管内皮细胞的精密调控至关重要。简要来说, S1P通过结合于S1P1受体, 可以启动Gi信号级联介导的Rac活化, 促进内皮细胞周边肌动蛋白环的形成, 加强细胞间连接和细胞与基底膜的局部连接, 显著降低血管的通透性[58, 59]。但是S1P通过作用于低表达的S1P3则会激活G12/13, 进而激活Rho通路介导应力纤维的产生[57], 促进内皮细胞的收缩从而降低内皮细胞的黏附连接, 增加血管的通透性[10, 60, 61]。因而在体内, 生理性配体S1P通过调节S1P1/S1P3信号通路的平衡维持血管内皮屏障的稳态。此外, 不同浓度的S1P可能产生截然相反的调控作用。在体外培养的人脐静脉内皮细胞(human umbilical vein endothelial cells, HUVEC)中, 较低浓度S1P (0.5或1 μmol·L-1)可显著增加VE-cadherin在细胞-细胞连接区域的分布, 促进黏附连接的形成; 然而高浓度S1P (10 μmol·L-1)则会破坏VE-cadherin的组装, 这可能与高浓度S1P介导S1P3激活, 从而使S1P1/S1P3平衡向S1P3偏移相关。
目前有关S1P与肺泡上皮细胞屏障的报道相对较少。研究表明, 血管内皮细胞高表达S1P1而肺泡上皮细胞则高表达S1P3。激活血管内皮细胞S1P1介导血管内皮屏障保护作用, 而激活肺泡上皮细胞S1P3则介导肺泡上皮屏障破坏作用[62] (图 2)。在肺组织中, 血管外间隙中S1P水平的升高可能会扰乱上皮完整性。Sammani等[7]发现通过气道给予低剂量S1P (< 0.3 mg·kg-1)会介导肺泡毛细血管内皮屏障保护作用, 降低LPS诱导的肺水肿, 较高剂量(0.5 mg·kg-1) S1P作用2 h则会导致屏障破坏, 而高剂量(> 2 mg·kg-1)气管滴注S1P可诱导小鼠死亡[7]。同时该团队还发现下调S1P3表达可以保护LPS诱导的屏障破坏。这提示S1P不同浓度下对屏障调节作用相反可能与S1P在高剂量激活血管内皮S1P3相关, 或者与气管滴注更易激活肺泡上皮细胞S1P3, 从而通过上皮细胞急性紧密连接开放诱导肺水肿相关[62]。Lin等[63]的研究亦显示, 人肺泡上皮细胞(human pulmonary alveolar epithelial cells, HPAEpiCs)暴露于高浓度S1P (10 μmol·L-1)后, 可诱导细胞间黏附分子-1 (intercellular adhesion molecule-1, ICAM-1)的表达, 促进肺损伤。
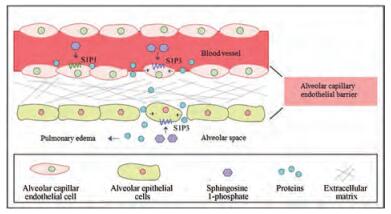
|
Figure 2 Effects of S1P on alveolar capillary endothelial barrier through activation of S1P1/S1P3. Activation of S1P1 on the alveolar capillary endothelial cells by S1P mediates barrier protection, while the barrier can be broken when S1P3 is stimulated by S1P. The large proteins and body fluids in the blood vessels thus enter into the lung interstitium. S1P can also regulate alveolar epithelial barrier. By activating S1P3 on the alveolar epithelial cells, S1P leads to epithelial barrier damage, which makes interstitial proteins and body fluids infiltrate into alveolar space and causes pulmonary edema |
这些研究表明S1P通过作用于不同受体和不同细胞, 起到不同的肺泡毛细血管屏障调节作用。因而, 开发高选择性的S1P1激动剂可能成为治疗多种肺部疾病的有效途径。
4 S1P1激动剂FTY720与血管内皮屏障FTY720 (fingolimod, GilenyaTM)是日本科学家在纯化改造冬虫夏草类真菌后得到的S1P结构类似物, 对S1P1/3/4/5均有较高的亲和性和激动活性[64, 65]。FTY720于2010年被FDA批准上市, 通过诱导淋巴细胞归巢[66]用于治疗复发缓解型多发性硬化[67, 68]。但在一项1 292例复发缓解型多发性硬化患者参与的12个月双盲临床试验中, 研究者发现FTY720会导致黄斑水肿[69]。经过深入研究发现, 与S1P相似, FTY720对血管屏障的调节与作用时间和浓度密切相关。一方面, FTY720长时间作用可加重肺损伤导致的血管渗漏[40], 并且加重博莱霉素诱导的小鼠肺纤维化和肺血管渗漏[70, 71]。另一方面, 0.1 mg·kg-1 FTY720可以显著降低脂多糖诱导的肺水肿和炎性肺损伤[45]。长时间大剂量给予FTY720导致的血管屏障破坏作用一方面可能与激活S1P3相关, 另一方面可能与诱导S1P1内陷后的泛素化降解相关[72]。基于此, 如何寻找高选择性的S1P1激动剂, 以获得更好的药效和更低的副作用成为药物研发的热点之一[73]。
5 小结与展望肺泡毛细血管内皮屏障的破坏在多种肺部疾病的发生发展中起着重要的促进作用, 如引起ALI以及ARDS的肺渗漏。该屏障的破坏还会导致液体在肺间质及肺泡中积聚, 并促进炎症反应以及进一步的肺组织坏死和纤维化。目前S1P对肺泡毛细血管内皮屏障的稳态调节作用已受到广泛关注, 但由于其复杂性, 具体的调节机制仍未完全阐明。不同浓度的S1P可能通过作用于不同的S1P受体亚型介导截然相反的生物学功能。基于S1P可以通过作用于肺泡毛细血管内皮细胞以及肺泡上皮细胞的S1P1从而发挥屏障保护作用, S1P1有望成为肺泡毛细血管内皮屏障稳态调节异常相关肺部疾病的潜在靶点, 高选择性的S1P1激动剂有望用于这些疾病的潜在治疗。
| [1] |
Murray JF. Pulmonary edema:pathophysiology and diagnosis[J]. Int J Tuberc Lung Dis, 2011, 15: 155-160. |
| [2] |
Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema[J]. N Engl J Med, 2005, 353: 2788-2796. DOI:10.1056/NEJMcp052699 |
| [3] |
Fein A, Grossman RF, Jones JG, et al. The value of edema fluid protein measurement in patients with pulmonary edema[J]. Am J Med, 1979, 67: 32-38. DOI:10.1016/0002-9343(79)90066-4 |
| [4] |
Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome[J]. N Engl J Med, 2017, 377: 562-572. DOI:10.1056/NEJMra1608077 |
| [5] |
Ince C, Mayeux PR, Nguyen T, et al. The endothelium in sepsis[J]. Shock, 2016, 45: 259-270. DOI:10.1097/SHK.0000000000000473 |
| [6] |
McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2012, 303: L364-381. DOI:10.1152/ajplung.00354.2011 |
| [7] |
Sammani S, Moreno-Vinasco L, Mirzapoiazova T, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung[J]. Am J Respir Cell Mol Biol, 2010, 43: 394-402. DOI:10.1165/rcmb.2009-0223OC |
| [8] |
Blé FX, Cannet C, Zurbruegg S, et al. Activation of the lung S1P(1) receptor reduces allergen-induced plasma leakage in mice[J]. Br J Pharmacol, 2009, 158: 1295-1301. DOI:10.1111/j.1476-5381.2009.00391.x |
| [9] |
Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome[J]. JCI Insight, 2019, 4: e124061. DOI:10.1172/jci.insight.124061 |
| [10] |
Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability[J]. Physiol Rev, 2006, 86: 279-367. DOI:10.1152/physrev.00012.2005 |
| [11] |
Xu RH, Fan H, Zhang L, et al. Effects of caffeoylquinic acids on LPS induced endothelial cell injury in HUVEC by regulating ERK/MAPK signaling pathway[J]. Acta Pharm Sin (药学学报), 2019, 54: 1207-1213. |
| [12] |
Parker JC, Yoshikawa S. Vascular segmental permeabilities at high peak inflation pressure in isolated rat lungs[J]. Am J Physiol Lung Cell Mol Physiol, 2002, 283: L1203-1209. DOI:10.1152/ajplung.00488.2001 |
| [13] |
Dejana E. Endothelial cell-cell junctions:happy together[J]. Nat Rev Mol Cell Biol, 2004, 5: 261-270. |
| [14] |
Birukov KG, Zebda N, Birukova AA. Barrier enhancing signals in pulmonary edema[J]. Compr Physiol, 2013, 3: 429-484. |
| [15] |
Carmeliet P, Lampugnani MG, Moons L, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis[J]. Cell, 1999, 98: 147-157. DOI:10.1016/S0092-8674(00)81010-7 |
| [16] |
Venkiteswaran K, Xiao K, Summers S, et al. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and β-catenin[J]. Am J Physiol Cell Physiol, 2002, 283: C811-821. DOI:10.1152/ajpcell.00417.2001 |
| [17] |
Bazzoni G, Dejana E. Endothelial cell-to-cell junctions:molecular organization and role in vascular homeostasis[J]. Physiol Rev, 2004, 84: 869-901. DOI:10.1152/physrev.00035.2003 |
| [18] |
Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions[J]. Annu Rev Cell Dev Biol, 1997, 13: 119-146. DOI:10.1146/annurev.cellbio.13.1.119 |
| [19] |
Stevenson BR, Siliciano JD, Mooseker MS, et al. Identification of ZO-1:a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia[J]. J Cell Biol, 1986, 103: 755. DOI:10.1083/jcb.103.3.755 |
| [20] |
Förster C. Tight junctions and the modulation of barrier function in disease[J]. Histochem Cell Biol, 2008, 130: 55-70. DOI:10.1007/s00418-008-0424-9 |
| [21] |
Parent RA. Comparative biology of the normal lung[M]. New York: Academic Press, 2015.
|
| [22] |
Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer[J]. Nature, 2014, 507: 190-194. DOI:10.1038/nature12930 |
| [23] |
Uhal BD. Cell cycle kinetics in the alveolar epithelium[J]. Am J Physiol, 1997, 272: L1031-1045. |
| [24] |
Bartis D, Mise N, Mahida RY, et al. Epithelial-mesenchymal transition in lung development and disease:does it exist and is it important?[J]. Thorax, 2014, 69: 760-765. DOI:10.1136/thoraxjnl-2013-204608 |
| [25] |
B rnthaler T, Maric J, Platzer W, et al. The role of PGE2 in alveolar epithelial and lung microvascular endothelial crosstalk[J]. Sci Rep, 2017, 7: 7923. DOI:10.1038/s41598-017-08228-y |
| [26] |
Neuhaus W, Samwer F, Kunzmann S, et al. Lung endothelial cells strengthen, but brain endothelial cells weaken barrier properties of a human alveolar epithelium cell culture model[J]. Differentiation, 2012, 84: 294-304. DOI:10.1016/j.diff.2012.08.006 |
| [27] |
Alemany R, van Koppen CJ, Danneberg K, et al. Regulation and functional roles of sphingosine kinases[J]. Naunyn Schmiedebergs Arch Pharmacol, 2007, 374: 413-428. DOI:10.1007/s00210-007-0132-3 |
| [28] |
Spiegel S, Merrill AH. Sphingolipid metabolism and cell growth regulation[J]. FASEB J, 1996, 10: 1388-1397. DOI:10.1096/fasebj.10.12.8903509 |
| [29] |
English D, Welch Z, Kovala AT, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis[J]. FASEB J, 2000, 14: 2255-2265. DOI:10.1096/fj.00-0134com |
| [30] |
Ancellin N, Colmont C, Su J, et al. Extracellular export of sphingosine kinase-1 enzyme:sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation[J]. J Biol Chem, 2002, 277: 6667-6675. DOI:10.1074/jbc.M102841200 |
| [31] |
Murata N, Sato K, Kon J, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions[J]. Biochem J, 2000, 352: 809-815. DOI:10.1042/bj3520809 |
| [32] |
Tiper IV, East JE, Subrahmanyam PB, et al. Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection[J]. Pathog Dis, 2016, 74: ftw063. DOI:10.1093/femspd/ftw063 |
| [33] |
Baeyens A, Fang V, Chen C, et al. Exit strategies:S1P signaling and T cell migration[J]. Trends Immunol, 2015, 36: 778-787. DOI:10.1016/j.it.2015.10.005 |
| [34] |
Takabe K, Paugh SW, Milstien S, et al. "Inside-out" signaling of sphingosine-1-phosphate:therapeutic targets[J]. Pharmacol Rev, 2008, 60: 181-195. DOI:10.1124/pr.107.07113 |
| [35] |
Marsolais D, Rosen H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules[J]. Nat Rev Drug Discov, 2009, 8: 297-307. DOI:10.1038/nrd2356 |
| [36] |
Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1[J]. Nature, 2004, 427: 355-360. DOI:10.1038/nature02284 |
| [37] |
Hecht JH, Weiner JA, Post SR, et al. Ventricular zone gene-1(Vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex[J]. J Cell Biol, 1996, 135: 1071-1083. DOI:10.1083/jcb.135.4.1071 |
| [38] |
Yamaguchi F, Tokuda M, Hatase O, et al. Molecular cloning of the novel human G protein-coupled receptor (GPCR) gene mapped on chromosome 9[J]. Biochem Biophys Res Commun, 1996, 227: 608-614. DOI:10.1006/bbrc.1996.1553 |
| [39] |
Liu CH, Hla T. The mouse gene for the inducible G-protein-coupled receptor Edg-1[J]. Genomics, 1997, 43: 15-24. DOI:10.1006/geno.1997.4759 |
| [40] |
Shea BS, Brooks SF, Fontaine BA, et al. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury[J]. Am J Respir Cell Mol Biol, 2010, 43: 662-673. DOI:10.1165/rcmb.2009-0345OC |
| [41] |
Takabatake N, Arao T, Sata M, et al. Involvement of pulmonary endothelial cell injury in the pathogenesis of pulmonary fibrosis:clinical assessment by 123I-MIBG lung scintigraphy[J]. Eur J Nucl Med Mol Imaging, 2005, 32: 221-228. DOI:10.1007/s00259-004-1663-1 |
| [42] |
Windh RT, Lee M-J, Hla T, et al. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins[J]. J Biol Chem, 1999, 274: 27351-27358. DOI:10.1074/jbc.274.39.27351 |
| [43] |
An S, Bleu T, Hallmark OG, et al. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid[J]. J Biol Chem, 1998, 273: 7906-7910. DOI:10.1074/jbc.273.14.7906 |
| [44] |
Okazaki H, Ishizaka N, Sakurai T, et al. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system[J]. Biochem Biophys Res Commun, 1993, 190: 1104-1109. DOI:10.1006/bbrc.1993.1163 |
| [45] |
Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury[J]. Am J Respir Crit Care Med, 2004, 169: 1245-1251. DOI:10.1164/rccm.200309-1258OC |
| [46] |
Simmons S, Erfinanda L, Bartz C, et al. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation[J]. J Physiol, 2019, 597: 997-1021. DOI:10.1113/JP276245 |
| [47] |
Tauseef M, Kini V, Knezevic N, et al. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells[J]. Circ Res, 2008, 103: 1164-1172. DOI:10.1161/01.RES.0000338501.84810.51 |
| [48] |
Wadgaonkar R, Patel V, Grinkina N, et al. Differential regulation of sphingosine kinases 1 and 2 in lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2009, 296: L603-613. DOI:10.1152/ajplung.90357.2008 |
| [49] |
Camerer E, Regard JB, Cornelissen I, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice[J]. J Clin Invest, 2009, 119: 1871-1879. |
| [50] |
Brinkmann V, Baumruker T. Pulmonary and vascular pharmacology of sphingosine 1-phosphate[J]. Curr Opin Pharmacol, 2006, 6: 244-250. DOI:10.1016/j.coph.2005.12.004 |
| [51] |
Ozaki H, Hla T, Lee MJ. Sphingosine-1-phosphate signaling in endothelial activation[J]. J Atheroscler Thromb, 2003, 10: 125-131. DOI:10.5551/jat.10.125 |
| [52] |
McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate:mechanistic insights[J]. Cell Signal, 2005, 17: 131-139. DOI:10.1016/j.cellsig.2004.08.006 |
| [53] |
Sanna MG, Wang SK, Gonzalez-Cabrera PJ, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo[J]. Nat Chem Biol, 2006, 2: 434-441. DOI:10.1038/nchembio804 |
| [54] |
Gaengel K, Niaudet C, Hagikura K, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2[J]. Dev Cell, 2012, 23: 587-599. DOI:10.1016/j.devcel.2012.08.005 |
| [55] |
Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation[J]. J Clin Invest, 2000, 106: 951-961. DOI:10.1172/JCI10905 |
| [56] |
Cai Y, Bolte C, Le T, et al. FOXF1 maintains endothelial barrier function and prevents edema after lung injury[J]. Sci Signal, 2016, 9: ra40. DOI:10.1126/scisignal.aad1899 |
| [57] |
Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate[J]. Cell, 1999, 99: 301-312. DOI:10.1016/S0092-8674(00)81661-X |
| [58] |
Li Q, Chen B, Zeng C, et al. Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells[J]. Exp Physiol, 2015, 100: 95-107. DOI:10.1113/expphysiol.2014.082149 |
| [59] |
Cerutti C, Ridley AJ. Endothelial cell-cell adhesion and signaling[J]. Exp Cell Res, 2017, 358: 31-38. DOI:10.1016/j.yexcr.2017.06.003 |
| [60] |
Ni X, Epshtein Y, Chen W, et al. Interaction of integrin beta4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement[J]. J Cell Biochem, 2014, 115: 1187-1195. DOI:10.1002/jcb.24770 |
| [61] |
Ebenezer DL, Fu P, Natarajan V. Targeting sphingosine-1-phosphate signaling in lung diseases[J]. Pharmacol Ther, 2016, 168: 143-157. DOI:10.1016/j.pharmthera.2016.09.008 |
| [62] |
Gon Y, Wood MR, Kiosses WB, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF[J]. Proc Natl Acad Sci U S A, 2005, 102: 9270-9275. DOI:10.1073/pnas.0501997102 |
| [63] |
Lin CC, Yang CC, Cho RL, et al. Sphingosine 1-phosphate-induced ICAM-1 expression via NADPH oxidase/ROS-dependent NF-kappaB cascade on human pulmonary alveolar epithelial cells[J]. Front Pharmacol, 2016, 7: 80. |
| [64] |
Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors[J]. J Biol Chem, 2002, 277: 21453-21457. DOI:10.1074/jbc.C200176200 |
| [65] |
Paugh SW, Payne SG, Barbour SE, et al. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2[J]. FEBS Lett, 2003, 554: 189-193. DOI:10.1016/S0014-5793(03)01168-2 |
| [66] |
Suzuki S, Enosawa S, Kakefuda T, et al. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation[J]. Transplantation, 1996, 61: 200-205. DOI:10.1097/00007890-199601270-00006 |
| [67] |
Jeffery DR, Rammohan KW, Hawker K, et al. Fingolimod:a review of its mode of action in the context of its efficacy and safety profile in relapsing forms of multiple sclerosis[J]. Expert Rev Neurother, 2016, 16: 31-44. DOI:10.1586/14737175.2016.1123094 |
| [68] |
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis[J]. N Engl J Med, 2010, 362: 402-415. DOI:10.1056/NEJMoa0907839 |
| [69] |
Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis[J]. N Engl J Med, 2010, 362: 387-401. DOI:10.1056/NEJMoa0909494 |
| [70] |
Oo ML, Chang SH, Thangada S, et al. Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice[J]. J Clin Invest, 2011, 121: 2290-2300. DOI:10.1172/JCI45403 |
| [71] |
Gendron DR, Lemay AM, Lecours PB, et al. FTY720 promotes pulmonary fibrosis when administered during the remodelling phase following a bleomycin-induced lung injury[J]. Pulm Pharmacol Ther, 2017, 44: 50-56. DOI:10.1016/j.pupt.2017.03.010 |
| [72] |
Chiba K, Hoshino Y, Ohtsuki M, et al. Immunosuppressive activity of FTY720, sphingosine 1-phosphate receptor agonist:I. prevention of allograft rejection in rats and dogs by FTY720 and FTY720-phosphate[J]. Transplant Proc, 2005, 37: 102-106. DOI:10.1016/j.transproceed.2004.12.286 |
| [73] |
Tian YL, Jin J, Wang XJ. Research progress of selective coleophor-1-phosphate receptor 1 agonist[J]. Acta Pharm Sin (药学学报), 2012, 47: 7-17. |
 2020, Vol. 55
2020, Vol. 55


