X染色体连锁凝血八因子(coagulation factor Ⅷ, FⅧ)基因缺陷导致甲型血友病, 在男性人群中发病率为五千分之一, 主要治疗方法为血浆和重组FⅧ蛋白替代疗法。FⅧ基因由于mRNA转录水平低、翻译后加工处理和跨细胞器转运等过程的复杂性, 致分泌量远低于与其具有结构同源性的凝血因子Ⅴ[1]。由于基因重组异源表达FⅧ水平低, 加之分离、纯化困难, 使得价格高并难以普及使用。虽然基于转基因技术的基因替代疗法有希望根治该病, 但FⅧ基因的表达和分泌水平低下依然是基因疗法的瓶颈; 而且过大的FⅧ基因cDNA难以被安全有效的腺相关病毒(adeno-associated virus, AAV)载体所承载。研究表明, FⅧ分子内部存在8对二硫键, 分别为A1区2对、A2区2对、A3区2对、C1区和C2区各1对。其中位于轻链A3区中Cys1899和Cys1903之间的二硫键的缺失突变不影响FⅧ功能, 并可提高其分泌性[2]。删除了位于FⅧ重链B区的缩减版FⅧ, 即B区缺失型FⅧ (B domain-deleted FⅧ, BDD-FⅧ), 具有与野生型FⅧ相似的生物学活性, 且由于其mRNA转录水平显著提高, 在制备基因工程蛋白质应用于临床治疗及基因治疗的研究中均采用BDD-FⅧ。本室双载体转BDD-FⅧ基因的研究证明, 重链A2区Cys662和轻链A3区Cys1828突变产生的链间二硫键可促进重、轻链二聚体的形成, 并可促进其分泌[3]。在此基础上, 本文通过Met662 > Cys和Asp1828 > Cys突变, 构建了可形成链间二硫键的BDD-FⅧ变构体F8C, 并将F8C的Cys1899和Cys1903分别突变为Gly, 构建了消除轻链A3区内源性二硫键并引入链间二硫键的BDD-FⅧ变构体F8CG。通过探讨两种变构体BDD-FⅧ转基因表达产物的分泌、活性以及对vWF (von Willebrand factor, vWF)结合力的影响, 将为进一步进行动物体内转基因实验奠定基础。
材料与方法 质粒与细胞系人BDD-FⅧ的真核表达质粒pCMV-F8由本实验室构建并保存。HEK293细胞和COS-7细胞购自中国科学院细胞库。
主要试剂Gel Extraction Kit、PCR Purification Kit、Spin Miniprep Kit和HiSpeed Plasmid Midi Kit (德国Qiagen公司); QuikChange XL-site directed mutagenesis kit (Stratagene公司); DMEM、Opti-MEM培养基和Lipofectamine 2000转染试剂盒(Invitrogen公司); 胎牛血清(Hyclone公司); 重组人FⅧ蛋白(BioChain公司); FⅧ重链多克隆抗体H-140 (Santa Cruz公司); FⅧ轻链单克隆抗体ESH8 (American Diagnostica公司); HRP标记兔抗人FⅧ多克隆抗体(Novus公司); FⅧ缺失的人血浆和正常人血浆(George King Biomedical公司); 兔抗人vWF多克隆抗体(A 0082, Dako公司); FⅧ活性检测的COATEST SP FⅧ试剂盒(Chromogenix公司)。其他试剂均为国产或进口分析纯。
真核重组表达质粒的构建pCMV-F8质粒为本室前期在人野生型Ⅷ (wild-type-Ⅷ, WT-Ⅷ)基础上构建的缺失B区大部分(Ile761~Asn1639)的BDD-FⅧ真核表达质粒。以pCMV-F8为模板, 用两对引物(重链A2区引入Met662→Cys突变的引物为5'-CCT TCAAACACAAATGCGTCTATGAAGACACACTC-3'和5'-GAGTGTGTCTTCATAGACTGCTTTGTGTTTGAAGG-3';轻链A3区引入Asp1828→Cys突变的引物为5'-CCCACTAAATGCGAGTTTGACTGCAAAGCC-3'和5'-GGCTTTGCAGTCAAACTCGCATTTAGTGGG-3'), 用QuikChange XL-site directed mutagenesis kit制备含Cys662和Cys1828突变的BDD-FⅧ真核表达质粒pCMV-F8C;进一步以质粒pCMV-F8C为模板, 用两对引物(轻链A3区引入Cys1899→Gly突变的引物为5'-GGAAAGAAACGGCAGGGCTCCCTGC-3'和5'-GCAGGGAGCCCTGCCGTTTCTTTCC-3'; A3区引入Cys1903→Gly突变的引物为5'-GCAGGGCTCCCGGCAATATCCAGATGG-3'和5'-CCATCTGGATATTGCCGGGAGCCCTGC-3')将pCMV-F8C中FⅧ轻链A3区的Cys1899和Cys1903突变为Gly, 得到真核表达质粒pCMV-F8CG。
HEK293和COS-7细胞的培养及基因转染HEK293和COS-7细胞用含10%胎牛血清的DMEM培养液贴壁培养于5% CO2、37 ℃培养箱。转染前1天用胰蛋白酶消化分散细胞, 按每孔5×105个细胞于2 mL DMEM培养液将细胞转接于6孔培养板, 待细胞生长融合至80%以上时, 用Lipofectamine 2000脂质体按试剂盒说明书将pCMV-F8、pCMV-F8C和pCMV-F8CG各6 μg分别转染HEK293和COS-7细胞。用空载体pcDNA3.1分别转染两种细胞作为阴性对照(Mock)。细胞转染质粒48 h后, 收集培养上清和转基因HEK293细胞。
Western blot方法观察转基因细胞的蛋白质表达用冻融法裂解所收集的转基因HEK293细胞, 提取细胞总蛋白, 用Bradford法进行蛋白质定量, 取12 μg总蛋白上样, 分别用还原性(加DTT)和非还原性(不加DTT) SDS-PAGE分离蛋白质, 半干电转系统将蛋白质转移至PVDF膜, 用5%脱脂奶粉溶液室温封闭2 h, 用1:1 000稀释的兔抗人FⅧ轻链单抗于37 ℃轻摇孵育1 h, 再用HRP-抗小鼠血清37 ℃轻摇孵育1 h, ECL plus法曝光X光胶片。
ELISA定量分析分泌至培养上清的FⅧ蛋白量参照本室基于文献[4]建立的细胞上清中FⅧ浓度测定的双夹心ELISA检测法[5], 用FⅧ轻链单抗包被酶标板, 用HRP标记FⅧ多抗作为检测抗体测定转基因细胞分泌至培养上清中的FⅧ浓度。用重组人FⅧ蛋白作为标准品制作标准曲线。
转基因细胞培养上清中FⅧ的生物活性分析用Coatest发色分析法, 参照文献[6], 按试剂盒说明书操作分析细胞培养上清中的FⅧ凝血生物活性。
FⅧ-vWF亲和力测定FⅧ-vWF亲和力测定方法为参照文献[7]进行。原理为将结合于特异性抗体的vWF与含FⅧ的样品反应, 之后用FⅧ特异性抗体检测结合于vWF的FⅧ量, 与FⅧ标准品(正常人血浆)比较得到FⅧ的vWF亲和力。用磷酸盐缓冲液(PBS)稀释的兔抗人vWF多克隆抗体(4 μg·mL-1)于4 ℃每孔加100 μL包被酶标板, 4 ℃孵育过夜, 用PBS-0.02% Tween 20洗3次, 每孔用200 μL PBS-1% BSA室温封闭1 h, 洗3次, 每孔加5%正常人血浆(PBS-0.1% BSA稀释) 100 μL, 室温孵育1 h, 洗3次, 加入CaCl2 (400 mmol·L-1) 100 μL, 室温放置30 min以去除与血浆中vWF结合的FⅧ。然后将细胞培养上清样品用PBS-0.1% BSA按1:20稀释, 每孔加100 μL, 室温孵育1 h。为探测结合的FⅧ, 洗板3次后每孔加100 μL浓度为2 μg·mL-1的FⅧ重链单克隆抗体ESH5, 37 ℃孵育1 h, 洗3次后加1:5 000稀释的HRP标记的山羊抗小鼠二抗, 37 ℃温育1 h后洗板10次, 加反应底物OPD溶液37 ℃温育30 min进行显色, 用2 mol·L-1 H2SO4终止反应, 490 nm读板。每个样品平行3孔。
统计学处理计量资料以x±s表示, 2样本均数比较采用t检验; 多样本均数比较采用单因素方差分析, 组间两两比较采用q检验, P < 0.05为差异具有统计学意义。
结果 1 突变体BDD-FⅧ的构建前期工作通过删除FⅧ大部分B区(del Ile761~Asn1639)构建了BDD-FⅧ (F8), 其中保留了B区N端的20个氨基酸残基(Ser741~Thr760)和C端的9个氨基酸残基(Pro1640~Arg1648)。通过点突变将F8的Met662和Asp1828分别突变为Cys, 得到突变体F8C。通过二者之间的二硫键, F8C被凝血酶(Ⅱa)水解活化形成异源三聚体, 包括重链A1区、A2区和轻链(A3-C1-C2), 其中A2区和轻链A3区之间的二硫键共价连接, 可防止A2区在生理条件下快速解离所导致的活化产物不稳定和活性下降。在此基础上, 将F8C中A3区的Cys1899和Cys1903突变为Gly, 可破坏二者之间的内源性二硫键, 得到F8CG。野生型BDD-FⅧ、两种BDD-FⅧ变构体及凝血酶活化产物的结构如图 1所示。
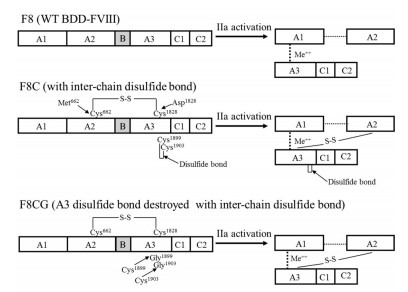
|
Figure 1 Schematic representation of B domain-deleted FⅧ (BDD-FⅧ) (F8), F8C, F8CG, and their active forms hydrolyzed by thrombin (Ⅱa). The BDD-FⅧ (F8) is generated from FⅧ by deleting most of its B-domain from Ile761-Asn1639. The F8C is resulting from F8 by Cys mutagenesis of Met662 in A2 and Asp1828 in A3 domain. The F8CG is derived from F8C with Cys1899 and Cys1903 replaced by Gly in its A3 domain, respectively. WT: Wild-type |
FⅧ轻链特异性单抗检测结果显示(图 2), 无论还原剂(DTT)存在与否, 野生型BDD-FⅧ可见细胞内加工产生少量二聚体BDD-FⅧ蛋白条带, 即离子键相连的重链(heavy chain, HC)和轻链(light chain, LC)二聚体(HC-LC)以及大量脱落的LC多肽, 说明HC-LC二聚体易于解聚。而含有Met662 > Cys/Asp1828 > Cys突变的BDD-FⅧ变构体F8C, 以及含有Met662 > Cys/Asp1828 > Cys突变和Cys1899 > Gly/Cys1903 > Gly突变的F8CG, 在非还原条件下(不加DTT)主要以二聚体形式存在, 只有少量未二聚体化的LC; 加DTT还原条件下二聚体形式消失, 表现为明显增多的解聚LC, 表明F8C和F8CG两种BDD-FⅧ变构体在细胞内加工后, 可形成链间二硫键共价结合的二聚体, 提示突变后的Cys662和Cys1828残基之间二硫键形成的高效性。

|
Figure 2 Western blot analysis for expressing products of transgenic HEK293 cells. Plasmids of F8, F8C, and F8CG were transfected into HEK293 cells, respectively. The total cellular proteins were collected at 48 h post-transfection and were separated by SDS-PAGE followed by Western blot. The blot was probed with a monoclonal antibody against human FⅧ light chain. The left half is non-reduced samples and the right half is samples reduced with dithiothreitol (DTT). HC: Heavy chain; LC: Light chain |
随后, 进行了在HC-LC间二硫键交联突变体基础上的A3区内源性二硫键缺失突变对异源表达FⅧ分泌活性的影响的研究。结果显示, HEK293和COS-7两种转基因细胞上清中, 二硫键缺失变构体FⅧ (F8CG)的分泌量分别为(331 ± 41)和(348 ± 47) ng·mL-1, 明显高于二硫键交联变构体FⅧ (F8C)的分泌量, 即(183 ± 29)和(199 ± 26) ng·mL-1。野生型BDD-FⅧ (F8)的HEK293和COS-7两种转基因细胞的分泌量分别为(96 ± 31)和(104 ± 32) ng·mL-1。两种变构体的分泌量均明显高于野生型, 表明轻链分子内二硫键的缺失可显著促进FⅧ的分泌(图 3)。
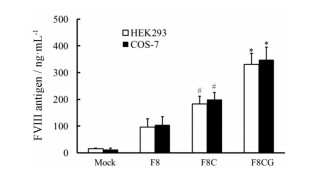
|
Figure 3 FⅧ antigen levels secreted into the culture supernatants of transgenic HEK293 and COS-7 cells. Plasmids of BDD-FⅧ (F8), F8C, F8CG, and mock control were transfected into HEK293 and COS-7 cells by Lipofectamine 2000 in triplicate. The amount of FⅧ antigen secreted into the culture supernatant at 48 h after transfection was measured by ELISA. n = 6, x±s. *P < 0.05 vs F8C group; #P < 0.05 vs F8 group |
转基因细胞培养上清的凝血生物活性结果显示(图 4), 在HEK293和COS-7两种转基因细胞上清中, F8CG活性分别为(2.29 ± 0.19)和(2.47 ± 0.35) U·mL-1, F8C活性分别为(1.19 ± 0.21)和(1.22 ± 0.25) U·mL-1, 野生型BDD-FⅧ (F8)活性分别为(0.69 ± 0.16)和(0.75 ± 0.15) U·mL-1。F8CG活性明显高于F8C活性, 两种变构体的活性均明显高于野生型活性。结合蛋白分泌水平实验结果可知, 含有轻链二硫键可抑制FⅧ分泌, 而缺失突变可通过改善分泌提高变构体F8CG的活性。而且, F8CG被凝血酶水解激活所形成的活化产物异源三聚体中的A2区与轻链间共价键相连, 减慢A2区解离, 进一步提高F8CG活性。
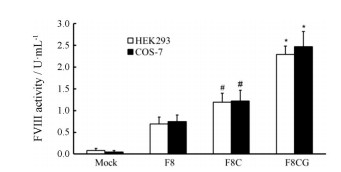
|
Figure 4 FⅧ biological activity of supernatant from transgenic HEK293 and COS-7 cells. Plasmids expressing for BDD-FⅧ (F8), F8C, F8CG and Mock control were transfected into HEK293 and COS-7 cells by Lipofectamine 2000 in triplicate. The FⅧ activity secreted into the culture supernatant at 48 h after transfection was measured by chromogenic method. n = 6, x±s. *P < 0.05 vs F8C group; #P < 0.05 vs F8 group |
vWF与FⅧ分子通过非共价结合达到稳定后者的作用, 没有结合vWF的FⅧ半衰期仅有1~2 h, 与vWF结合的FⅧ半衰期达12 h, 因此vWF对于提高FⅧ的生物利用度十分重要。转基因细胞培养上清FⅧ-vWF亲和力检测结果显示, 以野生型BDD-FⅧ (F8)转基因表达产物的vWF结合力为100%, F8CG的vWF结合力为96%~98%, F8C的vWF结合力为93%~97%, 而阴性对照细胞培养上清(Mock)未检测到与vWF结合的FⅧ。上述结果表明, FⅧ分子轻链内二硫键的缺失不影响其与vWF的结合(图 5)。

|
Figure 5 vWF binding capacity of transgene expressed products. The culture supernatants of transgenic HEK293 and COS-7 cells were collected, and the vWF affinity of the expressed F8, F8C, F8CG, and mock was analyzed by ELISA using the wild type F8 as reference (100% vWF binding capacity) |
FⅧ在内源性凝血途径中发挥重要作用。FⅧ基因遗传性缺陷引起的甲型血友病主要依靠血浆和重组蛋白输注治疗, 需要反复给药, 治疗费用高, 难以普及。作为单基因病, 基因疗法可望成为治愈该病的一个新途径, 但FⅧ表达和分泌低效以及FⅧ基因较大难以为AAV载体承载等因素影响疗效。另外, FⅧ在血浆中的稳定性有赖于其与vWF结合为复合物而防止被快速降解、细胞摄取和结合于活化血小板/内皮细胞表面, 没有与vWF结合的FⅧ半衰期将从12 h缩短至1~2 h[8]。提高FⅧ表达和分泌的研究包括去除B结构域、重链点突变和在B结构域氨基端添加N-糖基化修饰位点等。前期通过共转染vWF基因的研究也表明, vWF的增加可促进FⅧ分泌[9]。研究表明, FⅧ存在8对分子内二硫键[10], 二硫键通常被认为在维持蛋白质分子空间结构的稳定性和质量控制中具有重要作用。最近对此8个分子内二硫键的系统突变研究揭示, 其中7对二硫键的缺失突变导致FⅧ的分泌性降低, 并会降低其生物学活性。而通过Cys1903 > Gly突变去除A3区的1对二硫键时, 则不影响活性且分泌性提高2倍, 其可能机制为, Cys1903的突变可防止巯基与FⅧ分子中的其他游离巯基相互作用, 进而抑制分泌[11]。本研究通过在重、轻链间引入二硫键的Cys突变并在双载体培养细胞转FⅧ基因表明, 轻链可通过与重链间的二硫键连接促进重链分泌[12]。本文在BDD-FⅧ的重、轻链间引入二硫键的同时, 构建了链A3区内二硫键缺失变构体F8CG。细胞转基因结果显示, 其分泌水平均得到显著改善。生理性FⅧ在细胞内加工成重链和轻链, 以异源二聚体的形式分泌, 链间由二价金属离子相连, 易于解聚。而变构体F8C和F8CG中的Cys662和Cys1828间二硫键交联可有效防止二聚体解聚。与重链共价结合的轻链可有效促进二聚体化F8C和F8CG的分泌, 这种顺式促分泌作用和基于蛋白质剪接双载体共转重链和轻链基因后与重链肽键结合轻链顺式促进重链分泌的作用类似[13]。轻链A3区内源性二硫键缺失进一步加强了轻链对变构体F8CG的促分泌作用。F8CG活性的提高一方面源自其分泌量的增加, 另一方面由于被凝血酶水解活化后形成了异源三聚体产物(Al/A2/A3-C1-C2)中A2区和轻链A3区间的二硫键, 使A2区解离减慢, 从而使活化产物半衰期延长并活性提高, 而A2易于自发脱落是FⅧ活化产物不稳定且快速失活的主要原因[14]。
FⅧ重链和轻链二聚体分泌后与vWF的结合可保护FⅧ免于蛋白水解[15]。FⅧ与vWF结合的部位位于两个区域, 即轻链A3区氨基端的酸性区和C2区羧基端的2303~2332位残基。研究表明, 其中单独任何一个区域与vWF的结合力均显著低于完整的FⅧ二聚体, 即两个区域在空间构象上对FⅧ结合具有协同作用[16]。由于F8CG中同时引入了二硫键添加和缺失双重突变, 本文进一步检测了F8CG与vWF的亲和力, 结果显示其亲和力与野生型F8相近, 说明F8CG中的轻链二硫键缺失并不影响其与vWF的结合。变构体F8CG的分泌性和活性得到提高、且具有与vWF的结合力, 本研究为后续进一步动物体内活性及药动学实验奠定了基础。
| [1] |
Pittman DD, Tomkinson KN, Kaufman RJ. Post-translational requirements for functional factor Ⅴ and factor Ⅷ secretion in mammalian cells[J]. J Biol Chem, 1994, 269: 17329-17337. |
| [2] |
Selvaraj SR, Scheller AN, Miao HZ, et al. Bioengineering of coagulation factor Ⅷ for efficient expression through elimination of a dispensable disulfide loop[J]. J Thromb Haemost, 2012, 10: 107-115. DOI:10.1111/j.1538-7836.2011.04545.x |
| [3] |
Zhu F, Liu Z, Miao J, et al. Enhanced plasma factor Ⅷ activity in mice via cysteine mutation using dual vectors[J]. Sci China Life Sci, 2012, 55: 521-526. DOI:10.1007/s11427-012-4333-8 |
| [4] |
Gnatenko DV, Saenko EL, Jesty J, et al. Human factor Ⅷ can be packaged and functionally expressed in an adenoassociated virus background: applicability to haemophilia A gene therapy[J]. Br J Haematol, 1999, 104: 27-36. DOI:10.1046/j.1365-2141.1999.01137.x |
| [5] |
Zhu F, Liu Z, Chi X, et al. Protein trans-splicing based dual-vector delivery of the coagulation factor Ⅷ gene[J]. Sci China Life Sci, 2010, 53: 683-689. DOI:10.1007/s11427-010-4011-7 |
| [6] |
Pittman DD, Alderman EM, Tomkinson KN, et al. Biochemical immunological, and in vivo functional characterization of B-domain-deleted factor Ⅷ[J]. Blood, 1993, 81: 2925-2935. DOI:10.1182/blood.V81.11.2925.2925 |
| [7] |
De Meyer SF, Vanhoorelbeke K, Chuah MK, et al. Phenotypic correction of von Willebrand disease type 3 blood-derived endothelial cells with lentiviral vectors expressing von Willebrand factor[J]. Blood, 2006, 107: 4728-4736. DOI:10.1182/blood-2005-09-3605 |
| [8] |
De Meyer SF, Deckmyn H, Vanhoorelbeke K. von Willebrand factor to the rescue[J]. Blood, 2009, 113: 5049-5057. DOI:10.1182/blood-2008-10-165621 |
| [9] |
Zhu F, Liu Z, Miao J, et al. Propeptide-deleted von Willebrand factor improves heavy chain secretion and bioavtivity by split FⅧ gene Co-transfected cells[J]. J Chin Biotechnol (中国生物工程杂志), 2012, 32: 20-25. |
| [10] |
McMullen BA, Fujikawa K, Davie EW, et al. Locations of disulfide bonds and free cysteines in the heavy and light chains of recombinant human factor Ⅷ (antihemophilic factor A)[J]. Protein Sci, 1995, 4: 740-746. |
| [11] |
Selvaraj SR, Scheller AN, Miao HZ, et al. Bioengineering of coagulation factor Ⅷ for efficient expression through elimination of a dispensable disulfide loop[J]. J Thromb Haemost, 2012, 10: 107-115. DOI:10.1111/j.1538-7836.2011.04545.x |
| [12] |
Zhu F, Yang S, Liu Z, et al. Disulfide cross-linking enhances dual-vector based delivery of Split B-domain-deleted coagulation FactorⅧ gene[J]. Prog Biochem Biophys (生物化学与生物物理进展), 2012, 39: 181-187. |
| [13] |
Chen L, Zhu F, Li J, et al. The enhancing effects of the light chain on heavy chain secretion in split delivery of factor Ⅷ gene[J]. Mol Ther, 2007, 15: 1856-1862. DOI:10.1038/sj.mt.6300268 |
| [14] |
Fay PJ, Beattie TL, Regan LM, et al. Model for the factor Ⅷa-dependent decay of the intrinsic factor Xase. Role of subunit dissociation and factor IXa-catalyzed proteolysis[J]. J Biol Chem, 1996, 271: 6027-6032. DOI:10.1074/jbc.271.11.6027 |
| [15] |
Weiss HJ, Sussman Ⅱ, Hoyer LW. Stabilization of factor Ⅷ in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor Ⅷ and in patients with von Willebrand's disease[J]. J Clin Invest, 1977, 60: 390-404. DOI:10.1172/JCI108788 |
| [16] |
Saenko EL, Scandella D. The acidic region of the factor Ⅷ light chain and the C2 domain together form the high affinity binding site for von Willebrand factor[J]. J Biol Chem, 1997, 272: 18007-18014. DOI:10.1074/jbc.272.29.18007 |
 2020, Vol. 55
2020, Vol. 55


