2. 中国人民解放军总医院第五医学中心全军中医药研究所, 北京100039
2. China Military Institute of Chinese Medicine, the Fifth Medical Centre of Chinese PLA General Hospital, Beijing 100039, China
补骨脂为豆科植物补骨脂Psoralea corylifolia L.的干燥成熟果实, 具有温肾助阳, 纳气平喘, 温脾止泻的功效[1], 临床上常用于腰膝骨痛、骨质疏松、骨折、骨性关节炎等骨科疾病的治疗[2, 3]。然而, 随着补骨脂及其制剂的广泛使用, 临床上相继出现肝损伤病例报道[4-6], 国家食品药品监督管理总局已先后通报了含补骨脂的制剂(壮骨关节丸、仙灵骨葆、骨康胶囊)导致肝损伤的风险[7-9]。值得注意的是, 单方补骨脂注射剂在临床上有导致肝损伤的病例, 单独服用补骨脂代茶饮可导致急性肝损害[10], 提示补骨脂单独使用有引起肝损伤的风险。此外, 国际权威药物肝损伤信息网站Liver Tox也对补骨脂及其制剂进行了收录[11]。
不同实验室均在动物水平证实补骨脂药材和提取物长期给药时可引起肝脏损伤[12-14]。本课题组前期对含补骨脂的制剂壮骨关节丸进行拆方研究, 表明在免疫应激大鼠模型上单独给药补骨脂可引起肝脏损伤[15-18]。细胞实验表明, 补骨脂成分补骨脂二氢黄酮、补骨脂二氢黄酮甲醚在HepaRG肝细胞模型上均可引起肝细胞损伤[19, 20]。上述体内外实验结果提示, 补骨脂具有潜在的肝毒性作用, 临床应用补骨脂及其制剂应注意诱发肝损伤的风险。因此, 寻找有效的减毒方法, 是补骨脂临床应用及其相关制剂产业化发展亟待解决的重要课题。
目前补骨脂炮制方法主要为盐炙法。实验研究表明, 盐炙补骨脂仍具有一定的肝毒性[21], 提示盐炙法主要目的是改变药性和归经以增强药效[22], 可能并不主要是减毒。此外, 有相关企业对补骨脂采用水煮去毒的前处理方法, 但临床上仍出现肝损伤病例, 提示水溶性成分可能不是补骨脂主要的肝毒性物质基础。值得注意的是, 南北朝炮制经典著作《雷公炮炙论》记载了补骨脂药材的特殊修制(前处理)方法, 曰: “凡使, 性本大燥, 毒, 用酒浸一宿后, 漉出, 却用东流水浸三日夜, 却蒸从巳至申出, 日干用”, 可概括为酒浸水漂法。古人以“毒”记述补骨脂, 说明古人意识到补骨脂的潜在毒性, 并且指出在使用前应先以酒浸水漂法进行前处理来减毒。针对这一重要线索, 本文拟通过实验研究考察补骨脂酒浸水漂法减毒的客观真实性, 以及通过均匀设计法对比考察不同操作步骤的因素和水平, 筛选最优的修制减毒工艺, 为建立补骨脂减毒新方法的前处理工艺提供参考。相比于常规的2D细胞培养, 文献研究表明, HepaRG细胞3D培养获得的类器官模型能更好地模拟肝脏的结构和功能, 可以更为准确地评价药物肝毒性[23]。为此, 本文采用3D培养人源化肝脏类器官模型, 更加灵敏地检测和评价补骨脂修制前后的减毒效果。
材料与方法材料与仪器 补骨脂药材(北京绿野药业有限公司, 批号: 18052104);牛胰岛素、氢化可的松(Sigma公司); GlutaMAXTM、胎牛血清、William's E培养基(Gibco公司); Trypsin-EDTA (Macgene公司); Hoechst 33342、Calcein AM、EthD-1 (Thermo Fisher公司); Cell Titer-GloTM 3D细胞活力检测试剂、GloMax® Discover (Promega公司); TC10TM细胞自动计数器(Bio-Rad公司); 高内涵筛选分析仪(Thermo公司)。
修制品的制备 取同一批号补骨脂药材各约100 g, 采用U*12(108)均匀设计法, 以腺嘌呤核苷三磷酸(ATP)为响应指标, 以酒精浓度(A)、酒浸次数(B)、酒浸时间(C)、酒浸料液比(D)、水浸时间(E)、水浸次数(F)、水浸料液比(G)、蒸制时间(H)为试验因素。均匀设计因素水平表见表 1。
| 表 1 The table of factors and levels of Uniform design. A: Alcohol concentration; B: Times of alcohol soaking; C: Time of alcohol soaking; D: Ratio of alcohol and material in alcohol soaking; E: Time of water rinsing; F: Times of water rinsing; G: Ratio of water and material in water rinsing; H: Time of steaming |
不同修制品供试品溶液的制备 取修制品20 g, 精密称定, 置于锥形瓶, 加10倍量75%酒精, 超声处理1 h, 提取3次, 过滤, 合并滤液, 减压回收乙醇, 得补骨脂修制品稠浸膏, 冻干备用。取冻干粉适量, 配制质量浓度分数分别为0.5、1、2、4 mg·mL-1的药液, 以0.22 μm的微孔滤膜滤过, 备用。
类器官3D模型构建 HepaRG细胞由本实验室冻存。HepaRG细胞培养于含10%胎牛血清、100 μg·mL-1青链霉素的William's E培养基中, 放置于37 ℃, 5% CO2的培养箱中培养。以氢化可的松和二甲基亚砜(DMSO)诱导HepaRG细胞[24], 将诱导的HepaRG细胞以800个/孔接种于低吸附的96孔U型底板中, 静置10 min后置于培养箱中培养, 使细胞形成类器官3D结构。
不同修制品毒性检测 取培养4天的类器官球体, 将不同浓度的样品依次加入培养板内, 设置溶媒对照组, 置于培养箱中。24 h后将孔板中球体吸至384孔板中, 每孔加入CellTiter-GloTM试剂25 μL, 震荡混匀5 min, 室温孵育20 min, 采用酶标仪读取荧光值, 检测ATP合成量, 用于评价补骨脂修制品毒性大小。
高内涵成像分析 取荧光染料Hoechst 33342、Calcein AM、EthD-1按说明书配制待用。吸弃培养板内的液体, 磷酸盐缓冲液(PBS)冲洗3次, 每孔加入染液100 μL, 置于培养箱中孵育30 min。吸弃培养板孔内染液, 再加入PBS 100 μL, 待测。采用高内涵细胞成像对类器官的细胞进行定位和定量分析, 选用细胞毒性模块, 10倍物镜, 设定通道l检测波长为386 nm, 检测被Hoechst核酸染料染为蓝色的细胞核; 通道2检测波长为490 nm, 检测被Calcein AM染成绿色的活细胞; 通道3检测波长为528 nm, 检测被EthD-1染成红色的死细胞。采集荧光图像并对图像进行荧光强度的量化分析。
细胞ATP合成量测定 按均匀设计用表安排实验, 得12批不同修制条件的补骨脂, 修制品1到修制品12以X1~X12表示, 生品以S表示。采用CellTiter-GloTM3D试剂定量检测不同修制品处理后细胞中ATP合成量。
统计学方法 数据采用x ± s表示, 使用SPSS21.0软件进行统计分析, 组间比较采用单因素方差分析, P < 0.05表示差异具有显著性。
结果 1 不同修制品对肝脏类器官细胞的影响从宽场细胞核可以看出, 随补骨脂不同修制品给药浓度增加, 细胞核体积变小且细胞球体呈松散, 无规则状; X2修制品则无明显变化, 表明补骨脂不同修制品呈现出不同的减毒效果, X2修制品减毒效果较好。Hoechst 33342标记细胞核的染色结果显示, 给药24 h后X2修制品4个浓度(0.5、1、2、4 mg·mL-1)下细胞都出现均匀的蓝色荧光, 而生品和其他修制品随着给药浓度的增加, 细胞荧光强度出现不同程度的减弱, 提示不同修制品呈现出不同程度的减毒效果; Calcein AM标记活细胞的染色结果表明, 给药24 h后X2修制品4个浓度下细胞都出现较均匀的绿色荧光, 而生品随浓度的增加, 细胞的绿色荧光显著降低, 其余修制品随着浓度增加, 细胞荧光强度出现不同程度的减弱, 提示毒性均强于X2修制品; EthD-1标记的死细胞染色结果显示, 给药24 h后, 与生品相比X2修制品4个浓度下细胞出现较均匀的红色荧光, 生品和其他修制品随浓度增加, 细胞红色荧光逐渐增强, 提示毒性均强于X2修制品。结果如图 1和图 2。

|
图 1 Effects of different pre-processing products of Psoraleae Fructus on high content imaging of 3D organoid cells. X1-X12: Pre-processing products 1 to 12; S: Raw product |

|
图 2 The toxicity evaluation of different pre-processing products of Psoraleae Fructus on 3D organoid cells |
实验结果表明, 与生品相比, X2修制品在4个浓度下(0.5、1、2、4 mg·mL-1)细胞ATP释放量无显著差异, 提示补骨脂X2修制品有较好的减毒效果。均匀设计实验安排表和实验结果见表 2。
| 表 2 Evaluation of attenuation process conditions for pre-processing Psoraleae Fructus based on uniform design of U*12(108). Y1 - Y4: ATP release of cell in 4, 2, 1 and 0.5 mg·mL-1. Y: Comprehensive scores of ATP release in cell. Yi = Y1 × 0.25 + Y2 × 0.25 + Y3 × 0.25+Y4 × 0.25, i = 1, 2, 3, 4…12 |
采用DPS7.05软件进行线性相关分析和逐步回归分析, 拟合的线性回归方程和逐步回归方程经方差分析和检验均无统计学意义(P > 0.05), 因此线性拟合不合理。为此, 本实验通过二次多项式逐步回归分析方法, 对实验中的因素与响应数据进行分析, 建立二次多项式回归方程, 结合实验具体情况优化补骨脂修制工艺参数。二次多项式逐步回归非线性结果如下:
| $ \begin{array}{l} Y = - 1.36 - 0.546\;A + 3.004\;B + 0.082\;D + 0.71\;{A^2} + \\ 0.762\;{E^2} - 0.015\;5\;{F^2} - 0.008\;4\;B \times D - \\ 2.071\;B \times E + 0.551\;C \times G - 0.203\;C \times H\\ \left( {P < 0.01,{R^2} = 1} \right) \end{array} $ |
由方程可知, A (P = 0.000 1)、B (P = 0.000 1)、D (P = 0.006)、E (P = 0.000 1)和F (P = 0.000 8)为独立显著因素。最终确定补骨脂最优修制工艺为:酒精浓度80%、酒浸次数3次、酒浸料液比3倍、酒浸时间30 h、水浸料液比2倍、水浸次数3次、水浸时间12 h和蒸制时间5 h。
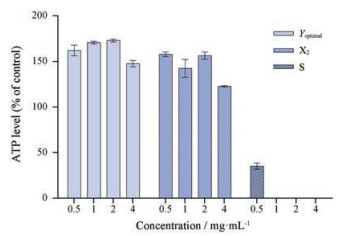
3 均匀设计结果验证取补骨脂药材100 g, 以上述最优工艺制得补骨脂最优修制品(Yoptimal)并制备冻干粉备用, 以肝脏类器官ATP释放量来评价修制品毒性。由图 3可知, 补骨脂生品给药浓度在4、2和1 mg·mL-1时细胞表现出明显毒性, 0.5 mg·mL-1时细胞毒性有所下降。补骨脂最优修制品在4个浓度下细胞未表现出明显肝毒性。结果表明, 补骨脂经酒浸水漂法处理后可以降低补骨脂的肝毒性, 提示酒浸水漂法修制减毒工艺科学合理。最终研究结果显示, 优化得到的修制工艺稳定可行, 为补骨脂最优修制减毒工艺。
|
图 3 ATP level of 3D organoid cells treated with the optimal pre-processing products of Psoraleae Fructus (Yoptimal), pre-processing product X2 and raw product (S). n = 3, x ± s |
南北朝《雷公炮炙论》中详细记载补骨脂“凡使, 性本大燥, 毒, 用酒浸一宿后, 漉出, 却用东流水浸三日夜, 却蒸从巳至申出, 日干用”, 提示《雷公炮炙论》所记载的补骨脂酒浸水漂法可能与补骨脂减毒相关, 但并未明确其为减毒方法, 且尚未有实验研究证明该方法减毒的客观真实性; 本文首次以实验证实古人所讲的酒浸水漂法可以降低补骨脂的肝细胞毒性。以U*12(108)的均匀设计法对补骨脂酒浸水漂法工艺进行优化研究, 最优修制减毒条件为:酒精浓度80%、酒浸次数3次、酒浸料液比3倍、酒浸时间30 h、水浸料液比2倍、水浸次数3次、水浸时间12 h和蒸制时间5 h。对最优实验结果进行验证, 结果证实最优实验条件在4个浓度下均无肝细胞毒性, 表明酒浸水漂法可降低补骨脂的潜在肝毒性。
补骨脂主要含有香豆素类、单萜酚类和黄酮类化合物[25, 26]。针对香豆素类成分补骨脂素和异补骨脂素, Wang等[27]发现小鼠连续灌胃28天补骨脂素(320 mg·kg-1)和异补骨脂素(160 mg·kg-1), 小鼠均未发生肝损伤; 而Zhou等[28]在小鼠单次灌胃给予补骨脂素800 mg·kg-1 24 h即引起小鼠肝脏损伤。针对黄酮类成分如补骨脂二氢黄酮和补骨脂二氢黄酮甲醚, 研究发现, 两个成分在HepaRG肝细胞模型上均可引起肝细胞损伤[19, 20]。单萜酚类成分如补骨脂酚, Li 等[29]在大鼠模型上连续口服给药6周, 发现补骨脂酚可引起胆汁淤积性肝损伤。然而, 值得注意的是, 上述这些与补骨脂潜在肝毒性相关的成分大多为醇溶性。此外, 相关研究发现, 补骨脂的醇提物具有肝毒性[30]; 另有研究表明, 补骨脂醇提物的肝毒性强于水提物[31]。均匀设计实验结果发现, 酒精浓度对减毒效果影响较大, 酒精浓度越高减毒效果越好, 这或许提示高酒精浓度可溶出更多的潜在毒性成分, 这与文献报道补骨脂醇提物的肝毒性强于水提物的结果基本一致。下一步将重点研究补骨脂修制减毒的化学成分变化规律和毒性物质基础。
《雷公炮炙论》对补骨脂的记载强调了“凡使”, 提示该方法是作为药材的前处理方法。该方法从南北朝一直沿用至清代。然而, 目前药典在补骨脂前处理仅为除去杂质, 并未采用酒浸水漂法的前处理工艺。本文研究结果表明, 历代沿用的酒浸水漂法前处理工艺可以有效降低补骨脂的潜在肝毒性, 深入研究该方法对肝毒性的降低作用及保留药效的作用, 将有利于降低补骨脂临床应用的肝损伤风险, 为提高患者用药安全提供参考。此外, 考虑到酒浸水漂法为药材前处理方法, 对于药监局已通报肝损伤风险的含补骨脂中成药的生产企业来说, 可在投料前处理补骨脂药材而不需要改变当前中成药生产工艺, 因此不涉及药品上市的再评价与注册, 故具有潜在的应用前景。作者还留意到, 本草文献有类似酒浸水漂法的传统修制减毒处理方法的记载, 如《伤寒论》中记载半夏使用之前要“水洗”除去半夏的毒性[32]。目前对传统修制方法的现代研究尚不多, 未来值得深入挖掘。
| [1] |
Chinese Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China (中华人民共和国药典)[S]. Part Ⅰ. Beijing: China Medical Science Press, 2015: 187-188.
|
| [2] |
Li J, Zhu AS, Xie WQ, et al. Study of the mechanism of benefiting kidney herbs in the prevention and treatment of osteoarthritis in vitro[J]. Chin J Osteoporos (中国骨质疏松杂志), 2016, 22: 877-882. |
| [3] |
Chai LJ, Zhou K, Wang SX, et al. Psoralen and bakuchiol ameliorate M-CSF plus RANKL-induced osteoclast differentiation and bone resorption via inhibition of AKT and AP-1 pathways in vitro[J]. Cell Physiol Biochem, 2018, 48: 2123-2133. DOI:10.1159/000492554 |
| [4] |
Li YJ, Huang YY. Drug-induced liver injury caused by Psoralea corylifolia:a case report[J]. Shanghai Med Pharm J (上海医药), 2016, 37: 41-42. |
| [5] |
Li A, Gao MH, Zhao N, et al. Acute liver failure associated with Fructus Psoraleae:a case report and literature review[J]. BMC Complement Altern Med, 2019. DOI:10.1186/s12906-019-2493-9 |
| [6] |
Wing IC, Man LT, Teresa N, et al. Liver injury associated with the use of Fructus Psoraleae (Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine[J]. Clin Toxicol, 2009, 47: 683-685. DOI:10.1080/15563650903059136 |
| [7] |
Tian WY, Lan S, Zhan L, et al. Safety evaluation and risk control measures of Psoralea corylifolia[J]. China J Chin Mater Med (中国中药杂志), 2017, 42: 4059-4066. |
| [8] |
Wang Y, Lin ZJ, Wan g X, et al. Analysis and pharmacovigilance thinking on Chinese patent medicine containing Psoraleae Fructus[J]. Chin J Pharmacovig (中国药物警戒), 2018, 15: 300. |
| [9] |
Liu YL, Ge FL, Zhu JX, et al. Re-evaluation of liver injury associated with Buguzhi Preparations based on passive monitoring data and hospital cases[J]. China J Chin Mater Med (中国中药杂志), 2019. DOI:10.19540/j.cnki.cjcmm.20190508.501 |
| [10] |
Nam SW, Baek JT, Lee DS, et al. A case of acute cholestatic hepatitis associated with the seeds of Psoralea corylifolia (Boh-Gol-Zhee)[J]. Clin Toxicol, 2005, 43: 589-591. DOI:10.1081/CLT-200068863 |
| [11] |
Jin R, Gu HY, Li LL, et al. Current status of Chinese herbal preparations in included in liver Tox database[J]. Chin J hepatol (中华肝脏病学杂志), 2016, 24: 817-823. |
| [12] |
Zhou K, Dai Z, Liu ZB, et al. Aqueous extract of Psoralea corylifolia induced liver injury in rats[J]. J Tianjin Univ TCM (天津中医药大学学报), 2013, 32: 221-224. |
| [13] |
Wang Z, Yang L, Lu GY, et al. Preliminary study on toxicity of Psoralen from different extractions in rats[J]. Lab Anim Sci (实验动物科学), 2019, 36: 6-12. |
| [14] |
Yang L, Wang ZX, Lu GY, et al. Study of three months long-term toxicity of rats with water extracts residue of Psoralea corylifolia[J]. Drug Evaluation Res (药物评价研究), 2019, 42: 1128-1134. |
| [15] |
Gao Y, Wang ZL, Tang JF, et al. New incompatible pair of TCM:Epimedii Folium combined with Psoraleae Fructus induces idiosyncratic hepatotoxicity under immunological stress conditions[J]. Front Med, 2019. DOI:10.1007/s11684-019-0690-z |
| [16] |
Tang JF, Wang XY, Weng Q, et al. Idiosyncratic hepatotoxicity evaluation of Zhuangguguanjie wan mediated by immune stress[J]. Acta Pharm Sin (药学学报), 2017, 52: 1033-1040. |
| [17] |
Tang JF, Wang XY, Li WX, et al. Correlation analysis on idiosyncratic hepatotoxicity of Zhuangguguanjie Wan and 27 cytokines based on related visualization[J]. Acta Pharm Sin (药学学报), 2018, 53: 1624-1633. |
| [18] |
Tang JF, Wang XY, Li WX, et al. Cytokine analysis of Zhuangguguanjie wan-induced idiosyncratic liver injury based on mathematical modeling[J]. Acta Pharm Sin (药学学报), 2018, 53: 574-584. |
| [19] |
Yang Y, Tang XL, Hao FR, et al. Bavachin induces apoptosis through mitochondrial regulated ER stress pathway in HepG2 cells[J]. Biol Pharm Bull, 2018, 41: 198-207. DOI:10.1248/bpb.b17-00672 |
| [20] |
Ji YB, Wang M, Wang S, et al. Study on mechanism of HepaRG cell injury induced by bavachinin[J]. Chin Pharm Bull (中国药理学通报), 2018, 34: 544-550. |
| [21] |
Cai TT, Huang NN, Wang L, et al. Experimental comparison study on acute toxicity of different processing methods of Psoraleae Fructus on normal mice[J]. Chin J Pharmacovig (中国药物警戒), 2017, 14: 730-736. |
| [22] |
Li N, Yan DM, Zhang JL, et al. Effect of different processing methods on the antiosteoporotic effective compositions of Psoralea corylifolia L[J]. Mod TCM Mater Med World Sci Technol (世界科学技术-中医药现代化), 2017, 19: 127-132. |
| [23] |
Li TT, Li RH, Liu ZX, et al. Three dimensional organoids-based evaluation for hepatotoxicity of the susceptible compound in Polygonum multiflorum Thunb[J]. Acta Pharm Sin (药学学报), 2017, 52: 1048-1054. |
| [24] |
Li PY, Li CY, Lu XH, et al. The three dimensional organoids-based high content imaging model for hepatotoxicity assessment[J]. Acta Pharm Sin (药学学报), 2017, 52: 1055-1062. |
| [25] |
Alam F, Khan GN, MHHB Asad. Psoralea corylifolia L:ethnobotanical, biological and chemical aspects:a review[J]. Phytother Res, 2018, 32: 597-615. DOI:10.1002/ptr.6006 |
| [26] |
Wang YF, Liu YN, Xiong W, et al. A UPLC-MS/MS method for in vivo and in vitro pharmacokinetic studies of psoralenoside, isopsoralenoside, psoralen and isopsoralen from Psoralea corylifolia extract[J]. J Ethnopharmacol, 2014, 151: 609-617. DOI:10.1016/j.jep.2013.11.013 |
| [27] |
Wang Y, Zhang H, Jiang JM, et al. Hepatotoxicity induced by psoralen and isopsoralen from Fructus Psoraleae:Wistar rats are more vulnerable than ICR mice[J]. Food Chem Toxicol, 2018, 125: 133-140. |
| [28] |
Zhou W, Chen X, Zhao GL, et al. Psoralen induced liver injury by attenuating liver regenerative capability[J]. Front Pharmacol, 2018, 9: 1179. DOI:10.3389/fphar.2018.01179 |
| [29] |
Li ZJ, Abudumijiti A, Zhao GL, et al. Bakuchiol contributes to the hepatotoxicity of Psoralea corylifolia in rats[J]. Phytother Res, 2017, 31: 1265-1272. DOI:10.1002/ptr.5851 |
| [30] |
Chen Y, Wang M, Song J, et al. Effect of extract ethanol and water decoction of Psoralea corylifolia L. on bone development and toxicities in zebrafish[J]. J Pharm Toxicol (中国药理学与毒理学杂志), 2017, 31: 661-669. |
| [31] |
Wu H, Song J, Zhong QX, et al. The liver toxicity and mechanism of Psoralen based on zebrafish[J]. J Nanjing Univ TCM (南京中医药大学学报), 2017, 33: 263-267. |
| [32] |
Li N, Gao F, Wang F. Processing methods of Chinese medicinals in Shanghanlun[J]. J Beijing Univ TCM (北京中医药大学学报), 2013, 36: 67-69. |
 2020, Vol. 55
2020, Vol. 55


