2. 复旦大学药学院, 智能化递药教育部和全军重点实验室, 上海 201203
2. Key Laboratory of Smart Drug Delivery, Ministry of Education and PLA, School of Pharmacy, Fudan University, Shanghai 201203, China
脂质体是临床上最具应用前景的纳米递药系统之一, 在抗肿瘤及抗真菌感染领域应用较广[1, 2]。脂质体是由磷脂双分子层构成的微型自组装囊泡体, 中央围成亲水区, 载药谱广:既可在内囊包载亲水性药物分子, 也可在双分子层内包载疏水性药物分子。脂质体具有较好的生物相容性[3], 粒径可控, 表面易修饰, 已成为近几十年纳米递药系统的研究热点[4-6]。
与其他纳米递药系统类似, 脂质体进入血循环后与血浆蛋白相互作用形成蛋白冠[7-10], 表面性质随之改变, 直接影响其体内性能, 如清除速率、组织分布和免疫原性等, 最终影响诊疗效果, 甚至产生毒副作用[11, 12]。特异性靶向肿瘤组织的脂质体制剂已有大量的基础研究, 但至今仍未有临床获批的靶向脂质体药物。有文献报道用于治疗恶性肿瘤的纳米药物中, 真正被递送至癌变部位的不足总量1%[13], 体内命运的不可预测性成为脂质体药物临床转化的绊脚石。深入研究表面蛋白冠对脂质体性能的调控作用与机制, 是指导靶向脂质体药物源头设计和推动临床转化的必要环节。
1 影响脂质体蛋白冠组分的因素血浆是最重要的生物介质, 其中血浆总蛋白浓度约为60~80 g·L-1, 已被鉴定的血浆蛋白有3 700余种[14]。脂质体入血后可在30 s内表面快速形成蛋白冠。血浆中蛋白浓度较高但结合活性较差的蛋白会快速与脂质体结合, 随循环时间延长, 浓度较低但结合活性较高的血浆蛋白逐渐置换已吸附在脂质体表面的血浆蛋白, 形成动态平衡(图 1)[15, 16]。根据蛋白冠中蛋白结合活性的强弱, 人为将其分为“hard层”和“soft层”, 现研究中提到的蛋白冠主要指“hard层”[17]。脂质体表面蛋白冠的组分, 不仅依赖于脂质体自身性质, 还与血浆成分也密切相关。吸附血浆蛋白后, 脂质体表面形成新的生物特征, 与最初的表面性质大不相同。明确脂质体与血浆蛋白间相互作用可指导脂质体药物的源头设计和临床转化。
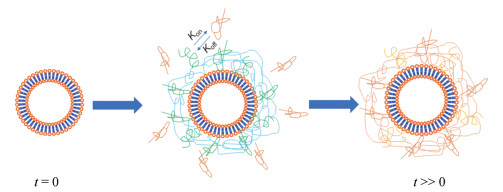
|
Figure 1 Dynamics of protein corona formation on liposome surface. Initially attached plasma proteins are subsequently exchanged by lower-abundance plasma proteins harboring higher affinity to achieve equilibrium |
血浆蛋白主要依赖氢键、范德华力、静电作用、堆积和疏水作用力等吸附在脂质体表面[18]。现研究中常用的脂质体粒径为80~120 nm左右, 比表面积大, 表面性质对蛋白冠的形成起决定作用[19]。
表面疏水的脂质体在水溶液甚至蛋白较丰富的体液内难以分散形式存在, 稳定性差, 需借助表面活性剂以减小脂质体的表面张力。表面活性剂通常是小分子或两亲性分子。与血浆接触后, 血中的蛋白会以表面活性剂类似的形式迅速吸附在脂质体表面以降低脂质体的表面张力[20]。除疏水作用外, 脂质体表面电荷也是影响其蛋白冠组分的重要因素之一, 荷电脂质体表面较易吸附血浆蛋白, 大部分血浆蛋白含荷电氨基酸。当制备脂质体的材料中有阳离子或阴离子基团时, 较中性磷脂材料的脂质体更易吸附血浆中补体蛋白, 而带等量电荷的脂质体, 阳离子脂质体比阴离子脂质体更易吸附血浆蛋白[21]。白蛋白是血浆中最丰富的一类蛋白, 荷负电, 可迅速吸附在带正电荷的脂质体表面。由阴离子和阳离子磷脂组成的脂质体分别倾向于吸附碱性和酸性血浆蛋白, 如:阴离子脂质体优先吸附等电离点(PI) > 5.5的血浆蛋白, 而阳离子脂质体易吸附PI < 5.5的血浆蛋白[22]。在表面电荷分布均匀的阳离子脂质体上, 随电荷密度的增加, 血浆蛋白与脂质体的亲和力增强, 脂质体表面吸附蛋白的量由亲和力与表面结合位点的数量等因素共同决定。
制备脂质体的材料直接影响脂质体的电位和亲/疏水性等理化性质, 是影响脂质体与血浆蛋白结合及蛋白冠组分的重要因素。氢化大豆磷脂酰胆碱(hydrogenated soy phosphatidylcholine, HSPC)、二硬脂酰卵磷脂(distearoylphosphatidylcholine, DSPC)、棕榈酸磷脂胆碱(palmitoyloleoylphosphatidylcholine, DOPC)、二油酰基磷脂酰乙醇胺(dioleoly-sn-glycero-phophoethanolamine, DOPE)和胆固醇(cholesterol)及阳离子材料(2, 3-二油酰基-丙基)-三甲胺[(2, 3-dioleoyloxy-propyl)-trimethylammonium, DOTAP]和DC-胆固醇是目前基础研究和商业化脂质体中最常用的磷脂材料[23-30]。Caracciolo课题组[31, 32]发现DOTAP和DC-胆固醇脂质倾向于吸附带负电荷的血浆蛋白, 如纤维蛋白原、凝血酶原、维生素K和玻璃体结合蛋白等。用带中性脂质材料代替DOTAP, 此类蛋白吸附量显著降低。而含DOPE的脂质体选择性吸附载脂蛋白及血清白蛋白(albumin), 胆固醇会促进脂质体对调理素的吸附, 如免疫球蛋白及补体蛋白等。此外, 当脂质体材料中插入较多惰性的、生物相容性好的亲水材料, 如神经节苷脂GM1、磷脂酰肌醇(phosphatidylinositol)和连接磷脂的聚乙二醇(polyethylene glycol, PEG)时, 脂质体表面会形成亲水保护层, 利用空间位阻减少脂质体表面吸附的蛋白总量, 显著降低调理素类蛋白的吸附[33, 34]。
靶向分子的修饰会直接影响脂质体表面性质。多肽及小分子类靶分子通常被修饰在脂质材料PEG-DSPE的亲水端后, 再制备脂质体, 而蛋白类则通过后修饰或后插入方式, 经化学键修饰在脂质体表面。靶分子的大小、荷电性、亲疏水性和修饰比例等, 均可影响蛋白冠组分[35, 36]。Guan等[37]前期研究发现, 在修饰了多肽的脑靶向脂质体中, 多肽长度、稳定性及多肽中带正电荷的数量会影响脂质体蛋白冠组分及关键血浆蛋白的丰度。当脂质体表面修饰了荷正电、稳定性长肽时, 脂质体进入血循环后会长时间吸附大量天然IgM。
脂质体中包载的药物也是影响脂质体蛋白冠组分的重要因素, 在人血浆中, 当脂质体内部包载DNA时, 会显著增加免疫球蛋白-γ蛋白(IgGs)的吸附[38]。此外, 脂质体表面曲率由粒径大小决定, 受表面曲率的影响, 血浆蛋白与脂质体表面结合的构象不同, 直接影响脂质体与血浆蛋白的亲和力和与特定蛋白的结合常数。
1.2 机体内环境对蛋白冠组分的影响体外蛋白冠组分分析对脂质体体内性能的预测具有重要意义。除自身理化性质外, 脂质体所处的生物环境也是影响蛋白冠组成的重要因素。胎牛血清、人血浆或血清常被用在体外蛋白冠的研究中, 并在细胞水平分析所形成的蛋白冠对脂质体性能的影响。血清与血浆成分不同可直接影响蛋白冠组分, 进而影响其与机体的相互作用。不同抗凝剂制备的血浆也会导致脂质体表面蛋白冠组分不同。体外蛋白冠的组分还会受温度、蛋白浓度和孵育时间等因素的影响[39]。机体内环境比体外更复杂, 在人体内血流速度从每秒几微米(毛细血管)到每秒60 cm (升主动脉)不等[40]。体内脂质体是处于动态过程, 血流剪切力的存在会显著影响蛋白冠的形貌[41, 42]。
影响体内蛋白冠的因素诸多, 如物种、年龄和健康状况等[43]。2014年的一项研究表明, 脂质体在小鼠血浆与人血浆中形成的蛋白冠相比, 电位、聚集状态和表面电位均有很大差异, 且蛋白冠中各蛋白的数量和种类与内环境密切相关。与人血浆比, 小鼠血浆的电性偏负, 同种脂质体在小鼠血浆中吸附的补体类调理素类蛋白较少, 免疫球蛋白类补体较多, 而吸附的载脂蛋白类偏高, 这也是很多脂质体药物在小鼠和人体内性能产生较大差异的重要原因, 限制了纳米药物的临床转化。同一种脂质体药物在不同个体(受年龄、疾病、性别、不同生理状况、生活方式及地域的影响)或疾病患者血浆中形成的蛋白冠也有明显差异。Caputo等[44]研究分析了两性霉素B脂质体在胃癌、乳腺癌、胰腺癌患者及健康人血清中形成蛋白冠的差异, 发现脂质体粒径在各组间无显著差异, 但在胰腺癌患者血中形成蛋白冠后的脂质体电位显著高于乳腺癌和胃癌患者。经SDS-PAGE分析表明, 患者形成的蛋白冠组分无显著差异, 仅吸附的分子质量约为37 kDa的血浆蛋白量(IgA和IgG)远高于其他组, 提示在脂质体药物研究过程中, 仅收集健康患者血浆对脂质体药物进行表征或研究有局限性。
2 蛋白冠对脂质体体内性能的影响脂质体作为纳米药物载体优势居多, 其载药效率高、载药谱广和生物相容性高, 表面修饰靶向分子后有望实现药物靶向递送[45]。因此, 延长脂质体血循环时间, 增加其在目标组织中的分布, 减少正常组织中蓄积以降低毒性, 是脂质体药物设计的关键因素, 应充分研究蛋白冠的形成及组分对脂质体体内性能的影响(图 2)[46]。
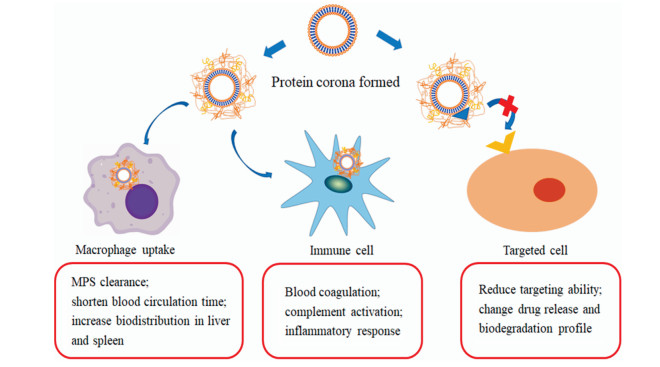
|
Figure 2 Effects of protein corona on in vivo performance of liposomes. Absorption of esoponins (such as albumin and apolipoproteins) would prolong the blood circulation time of liposomes, while opsonins (such as complements and immunoglobulins) reduce the circulation time. The absorption of protein corona also affects the interaction between liposomes and immune cells to induce immunogenicity. Protein corona would also reduce the targeting capability of liposomes, and affect burst release and biodegradation of liposomes |
在蛋白冠相关研究之前, 纳米药物进入血循环后被认为与细胞或组织直接作用, 体外设计的纳米药物理化性质即是进入体内后的生物性质。但表面形成的蛋白冠会迅速改变纳米药物的表面性质, 使其体内性能难以预期。纳米药物作为外源物质, 单核吞噬系统(mononuclear phagocytic system, MPS)识别后被迅速清除, 致使其体内循环时间短, 肝脏蓄积增加[47]。因此, 系统了解蛋白冠的形成对脂质体药动学性能的影响, 有助于预测其安全性及有效性。
脂质体与血浆蛋白的结合模式是影响其被MPS识别的关键因素。研究[48]表明, 非调理素类蛋白如血清白蛋白及载脂蛋白J (Apo J)吸附后可降低脂质体-细胞黏膜的相互作用, 减少巨噬细胞的摄取, 与血清白蛋白孵育也可显著抑制树突状细胞的摄取。相反, 当脂质体表面吸附较多调理素, 如补体及免疫球蛋白时, 吞噬细胞可快速识别脂质体, 进而加速脂质体被MPS清除。脂质体自身不能被吞噬细胞所结合或识别, 但当脂质体表面吸附较多调理素类分子后(包括免疫球蛋白类: IgG、IgA、IgM等; 补体系统的主要组分: C3、C4、C5及其他血浆蛋白:层黏连蛋白、纤连蛋白、C-reactive蛋白、Ⅰ型胶原蛋白等), 易通过3种方式被吞噬细胞识别[49]: ①调理素类蛋白吸附在表面后, 构象改变, 由静息状态被激活为活化形式, 借助表面暴露出的位点与吞噬细胞受体特异性结合; ②调理素类蛋白结构中大都含有疏水核心区, 与脂质体通过疏水作用相互作用后刺激吞噬细胞的摄取; ③通过激活补体(经典途径、旁路途径及凝集素途径)。Vu等[39]研究结果显示, 纳米药物表面吸附IgG后, 可激活C3补体裂解为C3a及C3b, 继而与单核细胞及巨噬细胞表面的Fc受体结合, 使其表面泛素化, 增加MPS的摄取。作者在前期研究中发现, 脂质体表面吸附了较多天然IgM后, 在小鼠体内清除加快, 体内循环时间缩短。
蛋白冠的形成也会改变巨噬细胞的吞噬机制[50]。清道夫受体(scavenger receptor class, SRs)在纳米药物的清除中发挥重要作用, 在巨噬细胞中, SRs可识别白蛋白或载脂蛋白在纳米表面形成的复合物。调理素类蛋白, 如纤维蛋白、免疫球蛋白和补体可分别被整合素、Fc受体和补体受体识别。减少或控制特定血浆蛋白的吸附, 延长血循环时间是脂质体药物设计的关键步骤。明确蛋白冠的形成对脂质体组织分布的影响也是药物设计的关键问题, 当脂质体被MPS摄取较多时, 可能增加药物在肝脾中的蓄积量, 产生毒副作用。Patel和Moghimi推测[51], 胆固醇丰富的脂质体在脾脏中蓄积较多, 与脂质体表面吸附较多的可以促进脾脏中吞噬细胞摄取的调理素, 而吸附促进kupffer细胞摄取脂质体的调理素类蛋白较少有关。
2.2 蛋白冠的形成对脂质体相容性和免疫安全性的影响脂质体主要由磷脂构成, 自身免疫原性低, 但作为纳米药物载体, 其尺寸与病毒相似, 是固有免疫系统(主要是补体C系统)的潜在靶点, 易引起超敏反应等不良反应。自多柔比星脂质体被FDA批准临床应用以来, 临床数据表明该脂质体第1次给药后, 约9%患者出现不同程度的超敏反应[52]。C3是补体C中含量最高的蛋白, 在人血浆中浓度约为1.2 mg·mL-1, 由α和β亚基构成, 补体激活后, C3裂解为过敏毒素C3a和C5a。在超敏反应中, 以凝血因子为始动因子, 凝血及补体激活的级联反应相互反馈, 但血浆中凝血因子含量极低, 凝血因子Ⅴ、Ⅶ和Ⅹ的浓度分别为20、10和200 nmol·L-1, 血浆纤维蛋白也仅有9 μmol·L-1。因此, 脂质体对血浆中关键因子极少量的吸附(非特异性吸附)会导致级联反应平衡发生变化。Vodovozova课题组[53]的研究表明, 载甲氨喋呤脂质体可引起凝血及补体激活(在血清中可检测到C3a), 引起超敏反应。本课题组研究数据显示, 脂质体表面吸附天然IgM后, 会增加骨髓来源树突状细胞(BMDCs)及淋巴结中MHC Ⅱ阳性抗原提呈细胞的摄取, 刺激B细胞产生抗体, 血清中特异性IgG及IgM含量显著升高。Vu等[39]研究也发现, 纳米药物表面吸附IgG后, 可通过补体C3激活C5和肥大细胞, 促进炎性反应。
此外, 蛋白冠的形成对血小板的活化、红细胞溶血和内皮细胞的摄取等均有一定程度的影响。体外研究表明, 荷正电脂质体(含有带正电荷的材料或修饰了带正电荷的靶向分子等)与细胞膜直接作用时, 会破坏细胞膜的连续性和完整性、损伤线粒体和细胞核, 引起炎症反应和氧化应激, 最终导致细胞死亡[14]。但荷正电脂质体进入体内血循环后, 优先吸附负电性较强的血浆蛋白, 缓解脂质体对细胞及器官的损伤, 减轻毒副作用。裸露的纳米药物与血小板接触后会迅速激活血小板, 启动凝血过程, 而形成蛋白冠后的纳米粒(孵育0.5 min)与血小板孵育后未见任何凝集反应[54]。
2.3 蛋白冠的形成对脂质体靶向效率的影响靶向脂质体药物是纳米递药系统研究的重点, 通过在脂质体表面修饰功能性分子, 引导脂质体在目标部位聚集, 减少在正常组织中的分布。多柔比星脂质体虽已被FDA批准上市多年, 至今仍未有可用于临床的靶向脂质体。靶向脂质体临床推动难度大, 与蛋白冠对其性能影响密不可分。蛋白冠影响脂质体靶向效果的方式有两种:阻碍或促进靶向分子与受体/抗原的结合。靶向分子与受体/抗原的特异性结合可显著提高脂质体与细胞或组织的结合活力, 但仅限于在0.5 nm距离内发挥作用, 因此蛋白冠中血浆蛋白含量和种类会直接影响脂质体的靶向效果。Corbo等[55]发现, 脂质体(含磷脂材料DPPC:DOPC:CHOL的摩尔比为6:3:1)与BALB/C小鼠血清孵育后, 乳腺癌4T1细胞的摄取效率显著增加。Kelly等[56]发现蛋白冠的形成可使修饰的转铁蛋白活性丧失, 在体内失去靶向效果。
此外, 蛋白冠的形成对脂质体释放速率和降解速率也产生显著影响。温敏感脂质体对癌症治疗有一定的前景, Al-Ahmady等[57]研究表明, 传统温敏脂质体(磷脂材料比: DPPC:HSPC:CHOL:DSPE-PEG2000 = 54:27:16:3)在CD-1大鼠体内形成蛋白冠后, 体内的温敏释放变缓且难以彻底释放, 但除去表面蛋白冠后, 包载在脂质体内的药物可迅速释放。
3 蛋白冠的应用蛋白冠成分复杂, 且直接影响脂质体的体内性能[58]。进一步明确影响脂质体表面吸附蛋白冠的因素和蛋白冠中关键血浆蛋白对脂质体性能的影响, 既可通过减少关键血浆蛋白的吸附, 维持脂质体的表面功能, 也可通过调节脂质体组成来操控表面蛋白冠的组分和特定血浆蛋白的丰度[59], 使脂质体在诊断及治疗领域发挥更重要作用, 推动临床转化(图 3)。

|
Figure 3 Strategies for development of liposomes enabled by mechanistic understanding of protein corona-liposome interactions. Liposomes inserted with stealth polymers (such as PEG) or coated by leukocyte-membrane reduce the opsonin absorption and increase the apolipoprotein coating, significantly prolonging circulation time of liposomes. To enhance targeting capability, liposomes can be modified to absorb more endogenous proteins with targeting capability. Disease-related proteins can be enriched on certain liposome surface and can be explored for early detection of diseases, such as specific IgA or IgG absorption in pancreatic cancer. PEG: Polyethylene glycol |
蛋白冠中调理素的吸附是导致脂质体体内清除速率加快的主要原因之一。研究表明, 当脂质体修饰有亲水聚合物如聚乙烯醇、肽类、低聚糖、糖蛋白或多糖时, 脂质体入血后循环时间显著延长[60]。PEG修饰后脂质体表面吸附的总蛋白量显著降低, 尤其是补体和免疫球蛋白等调理素类蛋白, 且蛋白冠中Apo J含量升高, 降低MPS的摄取, 循环时间延长[61]。
此外, 通过仿生学手段对脂质体进行“隐形”也是现研究中的常用手段。Rodriguez等[62]将CD47拟肽修饰在纳米药物表面, 向巨噬细胞传达一种“不要吞噬我”的信号, 可显著延缓肝脏和脾脏中吞噬细胞对纳米药物的清除速率, 该肽也有望延长脂质体药物的体内循环时间。Parodi等[63]利用仿生学手段, 制备了白细胞膜包被的脂质体(leukosomes), 通过减轻调理作用, 降低MPS对纳米药物的摄取。由于白细胞膜的包被, 脂质体表面吸附的总蛋白量可下降10倍左右(主要是IgG和白蛋白吸附量显著降低)。体外实验结果显示, 小鼠来源的巨噬细胞J774和人源的THP-1吞噬细胞摄取leukosomes量显著低于普通脂质体。白细胞膜包被的脂质体吸附血浆蛋白的模式与普通脂质体吸附模式亦不相同, 血浆中的补体蛋白及IgG与leukosomes表面受体结合, 可介导脂质体向炎症部位募集, 而非促进MPS对脂质体的清除(普通脂质体吸附调理素促进MPS清除)。
3.2 增加脂质体的靶向效率深入研究血浆成分对脂质体靶向效率的调控作用, 是实现靶向脂质体药物临床转化的关键步骤。血浆中含有大量可与组织和细胞表面特异性结合的配体, 蛋白冠的形成会对血浆中主要蛋白成分进行富集, 并与靶细胞或靶组织表面的受体特异性结合, 此类靶向治疗策略已有较广泛的研究。Arcella等[64]设计的阳离子脂质体表面大量吸附载脂蛋白、玻连蛋白和维生素K依赖蛋白, 这些蛋白可与血脑屏障(BBB)上过表达的受体(如Ⅰ型清道夫受体B和低密度脂蛋白受体等)结合, 通过转胞吞作用介导药物跨血脑屏障。DOTAP/DNA脂质体表面蛋白冠丰富, 借助NanoLC-MS/MS (nanoliquid chromatography tandem mass spectrometry)分析可识别蛋白冠中血浆蛋白组分的可能受体。蛋白冠中富含玻璃体蛋白, MDA-MB-435S细胞因表达相应受体而极易摄取DOTAP/DNA脂质体。借助流式细胞术及双色荧光共聚焦显微镜, 研究发现DOTAP/DNA脂质体在形成蛋白冠后, 可显著增加MDA-MB-435S细胞的摄取[27]。
脂蛋白复合物在细胞的胆固醇代谢中起关键作用, 且在细胞表面有多种受体, 载脂蛋白的富集可直接影响脂质体与细胞的相互作用, 提高靶向效果[65]。如载脂蛋白丰富的蛋白冠可靶向高表达清道夫B1受体(scavenger receptor class B type 1 receptor)的前列腺癌细胞(PC3与DU145细胞系), 载脂蛋白还可与低密度脂蛋白受体(low-density lipoprotein receptors)结合, 介导脂质体药物跨血脑屏障入脑, 用于脑部疾病的诊断及治疗。吸附载脂蛋白A-I (apolipoprotein A-I, Apo A-I)较多的阳离子脂质体可递送siRNA到小鼠肝脏细胞, 抑制丙型肝炎病毒的表达。Kim课题组[50, 51]研究制备的DOTAP-DOPE脂质体可特异性吸附Apo A-I, 递送蛋白药物到肺部, 治疗非小细胞性肺癌。载脂蛋白B (apolipoprotein B, Apo B)和载脂蛋白E (apolipoprotein E, Apo E)可增加肝细胞的摄取, 而载脂蛋白C-III (apolipoprotein C-III, Apo C-III)可削弱Apo E介导的HepG2细胞对胆固醇颗粒的摄取, 减轻其泡沫化水平。这些研究均表明蛋白冠的吸附可增加脂质体对特定细胞及组织的靶向功能。
3.3 探索疾病的生物标记物健康人和患者血浆成分有所不同, 如癌症患者血浆中特殊的细胞表型, 肽类、microRNA、代谢产物和某些蛋白等均可以作为生物标记物[66-68]。在特定条件下, 血浆蛋白的后修饰水平也可以作为同疾病的鉴定, 如补体C3、组氨酸富集的糖蛋白(histidine-rich glycoprotein)和激肽原-1 (kininogen-1)的糖基化与结肠癌的病理进程相关[69]; 某些血浆蛋白的羰基化(如VEGF-2、MMP-1及补体C5)与肥胖型2型糖尿病相关[70]; 炎症敏感型蛋白的升高与心肌梗死、中风和糖尿病等疾病密切相关, 尤其是α-纤维蛋白原、α-抗胰蛋白酶、C-反应蛋白与糖尿病密切相关[71], 而C-反应蛋白、肿瘤坏死因子-α和其他炎症因子可作为预测心血管疾病的重要标志物[72]。中度系统性炎症与慢性心衰的发病进程有关, 甚至会进一步引起心肌重构和心率失常等问题。流行病学研究已将较高的血浆炎症标记物和心血管疾病的发病率联系起来, 20年随访研究结果显示, 血浆中炎症相关蛋白(包括纤维蛋白原和α-1抗有胰蛋白酶)与心衰的住院率有关; 另一种受炎症影响的蛋白是白蛋白, 是血浆中最重要的一种血浆蛋白成分, 也是大多数纳米药物中最先被吸附的蛋白之一。白蛋白由肝脏合成, 是体内激素和维生素运输的基础。肝功能障碍或紊乱会直接影响血浆中白蛋白水平。白蛋白水平也是不同病理条件的指标, 如营养不良和感染等[73]。不仅如此, 神经系统紊乱的患者血浆蛋白水平也会有相应改变。在抑郁患者体内, 免疫球蛋白IgM、补体C3c、补体C4和α-抗胰蛋白酶水平升高, 而血浆中白蛋白和转铁蛋白水平比健康志愿者低[74]。阿尔茨海默病患者血浆中补体因子H和α-2巨球蛋白水平显著高于健康人群, 且含量与疾病程度密切相关[75-77]。
但疾病相关的生物标记分子在疾病早期浓度较低, 很难被检测到, 脂质体药物可对血浆中含量较低的分子起到富集作用。在进入血循环的瞬间, 脂质体首先会与含量较丰富的蛋白结合, 形成soft层, 但蛋白冠的吸附是一个动态过程, 随循环时间延长, 血浆中含量较低但结合活性较高的蛋白会置换含量较高的蛋白, 形成hard层, 使得血浆成分中含量较低的蛋白更容易被检测到。Corbo等[78]研究表明, 同一种纳米药物在不同疾病患者的血浆中形成的蛋白冠组分不同, 称之为个性化蛋白冠(personalized protein corona, PPC), 蛋白质组学研究结果表明, 纳米药物在健康人血浆及癌症患者的血浆中吸附的蛋白冠组分不同。Colapicchioni等[79]发现, 脂质体在胰腺癌患者血浆中形成的蛋白冠中IgA和IgG量显著升高, IgA和IgG是胰腺癌患者中产生的自噬抗体, 而自噬抗体在多种癌症被确诊前可检测到, 因此脂质体表面蛋白冠有望成为确诊早期癌症的生物标记物。
4 结论及展望脂质体已成为临床上常用的纳米递药系统(表 1)[80-126], 但其表面形成蛋白冠后, 其生物学特征异于体外设计, 影响MPS的摄取, 进而影响其在体内的清除速率、组织分布、靶向效率和免疫系统激活等, 具体机制及定量规律仍需进一步探讨, 对脂质体药物的安全性评价和临床转化具有重要意义。
| Table 1 Clinically used liposome-based products. US: United states; EU: European Union; HSPC: Hydrogenated soy phosphatidylcholine; PEG: Polyethylene glycol; DSPE: Distearoyl-sn-glycero-phosphoethanolamine; DSPC: Distearoylphosphatidylcholine; DOPC: Dioleoylphosphatidylcholine; DPPG: Dipalmitoylphosphatidylglycerol; EPC: Egg phosphatidylcholine; DOPS: Dioleoylphosphatidylserine; POPC: Palmitoyloleoylphosphatidylcholine; SM: Sphingomyelin; MPEG: Methoxy polyethylene glycol; DMPC: Dimyristoyl phosphatidylcholine; DMPG: Dimyristoyl phosphatidylglycerol; DSPG: Distearoylphosphatidylglycerol; DEPC: Dierucoylphosphatidylcholine; DOPE: Dioleoly-sn-glycero-phophoethanolamine |
靶向脂质体药物是纳米医学的重要研究方向, 在设计将治疗药物精准递送至病变部位时, 要全面地考虑到蛋白冠对靶向药物的影响, 减少MPS的清除作用以增加病变部位的蓄积。此外, 利用不同病理条件及生理条件下脂质体形成的蛋白冠组分不同, PPC为探索新靶向配体的设计提供了手段和依据, 也为筛选早期疾病标志物提供了可能。
脂质体药物的安全性是所有关键问题的重中之重, 在此前提下, 进一步明确脂质体与机体内环境的相互作用机制, 并加以规避和利用, 将有利于推动脂质体药物的临床转化。
致谢: 上海艾韦特医药科技有限公司李和文女士提供上市脂质体的相关信息。
| [1] |
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications[J]. Adv Drug Deliv Rev, 2013, 65: 36-48. DOI:10.1016/j.addr.2012.09.037 |
| [2] |
Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine[J]. ACC Chem Res, 2011, 44: 1094-1104. DOI:10.1021/ar200105p |
| [3] |
Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility[J]. J Control Release, 2011, 153: 198-205. DOI:10.1016/j.jconrel.2011.06.001 |
| [4] |
Nag OK, Yadav VR, Croft B, et al. Liposomes modified with superhydrophilic polymer linked to a nonphospholipid anchor exhibit reduced complement activation and enhanced circulation[J]. J Pharm Sci, 2015, 104: 114-123. DOI:10.1002/jps.24254 |
| [5] |
Nag OK, Awasthi V. Surface engineering of liposomes for stealth behavior[J]. Pharmaceutics, 2013, 5: 542-569. DOI:10.3390/pharmaceutics5040542 |
| [6] |
Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems[J]. Annu Rev Biomed Eng, 2012, 14: 1-16. DOI:10.1146/annurev-bioeng-071811-150124 |
| [7] |
Walkey CD, Olsen JB, Song F, et al. Protein corona fingerprin-ting predicts the cellular interaction of gold and silver nanoparticles[J]. ACS Nano, 2014, 8: 2439-2455. DOI:10.1021/nn406018q |
| [8] |
Bigdeli A, Palchetti S, Pozzi D, et al. Exploring cellular interactions of liposomes using protein corona fingerprints and physicochemical properties[J]. ACS Nano, 2016, 10: 3723-3737. DOI:10.1021/acsnano.6b00261 |
| [9] |
Bertrand N, Grenier P, Mahmoudi M, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics[J]. Nat Commun, 2017, 8: 777. DOI:10.1038/s41467-017-00600-w |
| [10] |
Karmali PP, Simberg D. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems[J]. Expert Opin Drug Deliv, 2011, 8: 343-357. DOI:10.1517/17425247.2011.554818 |
| [11] |
Saha K, Rahimi M, Yazdani M, et al. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona[J]. ACS Nano, 2016, 10: 4421-4430. DOI:10.1021/acsnano.6b00053 |
| [12] |
Chen D, Ganesh S, Wang W, et al. Plasma protein adsorption and biological identity of systemically administered nanoparticles[J]. Nanomedicine (Lond), 2017, 12: 2113-2135. DOI:10.2217/nnm-2017-0178 |
| [13] |
Soni S, Ruhela RK, Medhi B. Nanomedicine in central nervous system (CNS) disorders: a present and future prospective[J]. Adv Pharm Bull, 2016, 6: 319-335. DOI:10.15171/apb.2016.044 |
| [14] |
Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology[J]. Nat Nanotechnol, 2013, 8: 772-781. DOI:10.1038/nnano.2013.181 |
| [15] |
Yang ST, Liu Y, Wang YW, et al. Biosafety and bioapplication of nanomaterials by designing protein-nanoparticle interactions[J]. Small, 2013, 9: 1635-1653. DOI:10.1002/smll.201201492 |
| [16] |
Milani S, Bombelli FB, Pitek AS, et al. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard corona[J]. ACS Nano, 2012, 6: 2532-2541. DOI:10.1021/nn204951s |
| [17] |
Walkey CD, Chan WC. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment[J]. Chem Soc Rev, 2012, 41: 2780-2799. DOI:10.1039/C1CS15233E |
| [18] |
Hadjidemetriou M, Al-Ahmady Z, Buggio M, et al. A novel scavenging tool for cancer biomarker discovery based on the blood-circulating nanoparticle protein corona[J]. Biomaterials, 2019, 188: 118-129. DOI:10.1016/j.biomaterials.2018.10.011 |
| [19] |
Caracciolo G. Liposome-protein corona in a physiological environment: challenges and opportunities for targeted delivery of nanomedicines[J]. Nanomedicine, 2015, 11: 543-557. DOI:10.1016/j.nano.2014.11.003 |
| [20] |
Mahmoudi M, Lynch I, Ejtehadi MR, et al. Protein-nanoparticle interactions: opportunities and challenges[J]. Chem Rev, 2011, 111: 5610-5637. DOI:10.1021/cr100440g |
| [21] |
Roser M, Fischer D, Kissel T. Surface-modified biodegradable albumin nano- and microspheres. Ⅱ: effect of surface charges on in vitro phagocytosis and biodistribution in rats[J]. Eur J Pharm Biopharm, 1998, 46: 255-263. DOI:10.1016/S0939-6411(98)00038-1 |
| [22] |
Capriotti AL, Caracciolo G, Caruso G, et al. Differential analysis of "protein corona" profile adsorbed onto different nonviral gene delivery systems[J]. Anal Biochem, 2011, 419: 180-189. DOI:10.1016/j.ab.2011.08.003 |
| [23] |
Caracciolo G, Pozzi D, Capriotti AL, et al. Evolution of the protein corona of lipid gene vectors as a function of plasma concentration[J]. Langmuir, 2011, 27: 15048-15053. DOI:10.1021/la202912f |
| [24] |
Capriotti AL, Caracciolo G, Cavaliere C, et al. Do plasma proteins distinguish between liposomes of varying charge density?[J]. J Proteomics, 2012, 75: 1924-1932. DOI:10.1016/j.jprot.2012.01.003 |
| [25] |
Capriotti AL, Caracciolo G, Caruso G, et al. Label-free quantitative analysis for studying the interactions between nanoparticles and plasma proteins[J]. Anal Bioanal Chem, 2013, 405: 635-645. DOI:10.1007/s00216-011-5691-y |
| [26] |
Capriotti AL, Caracciolo G, Cavaliere C, et al. Shotgun proteomic analytical approach for studying proteins adsorbed onto liposome surface[J]. Anal Bioanal Chem, 2011, 401: 1195-1202. DOI:10.1007/s00216-011-5188-8 |
| [27] |
Caracciolo G, Pozzi D, Capriotti AL, et al. Factors determining the superior performance of lipid/DNA/protammine nanoparticles over lipoplexes[J]. J Med Chem, 2011, 54: 4160-4171. DOI:10.1021/jm200237p |
| [28] |
Caracciolo G, Cardarelli F, Pozzi D, et al. Selective targeting capability acquired with a protein corona adsorbed on the surface of 1, 2-dioleoyl-3-trimethylammonium propane/DNA nanoparticles[J]. ACS Appl Mater Interfaces, 2013, 5: 13171-13179. DOI:10.1021/am404171h |
| [29] |
Pozzi D, Colapicchioni V, Caracciolo G, et al. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells[J]. Nanoscale, 2014, 6: 2782-2792. DOI:10.1039/c3nr05559k |
| [30] |
Senior J, Gregoriadis G. Is half-life of circulating liposomes determined by changes in their permeability?[J]. Febs Lett, 1982, 145: 109-114. DOI:10.1016/0014-5793(82)81216-7 |
| [31] |
Damen J, Regts J, Scherphof G. Transfer and exchange of phospholipid between small unilamellar liposomes and rat plasma high density lipoproteins. Dependence on cholesterol content and phospholipid composition[J]. Biochim Biophys Acta, 1981, 665: 538-545. DOI:10.1016/0005-2760(81)90268-X |
| [32] |
Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors[J]. Proc Natl Acad Sci U S A, 1988, 85: 6949-6953. DOI:10.1073/pnas.85.18.6949 |
| [33] |
Allen TM, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system[J]. Febs Lett, 1987, 223: 42-46. DOI:10.1016/0014-5793(87)80506-9 |
| [34] |
Klibanov AL, Maruyama K, Torchilin VP, et al. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes[J]. Febs Lett, 1990, 268: 235-237. DOI:10.1016/0014-5793(90)81016-H |
| [35] |
Xiao W, Xiong J, Zhang S, et al. Influence of ligands property and particle size of gold nanoparticles on the protein adsorption and corresponding targeting ability[J]. Int J Pharm, 2018, 538: 105-111. DOI:10.1016/j.ijpharm.2018.01.011 |
| [36] |
Mahmoudi M, Abdelmonem AM, Behzadi S, et al. Temperature: the "ignored" factor at the NanoBio interface[J]. ACS Nano, 2013, 7: 6555-6562. DOI:10.1021/nn305337c |
| [37] |
Guan J, Shen Q, Zhang Z, et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption[J]. Nat Commun, 2018, 9: 2982. DOI:10.1038/s41467-018-05384-1 |
| [38] |
Yu K, Lai BF, Foley JH, et al. Modulation of complement activation and amplification on nanoparticle surfaces by glycopolymer conformation and chemistry[J]. ACS Nano, 2014, 8: 7687-7703. DOI:10.1021/nn504186b |
| [39] |
Vu VP, Gifford GB, Chen F, et al. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles[J]. Nat Nanotechnol, 2019, 14: 260-268. DOI:10.1038/s41565-018-0344-3 |
| [40] |
Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology[J]. Nat Nanotechnol, 2013, 8: 772-781. DOI:10.1038/nnano.2013.181 |
| [41] |
Palchetti S, Colapicchioni V, Digiacomo L, et al. The protein corona of circulating PEGylated liposomes[J]. Biochim Biophys Acta, 2016, 1858: 189-196. DOI:10.1016/j.bbamem.2015.11.012 |
| [42] |
Caracciolo G, Farokhzad OC, Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein corona[J]. Trends Biotechnol, 2017, 35: 257-264. DOI:10.1016/j.tibtech.2016.08.011 |
| [43] |
Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system[J]. Int J Pharm, 2018, 552: 328-339. DOI:10.1016/j.ijpharm.2018.10.011 |
| [44] |
Caputo D, Papi M, Coppola R, et al. A protein corona-enabled blood test for early cancer detection[J]. Nanoscale, 2017, 9: 349-354. DOI:10.1039/C6NR05609A |
| [45] |
Robson AL, Dastoor PC, Flynn J, et al. Advantages and limitations of current imaging techniques for characterizing liposome morphology[J]. Front Pharmacol, 2018, 9: 80. DOI:10.3389/fphar.2018.00080 |
| [46] |
Rideau E, Dimova R, Schwille P, et al. Liposomes and polymersomes: a comparative review towards cell mimicking[J]. Chem Soc Rev, 2018, 47: 8572-8610. DOI:10.1039/C8CS00162F |
| [47] |
Corbo C, Molinaro R, Parodi A, et al. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery[J]. Nanomedicine, 2016, 11: 81-100. DOI:10.2217/nnm.15.188 |
| [48] |
Nguyen VH, Lee BJ. Protein corona: a new approach for nanomedicine design[J]. Int J Nanomedicine, 2017, 12: 3137-3151. DOI:10.2147/IJN.S129300 |
| [49] |
Bouwens van der Vlis TAM, Kros JM, Mustafa D, et al. The complement system in glioblastoma multiforme[J]. Acta Neuropathol Commun, 2018, 6: 91. DOI:10.1186/s40478-018-0591-4 |
| [50] |
Agrahari V, Burnouf PA, Burnouf T, et al. Nanoformulation properties, characterization, and behavior in complex biological matrices: challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential[J]. Adv Drug Deliv Rev, 2019. |
| [51] |
Patel HM, Moghimi SM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system - the concept of tissue specificity[J]. Adv Drug Deliv Rev, 1998, 32: 45-60. DOI:10.1016/S0169-409X(97)00131-2 |
| [52] |
Ingen-Housz-Oro S, Pham-Ledard A, Brice P, et al. Immediate hypersensitivity reaction to pegylated liposomal doxorubicin: management and outcome in four patients[J]. Eur J Dermatol, 2017, 27: 271-274. |
| [53] |
Alekseeva AA, Moiseeva EV, Onishchenko NR, et al. Liposomal formulation of a methotrexate lipophilic prodrug: assessment in tumor cells and mouse T-cell leukemic lymphoma[J]. Int J Nanomedicine, 2017, 12: 3735-3749. DOI:10.2147/IJN.S133034 |
| [54] |
Jiang Z, Guan J, Qian J, et al. Peptide ligand-mediated targeted drug delivery of nanomedicines[J]. Biomater Sci, 2019, 7: 461-471. DOI:10.1039/C8BM01340C |
| [55] |
Corbo C, Molinaro R, Taraballi F, et al. Effects of the protein corona on liposome-liposome and liposome-cell interactions[J]. Int J Nanomedicine, 2016, 11: 3049-3063. DOI:10.2147/IJN.S109059 |
| [56] |
Kelly PM, Aberg C, Polo E, et al. Mapping protein binding sites on the biomolecular corona of nanoparticles[J]. Nat Nanotechnol, 2015, 10: 472-479. DOI:10.1038/nnano.2015.47 |
| [57] |
Al-Ahmady ZS, Hadjidemetriou M, Gubbins J, et al. Formation of protein corona in vivo affects drug release from temperature-sensitive liposomes[J]. J Control Release, 2018, 276: 157-167. DOI:10.1016/j.jconrel.2018.02.038 |
| [58] |
Oh JY, Kim HS, Palanikumar L, et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery[J]. Nat Commun, 2018, 9: 4548. DOI:10.1038/s41467-018-06979-4 |
| [59] |
Garcia-Alvarez R, Hadjidemetriou M, Sanchez-Iglesias A, et al. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape[J]. Nanoscale, 2018, 10: 1256-1264. DOI:10.1039/C7NR08322J |
| [60] |
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery[J]. Nat Biotechnol, 2015, 33: 941-951. DOI:10.1038/nbt.3330 |
| [61] |
Yuda T, Maruyama K, Iwatsuru M. Prolongation of liposome circulation time by various derivatives of polyethyleneglycols[J]. Biol Pharm Bull, 1996, 19: 1347-1351. DOI:10.1248/bpb.19.1347 |
| [62] |
Rodriguez PL, Harada T, Christian DA, et al. Minimal "Self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles[J]. Science, 2013, 339: 971-975. DOI:10.1126/science.1229568 |
| [63] |
Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions[J]. Nat Nanotechnol, 2013, 8: 61-68. DOI:10.1038/nnano.2012.212 |
| [64] |
Arcella A, Palchetti S, Digiacomo L, et al. Brain targeting by liposome-biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells[J]. ACS Chem Neurosci, 2018, 9: 3166-3174. DOI:10.1021/acschemneuro.8b00339 |
| [65] |
Barran-Berdon AL, Pozzi D, Caracciolo G, et al. Time evolution of nanoparticle-protein corona in human plasma: relevance for targeted drug delivery[J]. Langmuir, 2013, 29: 6485-6494. DOI:10.1021/la401192x |
| [66] |
Surinova S, Schiess R, Huttenhain R, et al. On the development of plasma protein biomarkers[J]. J Proteome Res, 2011, 10: 5-16. DOI:10.1021/pr1008515 |
| [67] |
Kiddle SJ, Steves CJ, Mehta M, et al. Plasma protein biomarkers of Alzheimer's disease endophenotypes in asymptomatic older twins: early cognitive decline and regional brain volumes[J]. Transl Psychiatry, 2015, 5: e584. DOI:10.1038/tp.2015.78 |
| [68] |
Schley G, Koberle C, Manuilova E, et al. Comparison of plasma and urine biomarker performance in acute kidney injury[J]. PLoS One, 2015, 10: e145042. |
| [69] |
Qiu Y, Patwa TH, Xu L, et al. Plasma glycoprotein profiling for colorectal cancer biomarker identification by lectin glycoarray and lectin blot[J]. J Proteome Res, 2008, 7: 1693-1703. DOI:10.1021/pr700706s |
| [70] |
Bollineni RC, Fedorova M, Bluher M, et al. Carbonylated plasma proteins as potential biomarkers of obesity induced type 2 diabetes mellitus[J]. J Proteome Res, 2014, 13: 5081-5093. DOI:10.1021/pr500324y |
| [71] |
Festa A, D'Agostino RJ, Tracy RP, et al. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study[J]. Dlabetes, 2002, 51: 1131-1137. |
| [72] |
Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women[J]. N Engl J Med, 2000, 342: 836-843. DOI:10.1056/NEJM200003233421202 |
| [73] |
Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition[J]. Semin Dial, 2004, 17: 432-437. DOI:10.1111/j.0894-0959.2004.17603.x |
| [74] |
Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls[J]. J Affect Disord, 1994, 30: 283-288. DOI:10.1016/0165-0327(94)90135-X |
| [75] |
Thambisetty M, Tripaldi R, Riddoch-Contreras J, et al. Proteome-based plasma markers of brain amyloid-beta deposition in non-demented older individuals[J]. J Alzheimers Dis, 2010, 22: 1099-1109. |
| [76] |
Hye A, Lynham S, Thambisetty M, et al. Proteome-based plasma biomarkers for Alzheimer's disease[J]. Brain, 2006, 129: 3042-3050. DOI:10.1093/brain/awl279 |
| [77] |
ManafiRad A, Farzadfar F, Habibi L, et al. Is amyloid-beta an innocent bystander and marker in Alzheimer's disease? Is the liability of multivalent cation homeostasis and its influence on amyloid-beta function the real mechanism?[J]. J Alzheimers Dis, 2014, 42: 69-85. DOI:10.3233/JAD-140321 |
| [78] |
Corbo C, Molinaro R, Tabatabaei M, et al. Personalized protein corona on nanoparticles and its clinical implications[J]. Biomater Sci, 2017, 5: 378-387. DOI:10.1039/C6BM00921B |
| [79] |
Colapicchioni V, Tilio M, Digiacomo L, et al. Personalized liposome-protein corona in the blood of breast, gastric and pancreatic cancer patients[J]. Int J Biochem Cell Biol, 2016, 75: 180-187. DOI:10.1016/j.biocel.2015.09.002 |
| [80] |
Zhao Y, Alakhova DY, Kim JO, et al. A simple way to enhance Doxil® therapy: drug release from liposomes at the tumor site by amphiphilic block copolymer[J]. J Control Release, 2013, 168: 61-69. DOI:10.1016/j.jconrel.2013.02.026 |
| [81] |
Batist G, Barton J, Chaikin P, et al. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy[J]. Expert Opin Pharmacother, 2002, 3: 1739-1751. DOI:10.1517/14656566.3.12.1739 |
| [82] |
Ren S, Dai Y, Li C, et al. Pharmacokinetics and pharmacodynamics evaluation of a thermosensitive chitosan based hydrogel containing liposomal doxorubicin[J]. Eur J Pharm Sci, 2016, 92: 137-145. DOI:10.1016/j.ejps.2016.07.002 |
| [83] |
No authors listed. FDA approves DaunoXome as first-line therapy for Kaposi's sarcoma. Food and Drug Administration[J]. J Int Assoc Physicians Aids Care, 1996, 2: 50-51. |
| [84] |
Stone NR, Bicanic T, Salim R, et al. Liposomal amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions[J]. Drugs, 2016, 76: 485-500. DOI:10.1007/s40265-016-0538-7 |
| [85] |
Adedoyin A, Bernardo JF, Swenson CE, et al. Pharmacokinetic profile of ABELCET (amphotericin B lipid complex injection): combined experience from phase Ⅰ and phase Ⅱ studies[J]. Antimicrob Agents Chemother, 1997, 41: 2201-2208. DOI:10.1128/AAC.41.10.2201 |
| [86] |
Clemons KV, Stevens DA. Comparative efficacies of four amphotericin B formulations--fungizone, amphotec (Amphocil), AmBisome, and Abelcet--against systemic murine aspergillosis[J]. Antimicrob Agents Chemother, 2004, 48: 1047-1050. DOI:10.1128/AAC.48.3.1047-1050.2004 |
| [87] |
Clemons KV, Stevens DA. Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis[J]. Antimicrob Agents Chemother, 1998, 42: 899-902. DOI:10.1128/AAC.42.4.899 |
| [88] |
Paterson DL, David K, Mrsic M, et al. Pre-medication practices and incidence of infusion-related reactions in patients receiving AMPHOTEC: data from the patient registry of amphotericin B cholesteryl sulfate complex for injection clinical tolerability (PRoACT) registry[J]. J Antimicrob Chemother, 2008, 62: 1392-1400. DOI:10.1093/jac/dkn394 |
| [89] |
Benesch M, Siegler N, Hoff K, et al. Safety and toxicity of intrathecal liposomal cytarabine (Depocyte) in children and adolescents with recurrent or refractory brain tumors: a multi-institutional retrospective study[J]. Anticancer Drugs, 2009, 20: 794-799. DOI:10.1097/CAD.0b013e32832f4abe |
| [90] |
Rueda DA, Olmos HD, Viciana GR, et al. Liposomal cytarabine (DepoCyte) for the treatment of neoplastic meningitis[J]. Clin Transl Oncol, 2005, 7: 232-238. DOI:10.1007/BF02710168 |
| [91] |
Gambling D, Hughes T, Martin G, et al. A comparison of depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery[J]. Anesth Analg, 2005, 100: 1065-1074. DOI:10.1213/01.ANE.0000145009.03574.78 |
| [92] |
Peravali R, Brock R, Bright E, et al. Enhancing the enhanced recovery program in colorectal surgery - use of extended-release epidural morphine (DepoDur®)[J]. Ann Coloproctol, 2014, 30: 186-191. DOI:10.3393/ac.2014.30.4.186 |
| [93] |
Pasero C, McCaffery M. Extended-release epidural morphine (DepoDur)[J]. J Perlanesth Nurs, 2005, 20: 345-350. DOI:10.1016/j.jopan.2005.07.004 |
| [94] |
Keck S, Glennon C, Ginsberg B. DepoDur extended-release epidural morphine: reshaping postoperative care[J]. Orthop Nurs, 2007, 26: 86-93. DOI:10.1097/01.NOR.0000265863.78103.78 |
| [95] |
Alam M, Hartrick CT. Extended-release epidural morphine (DepoDur): an old drug with a new profile[J]. Pain Pract, 2005, 5: 349-353. DOI:10.1111/j.1533-2500.2005.00048.x |
| [96] |
Burbridge M, Jaffe RA. Exparel®: a new local anesthetic with special safety concerns[J]. Anesth Analg, 2015, 121: 1113-1114. |
| [97] |
Oppenheimer AJ, Fiala T, Oppenheimer DC. Direct transversus abdominis plane blocks with exparel during abdominoplasty[J]. Ann Plast Surg, 2016, 77: 499-500. DOI:10.1097/SAP.0000000000000659 |
| [98] |
Ketonis C, Kim N, Liss F, et al. Wide awake trigger finger release surgery: prospective comparison of lidocaine, marcaine, and exparel[J]. Hand (N Y), 2016, 11: 177-183. DOI:10.1177/1558944715627618 |
| [99] |
Richard BM, Rickert DE, Doolittle D, et al. Pharmacokinetic compatibility study of lidocaine with EXPAREL in Yucatan miniature pigs[J]. ISRN Pharm, 2011, 2011: 582351. |
| [100] |
Surdam JW, Licini DJ, Baynes NT, et al. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients[J]. J Arthroplasty, 2015, 30: 325-329. DOI:10.1016/j.arth.2014.09.004 |
| [101] |
Soberon JJ, Sisco-Wise LE, Dunbar RM. Compartment syndrome in a patient treated with perineural liposomal bupivacaine (Exparel)[J]. J Clin Anesth, 2016, 31: 1-4. DOI:10.1016/j.jclinane.2015.11.001 |
| [102] |
Day KM, Nair NM, Sargent LA. Extended release liposomal bupivacaine injection (Exparel) for early postoperative pain control following palatoplasty[J]. J Craniofac Surg, 2018, 29: e525-e528. |
| [103] |
Day KM, Nair NM, Griner D, et al. Extended release liposomal bupivacaine injection (Exparel) for early postoperative pain control following pharyngoplasty[J]. J Craniofac Surg, 2018, 29: 726-730. |
| [104] |
Richard BM, Newton P, Ott LR, et al. The safety of EXPAREL® (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogs[J]. J Drug Deliv, 2012, 2012: 962101. |
| [105] |
Richard BM, Rickert DE, Newton PE, et al. Safety evaluation of EXPAREL (DepoFoam bupivacaine) administered by repeated subcutaneous injection in rabbits and dogs: species comparison[J]. J Drug Deliv, 2011, 2011: 467429. |
| [106] |
Glenn B, Drum M, Reader A, et al. Does liposomal bupivacaine (Exparel) significantly reduce postoperative pain/numbness in symptomatic teeth with a diagnosis of necrosis? a prospective, randomized, double-blind trial[J]. J Endod, 2016, 42: 1301-1306. DOI:10.1016/j.joen.2016.05.018 |
| [107] |
Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE Phase Ⅳ health economics trial[J]. J Pain Res, 2013, 6: 605-610. |
| [108] |
Marcet JE, Nfonsam VN, Larach S. An extended pain relief trial utilizing the infiltration of a long-acting multivesicular liPosome foRmulation of bupiVacaine, EXPAREL (IMPROVE): a phase Ⅳ health economic trial in adult patients undergoing ileostomy reversal[J]. J Pain Res, 2013, 6: 549-555. |
| [109] |
Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase Ⅳ health economic trial in adult patients undergoing open colectomy[J]. J Pain Res, 2012, 5: 567-572. |
| [110] |
Bupivacaine liposomal injection (Exparel) for post surgical pain[J]. Med Lett Drugs Ther, 2012, 54: 26-27.
|
| [111] |
Ang MJ, Silkiss RZ. The use of long-acting liposomal bupivacaine (Exparel) for postoperative pain control following enucleation or evisceration[J]. Ophthalmic Plast Reconstr Surg, 2018, 34: 599. |
| [112] |
Butz DR, Shenaq DS, Rundell VL, et al. Postoperative pain and length of stay lowered by use of exparel in immediate, implant-based breast reconstruction[J]. Plast Reconstr Surg Glob Open, 2015, 3: e391. DOI:10.1097/GOX.0000000000000355 |
| [113] |
Ilfeld BM, Viscusi ER, Hadzic A, et al. Safety and side effect profile of liposome bupivacaine (Exparel) in peripheral nerve blocks[J]. Reg Anesth Pain Med, 2015, 40: 572-582. DOI:10.1097/AAP.0000000000000283 |
| [114] |
Ichikawa K, Takeuchi Y, Yonezawa S, et al. Antiangiogenic photodynamic therapy (PDT) using visudyne causes effective suppression of tumor growth[J]. Cancer Lett, 2004, 205: 39-48. DOI:10.1016/j.canlet.2003.10.001 |
| [115] |
Gluck R, Walti E. Biophysical validation of Epaxal Berna, a hepatitis A vaccine adjuvanted with immunopotentiating reconstituted influenza virosomes (IRIV)[J]. Dev Biol (Basel), 2000, 103: 189-197. |
| [116] |
Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection[J]. Expert Rev Vaccines, 2008, 7: 1141-1150. DOI:10.1586/14760584.7.8.1141 |
| [117] |
Kursteiner O, Moser C, Lazar H, et al. Inflexal V - the influenza vaccine with the lowest ovalbumin content[J]. Vaccines, 2006, 24: 6632-6635. DOI:10.1016/j.vaccine.2006.05.084 |
| [118] |
Herzog C, Hartmann K, Kunzi V, et al. Eleven years of inflexal V - a virosomal adjuvanted influenza vaccine[J]. Vaccine, 2009, 27: 4381-4387. DOI:10.1016/j.vaccine.2009.05.029 |
| [119] |
Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production[J]. Vaccine, 2002, Suppl 5: B17-B23. |
| [120] |
Rodriguez MA, Pytlik R, Kozak T, et al. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: report of the pivotal phase 2 study[J]. Cancer, 2009, 115: 3475-3482. DOI:10.1002/cncr.24359 |
| [121] |
Cohen MR, Smetzer JL. New connectors coming for enteral feeding tubes; marqibo and risk of errors; angeliq is not a birth control pill[J]. Hosp Pharm, 2014, 49: 596-598. DOI:10.1310/hpj4907-596 |
| [122] |
Bedikian AY, Silverman JA, Papadopoulos NE, et al. Pharmacokinetics and safety of Marqibo (vincristine sulfate liposomes injection) in cancer patients with impaired liver function[J]. J Clin Pharmacol, 2011, 51: 1205-1212. DOI:10.1177/0091270010381499 |
| [123] |
No authors listed. FDA approves liposomal vincristine (Marqibo) for rare leukemia[J]. Oncology (Williston Park), 2012, 26: 841. |
| [124] |
Passero FJ, Grapsa D, Syrigos KN, et al. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy[J]. Expert Rev Anticancer Ther, 2016, 16: 697-703. DOI:10.1080/14737140.2016.1192471 |
| [125] |
No authors listed. In brief: liposomal irinotecan (Onivyde) for pancreatic cancer[J]. Med Lett Drugs Ther, 2016, 58: e76. |
| [126] |
Zhang H. Onivyde for the therapy of multiple solid tumors[J]. Onco Targets Ther, 2016, 9: 3001-3007. |
 2019, Vol. 54
2019, Vol. 54


