"肾精"是中医学的重要物质概念, 是生命的本原性物质, 主宰人体生长、发育、壮实、生殖、衰老、死亡全过程, 具有养骨, 充髓, 健脑, 化血, 生气, 生成生殖之精及御邪抗病的正气等生理功能[1]。但迄今为止, "肾精"的生物学物质基础及其作用机制尚未得到客观地阐释。
"补肾益精"中药治疗肾精亏虚证疾病临床应用甚广, 但其生物学物质基础及其作用机制至今未被阐释。现代医学临床中, 衰老、儿童发育不良、成人脑血管疾病、生殖功能低下、免疫功能低下、神经精神疾病、老年病、慢性消耗性疾病晚期、某些先天性与遗传性疾病、腰脊筋骨关节疾病等都属于肾精亏虚证范畴。上述疾病在中医临床多采用"补肾益精"治法与方药进行治疗, 经几千年实践检验疗效显著[2-5]。但其发挥作用的药物化学成分物质基础、疾病(证候)靶点的生物学物质基础, 及其两者的作用途径均未得到客观的阐释。由于肾精及其肾精亏虚证至今未找到客观依据, 导致补肾益精中药难以用客观指标进行药效学评价[6, 7]。
本研究选择具有"补肾益精填髓"显著功效的著名中药熟地黄、制首乌和黄精[8]作为补肾益精中药的代表, 选择在脑、骨、血、生殖等4个方面具有典型意义的阿尔茨海默症、原发性骨质疏松症、贫血症、不孕不育症和少精症作为肾精亏虚证疾病代表, 利用网络药理学的高通量筛选方法, 通过分析中药化学成分靶点与证候疾病靶点的交集群, 筛选获得"补肾益精中药"作用与"肾精亏虚证候"疾病的重要共有靶点, 并追踪这些靶点的生物学作用。最后采用肾精亏虚证的小鼠模型及灌胃模型小鼠熟地黄药液, 对比青年小鼠、模型小鼠和熟地黄灌胃小鼠体内重要靶点蛋白质含量变化, 对补肾益精中药治疗肾精亏虚证疾病的重要生物学靶点进行验证。
本文的探索旨在从补肾益精中药的化学物质基础(成分)、肾精亏虚证疾病生物学物质基础(病变)的客观性出发, 寻找并验证二者的重要交集点——药物作用于疾病的重要靶点。以期客观阐明补肾益精中药治疗肾精亏虚证疾病的生物学物质基础及其作用机制, 并为客观评价补肾益精中药的效果, 阐释肾精亏虚证候的本质甚至肾精的本质, 提供参考。
材料与方法
数据库 中药系统药理数据库(TCMSP, http://ibts.hkbu.edu.hk/LSP/tcmsp.php); 中医药综合数据库(TCMID, http://www.megabionet.org/tcmid/search/); 相似性基团数据库(SEA, http://sea.bkslab.org/); 在线人类孟德尔遗传数据库(OMIM, https://omim.org/); 基因-疾病关联数据库(DisGeNET, http://www.disgenet.org/); 蛋白质数据库(Uniprot, https://www.uniprot.org/); 人类蛋白参考数据库(HPRD, http://www.hprd.org/); 基因/蛋白相互作用检索工具(STRING, https://string-db.org/cgi/input.pl); 生物信息注释数据库(DAVID, https://david.ncifcrf.gov/)。
补肾益精中药潜在靶点的收集 以口服生物利用度(orally bioavailable, OB) ≥ 30%, Caco-2 ≥ -0.4, 类药性(drug-like, DL) ≥ 0.18为口服吸收筛选标准[9], 在TCMSP数据库、TCMID数据库中获得熟地黄(Rehmanniae radix praeparata, RRP)、制首乌(Polygoni multiflori radix praeparata, PMRP)、黄精(Polygonati rhizoma, PR)的药效成分及部分药物作用靶点的数据, 并查阅相关文献[10-14], 进行药物活性成分的找回或补充。最后, 利用SEA数据库预测该3种中药潜在作用靶点。
肾精亏虚证相关疾病潜在靶点的收集 分别以骨质疏松症、阿尔茨海默症、贫血症、不孕不育症和少精症为关键词, 检索OMIM数据库和DisGeNET数据库, 获得这5种肾精亏虚证相关疾病的靶点。
已收集靶点的名称转换 在Uniprot数据库中, 选择物种"人类(Homo sapiens)", 将上述收集到的所有靶点的名称校准为Uniprot官方基因名称(Official gene symbol)。
蛋白-蛋白相互作用数据的收集 为囊括药物的间接作用, 运用Cytoscape 3.6.1软件, 以人类蛋白质相互作用网络为背景(源自HPRD数据库[15]), 分别获取3种益精中药, 5种肾精亏虚证相关疾病全部靶点的邻近靶点, 并提取其靶点间相互关系数据。再利用Cytoscape 3.6.1软件, 获得3种益精中药与5种肾精亏虚证相关疾病的共有靶点, 并提取其靶点间相互关系数据。最后, 将上述收集到的所有靶点导入STRING数据库中, 获得靶点间关系和预测关系数据。
生物靶点网络的构建 运用Cytoscape 3.6.1软件, 构建3种益精中药的"药物-成分-靶点"网络, 5种肾精亏虚证相关疾病的"疾病-靶点"网络, 最后, 利用软件中Merge功能, 合并上述两个网络, 得到"(补肾益精功能)药物-成分-靶点-疾病(肾精亏虚证候)"网络(drug-compound-target-disease network, DCTD network)。
关键靶点的分析 提取"药物-成分-靶点-疾病"网络中益精中药与肾精亏虚证相关疾病的共有靶点网络, 以Closeness-Centrality和Degree两种拓扑参数均为平均值以上的靶点作为关键靶点, 即可能的肾精亏虚证的生物靶点, 记录相应靶点数据, 再通过DAVID数据库和Cytoscape 3.6.1软件中Clue GO + Clue Pedia插件对关键靶点进行GO富集分析和pathway注释。
肾精亏虚证小鼠模型对靶点的验证
药品与试剂 熟地黄免煎颗粒(1804002s, 华润三九医药股份有限公司)。丙二醛(MDA)、超氧化物歧化酶(SOD)、睾酮(T)、雌二醇(E2)、促肾上腺皮质激素(ACTH)、皮质酮(CORT)、三碘甲腺原氨酸(T3)、四碘甲腺原氨酸(T4)等ELISA试剂盒(上海优选生物科技有限公司); 异氟烷(217160701, 深圳市瑞沃德生命科技有限公司); 蛋白抽提试剂盒(P0013B)、细胞核蛋白与细胞浆蛋白抽提试剂盒(P0027) (碧云天生物技术研究所); SHC1抗体(武汉赛维尔生物科技有限公司); 促红细胞生成素(EPO)、促红细胞生成素受体(EPOR)抗体(北京博奥森生物技术有限公司)。其余抗体均购自万类生物科技有限公司。
动物 KM小鼠, 雄性, SPF级, 8月龄50只, 2月龄10只, 购于重庆医科大学动物实验中心, 生产许可证号:SCXK (渝) 2017-0001, 实验动物质量合格证号:NO.0005373;饲养于西南大学药学院实验动物中心, 实验动物使用许可证号:SYXK (渝) 2014-0002。动物房温度:18~22℃, 相对湿度:40%~60%, 12 h/12 h明暗交替。小鼠的饲养和使用均符合中国《实验动物管理条例》及西南大学药学院实验动物伦理委员会相关规定(伦理批件编号:0002183)。
靶点验证实验方法
自然衰老肾精亏虚证小鼠的造模方法 将8月龄KM小鼠50只, 常规饲养至12月龄。常规饲养条件:自由采食、饮水, 温度:18~22℃, 湿度:40%~60%, 12 h/12 h明暗交替。
自然衰老肾精亏虚证小鼠模型造模成功的判定标准 与青年小鼠(2月龄)相比, 每只老龄小鼠(12月龄)同时达到以下标准:①自主活动次数显著减少; ②血清氧化指标中SOD含量显著降低, MDA含量显著升高; ③血清内分泌指标中T、E2、ACTH、CORT、T3、T4含量显著减少, 则判定为自然衰老肾精亏虚证小鼠模型造模成功。
分组、给药及取材 将自然衰老肾精亏虚证模型成功小鼠随机分为自然衰老肾精亏虚证组、熟地黄4、8、16 g·kg-1组, 共4组, 每组10只。另取10只2月龄雄性KM小鼠作为青年组。根据熟地黄免煎颗粒与生药量的换算比(即1 g熟地黄免煎颗粒相当于熟地黄饮片3.3 g), 用蒸馏水将熟地黄免煎颗粒配置成生药浓度为0.2、0.4和0.8 g·mL-1药液, 每只小鼠灌胃给予熟地黄药液20 mL·kg-1, 每日1次, 连续给药4周。青年组、自然衰老肾精亏虚证组分别灌胃给予相应体积蒸馏水。末次给药后, 所有小鼠禁食不禁水12 h后, 异氟烷麻醉处死小鼠并取肾脏, 提取相应核蛋白与浆蛋白, 采用Western blot法检测相应靶点。
统计学分析 采用SPSS 21统计学软件包对两组间数据比较, 用t检验、多组间数据进行单因素方差分析。P < 0.05表示有显著性差异, P < 0.01表示有极显著性差异。
结果
1 3味补肾益精中药活性成分的筛选经TCMSP、TCMID数据库筛选及相关文献报道, 共得到化合物38个(表 1), 其中熟地黄有15个, 制首乌有12个, 黄精有11个。
| 表 1 38 active compounds screened from Rehmanniae radix praeparata (RRP), Polygoni multiflori radix praeparata (PMRP) and Polygonati rhizoma (PR) |
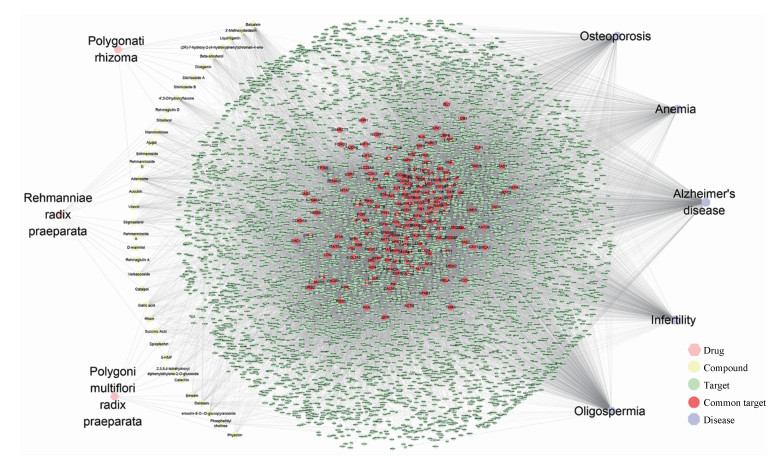
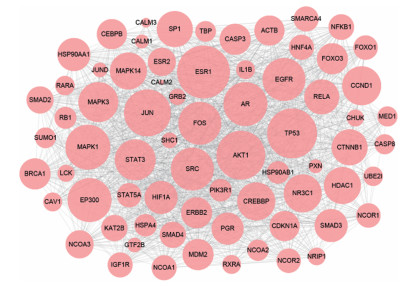
利用Cytoscape 3.6.1软件中的Merge功能, 将收集到的所有靶点进行整合, 构建出3味补肾益精中药与5种肾精亏虚证相关疾病的"(补肾益精功效)药物-成分-靶点-疾病(肾精亏虚证)"网络(图 1)。该网络由6 422个节点, 39 673条边组成。通过药物靶点映射疾病靶点, 共获得175个"药物-疾病"共同靶点。提取该共同靶点子网络, 并对该子网络进行拓扑参数筛选靶点, 分别设定参数值为Degree ≥ 33.554 3和Closeness-Centrality ≥ 0.530 0, 得到71个节点, 将该71个节点定义为"(补肾益精功效)药物-成分-靶点-疾病(肾精亏虚证)"网络的关键靶点, 提取关键靶点网络, 选择Degree值可视化关键靶点网络(图 2), 图中节点越大说明该节点对网络贡献越大, 所对应靶点越重要。
|
图 1 Drug-compound-target-disease (DCTD) network consisted of three representatives of traditional Chinese drugs of reinforcing kidney for supplementing essence (PR, RRP, PMRP) and five diseases related to deficiency of kidney essence |
|
图 2 Key targets network from DCTD network. The size of circles represented nodes degree value |
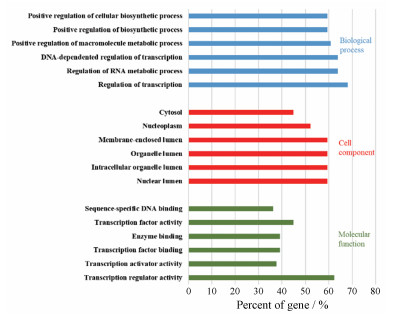
通过DAVID数据库和Clue GO数据库对71个关键靶点进行Gene Ontology (GO)分析, 图 3展示富集占比排名前6的生物学作用、细胞组分和分子功能分析结果。从图中可以得知, 该71个关键靶点对应的生物学作用主要涉及转录调节、RNA代谢过程的调控、DNA依赖的转录调控、大分子代谢过程正调控和生物合成过程正调控。细胞成分主要涉及核腔、细胞器内腔、膜封闭腔、核质和胞质溶胶等。分子功能主要涉及转录调节活性、转录激活子活性、酶结合、转录激活剂活性和蛋白质结合等。
|
图 3 Key target Gene Ontology(GO) enrichment analysis of key targets from DCTD network |
利用Cytoscape 3.6.1软件中Clue Go + Clue Pedia插件并结合DAVID数据库对关键靶点进行pathway通路分析。结果表明, 关键靶点共涉及雌激素信号通路、PPARα信号通路、Akt信号通路、MAPK信号通路、甲状腺激素信号通路、HIF-1信号通路、EPO信号通路等与肾精亏虚证表型、转录调节、能量代谢等功能相关的190条信号通路。选取拓扑参数前10的关键靶点构建其所参与的信号通路网络图(图 4), 由图中可知, 前10关键靶点富集出EPO信号通路。

|
图 4 Network of signaling pathways including the top 10 key targets. The red circles and ellipse represented key targets and signaling pathways, respectively |
依据本团队前期研究成果:① EPO可能为中医"肾精"的重要生物学基础[16]; ② EPO对脑细胞的生长促进作用可能是"肾精通于脑"的重要生物学作用机制[17]; ③ EPO对肾虚骨质疏松模型、肾虚免疫低下模型的改善作用可能是"肾精主骨生髓"和"肾精化气强卫"的现代生物学解释[18, 19]。因此, 选取"EPO信号通路"中富集的靶点及EPO上游重要调控靶点HIF-1α进行实验验证。EPO信号通路富集到的关键靶点为:SHC1、GRB2、MAPK3、JUN、STAT5A和FOS (表 2)。
| 表 2 Key targets in erythropoietin (EPO) pathway |
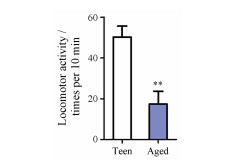
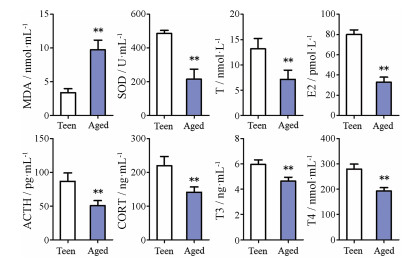
2月龄青年小鼠活泼好动、反应灵敏、毛发黑亮、较少脱毛。与青年小鼠相比, 造模12月龄自然衰老小鼠畏寒怕冷、蜷缩扎堆、精神萎靡、弓背少动、毛发枯燥、光泽暗淡、脱毛严重; 自主活动次数显著减少(图 5), 血清MDA显著升高, SOD、T、E2、ACTH、CORT、T3和T4显著下降, 表明自然衰老致肾精亏虚证小鼠模型造模成功(图 6)。50只8月龄小鼠常规饲养至12月, 自然衰老死亡5只, 未达到模型成功指标1只, 成功44只, 成功率88%。
|
图 5 Locomotor activity testing for teen group and aged group. n =6, |
|
图 6 The variation of serum malondialdehyde (MDA), superoxide dismutase (SOD), testosterone (T), estradiol (E2), adreno-cortico-tropic-hormone (ACTH), corticosterone (CORT), triiodothyronine (T3) and thyroxine (T4) between teen group and aged group. n =6, |
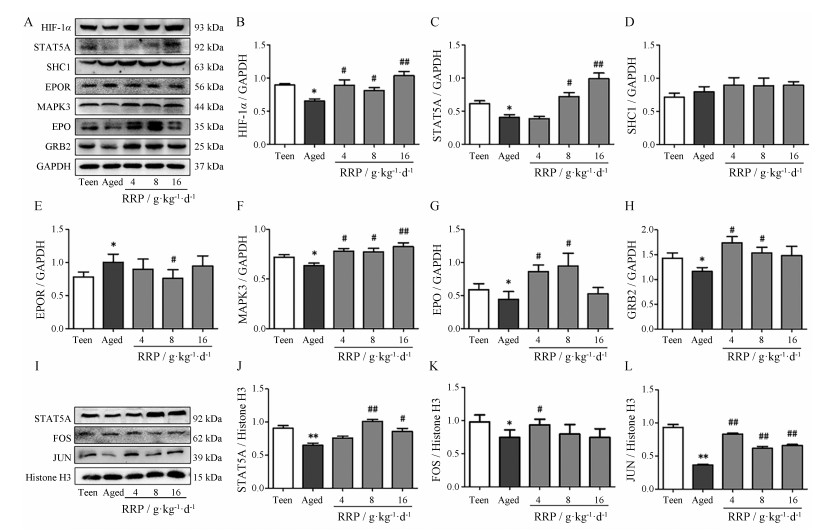
Western blot结果显示(图 7):在预测的7个靶点中, 与青年组相比, 自然衰老肾精亏虚证组HIF-1α、GRB2、MAPK3、STAT5A、JUN和FOS表达量均具有显著下降, SHC1没有显著性差异。另检测EPO通路中EPO和EPOR, 与青年组相比, 自然衰老肾精亏虚证组EPO下调, 而EPOR上调。提示自然衰老肾精亏虚证小鼠体内EPO通路相关蛋白显著降低。
|
图 7 the protein express of key targets in EPO pathway were determined by Western blot analysis. A:Western blot bands for kidney cytoplasm HIF-1α, STAT5A, SHC1, EPOR, MAPK3, EPO and GRB2; B-H:Quantification of kidney cytoplasm HIF-1α, STAT5A, SHC1, EPOR, MAPK3, EPO and GRB2 Western blot analyses in A; I:Western blot bands for kidney nucleus STAT5A, FOS and JUN; J-L:Quantification of kidney nucleus STAT5A, FOS and JUN Western blot analyses in I. n =3, |
在预测的7个靶点中, 与自然衰老肾精亏虚证组相比, 熟地黄组HIF-1α、GRB2、MAPK3、STAT5A、JUN、FOS表达量具有显著性的上调(图 7), SHC1无显著性差异, 但SHC1有上升趋势。与自然衰老肾精亏虚证组相比, 熟地黄4和8 g·kg-1组EPO表达量显著升高, 而16 g·kg-1组EPO升高但无显著性差异; 熟地黄8 g·kg-1组EPOR表达量显著降低。结果提示, 补肾益精的典型中药熟地黄治疗自然衰老肾精亏虚证小鼠的作用机制, 与逆转EPO信号通路蛋白的降低显著相关。
以上结果表明, 肾精亏虚证的证候生物学物质基础可能与EPO信号通路中的HIF-1α、GRB2、MAPK3、STAT5A、JUN、FOS、EPO、EPOR蛋白密切相关, 与SHC1也有一定的关联, 提示EPO信号通路可能是补益肾精中药治疗肾精亏虚证的机制之一。
讨论
"肾精亏虚证"相关疾病的共同病理基础是"补肾益精中药"治疗相关疾病的共同靶点。中西医结合的研究证明, 相同证候的不同疾病(异病同证)具有共同的病理基础[20], 这些共同病理基础正是该类中药治疗该种证候不同疾病的共同靶点。因此, 建立"(同类功能)中药-成分-靶点-疾病(相同证候)"的关联网络, 从中获取的交集靶点群, 即是异病同证的共同病理基础, 亦称"疾病-证候-功效-治法"的共同作用靶点群[21]。本研究依据此思路去寻找中药"补肾益精"治疗肾精亏虚证多种疾病的共同生物物质基础, 具有理论依据。本研究获得骨质疏松症、阿尔茨海默症、贫血、不孕不育症和少精症5种肾精亏虚证相关疾病与熟地黄、制首乌、黄精3味经典补肾益精中药共有生物靶点175个。经拓扑网络参数分析, 关键相同靶点有71个。为中医理论"异病同治"和"对证治疗"提供了现代病理学基础依据。
本研究获得的关键靶点的生物学作用能够阐释肾精亏虚证患者临床表现:体质虚弱、少气无力、生长发育迟缓、骨质疏松、贫血、健忘、生殖能力低下、早衰、环境适应力差和易感病等症状。对于肾精亏虚证的疾病病理靶点有研究[22]报道表明, 衰老、骨质疏松症、不孕不育症和少精症等肾精亏虚证患者早期表现为下丘脑-垂体-性腺轴的下调。其中, 骨质疏松症、不孕不育症和少精症的发病与性腺轴中雄激素受体(AR)下调或失活相关, AR活化后可通过BMP/Smad途径调节肝脏hepcidin的转录从而促进影响铁离子进入红细胞, 提高血红蛋白含量, 改善贫血症状[23]。阿尔茨海默症的研究中发现性腺轴相关蛋白雌激素受体(ESR1)可能与其发病相关[24]。而补肾阳中药调节性腺轴药效明显, 其关键作用靶点有转录相关因子TP53、JUN和EP300, 性激素调节相关蛋白ESR1、AR和信号转导因子MAPK1等[25, 26]。除性腺轴相关靶点变化外, 肾精亏虚骨质疏松患者的基因芯片检测发现, 细胞骨架蛋白SRC、信号转导因子STAT、转录因子JUN的表达量显著下降[27]; 少精症患者精液中存在炎症信号转导因子MAPK1、MAPK3含量的显著上升[28]。对于肾精亏虚证的疾病病理靶点所参与的通路报道显示, 病理靶点主要参与如MAPK通路类似的细胞内跨膜信号转导系统。MAPK-ERK1/2通路与AKT通路上调可以抗细胞凋亡, 促进细胞增殖和对钙的吸收, 也是补肾益精中药发挥促成骨细胞骨形成作用的两条途径[29, 30], p38MAPK和JNK通路下调可降低炎症水平, 改善阿尔茨海默症状[31], 提高少精症患者精子活力[28]。补肾益精方药可以逆转肾精亏虚骨质疏松患者JAK-STAT通路的下调, 改善贫血症状及内分泌紊乱, 活化AKT通路促进精原细胞增殖[27, 32]。上述报道与"肾精主骨生髓"、"肾精通于脑"、"肾精化生肝血"和"肾精主生殖"等中医学重要学术理论内涵相吻合。本研究也发现71个关键相同靶点涉及生物过程和功能为转录调节、转录激活、转录因子活性、大分子代谢过程正调控和生物合成过程正调控等; pathway分析显示, 这些关键靶点参与EPO、MAPK、AKT、PPARα、JAK-STAT等细胞生长、增殖、分化, 糖代谢, 信号转导等相关的信号通路的调控。其中, 对关联网络贡献最高的前10个关键靶点有:转录相关蛋白TP53、CREBBP、JUN和EP300;性腺轴相关蛋白ESR1和AR, 细胞骨架、生存、信号转导等相关蛋白SRC、AKT1、MAPK1和MAPK3。本文网络药理学分析结果与研究报道相呼应, 表明肾精亏虚证的生物学物质基础涉及多种靶点、通路的改变, 与中药"多成分-多靶点-多途径"的作用方式相符。根据这些靶点及相关通路的生物学作用, 与"肾精亏虚证临床表征以机体多种生理功能衰弱为特点"相对应, 可初步解释补肾益精中药治疗肾精亏虚证是通过正向调控机体细胞转录过程, 增强细胞自身活力与功能, 促进生物分子合成, 改善机体虚弱状态。
肾精亏虚证与EPO信号通路的关键靶点密切相关。EPO信号通路是由EPO与其受体EPOR结合后, 引起JAK2、STAT、SHC、GRB2和MAPK3等下游因子活化, 从而发挥多种生物功能的信号转导机制。文献资料报道, 肾精亏虚自然衰老大鼠与青年大鼠的基因芯片检测结果显示, 睾丸组织内EPO信号通路显著下调[33]。EPO信号通路可以调控T细胞等免疫细胞, 从而发挥调节免疫功能、抗炎等作用[34-36]。在自然衰老大鼠模型和D-半乳糖致衰老大鼠模型中, 外源性EPO可以促进脑源性神经营养因子(BDNF)的表达, 从而发挥抗神经系统衰老的作用[37]。EPO通路下游的SHC/GRB2/FOS/JUN途径可以影响"衰老信号"的传递[38]。细胞衰老模型中存在JAK-STAT通路的下调和MAPK通路的上调[39]。本团队前期根据肾精的概念内涵研究发现:①肾精通于脑:在肾精亏虚证自然衰老大鼠、自然衰老小鼠模型和D-半乳糖致亚急性衰老模型中, 均能观察到其脑内EPO含量的下降[16, 19, 37]; ②肾精主骨生髓:EPO本身可刺激骨髓中红系祖细胞增殖、分化; 在肾精亏虚证骨质疏松模型中发现HIF-1α、HIF-2α和EPO表达量显著下降[18]; ③肾精化气强卫:在肾精亏虚证免疫低下模型中发现EPO、STAT5、p-STAT5均显著降低[19]; ④肾精主生殖:在腺嘌呤致肾精亏虚证生殖障碍模型中, HIF-1α与EPO存在显著性降低, EPOR与STAT5反馈性升高[40]。并且, 在"(补肾益精功能)药物-成分-靶点-疾病(肾精亏虚证候)"网络的关键靶点中, 存在于EPO信号通路的关键靶点HIF-1α、GRB2、MAPK3、STAT5A、JUN和FOS均在自然衰老肾精亏虚证模型中发生显著性降低。因此, 推测自然衰老肾精亏虚证的发病机制与EPO通路下调有关。
补肾益精中药治疗肾精亏虚证的重要生物学基础可能是EPO通路中的相关靶点, 肾精亏虚证相关疾病中皆有其相应靶点变化的报道。熟地黄水煎液可通过调节EPO通路下游JUN, 改善雷公藤多苷诱导的小鼠肾虚生殖障碍模型[41]、MSG毁损的下丘脑弓状核大鼠的学习记忆能力[42]。重用熟地黄的补肾益精名方右归饮可通过EPO/JAK2/STAT5及EPO/JAK2/NF-κB途径纠正肾精亏虚证免疫低下模型异常的肾虚与免疫指标[19]; 经由HIF-1α/EPO/EPOR/STAT5途径改善肾精亏虚证生殖障碍模型紊乱的肾虚与生殖指标[40]。Zheng等[43]在少精症大鼠模型中发现STAT5基因下调, 而以黄精为主的黄精赞育胶囊可使其上调。自然衰老肾精亏虚大鼠模型与D-半乳糖诱导的衰老大鼠模型均显示, 肾精亏虚大鼠脑内EPO和EPOR表达量下降, 并与MDA呈负相关。而熟地黄、制首乌和右归饮分别给药后, 均能使肾精亏虚大鼠脑内EPO、EPOR表达量显著上升[44]。EPO通路活化可改善阿尔茨海默症模型小鼠一般活动和记忆能力, 减少皮质和海马区Aβ沉积[45]。损伤脑神经血管单位(NVU)模型后, EPO/EPOR表达显著下调, 而补肾益精中药能逆转其下调[20, 21, 46]。小鼠贫血模型中存在明显的EPOR下调, 制首乌总多糖则能使EPOR受体密度增加, 增强EPOR下游GATA-1表达从而展现受体间正协同作用[47]。骨质疏松症患者外周血白细胞中JAK/STAT通路整体下调, 六味地黄丸则能整体上调此通路[48]。此外, 补肾益精中药可以经由MAPK3、JUN和FOS等靶点的下游通路发挥促骨形成作用[49]。上述报道表明, 补肾益精方药治疗肾精亏虚证相关疾病皆与EPO信号通路相关, EPO信号通路中的蛋白靶点可能是其治疗作用的重要生物学物质基础。
本文研究结果结合文献报道可以推测, 补肾益精中药治疗肾精亏虚证的机制很可能是经由EPO/EPOR介导的信号通路, 即补肾益精中药可能通过活化EPO信号通路而发挥"补肾精"作用。
| [1] |
Shi SB. Study on the Connotation and Origin of Core Concepts in the Theory of "the Kidneys Stores The Essence" ("肾藏精"藏象基础理论核心概念诠释)[D]. Shenyang: Liaoning University of Traditional Chinese Medicine, 2013.
|
| [2] |
Masters CL, Bateman R, Blennow K, et al. Alzheimer's disease[J]. Nat Rev Dis Primers, 2015, 1: 15056. DOI:10.1038/nrdp.2015.56 |
| [3] |
Bai BH, Xie XW, Li DP, et al. Research status of correlation between TCM constitution and osteoporosis in recent five years[J]. Chin J Osteoporosis (中国骨质疏松杂志), 2018, 24: 1229-1235. |
| [4] |
He R, Wang HJ, Zhou Y, et al. Siwu decoction improves iron deficiency anemia in infant rats and regulates iron metabolis[J]. China J Chin Mater Med (中国中药杂志), 2017, 42: 944-950. |
| [5] |
Hu X, Mu Y, Wu YX, et al. Research progress on the role of heat shock protein 70 in infertility[J]. Chin J Reprod Contracep (中华生殖与避孕杂志), 2018, 38: 252-255. |
| [6] |
Xu XY. Establishment of screening and potency evaluation for drugs with healthy effect on brain via enhancing kidney and essence[C]//Papers Collection of the Seventh Symposium on Clinical Traditional Chinese Medicine of the Chinese Society of Traditional Chinese Medicine in 2014(中华中医药学会2014第七次临床中药学术研讨会论文集). Chongqing: China Association of Chinese Medicine, 2014: 3.
|
| [7] |
Zheng LM, Ao HQ, Xu ZW. Analysis of literatures about methods for preparing kidney essence insufficient models[J]. Chin J Basic Med Tradit Chin Med (中国中医基础医学杂志), 2015, 21: 1323-1326. |
| [8] |
Zhang SF, Xu SJ. Discussion on essence-filling drug[J]. Chin J Basic Med Tradit Chin Med (中国中医基础医学杂志), 2019, 25: 98-99. |
| [9] |
Chen Y, Wei JH, Zhang Y, et al. Anti-endometriosis mechanism of Jiawei Foshou San based on network pharmacology[J]. Front Pharmacol, 2018, 9: 811. DOI:10.3389/fphar.2018.00811 |
| [10] |
Meng XL, Ma JN, Zhang SS, et al. Content changes of chemical components and their effect of adjuvants during the process of Rehmanniae Radix Praeparata (steamed for nine times and shined for nine times)[J]. Chin Tradit Herbal Drugs (中草药), 2016, 47: 752-759. |
| [11] |
Xu P, Su S, Tan C, et al. Effects of aqueous extracts of Ecliptae herba, Polygoni multiflori radix praeparata and Rehmanniae radix praeparata on melanogenesis and the migration of human melanocytes[J]. J Ethnopharmacol, 2017, 195: 89-95. DOI:10.1016/j.jep.2016.11.045 |
| [12] |
Yu YM. Studies on the Active Constituents of Polygonatum sibiricum (黄精的活性成分研究)[D]. Tianjing: Tianjing Medical University, 2017.
|
| [13] |
Jiang CX, Zhang TJ, Chen CQ, et al. Research progress in Molygonati ohizoma and predictive analysis on Q-marker[J]. Chin Tradit Herbal Drugs(中草药), 2017, 48: 1-16. |
| [14] |
Zhao MJ, Gong XH, Dang Y, et al. Effect of processing time of Polygoni Multiflori Radix on content changes of 16 components[J]. China J Chin Mater Med (中国中药杂志), 2017, 42: 140-145. |
| [15] |
Keshava PTS, Goel R, Kandasamy K, et al. Human protein reference database-2009 update[J]. Nucleic Acids Res, 2009, 37: 767-772. DOI:10.1093/nar/gkn892 |
| [16] |
Li X. The Verification of Similarity Between EPO and Shen-Jing In Vivo and Study on Neuroprotection Effect and Mechanism of Tonic Herbs in Aging Rats (EPO与"肾精"相似性的体内验脑作用机制研究)[D]. Chongqing: Southwest University, 2016.
|
| [17] |
Yang S. Promotive Effects of EPO on Neurovascular Unit Cell Growth and Bushen Yijing Recipe on EPO (EPO促脑神经血管单元细胞生长及补肾益精方药促EPO生成作用研究)[D]. Chongqing: Southwest University, 2016.
|
| [18] |
Xiao YP. Relationship Between Kidney-Deficiency Osteoporosis Mice and EPO and Improvement Effect of You-gui-yin on Them (肾虚骨质疏松与EPO的相关性及右归饮的改善作用)[D]. Chongqing: Southwest University, 2018.
|
| [19] |
Li JJ, Wu C, Ke H, et al. Changes of EPO in rats with chronic renal failure and low immunity and reversal effects of Yougui Yin and exogenous EPO[J]. China J Chin Mater Med (中国中药杂志), 2019, 44: 1246-1257. |
| [20] |
Zhao Y, Wei SS, Xu JK, et al. Research on the biological basis of TCM syndrome:from different diseases with same syndrome[J]. J Tradit Chin Med (中医杂志), 2014, 55: 829-831. |
| [21] |
Shen ZY. "Kidney Research" keeps forging ahead through "keeping pace with the times"[J]. Chin J Integr Tradit West Med (中国中西医结合杂志), 2015, 35: 946-949. |
| [22] |
Shen ZY. Regulation of HPAT axis molecular network in senility-physiological kidney deficiency syndrome[J]. Chin J Integr Tradit West Med (中国中西医结合杂志), 2004, 24: 841-843. |
| [23] |
Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells[J]. Aging Cell, 2013, 12: 280-291. DOI:10.1111/acel.12052 |
| [24] |
Zhang BY, Pang XC, Jia H, et al. Repositioning drug discovery for Alzheimer's disease based on global marketed drug data[J]. Acta Pham Sin (药学学报), 2019, 54: 1214-1224. |
| [25] |
Jana S, Soňa L, Eva R, et al. Mutations in the TP53 gene affected recruitment of 53BP1 protein to DNA lesions, but level of 53BP1 was stable after γ-irradiation that depleted MDC1 protein in specific TP53 mutants[J]. Histochem Cell Biol, 2017, 148: 239-255. DOI:10.1007/s00418-017-1567-3 |
| [26] |
Zhou JL, Li AY, Wang QM, et al. Mechanism of Yang-tonifying herbs distributing along kidney meridians in molecular level by network pharmacology[J]. Chin Tradit Herbal Drugs (中草药), 2019, 50: 1838-1847. |
| [27] |
Xie LH, Chen J, Li SQ, et al. Effect of Liuweidihuang pill on JAK/STAT signaling pathway gene expression in postmenopausal osteoporosis with the kidney yin deficiency[J]. Chin J Osteoporosis (中国骨质疏松杂志), 2014, 20: 741-746. |
| [28] |
Di Q, Guo J, Wang F, et al. Effect of Jiawei Tianxiong powder on sperm P38MAPK/ERK signaling pathway in asthenospermia patients[J]. Chin J Basic Med Tradit Chin Med (中国中医基础医学杂志), 2016, 22: 788-791. |
| [29] |
Zhou CC, Hong GJ, Yan HY. Predicting and validating the mechanism of Trichosanthes mediated anti-myocardial ischemia-reperfusion injury by network pharmacology[J]. Acta Pham Sin (药学学报), 2019, 54: 1234-1240. |
| [30] |
Fan CL, Ge JR. Resarch progress of the effect of kidney-tonifying herbs on the related signal transduction pathways of osteoporosis[J]. Chin J Osteoporosis (中国骨质疏松杂志), 2014, 20: 446-451. |
| [31] |
Sun LM, Liu LF, Zhu HX, et al. Network pharmacology-based study on intervention mechanism of Huanglian Jiedu decoction in the treatment of Alzheimer's disease[J]. Acta Pham Sin (药学学报), 2017, 52: 1268-1275. |
| [32] |
Liu ZC, Zhao HX, Ma N, et al. Effect of Wuzi Yanzong Fang on proliferation of spermatogonial stem cells in aging rats through PI3K/Akt signaling pathway[J]. Chin J Exp Tradit Med Formulae (中国实验方剂学杂志), 2018, 24: 119-125. |
| [33] |
Hui CH, Liu HK, Du YF, et al. Screening of differentially expressed genes in testicular tissue of aging rats regulated by Polygonum multiflorum drink[J]. Lishizhen Med Mater Med Res (时珍国医国药), 2017, 28: 1583-1586. |
| [34] |
Cravedi P, Manrique J, Hanlon KE, et al. Immunosuppressive effects of erythropoietin on human alloreactive T cells[J]. J Am Soc Nephrol, 2014, 25: 2003-2015. DOI:10.1681/ASN.2013090945 |
| [35] |
Cadman ET, Thysse KA, Bearder S, et al. Eosinophils are important for protection, immunoregulation and pathology during infection with Nematode Microfilariae[J]. PLoS Pathog, 2014, 10: 1003988. DOI:10.1371/journal.ppat.1003988 |
| [36] |
Icardi A, Paoletti E, De NL, et al. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency:the potential role of inflammation[J]. Nephrol Dial Transplant, 2013, 28: 1672-1679. DOI:10.1093/ndt/gft021 |
| [37] |
Wang HQ, Gao Z, Chen MY, et al. Effects of recombinant human erythropoietin on brain-derived neurotrophic factor expression in different brain regions of aging rats[J]. J South Med Univ (南方医科大学学报), 2017, 37: 551-554, 562. |
| [38] |
Pérez H, Finocchietto PV, Alippe Y, et al. p66 Inactivation modifies RNS production, regulates Sirt3 activity, and improves mitochondrial homeostasis, delaying the aging process in mouse brain[J]. Oxid Med Cell Longev, 2018, 2018: 1-13. |
| [39] |
Wang Y, Zhu ZX, Cai QP. Senescence-associated secretory phenotype and its complex regulation networks:a review of molecular mechanisms[J]. Acad J Second Mil Med Univ (第二军医大学学报), 2018, 39: 80-85. |
| [40] |
Li ZH, Wu C, Ke H, et al. You-Gui-Yin improved the reproductive dysfunction of male rats with chronic kidney disease via regulating the HIF1α-STAT5 pathway[J]. J Ethnopharmacol, 2019, 246: 112240. |
| [41] |
Zhang XX, Huang D, Liu NN, et al. GTW-induced abnormal expressions of testicular reproduction-related genes and intervention with kidney-tonifying Chinese herbs[J]. Nat J Androl (中华男科学杂志), 2012, 18: 466-471. |
| [42] |
Cui Y, Hou SL, Yan ZH, et al. Effect of Shu Di-huang on the expression of c-fos and NGF in hippocampi and learning and memory of rats damaged thalamic arcuate nucleus[J]. China J Chin Mater Med (中国中药杂志), 2003, 29: 77-80. |
| [43] |
Zheng Y, Liu B, Ji W, et al. Therapeutic effect of Huangjingzanyu optimized formula on sperm quality and activities of succinic dehydrogenase and lactate dehydrogenase-C4 in rat asthenospermia model[J]. Pak J Zool, 2014, 46: 223-230. |
| [44] |
Li X, Chen YB, Shao SY, et al. Oxidative stress induces the decline of brain EPO expression in aging rats[J]. Exp Gerontol, 2016, 83: 89-93. DOI:10.1016/j.exger.2016.07.012 |
| [45] |
Rodríguez CY, Strehaiano M, Rodríguez OT, et al. An intranasal formulation of erythropoietin (neuro-EPO) prevents memory deficits and amyloid toxicity in the APPSwe transgenic mouse model of Alzheimer's disease[J]. J Alzheimer's Dis, 2016, 12: 1022. |
| [46] |
Qiang X, Yang L, Ran HE, et al. Lyophilized powder of catalpol and puerarin protects neurovascular unit from stroke[J]. Int J Biol Sci, 2016, 12: 367-380. DOI:10.7150/ijbs.14059 |
| [47] |
Feng XM, Zhu BD, Lv Y. Effect of polysaccharide of Radix Polygoni Multiflori on the expression of erythropoietin receptor and transcription factor GATA-1 in spleen of mice with myelosuppressive anemia[J]. Chin Tradit Herb Drugs (中草药), 2010, 41: 93-96. |
| [48] |
Chen J, Xie LH, Li SQ, et al. Relationship between JAK/STAT pathway and immunomodulatory effects of Liuwei Dihuang pills on postmenopausal osteoporosis patients with syndrome of deficiency of kidney yin[J]. Chin J Tradit Chin Med Pharm (中华中医药杂志), 2017, 32: 1747-1750. |
| [49] |
Xiao YP, Zeng J, Jiao LN, et al. Review for treatment effect and signaling pathway regulation of kidney-tonifying traditional Chinese medicine on osteoporosis[J]. China J Chin Mater Med (中国中药杂志), 2018, 43: 21-30. |
 2020, Vol. 55
2020, Vol. 55


