2. 福建医科大学药学院, 福建省药物靶点发现与结构功能研究重点实验室, 福建 福州 350122
2. Fujian Key Laboratory of Drug Target Discovery and Structural and Functional Research, College of Pharmacy, Fujian Medical University, Fuzhou 350122, China
PI3K/Akt/mTOR信号通路是由磷脂酰肌醇-3激酶(PI3Ks)及其下游介质Akt和哺乳动物雷帕霉素靶蛋白(mTOR)构成PI3K/Akt/mTOR信号级联、调节细胞增殖、生存和代谢的核心成分[1], 是连接癌基因和多种受体与许多重要细胞功能的关键信号转导系统, 可能是人类癌症中最常激活的信号通路[2]。磷脂酰肌醇3-激酶(PI3Ks)是PI3K/Akt/mTOR信号通道中的关键激酶, 它整合来自生长因子、细胞因子和其他环境线索的信号, 将其转化为调节多种信号通路的细胞内信号。这些通路控制许多生理功能和细胞过程, 包括细胞增殖、生长、存活、运动和代谢, 且PI3K的激活常见于各种癌症中[2, 3]。PI3Ks作为酪氨酸激酶受体(RTKs)和G蛋白偶联受体(GPCRs)下游的主要效应物, 是PI3K/Akt/mTOR信号通道中关键性激酶, 能够使磷脂酰肌醇4, 5-二磷酸(PIP2)的3′-羟基磷酸化生成磷脂酰肌醇3, 4, 5-三磷酸(PIP3), 这种脂质产物作为“第二信使”将各种生长因子和细胞因子的信号转导到细胞内, 进而激活丝氨酸/苏氨酸激酶Akt等下游效应通路[4]。最终作用细胞核内翻译起始因子4E-BP、S6Ks, 进而影响细胞的翻译、转录、生长和增殖。
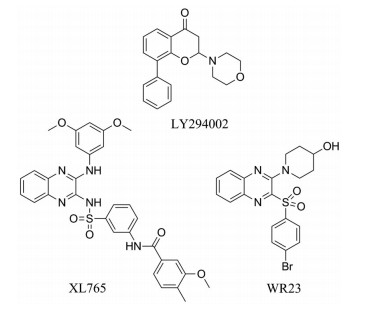
LY294002 (图 1)是第一个可逆的靶向PI3K家族的小分子抑制剂(对PI3Kα、PI3Kδ和PI3Kβ半数抑制率IC50分别为0.50、0.57、0.97 μmol·L-1)。但是两者对单个PI3K亚型几乎没有选择性, 在动物实验中也具有相当大的毒性[5, 6]。XL765 (图 1)是一个含喹喔啉母环的高选择性的强ATP竞争性, 可逆泛I类PI3K抑制剂和mTOR双重抑制剂[7]。它作用于p110α、p110β、p110δ、p110γ和mTOR的IC50分别为39、113、9、43、157 nmol·L-1。XL765的药效学活性与抑制迁移性肿瘤细胞的增殖、抑制肿瘤血管生成、诱导细胞凋亡有关, 在多种人体肿瘤模型中均有明显的肿瘤生长抑制作用[8, 9]。WR23 (图 1)也是含有喹喔啉母核的PI3K抑制剂, 它是由Wu等[10-12]发现的含有吗啉环喹喔啉类PI3Kα抑制剂, 对PI3Kα半数抑制率IC50为0.025 μmol·L-1。Corona等[13]研究表明, 根据电子等排体将2位链接分别替换为O、S时, 同时在60种人类癌细胞上分别测试2位链接为N、O、S 3种类型的新化合物的抑制活性, 研究结果显示O链接活性表现最高。本文以喹喔啉为母核, 以PI3K为作用靶点, 设计并合成了22个喹喔啉类衍生物, 通过1H NMR、13C NMR、ESI-MS进行结构确证, 并对其进行抗肿瘤活性筛选, 初步探究其构效关系, 为该类化合物作为PI3K抑制剂进一步的探索及其结构优化研究提供依据。
|
Figure 1 Chemical structures of LY294002, XL765 and WR23 |
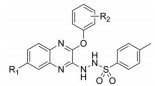
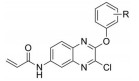
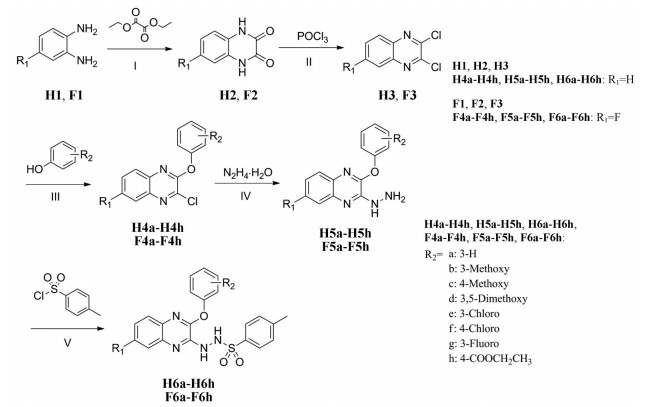
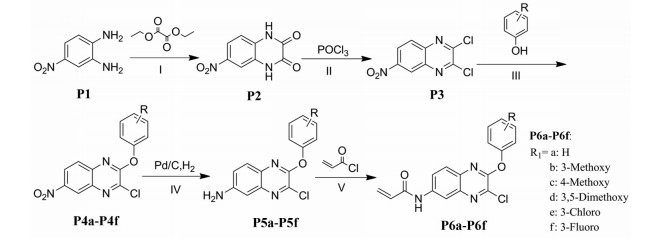
以邻苯二胺和4-氟邻苯二胺为起始原料, 与草酸二乙酯环合得到喹喔啉酮(H2)和6-氟喹喔啉酮(F2), 经三氯氧磷氯化得到2, 3-二氯喹喔啉(H3)和6-氟-2, 3-二氯喹喔啉(F3), 再与取代苯酚在弱碱性条件下发生亲核取代反应生成2-苯氧基类喹喔啉衍生物(H4a~H4h, F4a~F4h), 与水合肼反应后再与对甲苯磺酰氯酰化最终得到16个磺酰肼类喹喔啉衍生物(H6a~H6h, F6a~F6h) (合成路线1)。以4-硝基邻苯二胺为起始原料, 经三氯氧磷氯化和取代苯酚的亲核取代反应得到6-硝基-3-取代苯氧基喹喔啉衍生物(P4a~P4f), 再经Pd/C/H2还原后与丙烯酰氯反应得到6个丙烯酰胺类喹喔啉衍生物(P6a~P6f) (合成路线2)。目标化合物经1H NMR、13C NMR和ESI-MS确证其结构, 数据见实验部分。
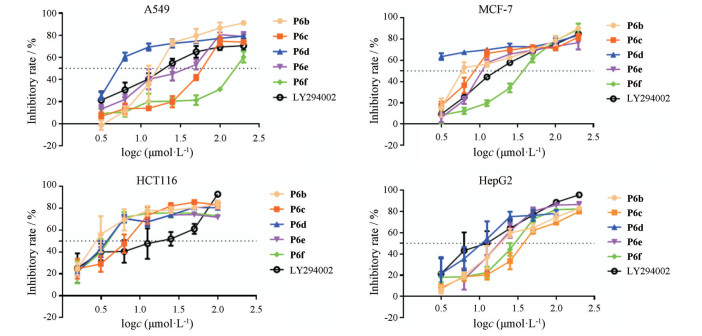
2 生物活性评价以人非小细胞肺癌A549、人乳腺癌细胞MCF-7、人结肠癌细胞HCT-116、人肝癌细胞Hep-G2为实验对象, 通过MTT法对所得22个目标化合物进行体外抗肿瘤细胞增殖实验, 并以LY294002为阳性对照, 结果见表 1、2和图 2。其中P6b、P6e、P6f对HCT116活性较好(IC50=3.24、4.78和4.50 μmol·L-1), P6d对MCF-7具有较强抑制作用(IC50=0.228 7 μmol·L-1)。
| 表 1 Antitumor evaluation of quinoxaline derivatives of aromatic sulfonyl hydrazide in vitro |
| 表 2 Antitumor evaluation of quinoxaline derivatives of acrylamide in vitro |
|
Figure 2 Inhibitory rate curve of compound P6b-P6f, LY294002 on A549, MCF-7, HCT116 and HepG2 |
利用SYBYL/Surflex-Dock模块, 以PI3Kα和抑制剂GCD-0326的晶体复合物(PDB: 5DXT)的蛋白数据为基础, 对化合物进行分子模拟对接进行简单初步分析。首先对配体分子进行Tripos力场能量最小化计算, 最大重复次数(Max Iterations)设置为1 000, Gradient能量差设置为0.005, 得到最低能量构象的小分子用于对接分析。结果表明, 化合物都能与靶点位置形成较好的氢键作用, P6d对接示意图显示(图 3), 苯氧基上3, 5-二甲氧基分别与Lys802、Tyr836形成氢键; 喹喔啉母核与丙烯酰胺分别与Ser774、Thr856形成氢键作用。根据活性筛选结果以及对接结果可得出, 苯氧基上3, 5-二甲氧基取代能明显提高活性, 丙烯酰胺的引入也对活性有一定的影响。
|
Scheme 1 Synthesis of H6a-H6h, F6a-F6h. Reagents and conditions: (Ⅰ) HCl, H2O, 90 ℃, 6 h; (Ⅱ) POCl3, DMF, 110 ℃, 4 h; (Ⅲ) DMF, 70 ℃, 1 h; (Ⅳ) H2O, rt, 24 h; (Ⅴ) DCM, rt, 4 h |
|
Scheme 2 Synthesis of P6a-P6f. Reagents and conditions: (Ⅰ) HCl, H2O, 90 ℃, 6 h; (Ⅱ) POCl3, DMF, 110 ℃, 4 h; (Ⅲ) DMF, 70 ℃, 1 h; (Ⅳ) Ethanol, rt, 4 h; (Ⅴ) DCM, rt, 2 h |

|
Figure 3 Interaction between PI3Kα with P6d (left) and protein surface (right) PDB:5DXT |
本文以喹喔啉母核为结构基础, 在喹喔啉母核上2位引入取代苯氧基片段, 延长3位连接链改为磺酰肼或去掉3位取代, 并在6位加入一系列取代基团, 设计并合成了22个目标化合物, 并通过1H NMR、13C NMR、ESI-MS进行了确证。采用MTT法进行抗肿瘤活性测试, 结果显示丙烯酰胺类喹喔啉衍生物具有相对较好的活性, 并以3, 5-二甲氧基取代(P6d)活性最佳(IC50=0.228 7 μmol·L-1)。利用计算机辅助药物设计进行分子对接分析, 结果显示目标化合物与作用靶点具有较好的结合作用, 生物活性实验也证实了其具有良好的相关性。本研究结果表明, 该类化合物可进行进一步的探索及其结构优化研究, 对针对PI3K信号通路的小分子抗肿瘤药物的开发具有一定的意义和价值。
实验部分RE52-98型旋转蒸发仪; WRS-1B数字熔点仪; 核磁共振波谱仪: Bruker AVANCE Ⅲ 400M、Bruker ascend 500M、Bruker AVANCE ⅢHD 600 MHz; 质谱仪: Thermo Finnigan; Thermol Labsystems酶标仪: Multiskan Mk3; 4-硝基邻苯二胺(G1517071)、苯酚(H1820089)、3-氯苯酚(L1712072)、4-氯苯酚(J1631082)、3-氟苯酚(G1528032)、尼泊金乙酯(K1715100)、对甲基苯磺酰氯(L1606048)、草酸二乙酯(F1526034), 阿拉丁生化科技股份有限公司; 4-氟邻苯二胺(C10078939), 上海麦克林生化科技有限公司; 水合肼(20160510)、三氯氧磷(20180111)、N, N-二甲基甲酰胺(20180117), 国药集团药业股份有限公司; 3-甲氧基苯酚(M71420GGQ0), 天津希恩思生化科技有限公司; 4-甲氧基苯酚(EH030124)、丙烯酰氯(GD270002)、3, 5-二甲氧基苯酚(FJ210258), 萨恩化学技术(上海)有限公司。
1 化合物的合成 4-1-1 1, 4-二氢喹喔啉-2, 3-二酮(H2)的合成邻苯二胺100 mmol、草酸二乙酯100 mmol和4 mol·L-1盐酸水溶液80 mL, 90 ℃搅拌6 h。反应液静置冷却析出紫红色晶体, 抽滤, 水洗, 干燥, 得紫红色晶体14.29 g, 收率88%。同法, 用4-氟邻苯二胺代替邻苯二胺合成F2, 得亮黑色晶体, 收率92%。同法用4-硝基邻苯二胺替代邻苯二胺得到P2, 紫红色固体, 收率95%。
1.2 2, 3-二氯-喹喔啉(H3)的合成1, 4-二氢喹喔啉-2, 3-二酮(H2) 60 mmol、新蒸POCl3 120 mL、10 mL DMF, 回流搅拌4 h。反应液倒入800 mL冰水中, 析出大量灰色固体, 抽滤, 水洗, 干燥后得灰色固体, 正丁醇重结晶得白色固体粉末10.77 g, 收率90%。
同法合成F3, 白色固体粉末, 收率91%; P3, 白色固体粉末, 收率92%。
1.3 2-氯-3-苯氧基喹喔啉(H4a)的合成2, 3-二氯喹喔啉(H3) 6.00 mmol溶于10 mL DMF中, 加K2CO3 7.20 mmol, 滴加入苯酚(6 mmol)的DMF (8 mL)溶液, 70 ℃反应1 h。反应液倒入40 mL饱和食盐水中, 静置, 过滤, 乙醇重结晶得浅黄色固体0.85 g, 收率55%。
同法合成H4b~H4h、F4a~F4h、P4a~P4f。
1.4 2-肼基-3-苯氧基喹喔啉(H5a)的合成2-氯-3-苯氧基喹喔啉2.00 mmol (H4a)、80 mL乙醇、1.5 mL 85%水合肼, 常温搅拌24 h, 过滤得棕黄色固体0.46 mg, 收率91%。同法合成H5b~H5h、F5a~F5h。
4-1-5 N'-(3-苯氧基喹喔啉)-4-甲基苯磺酰肼(H6a)的合成2-肼基-3-苯氧基喹喔啉(H5a) 1.55 mmol溶于30 mL二氯甲烷中, 加入对甲苯磺酰氯1.86 mmol, 吡啶两滴, 常温搅拌4 h, 反应液拌硅胶, 硅胶柱分离, 得浅黄色固体0.41 g, 收率65%, mp: 173.6~175.1 ℃; ESI-MS (m/z): 405.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H), 9.84 (s, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.53~7.45 (m, 2H), 7.39 (dd, J = 12.1, 5.6 Hz, 2H), 7.32 (d, J = 7.8 Hz, 3H), 7.26 (t, J = 7.7 Hz, 2H), 7.22 (d, J = 8.3 Hz, 2H), 2.15 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 152.60, 147.59, 143.44, 143.41, 138.37, 137.78, 135.20, 130.16, 129.38, 128.28, 127.54, 126.60, 126.01, 125.84, 125.69, 122.31, 21.26。
同法合成H6b~H6h、F6a~F6h。
N'-(3-(3-甲氧基苯氧基)喹喔啉-2-基)-4-甲基-苯磺酰肼(H6b), 浅黄色固体, 收率63%, mp: 159.3~161.7 ℃; ESI-MS (m/z): 435.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.95 (d, J = 1.8 Hz, 1H), 9.83 (s, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 7.8 Hz, 2H), 7.38 (d, J = 8.2 Hz, 1H), 7.30 (d, J = 7.3 Hz, 1H), 7.24 (dd, J = 12.5, 8.1 Hz, 3H), 6.91 (dd, J = 14.1, 5.3 Hz, 3H), 3.79 (s, 3H), 2.17 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 160.72, 153.62, 147.47, 143.43, 143.41, 138.38, 137.81, 135.25, 130.55, 129.37, 128.29, 127.56, 126.66, 125.84, 125.70, 114.34, 111.69, 108.26, 55.86, 21.26。
N'-(3-(4-甲氧基苯氧基)喹喔啉-2-基)-4-甲基-苯磺酰肼(H6c), 浅黄色固体, 收率65%, mp: 177.0~178.6 ℃; ESI-MS (m/z): 435.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.93 (s, 1H), 9.81 (s, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 7.8 Hz, 2H), 7.29 (d, J = 7.9 Hz, 1H), 7.24 (dd, J = 14.1, 5.4 Hz, 5H), 7.03 (d, J = 9.0 Hz, 2H), 3.80 (s, 3H), 2.16 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 157.12, 147.95, 145.79, 143.41, 143.40, 138.28, 137.79, 135.29, 129.37, 128.28, 127.38, 126.53, 125.81, 125.64, 123.27, 115.03, 55.87, 21.26。
N'-(3-(3, 5-甲氧基苯氧基)喹喔啉-2-基)-4-甲基-苯磺酰肼(H6d), 浅黄色固体, 收率65%, mp: 135.1~137.4 ℃; ESI-MS (m/z): 465.15 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H), 9.81 (s, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.1 Hz, 1H), 7.41 (d, J = 6.9 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.25 (d, J = 9.8 Hz, 2H), 7.22 (s, 1H), 6.54 (d, J = 2.1 Hz, 2H), 6.46 (t, J = 2.1 Hz, 1H), 3.76 (s, 6H), 2.17 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 161.34, 154.24, 147.35, 143.40, 143.39, 138.37, 137.85, 135.26, 129.37, 128.28, 127.58, 126.72, 125.82, 125.70, 100.81, 98.01, 55.96, 21.27。
N'-(3-(3-氯苯氧基)喹喔啉-2-基)-4-甲基-苯磺酰肼(H6e), 浅黄色固体, 收率67%, mp: 148.7~150.9 ℃; ESI-MS (m/z): 439.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.99 (d, J = 2.0 Hz, 1H), 9.86 (d, J = 1.7 Hz, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.57~7.50 (m, 2H), 7.47~7.38 (m, 3H), 7.36 (dd, J = 8.1, 2.2 Hz, 1H), 7.33~7.28 (m, 1H), 7.28~7.24 (m, 1H), 7.22 (d, J = 8.1 Hz, 2H), 2.16 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 153.32, 147.18, 143.41, 143.32, 138.53, 137.81, 135.03, 133.91, 131.55, 129.38, 128.29, 127.77, 126.70, 126.13, 125.86, 125.76, 122.61, 121.24, 21.26。
N'-(3-(4-氯苯氧基)喹喔啉-2-基)-4-甲基-苯磺酰肼(H6f), 浅黄色固体, 收率62%, mp: 189.2~189.8 ℃; ESI-MS (m/z): 439.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.96 (d, J = 2.1 Hz, 1H), 9.85 (d, J = 2.0 Hz, 1H), 7.76 (d, J = 8.2 Hz, 2H), 7.56~7.52 (m, 2H), 7.40 (s, 1H), 7.39 (d, J = 2.4 Hz, 2H), 7.37 (s, 1H), 7.31~7.26 (m, 1H), 7.24 (d, J = 8.3 Hz, 1H), 7.20 (d, J = 8.1 Hz, 2H), 2.14 (s, 3H); 13CNMR (151 MHz, DMSO-d6) δ 151.35, 147.32, 143.42, 143.34, 138.46, 137.79, 135.06, 130.05, 129.38, 128.28, 127.70, 126.64, 125.85, 125.75, 124.27, 21.26。
N'-(3-(3-氟苯氧基)喹喔啉-2-基)-4-甲基-苯磺酰肼(H6g), 浅黄色固体, 收率68%, mp: 146.3~148.5 ℃; ESI-MS (m/z): 424.10 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 9.98 (d, J = 1.9 Hz, 1H), 9.86 (d, J = 1.8 Hz, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.54 (dd, J = 15.2, 8.2 Hz, 1H), 7.43 (t, J = 7.4 Hz, 2H), 7.37~7.28 (m, 2H), 7.26 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 8.1 Hz, 3H), 7.20~7.14 (m, 1H), 2.16 (s, 3H); 13CNMR (126 MHz, DMSO-d6) δ 162.80 (d, J = 244.6 Hz), 153.60 (d, J = 11.3 Hz), 147.15, 143.42, 143.36, 138.54, 137.83, 135.08, 131.36 (d, J = 9.5 Hz), 129.39, 128.29, 127.78, 126.73, 125.87, 125.77, 118.49 (d, J = 3.0 Hz), 112.95 (d, J = 20.9 Hz), 110.21 (d, J = 24.5 Hz), 21.25。
4-((3-对甲苯磺酰肼)喹喔啉-2-基)氧基苯甲酸乙酯(H6h), 白色固体, 收率65%, mp: 154.0~156.1 ℃; ESI-MS (m/z): 477.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 10.00 (d, J = 2.2 Hz, 1H), 9.92 (d, J = 2.1 Hz, 1H), 8.09 (d, J = 8.7 Hz, 2H), 7.78 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.7 Hz, 2H), 7.48~7.39 (m, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.27 (d, J = 8.2 Hz, 1H), 7.22 (d, J = 8.1 Hz, 2H), 4.35 (q, J = 7.1 Hz, 2H), 2.16 (s, 3H), 1.35 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, DMSO-d6) δ 165.55, 156.49, 146.96, 143.43, 138.59, 137.77, 134.98, 132.16, 131.41, 129.38, 129.13, 128.28, 127.90, 127.43, 126.72, 122.42, 61.30, 21.26, 14.66。
N'-(3-苯氧基-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6a), 白色固体, 收率70%, mp: 183.5~184.4 ℃; ESI-MS (m/z): 423.00 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.97 (s, 1H), 9.87 (s, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.50 (t, J = 7.8 Hz, 2H), 7.33 (d, J = 7.2 Hz, 4H), 7.27 (d, J = 6.1 Hz, 1H), 7.24 (s, 1H), 7.23~7.17 (m, 2H), 2.17 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 159.80 (d, J = 242.0 Hz), 152.46, 148.39, 143.43, 143.08, 137.82, 135.78 (d, J = 13.0 Hz), 135.30, 130.20, 129.38, 128.29, 127.43 (d, J = 9.7 Hz), 126.17, 122.30, 116.29 (d, J = 24.0 Hz), 111.14 (d, J = 22.8 Hz), 21.26。
N'-(3-(3-甲氧基苯氧基)-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6b), 白色固体, 收率75%, mp: 177.6~180.4 ℃; ESI-MS (m/z): 453.05 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.93 (s, 1H), 9.83 (s, 1H), 7.78 (d, J = 7.5 Hz, 2H), 7.40 (t, J = 8.0 Hz, 1H), 7.36~7.30 (m, 1H), 7.30~7.26 (m, 1H), 7.24 (d, J = 7.3 Hz, 3H), 6.97~6.86 (m, 3H), 3.80 (s, 3H), 2.19 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 159.81 (d, J = 239.0 Hz), 153.46, 148.28, 143.42, 143.07, 137.85, 135.82 (d, J = 12.8 Hz), 135.32, 130.59, 129.38, 129.13, 128.30, 127.42 (d, J = 9.7 Hz), 116.31 (d, J = 24.3 Hz), 114.32, 111.90, 111.20 (d, J = 22.4 Hz), 108.26, 55.89, 21.25。
N'-(3-(4-甲氧基苯氧基)-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6c), 白色固体, 收率72%, mp: 171.1~172.8 ℃; ESI-MS (m/z): 453.05 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.92 (s, 1H), 9.83 (s, 1H), 7.78 (d, J = 7.6 Hz, 2H), 7.29 (d, J = 8.0 Hz, 3H), 7.26 (d, J = 13.3 Hz, 2H), 7.20 (d, J = 9.3 Hz, 4H), 2.36 (s, 3H), 2.18 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 159.80 (d, J = 241.8 Hz), 150.18, 148.56, 143.41, 143.07, 137.82, 135.85 (d, J = 12.8 Hz), 135.32, 135.25, 130.54, 129.37, 129.13, 128.29, 127.40 (d, J = 9.8 Hz), 122.05, 116.17 (d, J = 24.5 Hz), 111.10 (d, J = 22.5 Hz), 20.92。
N'-(3-(3, 5-二甲氧基苯氧基)-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6d), 白色固体, 收率75%, mp: 186.4~187.1 ℃; ESI-MS (m/z): 483.10 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H), 9.82 (s, 1H), 7.77 (d, J = 8.1 Hz, 2H), 7.32 (t, J = 7.6 Hz, 1H), 7.27 (dd, J = 13.2, 7.4 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 6.54 (s, 2H), 6.46 (s, 1H), 3.76 (s, 6H), 2.19 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 161.37, 159.80 (d, J = 242.2 Hz), 154.05, 148.16, 143.40, 143.01, 137.88, 135.78, 135.30, 129.37, 128.29, 127.41, 116.40, 111.34, 100.82, 98.18, 55.97, 21.26。
N'-(3-(3-氯苯氧基)-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6e), 白色固体, 收率70%, mp: 186.1~186.7 ℃; ESI-MS (m/z): 457.00 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.96 (s, 1H), 7.78 (d, J = 7.3 Hz, 2H), 7.57~7.50 (m, 2H), 7.41 (d, J = 8.0 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.33 (d, J = 8.3 Hz, 1H), 7.27 (t, J = 7.9 Hz, 2H), 7.23 (d, J = 7.7 Hz, 2H), 2.18 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 159.83 (d, J = 242.2 Hz), 153.17, 148.02, 143.43, 142.97, 137.84, 135.62 (d, J = 12.9 Hz), 135.49, 133.97, 131.58, 129.38, 128.31, 127.46 (d, J = 9.8 Hz), 126.30, 122.65, 121.26, 116.54 (d, J = 24.5 Hz), 111.25 (d, J = 22.5 Hz), 21.25。
N'-(3-(4-氯苯氧基)-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6f), 白色固体, 收率70%, mp: 199.4~200.2 ℃; ESI-MS (m/z): 457.00 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H), 9.87 (s, 1H), 7.78 (d, J = 7.9 Hz, 2H), 7.56 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 7.33 (t, J = 8.7 Hz, 1H), 7.29 (d, J = 6.2 Hz, 1H), 7.23 (d, J = 7.8 Hz, 3H), 2.18 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 159.83 (d, J = 242.0 Hz), 151.21, 148.14, 143.43, 142.98, 137.82, 135.64 (d, J = 12.7 Hz), 135.42, 130.24, 130.10, 129.38, 128.30 (s), 127.46 (d, J = 9.8 Hz), 124.28, 116.46 (d, J = 24.5 Hz), 111.18 (d, J = 22.9 Hz), 21.25。
N'-(3-(3-氟苯氧基)-7-氟喹喔啉-2-基)-4-甲基-苯磺酰肼(F6g), 白色固体, 收率72%, mp: 174.2~175.6 ℃; ESI-MS (m/z): 441.00 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.99 (s, 1H), 9.88 (s, 1H), 7.77 (d, J = 7.9 Hz, 2H), 7.54 (dd, J = 15.3, 8.1 Hz, 1H), 7.34 (t, J = 9.8 Hz, 2H), 7.27 (d, J = 5.1 Hz, 1H), 7.26~7.15 (m, 5H), 2.18 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 162.80 (d, J = 244.7 Hz), 159.84 (d, J = 242.2 Hz), 153.40 (d, J = 11.3 Hz), 147.97, 143.43, 142.96, 137.85, 135.63 (d, J = 12.8 Hz), 135.47, 131.40 (d, J = 9.6 Hz), 129.39, 128.30, 127.46 (d, J = 9.9 Hz), 118.51 (d, J = 3.1 Hz), 116.54 (d, J = 24.4 Hz), 113.13 (d, J = 21.0 Hz), 111.25 (d, J = 22.5 Hz), 110.27 (d, J = 24.6 Hz), 21.25。
4-甲基-((3-(2-苯磺酰肼)-6-氟喹喔啉-2-基)-4-氧基)苯磺酰肼(F6h), 白色固体, 收率70%, mp: 172.2~173.8 ℃; ESI-MS (m/z): 495.05 [M-H]-; 1H NMR (400 MHz, DMSO-d6) δ 9.97 (s, 1H), 9.92 (s, 1H), 8.09 (d, J = 7.8 Hz, 2H), 7.78 (d, J = 7.5 Hz, 2H), 7.52 (d, J = 7.6 Hz, 2H), 7.34 (t, J = 9.4 Hz, 1H), 7.30 (d, J = 6.5 Hz, 1H), 7.25 (s, 1H), 7.23 (d, J = 7.9 Hz, 2H), 4.36 (q, J = 13.9, 7.0 Hz, 2H), 2.18 (s, 3H), 1.36 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) δ 165.55, 159.84 (d, J = 242.3 Hz), 156.29, 147.81, 143.45, 143.01, 137.82, 135.57 (d, J = 7.6 Hz), 135.54, 131.43, 129.39, 128.30, 127.64, 127.50 (d, J = 9.9 Hz), 122.49, 116.69 (d, J = 24.6 Hz), 111.23 (d, J = 22.5 Hz), 61.32, 21.25, 14.66。
1.6 3-氯-2-苯氧基-6-氨基喹喔啉(P5a)的合成3-氯-2-苯氧基-6-硝基喹喔啉6.00 mmol (P4a), 100 mL乙醇, 加入5%钯炭, N2将空气排尽, 再缓慢通入H2, 常温搅拌过夜, 反应液过滤, 滤液拌硅胶, 硅胶柱分离, 得亮黄色固体粉末, 收率41%。
同法合成P5b~P5f。
1.7 N-(2-苯氧基-3-氯喹喔啉-6基)丙烯酰胺(P6a)的合成3-氯-2-苯氧基-6-氨基喹喔啉0.30 mmol (P5a), 10 mL二氯甲烷, 于0 ℃下加入丙烯酰氯0.45 mmol, 移置室温搅拌2 h, 反应液, 硅胶柱分离, 得浅黄色固体(P6a), 收率65%, mp: 154.8~156.2 ℃, ESI-MS (m/z): 324.10 [M-H]-, 1H NMR (400 MHz, DMSO-d6) δ 11.11 (s, 1H), 8.99 (s, 1H), 8.40 (d, J = 9.2 Hz, 1H), 8.20 (d, J = 9.0 Hz, 1H), 8.01 (d, J = 7.6 Hz, 2H), 7.90~7.83 (m, 3H), 7.00 (dd, J = 16.9, 10.0 Hz, 1H), 6.86 (d, J = 16.5 Hz, 1H), 6.36 (d, J = 10.1 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 164.13, 153.05, 152.38, 139.65, 139.58, 139.49, 135.56, 131.96, 130.31, 128.26, 127.69, 126.22, 124.41, 122.03, 115.60。
同法合成P6b~P6f。
N-(2-(3-甲氧基苯氧基)-3-氯喹喔啉-6基)丙烯酰胺(P6b), 浅黄色固体, 收率65%, mp: 151.4~152.7 ℃, ESI-MS (m/z): 354.00 [M-H]-, 1H NMR (400 MHz, DMSO-d6) δ 10.59 (s, 1H), 8.49 (s, 1H), 7.91 (d, J = 9.1 Hz, 1H), 7.73 (d, J = 8.3 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 6.96 (d, J = 12.3 Hz, 1H), 6.92 (s, 2H), 6.50 (dd, J = 16.5, 10.1 Hz, 1H), 6.36 (d, J = 16.7 Hz, 1H), 5.86 (d, J = 9.8 Hz, 1H), 3.79 (s, 3H); 13CNMR (126 MHz, DMSO-d6) δ 164.13, 160.91, 154.08, 152.32, 139.62, 139.59, 139.50, 135.62, 131.96, 130.70, 128.27, 127.74, 124.41, 115.58, 114.02, 112.01, 107.99, 55.89。
N-(2-(4-甲氧基苯氧基)-3-氯喹喔啉-6基)丙烯酰胺(P6c), 浅黄色固体, 收率67%, mp: 158.4~159.7 ℃, ESI-MS (m/z): 354.00 [M-H]-, 1H NMR (400 MHz, DMSO-d6) δ 10.58 (s, 1H), 8.47 (s, 1H), 7.90 (d, J = 8.9 Hz, 1H), 7.69 (d, J = 9.2 Hz, 1H), 7.28 (d, J = 7.7 Hz, 2H), 7.05 (d, J = 7.9 Hz, 2H), 6.50 (dd, J = 16.8, 10.0 Hz, 1H), 6.35 (d, J = 16.9 Hz, 1H), 5.85 (d, J = 10.0 Hz, 1H), 3.82 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 164.11, 157.31, 152.73, 146.25, 139.59, 139.42, 139.34, 135.60, 131.97, 128.22, 127.60, 124.34, 123.05, 115.61, 115.19, 55.91。
N-(2-(3, 5-二甲氧基苯氧基)-3-氯喹喔啉-6基)丙烯酰胺(P6d), 浅黄色固体, 收率60%, mp: 147.9~149.5 ℃, ESI-MS (m/z): 384.00 [M-H]-, 1H NMR (400 MHz, DMSO-d6) δ 10.60 (s, 1H), 8.49 (s, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.77 (d, J = 9.1 Hz, 1H), 6.56 (s, 2H), 6.55~6.48 (m, 1H), 6.47 (s, 1H), 6.36 (d, J = 16.8 Hz, 1H), 5.86 (d, J = 10.3 Hz, 1H), 3.77 (s, 6H); 13C NMR (126 MHz, DMSO-d6) δ 164.14, 161.55, 154.74, 152.25, 139.61, 139.52, 135.69, 131.96, 131.90, 128.28, 127.82, 124.42, 115.57, 100.54, 98.30, 55.98。
N-(2-(3-氯苯氧基)-3-氯喹喔啉-6基)丙烯酰胺(P6e), 浅黄色固体, 收率70%, mp: 174.5~176.2 ℃, ESI-MS (m/z): 359.95 [M-H]-, 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 8.50 (s, 1H), 7.91 (d, J = 9.2 Hz, 1H), 7.75 (d, J = 9.2 Hz, 1H), 7.63~7.48 (m, 2H), 7.40 (dd, J = 18.3, 8.1 Hz, 2H), 6.50 (dd, J = 16.8, 10.1 Hz, 1H), 6.36 (d, J = 16.8 Hz, 1H), 5.86 (d, J = 10.3 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 164.17, 153.78, 152.17, 139.78, 139.67, 139.54, 135.45, 134.10, 131.95, 131.73, 128.34, 127.81, 126.39, 124.49, 122.47, 121.05, 115.56。
N-(2-(3-氟苯氧基)-3-氯喹喔啉-6基)丙烯酰胺(P6f), 浅黄色固体, 收率68%, mp: 167.8~168.5 ℃, ESI-MS (m/z): 341.95 [M-H]-, 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 8.50 (s, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 9.0 Hz, 1H), 7.56 (dd, J = 15.3, 8.0 Hz, 1H), 7.36 (d, J = 9.8 Hz, 1H), 7.22 (dd, J = 19.1, 8.9 Hz, 2H), 6.50 (dd, J = 16.9, 10.1 Hz, 1H), 6.36 (d, J = 16.6 Hz, 1H), 5.86 (d, J = 10.1 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 164.16, 162.92 (d, J = 244.9 Hz), 154.04 (d, J = 11.4 Hz), 152.08, 139.78, 139.69, 139.53, 135.47, 131.95, 131.55 (d, J = 9.6 Hz), 128.32, 127.82, 124.49, 118.27, 115.55, 113.20 (d, J = 20.9 Hz), 110.03 (d, J = 24.6 Hz)。
1.8 中间体核磁共振氢谱2, 3-二氯喹喔啉(H3), 1H NMR (400 MHz, DMSO-d6) δ 8.13~8.05 (m, 2H), 7.99~7.92 (m, 2H)。
2-氯-3-苯氧基喹喔啉(H4a), 1H NMR (400 MHz, DMSO-d6) δ 8.06~7.98 (m, 1H), 7.80~7.66 (m, 3H), 7.53 (t, J = 7.9 Hz, 2H), 7.36 (dd, J = 15.0, 7.7 Hz, 3H)。
2-肼基-3-苯氧基喹喔啉(H5a), 1H NMR (400 MHz, DMSO-d6) δ 8.95 (s, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 7.9 Hz, 2H), 7.46~7.39 (m, 2H), 7.32 (dd, J = 13.1, 7.6 Hz, 3H), 7.24 (t, J = 7.1 Hz, 1H), 4.59 (s, 2H)。
2-氯-3-(3-甲氧基苯氧基)喹喔啉(H4b), 1H NMR (400 MHz, DMSO-d6) δ 8.14~7.88 (m, 1H), 7.80~7.71 (m, 3H), 7.41 (t, J = 8.2 Hz, 1H), 7.00 (t, J = 2.2 Hz, 1H), 6.93 (td, J = 8.6, 2.2 Hz, 2H), 3.78 (s, 3H)。
2-肼基-3-(3-甲氧基苯氧基)喹喔啉(H5b), 1H NMR (400 MHz, DMSO-d6) δ 8.92 (s, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.45~7.40 (m, 2H), 7.38 (t, J = 8.2 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 6.92 (t, J = 5.2 Hz, 2H), 6.90~6.85 (m, 1H), 4.57 (s, 2H), 3.78 (s, 3H)。
2-氯-3-(4-甲氧基苯氧基)喹喔啉(H4c), 1H NMR (400 MHz, DMSO-d6) δ 8.01 (d, J = 7.5 Hz, 1H), 7.74 (dt, J = 14.6, 7.4 Hz, 3H), 7.29 (d, J = 9.0 Hz, 2H), 7.05 (d, J = 9.0 Hz, 2H), 3.81 (s, 3H)。
2-肼基-3-(4-甲氧基苯氧基)喹喔啉(H5c), 1H NMR (400 MHz, DMSO-d6) δ 8.91 (s, 1H), 7.59 (d, J = 7.9 Hz, 1H), 7.41 (t, J = 8.5 Hz, 2H), 7.24 (dd, J = 12.7, 6.3 Hz, 3H), 7.03 (d, J = 9.0 Hz, 2H), 4.57 (s, 2H), 3.80 (s, 3H)。
2-氯-3-(3, 5-二甲氧基苯氧基)喹喔啉(H4d), 1H NMR (400 MHz, DMSO-d6) δ 8.02 (d, J = 6.9 Hz, 1H), 7.83~7.68 (m, 3H), 6.58 (d, J = 2.0 Hz, 2H), 6.48 (s, 1H), 3.76 (s, 6H)。
2-肼基-3-(3, 5-二甲氧基苯氧基)喹喔啉(H5d), 1H NMR (400 MHz, DMSO-d6) δ 8.91 (s, 1H), 7.60 (d, J = 8.1 Hz, 1H), 7.45 (dd, J = 17.1, 7.6 Hz, 2H), 7.26 (dd, J = 11.0, 4.1 Hz, 1H), 6.55 (d, J = 2.2 Hz, 2H), 6.45 (t, J = 2.2 Hz, 1H), 4.57 (s, 2H), 3.76 (s, 6H)。
2-氯-3-(3-氯苯氧基)喹喔啉(H4e), 1H NMR (400 MHz, DMSO-d6) δ 8.03 (d, J = 8.3 Hz, 1H), 7.76 (d, J = 6.1 Hz, 2H), 7.59 (d, J = 2.1 Hz, 1H), 7.55 (d, J = 8.1 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.39 (dd, J = 8.2, 2.2 Hz, 1H)。
2-肼基-3-(3-氯苯氧基)喹喔啉(H5e), 1H NMR (400 MHz, DMSO-d6) δ 8.97 (s, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.53 (t, J = 8.2 Hz, 2H), 7.45 (d, J = 7.9 Hz, 2H), 7.38 (t, J = 9.2 Hz, 2H), 7.25 (t, J = 7.7 Hz, 1H), 4.60 (s, 2H)。
2-氯-3-(3-氯苯氧基)喹喔啉(H4f), 1H NMR (400 MHz, DMSO-d6) δ 8.03 (d, J = 7.7 Hz, 1H), 7.80~7.71 (m, 3H), 7.60~7.57 (m, 2H), 7.47~7.41 (m, 2H)。
2-肼基-3-(4-氯苯氧基)喹喔啉(H5f), 1H NMR (400 MHz, DMSO-d6) δ 8.98 (s, 1H), 7.61 (d, J = 8.6 Hz, 1H), 7.58~7.53 (m, 2H), 7.43 (d, J = 7.6 Hz, 2H), 7.42~7.39 (m, 2H), 7.25 (dd, J = 11.1, 4.0 Hz, 1H), 4.59 (s, 2H)。
2-氯-3-(3-氟苯氧基)喹喔啉(H4g), 1H NMR (400 MHz, DMSO-d6) δ 8.04 (d, J = 8.5 Hz, 1H), 7.78 (s, 1H), 7.70 (dd, J = 6.2, 3.5 Hz, 1H), 7.62~7.54 (m, 2H), 7.39 (dd, J = 8.9, 6.9 Hz, 1H), 7.29 (d, J = 10.4 Hz, 1H), 7.21 (t, J = 8.5 Hz, 1H)。
2-肼基-3-(3-氟苯氧基)喹喔啉(H5g), 1H NMR (400 MHz, DMSO-d6) δ 8.97 (s, 1H), 7.61 (d, J = 7.4 Hz, 1H), 7.54 (dd, J = 15.1, 8.1 Hz, 1H), 7.48~7.42 (m, 2H), 7.34 (d, J = 10.1 Hz, 1H), 7.25 (dd, J = 13.3, 7.0 Hz, 2H), 7.18 (t, J = 8.4 Hz, 1H), 4.59 (s, 2H)。
4-((3-氯喹喔啉-2-基)氧基)苯甲酸乙酯(H4h), 1H NMR (400 MHz, DMSO-d6) δ 8.13~8.09 (m, 2H), 8.04 (dd, J = 6.7, 2.8 Hz, 1H), 7.82~7.72 (m, 3H), 7.57~7.52 (m, 2H), 4.36 (q, J = 7.1 Hz, 2H), 1.35 (t, J = 7.1 Hz, 3H)。
4-((3-肼基喹喔啉-2-基)氧基)苯甲酸乙酯(H5h), 1H NMR (400 MHz, DMSO-d6) δ 9.02 (s, 1H), 8.08 (d, J = 8.6 Hz, 2H), 7.62 (d, J = 8.2 Hz, 1H), 7.51 (d, J = 8.6 Hz, 2H), 7.48~7.41 (m, 2H), 7.27 (d, J = 7.0 Hz, 1H), 4.60 (s, 2H), 4.34 (q, J = 7.1 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H)。
2, 3-二氯-6-氟喹喔啉(F3), 1H NMR (400 MHz, DMSO-d6) δ 8.19 (dd, J = 7.7, 6.1 Hz, 1H), 7.96 (d, J = 9.2 Hz, 1H), 7.90 (t, J = 8.9 Hz, 1H)。
3-氯-6-氟-2-苯氧基喹喔啉(F4a), 1H NMR (400 MHz, DMSO-d6) δ 8.10 (t, J = 7.0 Hz, 1H), 7.66 (t, J = 8.8 Hz, 2H), 7.54 (dd, J = 14.6, 8.0 Hz, 3H), 7.39 (d, J = 6.9 Hz, 2H), 7.34 (s, 1H)。
3-肼基-6-氟-2-苯氧基喹喔啉(F5a), 1H NMR (400 MHz, DMSO-d6) δ 8.95 (s, 1H), 7.66~7.60 (m, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.42~7.26 (m, 4H), 7.22 (d, J = 10.2 Hz, 1H), 4.57 (s, 2H)。
3-氯-2-(3-甲氧基苯氧基)-6-氟喹喔啉(F4b), 1H NMR (400 MHz, DMSO-d6) δ 8.10 (dd, J = 9.2, 5.9 Hz, 1H), 7.66 (td, J = 8.8, 2.8 Hz, 1H), 7.60 (dd, J = 9.8, 2.7 Hz, 1H), 7.42 (t, J = 8.2 Hz, 1H), 7.00 (t, J = 2.3 Hz, 1H), 6.93 (td, J = 7.8, 2.3 Hz, 2H), 3.78 (s, 3H)。
3-肼基-2-(3-甲氧基苯氧基)-6-氟喹喔啉(F5b), 1H NMR (400 MHz, DMSO-d6) δ 8.93 (s, 1H), 7.62 (dd, J = 9.0, 5.9 Hz, 1H), 7.39 (t, J = 8.2 Hz, 1H), 7.32 (td, J = 8.8, 2.9 Hz, 1H), 7.25 (dd, J = 9.8, 2.9 Hz, 1H), 6.96~6.87 (m, 3H), 4.56 (s, 2H), 3.78 (s, 3H)。
3-氯-2-(4-甲氧基苯氧基)-6-氟喹喔啉(F4c), 1H NMR (400 MHz, DMSO-d6) δ 8.21 (dd, J = 9.3, 5.9 Hz, 1H), 8.03~7.96 (m, 1H), 7.69~7.61 (m, 1H), 7.31 (d, J = 8.2 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H), 2.37 (s, 3H)。
3-肼基-2-(4-甲氧基苯氧基)-6-氟喹喔啉(F5c), 1H NMR (400 MHz, DMSO-d6) δ 8.94 (s, 1H), 7.61 (dd, J = 9.0, 5.9 Hz, 1H), 7.35~7.30 (m, 1H), 7.30~7.26 (m, 2H), 7.24~7.15 (m, 3H), 4.55 (s, 2H), 2.36 (s, 3H)。
3-氯-2-(3, 5-二甲氧基苯氧基)-6-氟喹喔啉(F4d), 1H NMR (400 MHz, DMSO-d6) δ 8.20~7.99 (m, 1H), 7.73~7.53 (m, 1H), 6.57 (s, 2H), 6.47 (s, 1H), 3.75 (s, 6H)。
3-肼基-2-(3, 5-二甲氧基苯氧基)-6-氟喹喔啉(F5d), 1H NMR (400 MHz, DMSO-d6) δ 8.88 (s, 1H), 7.69~7.55 (m, 1H), 7.30 (dd, J = 16.4, 9.0 Hz, 2H), 6.55 (s, 2H), 6.45 (s, 1H), 4.53 (s, 2H), 3.75 (s, 6H)。
3-氯-2-(3-氯苯氧基)-6-氟喹喔啉(F4e), 1H NMR (400 MHz, DMSO-d6) δ 8.11 (dd, J = 9.1, 5.8 Hz, 1H), 7.72~7.65 (m, 1H), 7.62 (dd, J = 9.7, 2.7 Hz, 1H), 7.58 (t, J = 2.0 Hz, 1H), 7.55 (d, J = 8.1 Hz, 1H), 7.47~7.42 (m, 1H), 7.39 (dd, J = 7.8, 1.8 Hz, 1H)。
3-肼基-2-(3-氯苯氧基)-6-氟喹喔啉(F5e), 1H NMR (400 MHz, DMSO-d6) δ 8.96 (s, 1H), 7.67~7.61 (m, 1H), 7.54 (t, J = 8.1 Hz, 2H), 7.43~7.36 (m, 2H), 7.33 (d, J = 9.4 Hz, 1H), 7.27 (d, J = 9.7 Hz, 1H), 4.62 (s, 2H)。
3-氯-2-(3-氯苯氧基)-6-氟喹喔啉(F4f), 1H NMR (400 MHz, DMSO-d6) δ 8.10 (dd, J = 9.2, 5.8 Hz, 1H), 7.67 (td, J = 8.8, 2.8 Hz, 1H), 7.60 (dd, J = 6.6, 4.5 Hz, 3H), 7.47~7.40 (m, 2H)。
3-肼基-2-(3-氯苯氧基)-6-氟喹喔啉(F5f), 1H NMR (400 MHz, DMSO-d6) δ 8.98 (s, 1H), 7.67~7.59 (m, 1H), 7.56 (d, J = 8.7 Hz, 2H), 7.41 (d, J = 8.8 Hz, 2H), 7.37~7.30 (m, 1H), 7.25 (d, J = 10.2 Hz, 1H), 4.65 (s, 2H)。
3-氯-2-(3-氟苯氧基)-6-氟喹喔啉(F4g), 1H NMR (400 MHz, DMSO-d6) δ 8.15~8.08 (m, 1H), 7.69 (t, J = 8.9 Hz, 1H), 7.62 (d, J = 9.8 Hz, 1H), 7.60~7.54 (m, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.29~7.21 (m, 2H)。
3-肼基-2-(3-氟苯氧基)-6-氟喹喔啉(F5g), 1H NMR (400 MHz, DMSO-d6) δ 8.97 (s, 1H), 7.70~7.58 (m, 1H), 7.54 (t, J = 8.1 Hz, 2H), 7.44~7.36 (m, 2H), 7.36~7.31 (m, 1H), 7.27 (d, J = 10.8 Hz, 1H), 4.65 (s, 2H)。
4-((3-氯-6-氟喹喔啉-2-基)氧基)苯甲酸乙酯(F4h), 1H NMR (400 MHz, DMSO-d6) δ 8.12 (t, J = 7.5 Hz, 3H), 7.69 (t, J = 8.8 Hz, 1H), 7.56 (t, J = 10.1 Hz, 3H), 4.36 (q, J = 6.9 Hz, 2H), 1.36 (t, J = 7.0 Hz, 3H)。
4-[(3-肼基-6-氟喹喔啉-2-基)氧基]苯甲酸乙酯(F5h), 1H NMR (400 MHz, DMSO-d6) δ 9.00 (s, 1H), 8.17~8.04 (m, 2H), 7.73~7.66 (m, 1H), 7.66~7.58 (m, 1H), 7.58~7.49 (m, 2H), 7.40~7.21 (m, 1H), 4.59 (s, 2H), 4.37 (q, 2H), 1.36 (t, J = 7.1 Hz, 3H)。
2, 3-二氯-6-硝基喹喔啉(P3), 1H NMR (400 MHz, DMSO-d6) δ 8.95~8.86 (m, 1H), 8.62 (dd, J = 9.2, 0.7 Hz, 1H), 8.33 (d, J = 9.2 Hz, 1H)。
3-氯-6-硝基-2-苯氧基喹喔啉(P4a), 1H NMR (400 MHz, DMSO-d6) δ 8.83 (d, J = 2.4 Hz, 1H), 8.44 (dd, J = 9.2, 2.6 Hz, 1H), 7.90 (d, J = 9.2 Hz, 1H), 7.59~7.50 (m, 2H), 7.41 (d, J = 7.7 Hz, 2H), 7.37 (d, J = 7.4 Hz, 1H)。
3-氯-2-(3-甲氧基苯氧基)-6-硝基喹喔啉(P4b), 1H NMR (400 MHz, DMSO-d6) δ 8.84 (d, J = 2.5 Hz, 1H), 8.45 (dd, J = 9.2, 2.6 Hz, 1H), 7.94 (d, J = 9.2 Hz, 1H), 7.44 (t, J = 8.2 Hz, 1H), 7.02 (t, J = 2.3 Hz, 1H), 7.00~6.93 (m, 2H), 3.78 (s, 3H)。
3-氯-2-(4-甲氧基苯氧基)-6-硝基喹喔啉(P4c), 1H NMR (400 MHz, DMSO-d6) δ 8.82 (s, 1H), 8.47~8.41 (m, 1H), 7.90 (d, J = 9.2 Hz, 1H), 7.32 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 9.0 Hz, 2H), 3.82 (s, 3H)。
3-氯-2-(3, 5-二甲氧基苯氧基)-6-硝基喹喔啉(P4d), 1H NMR (400 MHz, DMSO-d6) δ 8.84 (d, J = 2.5 Hz, 1H), 8.45 (dd, J = 9.2, 2.6 Hz, 1H), 7.98 (d, J = 9.2 Hz, 1H), 6.62 (s, 1H), 6.61 (s, 1H), 6.51 (t, J = 2.2 Hz, 1H), 3.77 (s, 6H)。
3-氯-2-(3-氯苯氧基)-6-硝基喹喔啉(P4e), 1H NMR (400 MHz, DMSO-d6) δ 8.85 (d, J = 2.5 Hz, 1H), 8.46 (dd, J = 9.2, 2.6 Hz, 1H), 7.96 (d, J = 9.2 Hz, 1H), 7.62~7.60 (m, 1H), 7.58 (d, J = 8.1 Hz, 1H), 7.47 (ddd, J = 8.1, 2.0, 0.9 Hz, 1H), 7.42 (ddd, J = 8.2, 2.2, 0.9 Hz, 1H)。
3-氯-2-(3-氟苯氧基)-6-硝基喹喔啉(P4f), 1H NMR (400 MHz, DMSO-d6) δ 8.85 (d, J = 2.5 Hz, 1H), 8.46 (dd, J = 9.2, 2.6 Hz, 1H), 7.95 (d, J = 9.2 Hz, 1H), 7.60 (td, J = 8.3, 6.8 Hz, 1H), 7.40 (dt, J = 9.9, 2.3 Hz, 1H), 7.32~7.22 (m, 2H)。
3-氯-2-苯氧基6-氨基喹喔啉(P5a), 1H NMR (400 MHz, DMSO-d6) δ 7.55 (d, J = 2.5 Hz, 1H), 7.42 (dd, J = 6.2, 2.6 Hz, 1H), 6.80 (d, J = 7.2 Hz, 1H), 6.64~6.60 (m, 2H), 6.41 (d, J = 7.2 Hz, 2H), 6.37 (d, J = 6.4 Hz, 1H), 5.94 (s, 2H)。
3-氯-2-(3-甲氧基苯氧基)-6-氨基喹喔啉(P5b), 1H NMR (400 MHz, DMSO-d6) δ 7.46 (d, J = 9.0 Hz, 1H), 7.36 (t, J = 7.9 Hz, 1H), 7.16 (d, J = 8.8 Hz, 1H), 6.90 (d, J = 12.4 Hz, 2H), 6.82 (d, J = 8.5 Hz, 2H), 5.96 (s, 2H), 3.78 (s, 3H)。
3-氯-2-(4-甲氧基苯氧基)-6-氨基喹喔啉(P5c), 1H NMR (400 MHz, DMSO-d6) δ 7.41 (d, J = 9.1 Hz, 1H), 7.21 (d, J = 7.8 Hz, 2H), 7.13 (d, J = 9.2 Hz, 1H), 7.01 (d, J = 7.7 Hz, 2H), 6.90 (s, 1H), 5.91 (s, 2H), 3.80 (s, 3H)。
3-氯-2-(3, 5-二甲氧基苯氧基)-6-氨基喹喔啉(P5d), 1H NMR (400 MHz, DMSO-d6) δ 7.48 (d, J = 8.7 Hz, 1H), 7.15 (d, J = 9.0 Hz, 1H), 6.89 (s, 1H), 6.44 (s, 2H), 6.41 (s, 1H), 5.95 (s, 2H), 3.74 (s, 6H)。
3-氯-2-(3-氯苯氧基)-6-氨基喹喔啉(P5e), 1H NMR (400 MHz, DMSO-d6) δ 7.51 (d, J = 8.3 Hz, 1H), 7.47 (d, J = 7.2 Hz, 2H), 7.36 (d, J = 8.2 Hz, 1H), 7.28 (d, J = 8.3 Hz, 1H), 7.17 (d, J = 9.2 Hz, 1H), 6.91 (s, 1H), 5.99 (s, 2H)。
3-氯-2-(3-氟苯氧基)-6-氨基喹喔啉(P5f), 1H NMR (400 MHz, DMSO-d6) δ 7.55~7.49 (m, 1H), 7.48 (d, J = 9.1 Hz, 1H), 7.26 (d, J = 10.3 Hz, 1H), 7.16 (t, J = 9.0 Hz, 3H), 6.91 (s, 1H), 6.00 (s, 2H)。
2 生物活性测试用LY294002作为阳性对照化合物, 采用MTT法, 以人非小细胞肺癌A549、人乳腺癌细胞MCF-7、人结肠癌细胞HCT-116和人肝癌细胞HepG2 4种癌细胞为筛选对象, 分别对22个目标化合物进行体外抗肿瘤活性测试。于96孔板中接种处于对数期的癌细胞每孔5×103个, 培养24 h后加入待测化合物。继续培养48 h后加入MTT溶液20 μL, 培养4 h后弃上清液, 加入DMSO 200 mL, 振匀, 酶标仪570 nm波长测OD值, 重复3次, 取平均值, 计算得IC50。抑制率(%) = ((对照组OD值-给药组OD值)/对照组OD值)×100%。
| [1] |
Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination[J]. Development, 2016, 143: 3050-3060. DOI:10.1242/dev.137075 |
| [2] |
Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer[J]. Nat Rev Drug Discov, 2009, 8: 627-644. DOI:10.1038/nrd2926 |
| [3] |
Bar V, Julie GG, Mariona G, et al. The emerging mechanisms of isoform-specific PI3K signalling[J]. Nat Rev Mol Cell Biol, 2010, 11: 329-341. |
| [4] |
Zhao W, Qiu Y, Kong D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy[J]. Acta Pharm Sin B, 2017, 7: 27-37. DOI:10.1016/j.apsb.2016.07.006 |
| [5] |
Knight ZA, Shokat KM. Chemically targeting the PI3K family[J]. Biochem Soc Trans, 2007, 35: 245-249. DOI:10.1042/BST0350245 |
| [6] |
Marone R, Cmiljanovic V, Giese B, et al. Targeting phosphoinositide 3-kinase—moving towards therapy[J]. Biochim Biophys Acta, 2008, 1784: 159-185. DOI:10.1016/j.bbapap.2007.10.003 |
| [7] |
Ben M, Rodrigo D, Josep T. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs[J]. Oncotarget, 2010, 1: 530-543. |
| [8] |
Prasad G, Sottero T, Yang X, et al. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with erlotinib, temozolomide, and radiation[J]. Neuro Oncol, 2011, 13: 384-392. DOI:10.1093/neuonc/noq193 |
| [9] |
Dienstmann R, Rodon J, Serra V, et al. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors[J]. Mol Cancer Ther, 2014, 13: 1021-1031. DOI:10.1158/1535-7163.MCT-13-0639 |
| [10] |
Wu P, Su Y, Liu X, et al. Synthesis and biological evaluation of novel 2-arylamino-3-(arylsulfonyl) quinoxalines as PI3Kα inhibitors[J]. Eur J Med Chem, 2011, 46: 5540-5548. DOI:10.1016/j.ejmech.2011.09.015 |
| [11] |
Peng W, Yi S, Liu X, et al. Discovery of novel 2-piperidinol-3-(arylsulfonyl) quinoxalines as phosphoinositide 3-kinase α (PI3Kα) inhibitors[J]. Bioorg Med Chem, 2012, 20: 2837-2844. DOI:10.1016/j.bmc.2012.03.026 |
| [12] |
Wu P, Su Y, Liu X, et al. Discovery of novel morpholino-quinoxalines as PI3Kα inhibitors by pharmacophore-based screening[J]. Med Chem Commun, 2012, 3: 659-662. DOI:10.1039/c2md00255h |
| [13] |
Corona P, Carta A, Loriga M, et al. Synthesis and in vitro antitumor activity of new quinoxaline derivatives[J]. Eur J Med Chem, 2009, 44: 1579-1591. DOI:10.1016/j.ejmech.2008.07.025 |
 2020, Vol. 55
2020, Vol. 55