胰岛素抵抗(insulin resistance)是2型糖尿病的发病基础, 也贯穿多种代谢相关疾病的发生发展[1]。肝脏是机体进行脂质代谢的重要器官:正常状态下, 胰岛素促进肝脏脂肪合成, 同时抑制糖异生进而降低肝脏葡萄糖产生; 胰岛素抵抗状态下, 糖异生途径对胰岛素不敏感, 胰岛素抑制肝脏葡萄糖产生的作用降低, 导致肝脏葡萄糖产生增多, 然而肝脏脂肪合成仍保持较高水平, 表现在肝脏及血浆脂肪酸(FA)和甘油三酯(TAG)水平异常增高。胰岛素抵抗状态下, 胰岛素失去抑制肝脏葡萄糖产生的同时仍促进肝脏脂质合成近年引起广泛关注[2, 3]。本文主要通过固醇调节因子结合蛋白1c (SREBP1c)、哺乳动物雷帕霉素靶复合体1 (mTORC1)、内质网应激、FoxO1、脂质合成底物调节脂质合成的作用特征, 综述胰岛素抵抗状态下肝脏脂质合成增加的分子机制研究进展。
1 正常脂质合成过程肝脏中脂肪合成通过FA与甘油骨架缩合产生TAG。FA主要存在两种来源:一是肝脏摄取循环中源于食物和脂肪组织的游离脂肪酸(FFA); 二是通过脂质的从头合成(DNL, de novo lipogenesis)。DNL的直接原料来自乙酰辅酶A (acetyl-CoA), 乙酰辅酶A经一系列酶促反应最终合成脂肪酸, 这些脂肪酸酯化后形成TAG[4]。肝脏和脂肪组织中均存在DNL, 而肝脏DNL在维持血清TAG稳态方面发挥关键作用[5]。
一系列酶促反应调控DNL的形成。首先, 线粒体内的乙酰辅酶A与草酰乙酸缩合形成柠檬酸, 柠檬酸被线粒体内膜上的载体转运至细胞质, 在胞质ATP-柠檬酸裂解酶(ATP-citrate lyase, ACLY)的作用下, 柠檬酸裂解释放出乙酰辅酶A。随后, 乙酰辅酶A在乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC)作用下生成丙二酸单酰辅酶A, 后者在脂肪酸合酶(fatty acid synthase, FAS)的作用下经连续7次重复加成反应, 最终形成DNL的主要产物棕榈酸。上述过程同时伴随产生一系列短链脂肪酸等代谢产物(图 1)。

|
Figure 1 Liver lipogenesis process in insulin resistance. (1) Triglyceride (TAG) synthesis: glucose taken up by the cell is converted to pyruvate through glycolysis. Pyruvate is further converted into acetyl-CoA and enters the tricarboxylic acid (TCA) cycle. Excess acetyl-CoA converted to citrate can exit the mitochondria and become the substrate for lipogenic enzymes. Fatty acids (FA) that spill over from lipolysis of white adipose tissue also contribute to hepatic lipid synthesis via esterification and β oxidation. (2) Liver lipogenesis remains at a high level in insulin resistance: Increased liver lipogenesis is followed with a significant increase in the expression levels of key enzymes and enzyme activities, such as ACLY (ATP-citrate lyase), ACC (acetyl-CoA carboxylase) and FAS (fatty acid synthase). The rates of adipose tissue lipolysis are also increased, resulting in increased FA delivery to liver, which results in increased hepatic esterification of FA to TAG |
最近的研究发现, 60%高脂饲料所致肥胖小鼠的肝脏脂肪酸合成速率比正常饮食小鼠增加约2~4倍[6]。DNL产生的脂质在正常小鼠占肝内脂肪的25%, 然而在瘦素受体敲除的糖尿病小鼠(db/db小鼠)体内则高达60%[7]。Wiegman等[8]研究发现, DNL在瘦素敲除的糖尿病小鼠(ob/ob小鼠)比野生型小鼠增加约10倍左右。临床研究发现, 健康人群DNL产生的脂质约占肝脏内脂肪的5%, 但非酒精性脂肪肝(NAFLD)患者的这一比例提升至26%[9]。大量研究证实, 胰岛素抵抗患者的肝脏DNL能够增加两倍以上[10]。这些研究结果表明, 胰岛素抵抗状态下的肝脏脂质合成长期保持亢进状态[3]。
脂肪生成通过脂肪生成酶催化系列反应而成。参与DNL的关键酶主要包括ACLY、ACC、FAS、长链脂肪酸延长酶5 (ELOVL5)。Guan等[6]研究发现, 高脂饮食喂养的肥胖小鼠与野生型小鼠相比参与DNL的关键酶基因在肝脏表达水平显著增加, 表现在1~2倍ACLY、1~2倍ACC、2~3倍FAS以及3~5倍ELOVL5的基因表达增加。与野生型小鼠相比, db/db小鼠肝脏中ACLY的基因表达水平升高约4~5倍, 蛋白水平增加约2.5倍, 酶活性提升约2.5倍左右[11]。在ob/ob小鼠与正常小鼠的对比实验发现: ob/ob小鼠肝脏中ACC的基因表达水平增加约4倍, 酶活性提升约2.5倍[12]; FAS的基因表达水平增加约11倍, 酶活性提升约6倍。与正常人群相比较, 肥胖且伴有2型糖尿病的患者肝脏FAS基因表达增加2.9倍, 肥胖但未患糖尿病的受试者肝脏中FAS的基因表达亦增加2.4倍[13]。这些实验动物以及临床研究的结果提示, 胰岛素抵抗状态下肝脏DNL过程伴随着关键酶的表达水平和酶活性显著升高, 这也是肝脏脂质合成显著增加的关键原因(图 1)。
3 肝脏脂质合成依赖胰岛素信号通路胰岛素主要通过磷酸肌醇-3-磷酸激酶(PI3K)/蛋白激酶B (Akt)调节脂质合成。胰岛素结合胰岛素受体(IR)后, 激活胰岛素受体底物(IRS)。IRS招募并激活PI3K, 并产生PIP3。PIP3募集Akt, 通过3-磷酸肌醇依赖性蛋白激酶1 (PDK1), 首先催化Akt蛋白的Thr308位点发生磷酸化, 再通过哺乳动物雷帕霉素靶复合体2 (mTORC2)丝氨酸磷酸化Akt (Ser473)进而激活Akt[14]。Akt信号通路是胰岛素控制肝脏脂质代谢的关键通路, 过表达组成型活化Akt显著促进肝脏肿大以及肝脏内的脂肪堆积[15, 16]。与此类似, Leavens等[17]在遗传或饮食诱导的肥胖及胰岛素抵抗小鼠上研究发现, 肝脏特异性敲除Akt2能够抑制脂肪肝的形成。
胰岛素抵抗情况下, 肝脏内存在的胰岛素和胰岛素受体对于肝脏脂质代谢的调节具有重要作用[14, 18]。研究发现, 肝细胞胰岛素受体敲除的小鼠葡萄糖稳态虽然发生显著变化并产生明显的胰岛素抵抗, 但是此类型小鼠的脂质代谢未出现异常, 从而免受胰岛素抵抗所致脂质代谢异常。给予高脂饮食后, 不会造成肝内累积过量甘油三酯, 避免形成脂肪肝。与正常对照组小鼠相比较, 肝细胞胰岛素受体敲除的小鼠的血清甘油三酯降低、脂肪酸氧化增加, 但是DNL无显著增加。因此, 完整的胰岛素信号对于肝脏脂质合成增加必不可少。临床研究的结果也发现, 肝脏脂质合成依赖胰岛素信号, 胰岛素受体功能突变患者表现出严重的胰岛素抵抗和高血糖状态, 但患者并不会发展为脂肪肝[18]。上述研究提示, 尽管在胰岛素抵抗状态肝脏胰岛素信号下调, 但足以维持肝脏的脂质合成。
4 胰岛素抵抗状态下脂质合成增加的分子机制 4.1 固醇调节因子结合蛋白1c (SREBP1c)普遍存在的转录因子SREBP1c在脂肪酸、甘油三酯和胆固醇的合成中发挥关键作用[19] (图 2)。SREBP1c的表达受多种营养因素以及激素调控, 其中胰岛素是最为有效的激活因子之一。胰岛素促进SREBP1c的基因表达及翻译后修饰, 进而调节脂质合成过程。在肝细胞中, 胰岛素激活Akt并促进SREBP亚型的合成和加工, 促使SREBP1c成熟。成熟的SREBP1c定位至细胞核后, 促进包括FAS、ACC、硬脂酸辅酶A脱氢酶1 (SCD1)、长链脂肪酸延伸家族成员6 (ELOVL6)等多个脂质合成相关基因的转录。甾醇调节元件结合蛋白剪接激活蛋白(SCAP)行使分子伴侣功能, 促进SREBP蛋白从内质网转运至高尔基体进行加工。肝脏特异性敲除SCAP的转基因小鼠, 无论给予高脂饮食诱导的肥胖动物或瘦素缺陷的自发肥胖小鼠模型, 均可避免形成肝脂肪变性[20]。这些结果提示, SREBP1c在调节胰岛素下游的肝脂质积累发挥关键作用。的确, Shimonmura等[21]在胰岛素抵抗小鼠(如HFD小鼠和ob/ob糖尿病小鼠)模型中发现, 肝脏SREBP1c表达水平显著增加并明显促进脂质合成。
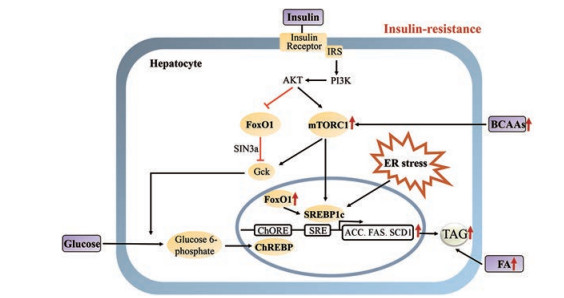
|
Figure 2 Molecular mechanism of increased hepatic lipogenesis in insulin resistance (1) Insulin resistance is followed with an activity increase of the lipogenic transcription factor SREBP1c, which in turn activates the lipogenic gene program. (2) ER stress activates SREBP1c that enhanced lipid accumulation. (3) Circulating levels of branched chain amino acids (BCAAs) are increased in insulin resistant. BCAAs are potent activators of mTORC1. Activated mTORC1 promotes SREBP1c maturation, transcription and promotes lipogenesis. (4) Insulin resistance increase FoxO1 nuclear translocation and the subsequent increase in the transcription of lipogenic genes. (5) In insulin resistance, FA from the adipose tissue is increased, and is transported to the liver for lipogenesis |
在胰岛素信号通路中, mTORC1位于Akt下游以及S6激酶(S6K)上游, 主要发挥调节蛋白翻译和细胞生长等生物学作用[22-24] (图 2)。体外研究结果发现, mTORC1的激活是Akt诱导的SREBP1c成熟、转录过程所必需。动物实验研究结果显示, 单独激活mTORC1不足以诱导SREBP1c介导的DNL, 必须同时伴随叉头转录因子O1 (FoxO1)的抑制效应[25, 26]。这些研究证据提示, 肝脏依赖于Akt通路中mTOC1活化和FoxO1抑制完成DNL, 进而增加糖酵解、促进CHREBP活化, 同时伴随SREBP1c激活和脂质合成关键基因的转录激活[27]。
研究发现, 胰岛素抵抗状态下mTORC1处于过度活化状态[28, 29]。胰岛素抵抗性肥胖和2型糖尿病患者血清支链氨基酸(BCAAs)水平显著增加[30, 31]。临床研究发现, BCAAs水平升高与胰岛素抵抗程度呈正相关[32]。BCAAs包含具有非线性脂肪族侧链的3种必需氨基酸, 包括亮氨酸、异亮氨酸和缬氨酸。这些氨基酸都是有效的mTORC1活化剂[33]。活化的mTORC1能够通过非依赖胰岛素信号通路促进SREBP1c成熟、转录进而促进脂质合成。
4.3 内质网应激(ER stress)内质网是合成分泌型及胞膜型蛋白质的重要细胞器。在分子伴侣的帮助下, 蛋白质在内质网完成折叠和组装。内质网稳态异常包括钙离子浓度变化干扰、糖基化或合成过多的分泌蛋白等, 稳态异常诱导的适应性反应称为内质网应激[34]。肥胖的胰岛素抵抗患者表现出高血糖、高胰岛素血症[35], 同时促炎因子[36]及游离脂肪酸[37]显著增加, 这些因素均能破坏内质网稳态进而诱导内质网应激的产生。内质网应激增强脂质合成进一步促进胰岛素抵抗的发展进程, 主要通过非胰岛素信号通路促进SREBP1c蛋白的剪接, 进而激活脂质合成程序[38, 39] (图 2)。值得一提的是, 在胰岛素抵抗状态下经FA、甘油合成TAG过程非常活跃。内质网作为TAG合成并被包装为脂质颗粒的细胞器, 需要重新形成内质网膜并不断延伸。这一过程对内质网本身也是一种应激反应, 进一步促进脂质合成并形成反馈循环[38]。
4.4 FoxO1在肝脏中, 胰岛素激活Akt后能引起FoxO1磷酸化, 导致FoxO1驻留细胞质、不能进入细胞核行使转录因子的功能[40]。研究发现, 在肝脏中过表达不能被Akt磷酸化的FoxO1 (FoxOAAA), 即持续性激活型FoxO1的转基因小鼠, 表现出DNL和TAG基础分泌减少, 同时饮食无法诱导脂质合成基因增加。与此类似, 肝脏缺失3种亚型的FoxO均可诱导DNL、肝脏脂肪变性和脂肪合成基因表达增加[41, 42]。这些研究表明FoxO1具有抑制脂质合成的功能, 但确切的分子机制尚不明确。Gck作为葡萄糖代谢的限速酶, 而葡萄糖代谢产物乙酰辅酶A则是DNL的关键原料。FoxO1可通过SIN3a介导的共抑制机制降低葡萄糖激酶(Gck)的表达, 进而减少肝脏脂质合成[43]。研究发现, 原代肝细胞中过表达SIN3a能够减弱胰岛素诱导的脂质合成增加。然而, 在肝脏特异性SIN3a敲除小鼠的原代肝细胞上, 胰岛素则失去诱导脂质合成的能力。
在胰岛素抵抗状态下, 肝脏FoxO1的产生及活性均增加, 并且细胞核内分布增加[44]。增加的FoxO1促进肝脏过氧化物酶体增殖物激活受体-γ协同激活因子1β (PGC1β)表达, 而SREBP1c的共激活因子PGC1β亦是SREBP1c介导肝脏脂肪生成所必需[45]。因此, 在胰岛素抵抗情况下, FoxO1能够通过胰岛素信号非依赖方式促进肝脂质合成。
4.5 脂质合成底物研究发现, 在胰岛素抵抗状态下胰岛素抑制脂肪组织脂解作用能力减弱。同时, 巨噬细胞浸润的白色脂肪组织中脂解作用增强, 更多的脂肪酸被转运至肝脏中用于合成甘油三酯, 正是这种底物量的增加进一步促使肝脏脂肪合成亢进。
5 结语胰岛素信号通路活化对于肝脏脂质合成增加是必需。在胰岛素抵抗发生时, 肝脏胰岛素信号通路中关键因子Akt磷酸化水平降低, 但肝脏脂质合成依然增加。因此, 胰岛素抵抗状态下胰岛素信号通路减弱但未被完全阻断, 存在非依赖胰岛素信号通路的因素促进脂质合成亢进。ER stress、mTORC1和FoxO1均能通过非依赖胰岛素信号方式激活SREBP1c, 进而促进脂质合成。同时, 肝脏中脂质合成底物的来源显著增加, 亦是脂质合成增加的关键原因。这些因素共同导致肝脏胰岛素抵抗和脂质合成亢进并存的现象, 进一步阐明其发生的分子机制有助于发现降低肝脏脂质合成亢进、改善胰岛素抵抗的新药靶。
| [1] |
Guilherme A, Henriques F, Bedard AH, et al. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus[J]. Nat Rev Endocrinol, 2019, 15: 207-225. DOI:10.1038/s41574-019-0165-y |
| [2] |
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism[J]. Nat Rev Mol Cell Biol, 2018, 19: 654-672. DOI:10.1038/s41580-018-0044-8 |
| [3] |
Sanders FWB, Griffin JL. De novo lipogenesis in the liver in health and disease:more than just a shunting yard for glucose[J]. Biol Rev, 2016, 91: 452-468. DOI:10.1111/brv.12178 |
| [4] |
Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance[J]. Trends Endocrinol Metab, 2011, 22: 353-363. DOI:10.1016/j.tem.2011.04.007 |
| [5] |
Bjorntorp P, Sjostrom L. Carbohydrate storage in man:speculations and some quantitative considerations[J]. Metab Clin Exp, 1978, 27: 1853-1865. DOI:10.1016/S0026-0495(78)80004-3 |
| [6] |
Guan D, Xiong Y, Borck PC, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes[J]. Cell, 2018, 174: 831-42. DOI:10.1016/j.cell.2018.06.031 |
| [7] |
Trayhurn P, Wusteman MC. Lipogenesis in genetically diabetic (db/db) mice:developmental changes in brown adipose tissue, white adipose tissue and the liver[J]. Biochim Biophys Acta, 1990, 1047: 168-174. DOI:10.1016/0005-2760(90)90043-W |
| [8] |
Wiegman CH, Bandsma RH, Ouwens M, et al. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin[J]. Diabetes, 2003, 52: 1081-1089. DOI:10.2337/diabetes.52.5.1081 |
| [9] |
Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease[J]. J Clin Investig, 2005, 115: 1343-1351. DOI:10.1172/JCI23621 |
| [10] |
Baothman OA, Zamzami MA, Taher I, et al. The role of gut microbiota in the development of obesity and diabetes[J]. Lipids Health Dis, 2016, 15: 108. DOI:10.1186/s12944-016-0278-4 |
| [11] |
Wang Q, Jiang L, Wang J, et al. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice[J]. Hepatology, 2009, 49: 1166-1175. DOI:10.1002/hep.22774 |
| [12] |
Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice[J]. Am J Physiol Endocrinol Metab, 2006, 291: E358-364. DOI:10.1152/ajpendo.00027.2006 |
| [13] |
Eissing L, Scherer T, Todter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health[J]. Nat Commun, 2013, 4: 1528. DOI:10.1038/ncomms2537 |
| [14] |
Fon Tacer K, Rozman D. Nonalcoholic Fatty liver disease:focus on lipoprotein and lipid deregulation[J]. J Lipids, 2011, 2011: 783976. |
| [15] |
Ono H, Shimano H, Katagiri H, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement[J]. Diabetes, 2003, 52: 2905-2913. DOI:10.2337/diabetes.52.12.2905 |
| [16] |
Horie Y, Suzuki A, Kataoka E, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas[J]. J Clin Investig, 2004, 113: 1774-1783. DOI:10.1172/JCI20513 |
| [17] |
Leavens KF, Easton RM, Shulman GI, et al. Akt2 is required for hepatic lipid accumulation in models of insulin resistance[J]. Cell Metab, 2009, 10: 405-418. DOI:10.1016/j.cmet.2009.10.004 |
| [18] |
Biddinger SB, Hernandez-Ono A, Rask-Madsen C, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis[J]. Cell Metab, 2008, 7: 125-134. DOI:10.1016/j.cmet.2007.11.013 |
| [19] |
Horton JD, Goldstein JL, Brown MS. SREBPs:activators of the complete program of cholesterol and fatty acid synthesis in the liver[J]. J Clin Investig, 2002, 109: 1125-1131. DOI:10.1172/JCI0215593 |
| [20] |
Moon YA, Liang G, Xie X, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals[J]. Cell Metab, 2012, 15: 240-246. DOI:10.1016/j.cmet.2011.12.017 |
| [21] |
Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus[J]. J Biol Chem, 1999, 274: 30028-30032. DOI:10.1074/jbc.274.42.30028 |
| [22] |
Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver:mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis[J]. Proc Natl Acad Sci U S A, 2010, 107: 3441-3446. DOI:10.1073/pnas.0914798107 |
| [23] |
Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth[J]. Cell Metab, 2008, 8: 224-236. DOI:10.1016/j.cmet.2008.07.007 |
| [24] |
Tang Y, Wallace M, Sanchez-Gurmaches J, et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism[J]. Nat Commun, 2016, 7: 11365. DOI:10.1038/ncomms11365 |
| [25] |
Wan M, Leavens KF, Saleh D, et al. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c[J]. Cell Metab, 2011, 14: 516-527. DOI:10.1016/j.cmet.2011.09.001 |
| [26] |
Yecies JL, Zhang HH, Menon S, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways[J]. Cell Metab, 2011, 14: 21-32. DOI:10.1016/j.cmet.2011.06.002 |
| [27] |
Titchenell PM, Quinn WJ, Lu M, et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production[J]. Cell Metab, 2016, 23: 1154-1166. DOI:10.1016/j.cmet.2016.04.022 |
| [28] |
Tremblay F, Jacques H, Marette A. Modulation of insulin action by dietary proteins and amino acids:role of the mammalian target of rapamycin nutrient sensing pathway[J]. Curr Opin Clin Nutr Metab Care, 2005, 8: 457-462. DOI:10.1097/01.mco.0000172589.55434.03 |
| [29] |
Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1[J]. Cell Metab, 2006, 3: 393-402. DOI:10.1016/j.cmet.2006.05.003 |
| [30] |
Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance[J]. Cell Metab, 2009, 9: 311-326. DOI:10.1016/j.cmet.2009.02.002 |
| [31] |
McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents[J]. Pediatr Obes, 2013, 8: 52-61. DOI:10.1111/j.2047-6310.2012.00087.x |
| [32] |
Badoud F, Lam KP, DiBattista A, et al. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese[J]. J Proteome Res, 2014, 13: 3455-3466. DOI:10.1021/pr500416v |
| [33] |
Hara K, Yonezawa K, Weng QP, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism[J]. J Biol Chem, 1998, 273: 14484-14494. DOI:10.1074/jbc.273.23.14484 |
| [34] |
Winnay JN, Kahn CR. PI 3-kinase regulatory subunits as regulators of the unfolded protein response[J]. Methods Enzymol, 2011, 490: 147-158. DOI:10.1016/B978-0-12-385114-7.00009-X |
| [35] |
Boden G, Cheung P, Salehi S, et al. Insulin regulates the unfolded protein response in human adipose tissue[J]. Diabetes, 2014, 63: 912-922. DOI:10.2337/db13-0906 |
| [36] |
Denis RG, Arruda AP, Romanatto T, et al. TNF-alpha transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus[J]. Neuroscience, 2010, 170: 1035-1044. DOI:10.1016/j.neuroscience.2010.08.013 |
| [37] |
Boden G. Endoplasmic reticulum stress:another link between obesity and insulin resistance/inflammation?[J]. Diabetes, 2009, 58: 518-519. DOI:10.2337/db08-1746 |
| [38] |
Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice[J]. J Clin Investig, 2009, 119: 1201-1215. DOI:10.1172/JCI37007 |
| [39] |
Ferre P, Foufelle F. Hepatic steatosis:a role for de novo lipogenesis and the transcription factor SREBP-1c[J]. Diabetes Obes Metab, 2010, 12 Suppl 2: 83-92. |
| [40] |
Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism[J]. Curr Diabetes Rep, 2009, 9: 208-214. DOI:10.1007/s11892-009-0034-5 |
| [41] |
Rena G, Guo S, Cichy SC, et al. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B[J]. J Biol Chem, 1999, 274: 17179-17183. DOI:10.1074/jbc.274.24.17179 |
| [42] |
Rena G, Woods YL, Prescott AR, et al. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion[J]. EMBO J, 2002, 21: 2263-2271. DOI:10.1093/emboj/21.9.2263 |
| [43] |
Langlet F, Haeusler RA, Linden D, et al. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling[J]. Cell, 2017, 171: 824-35. DOI:10.1016/j.cell.2017.09.045 |
| [44] |
Qu S, Altomonte J, Perdomo G, et al. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism[J]. Endocrinology, 2006, 147: 5641-5652. DOI:10.1210/en.2006-0541 |
| [45] |
Lin J, Yang R, Tarr PT, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP[J]. Cell, 2005, 120: 261-273. DOI:10.1016/j.cell.2004.11.043 |
 2020, Vol. 55
2020, Vol. 55


