2017年2月, WHO发布全球首份急需研发新抗生素的重点病原体清单, 其中1类重点极为重要(critical)包括3种:碳青霉烯类药物耐药鲍曼不动杆菌、碳青霉烯类药物耐药铜绿假单胞菌以及碳青霉烯类药物耐药和三代头孢菌素耐药肠杆菌科细菌, 这些多药耐药革兰氏阴性菌对碳青霉烯类抗生素具有耐药性, 因此, 临床急需新的抗生素来治疗这些严重的耐药菌。多黏菌素对该类阴性菌具有很好疗效, 因此多黏菌素成为治疗多药耐药革兰氏阴性菌的“最后一道防线”[1]。多黏菌素首次报道于1947年[2], 是不同多黏芽孢类杆菌发酵产生的不同结构类型的环状抗菌脂肽, 包括多黏菌素B、E、A、C、D、F、K、M、P、S和T等[3, 4]。其中, 临床使用的是多黏菌素B和黏菌素甲磺酸盐(colistimethate sodium, CMS)。多黏菌素类抗生素的共同结构特征为:由环状七肽、线性三肽和与线性三肽连接的N-脂肪酰基链三部分组成, 其中七肽环由4位氨基酸L-Dab (α, γ-二氨基丁酸)与10位氨基酸L-Thr (或L-Leu)缩合形成。根据N-取代脂肪酰基链或7位氨基酸不同, 同类型结构的多黏菌素中一般又含有两种以上的不同组分。20世纪50年代初, 多黏菌素B应用不久, 就发现全身给药常发生严重的不良反应, 包括显著的肾毒性, 20世纪70年代后临床使用逐渐减少[5-7]。最近临床研究发现CMS诱导的患者急性肾损伤发生率高达60.4%, 多黏菌素B的发生率为41.8%, 多黏菌素的肾毒性严重限制了临床使用[8, 9]。因此, 急需研发新型高效、低毒的多黏菌素衍生物。
多黏菌素B的抗菌作用与其两亲性的结构有关, 带正电的L-Dab残基(α, γ-二氨基丁酸)与脂质A (lipid A)带负电的磷酸基团产生静电相互作用; 多黏菌素的N-取代脂肪酰基链和6、7位疏水氨基酸插入到细菌外膜导致外膜膨胀[1]。20世纪70年代以来, 很多研究人员通过修饰多黏菌素的结构来研究其构效关系。除了改变N-取代脂肪酰基链的长度, 很多芳基、环烷基也用来替换N-取代脂肪酰基链, 特别是含有杂原子的芳基、环烷基替换得到的衍生物CB-182804、5x和CA824的抗菌活性接近或相当于多黏菌素B[10-12], 因此希望通过在N-取代脂肪酰基链中引入氮原子、氧原子来考察其对抗菌活性的影响。此外, 之前对6位氨基酸研究较少, 最近研究表明辛烷基甘氨酸(D-octylglycine)或联苯基丙氨酸(bipheyl-D-alanine)替换6位D-Phe后抗菌活性保持[13, 14]。因此, 希望通过在D-Phe的苯环上引入不同极性或疏水性的取代基, 考察其对抗菌活性的影响。
本研究通过改变N-取代脂肪酰基链或6位D-Phe的疏水性和体积设计合成两类多黏菌素B衍生物20个(表 1), 衍生物的合成如合成路线1所示[15]。首先评价所有衍生物的体外抗菌活性, 接着选择活性较好的衍生物评价其肾细胞毒性。与多黏菌素B相比, 大部分衍生物显示出有效的抗菌活性, 同时衍生物7e的肾细胞毒性相当、7l的肾细胞毒性降低。
| Table 1 Chemical structures of polymyxin B analogues. atert-butyl; bbenzoyl |
本文共合成多黏菌素B衍生物20个, 化合物结构经高分辨质谱和核磁共振氢谱确证。详细数据见实验部分。
2 活性评价体外抗菌试验表明(表 2), 大部分化合物对测定的阴性菌显示出有效的抗菌活性。衍生物7a (2~4 μg·mL-1)和7b (2~8 μg·mL-1)与多黏菌素B (0.5~2 μg·mL-1)相比, 体外抗菌活性降低; 表明N-取代脂肪酰基链替换为芳香疏水性基团抗菌活性下降。衍生物7c对大肠埃希菌、肺炎克雷伯菌和鲍曼不动杆菌的抗菌活性下降, 对铜绿假单胞菌(1 μg·mL-1)的抗菌活性保持; 表明减小N-取代脂肪酰基链的疏水性使活性降低。衍生物7d (0.5~2 μg·mL-1)对大肠埃希菌、鲍曼不动杆菌和铜绿假单胞菌的抗菌活性保持, 对肺炎克雷伯菌的抗菌活性下降。衍生物7e (0.5~4 μg·mL-1)对测定的阴性菌抗菌活性保持。值得注意的是, N-取代脂肪酰基链疏水性降低的衍生物7c~7e对铜绿假单胞菌的抗菌活性保持。衍生物7f~7p、7s和7t (≤4 μg·mL-1)抗菌活性保持, 特别是衍生物7l (0.25~2 μg·mL-1)体外抗菌活性增强; 表明6位D-Phe苯环上引入小体积的极性基团对抗菌活性影响较小。衍生物7q (4~16 μg·mL-1)和7r (2~8 μg·mL-1)对测定阴性菌的体外抗菌活性下降; 表明在D-Phe的苯环上引入大体积的疏水性基团对活性不利。选择有抗菌活性的化合物(MIC≤4 μg·mL-1)测定其对非洲绿猴肾细胞(Vero)的细胞毒性(表 2)。与多黏菌素B (120.3 ± 6.0 μg·mL-1)相比, 衍生物7e (128.1 ± 10.6 μg·mL-1)的肾细胞毒性相当(P > 0.05), 衍生物7l (217.1 ± 13.2 μg·mL-1)的肾细胞毒性降低(P < 0.01), 其他化合物的肾细胞毒性增大。
| Table 2 Minimum inhibitory concentration (MIC: μg·mL-1) and cytotoxicity (CC50: μg·mL-1) of polymyxin B analogues. aAmerican Type Culture Collection. bClinical isolate. c50% cytotoxic concentration. dPolymyxin B. eNot determined. ESBLs: Extended-spectrum β-lactamases. NDM-1: New Delhi Metallo-beta-lactamase-1 |
本文通过固相合成方法, 高效合成多黏菌素B衍生物, 所合成衍生物结构经高分辨质谱和核磁共振氢谱确证。其中衍生物7e的抗菌活性(0.5~4 μg·mL-1)和肾细胞毒性(128.1 ± 10.6 μg·mL-1)与阳性对照药多黏菌素B相当, 衍生物7l的体外抗菌活性(0.25~2 μg·mL-1)增强, 肾细胞毒性(217.1 ± 13.2 μg·mL-1)降低。构效关系表明, N-取代脂肪酰基链替换为芳香疏水性基团抗菌活性下降, 降低N-取代脂肪酰基链的疏水性抗菌活性也降低; 6位D-Phe苯环上引入大体积的疏水性基团对活性不利, 苯环上引入小体积的极性取代基抗菌活性保持。研究结果表明, 通过对多黏菌素B的结构改造, 有可能获得高效、低毒的多黏菌素衍生物。
实验部分CS136xt型全自动多肽合成仪(美国CS Bio公司); 液质联用仪为日本岛津LC-MS 2020, 分析柱Shim-pack VP-ODS (2.0 mm×150 mm, 5 μm); 高效液相为美国安捷伦1200 series型分析型高效液相色谱系统, 分析柱Eclipse XDB-C18 (4.6 mm×150 mm, 5 μm); 分离纯化为日本岛津LC-20AT型半制备型高效液相色谱系统, 半制备柱Shim-pack PREP-ODS (20 mm×250 mm ID, 10 μm); 旋光为美国Autopol IV旋光仪测定; 高分辨质谱(HR-MS)为美国Thermo Scientific LTQ Orbitrap XL instrument测定; 1H NMR采用Bruker 500 MHz核磁共振波谱仪测定。
氨基酸、6-氯苯并三氮唑-1, 1, 3, 3-四甲基脲六氟磷酸盐(HCTU)购自吉尔生化(上海)有限公司; (3H-1, 2, 3-三唑并[4, 5-b]吡啶-3-氧基)三-1-吡咯烷基鏻六氟磷酸盐(PyAOP)、1-羟基-7-偶氮苯并三氮唑(HOAt)和四(三苯基膦)钯(Pd(PPh3)4)购自上海迈瑞尔化学技术有限公司; 色谱纯乙腈、色谱纯三氟乙酸(TFA)、(S)-(+)-6-甲基-1-辛醇、4-苯氧基苯甲酸、4-(4-甲基苯氧基)苯甲酸、5-(二甲氨基)戊酸、6-甲氧基己酸、3-氧代庚酸甲酯、N-甲基吗啉(NMM)、三异丙基硅烷(TIS)、苯硅烷(PhSiH3)、N, N-二异丙基乙胺(DIPEA)购自J & K百灵威科技有限公司; 哌啶、无水乙醚、水合肼(40%)、甲醇(MeOH)、二氯甲烷(DCM)等购自北京化工厂; N, N-二甲基甲酰胺(DMF)购自北京博迈杰科技有限公司; 2CTC resin (0.53 mmol·g-1)购自天津南开和成科技有限公司, (S)-(+)-6-甲基-1-辛酸为实验室制备[15]。

|
Scheme 1 Synthetic route of polymyxin B analogues
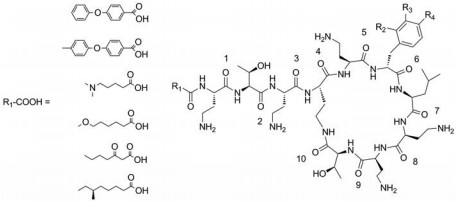
Compounds 7a-7e: R2=H, R3=H, R4=H. 7a: R1COOH=4-phenoxybenzoic acid; 7b: R1COOH=4-(4-methylphenoxy)benzoic acid; 7c: R1COOH=5-(dimethylamino)pentanoic acid; 7d: R1COOH=6-methoxyhexanoic acid; 7e: R1COOH=3-oxoheptanoic acid. Compounds 7f-7t: R1COOH=(S)-6-methyloctanoic acid. 7f: R2=H, R3=H, R4=F; 7g: R2=H, R3=H, R4=Cl; 7h: R2=H, R3=H, R4=Br; 7i: R2=H, R3=H, R4=NO2; 7j: R2=H, R3=H, R4=OH; 7k: R2=H, R3=H, R4=NH2; 7l: R2=H, R3=H, R4=CN; 7m: R2=H, R3=H, R4=CH3; 7n: R2=H, R3=H, R4=CF3; 7o: R2=H, R3=H, R4=OCH3; 7p: R2=H, R3=H, R4=OCH2CH3; 7q: R2=H, R3=H, R4=tBu; 7r: R2=H, R3=H, R4=Bz; 7s: R2=H, R3=Cl, R4=H; 7t: R2=Cl, R3=H, R4=H. Reagents and conditions: (a) Fmoc-L-Dab-OAll, DIPEA, DCM, 2 h, MeOH; (b) (ⅰ) 20% piperidine in DMF, 5 min and 10 min; (ⅱ) appropriate amino acid, HCTU, DIPEA, DMF, 1 h; (ⅲ) For compounds 7a to 7d and 7f to 7t: corresponding carboxylic acid, HCTU, DIPEA, DMF, 1 h; For compound 7e: corresponding carboxylic acid, DIC, HOBt, DMF, 2 h; (c) 3% hydrazine in DMF, 2×30 min; (d) Fmoc-L-Thr(tBu)-OH, HCTU, DIPEA, DMF, 1 h; (e) (ⅰ) 20% piperidine in DMF, 5 min and 10 min; (ⅱ) PhSiH3, Pd(PPh3)4, 50% DCM/50% DMF, 2 h; (f) PyAOP, HOAt, NMM, DMF, 3 h; (g) TFA:TIS:H2O (95:2.5:2.5), 2 h; followed by reverse phase HPLC purification. |
3-氧代庚酸甲酯(3.2 mL, 20 mmol)溶于15 mL MeOH, 加入3 mol·L-1 NaOH溶液30 mL, 室温搅拌2 h。减压浓缩, 冰浴下用2 mol·L-1盐酸酸化至pH=2。乙醚萃取3次(3×30 mL), 无水硫酸钠干燥, 过滤, 减压浓缩, 得白色固体2.8 g, 收率97%[16]。ESI-MS: 143[M-H]-。
1.2 多黏菌素B衍生物的合成步骤a.将2CTC resin (0.95 g, 0.53 mmol·g-1, 0.5 mmol)加入多肽合成管中, 加入DCM溶胀5 min, DCM溶解Fmoc-L-Dab-OAll (2 mmol, 4 eq)和DIPEA (4 mmol, 8 eq)加入到树脂中, 室温反应2 h。加入0.5 mL MeOH反应30 min, 用DCM、DMF分别洗涤树脂5次, 得1。
步骤b.采用Fmoc策略, 合成线性肽-树脂。脱除Fmoc保护基, 用20%哌啶/DMF处理两次(5 min, 10 min), 用DCM、DMF分别洗涤树脂5次; 缩合反应, 将8位氨基酸Fmoc-L-Dab(Boc)-OH (2 mmol, 4 eq)、HCTU (2 mmol, 4 eq)和DIPEA (4 mmol, 8 eq)的DMF溶液, 与树脂样品反应1 h, 用DCM、DMF分别洗涤树脂5次。用相同方法脱除Fmoc保护基以及缩合7位氨基酸Fmoc-L-Leu-OH、6位氨基酸、5位氨基酸Fmoc-L-Dab(Boc)-OH、4位氨基酸Fmoc-L-Dab(Dde)-OH、3位氨基酸Fmoc-L-Dab(Boc)-OH、2位氨基酸Fmoc-L-Thr(tBu)-OH、1位氨基酸Fmoc-L-Dab(Boc)-OH、侧链羧酸(衍生物1e除外), 得2。衍生物1e的侧链羧酸缩合反应为3-氧代庚酸(2 mmol, 4 eq)、DIC (2 mmol, 4 eq)和HOBt (2 mmol, 4 eq)的DMF溶液, 与树脂样品反应2 h, 用DCM、DMF分别洗涤树脂5次, 得2。
步骤c.用2%水合肼/DMF处理(2×30 min)脱除Dde保护基, 用DCM、DMF分别洗涤树脂5次, 得3。
步骤d.将10位氨基酸Fmoc-L-Thr(tBu)-OH (2 mmol, 4 eq)、HCTU (2 mmol, 4 eq)和DIPEA (4 mmol, 8 eq)的DMF溶液, 与树脂样品反应1 h, 用DCM、DMF分别洗涤树脂5次, 得4。
步骤e.脱除Fmoc保护基, 用20%哌啶/DMF处理两次(5 min, 10 min), 用DMF洗涤树脂5次; PhSiH3 (3.5 mmol, 7 eq)与Pd(PPh3)4 (0.1mmol, 0.2 eq)溶于50% DCM/50% DMF, 避光反应2 h, 0.5%二乙基二硫代氨基甲酸钠/DMF溶液洗涤3次, 用DCM、DMF分别洗涤树脂5次, 得5。
步骤f.将PyAOP (2 mmol, 4 eq)、HOAt (2 mmol, 4 eq)和NMM (4 mmol, 8 eq), 与树脂样品反应3 h, 使9位L-Dab的羧基与10位L-Thr的氨基缩合, 实现固相环合, 用DCM、DMF分别洗涤树脂5次, 甲醇洗涤1次, 干燥, 得6。
步骤g.用10 mL TFA/TIS/H2O (95:2.5:2.5)进行肽的裂解和脱除全部保护基, 滤去树脂, 将获得的裂解液加入到冷乙醚中以析出肽, 离心, 半制备液相分离纯化, 冷冻干燥得7a~7t, HPLC检测纯度。
衍生物 7a 产率为29%, 白色固体, 分子式为C60H90O14N16, ESI-MS: m/z 1 259.690 6 [M+H]+, 液相含量96.6%, [α]D25-41.8 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.83 (d, J = 8.5 Hz, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.43~7.23 (m, 6H), 7.14 (m, 4H), 4.71 (dd, J = 8.8, 5.3 Hz, 1H), 4.57 (t, J = 8.2 Hz, 1H), 4.54~4.46 (m, 2H), 4.28 (m, 8H), 3.41~3.31 (m, 1H), 3.24~3.00 (m, 11H), 2.94~2.75 (m, 2H), 2.39~1.83 (m, 12H), 1.54~1.36 (m, 2H), 1.20 (m, 6H), 0.76 (s, 4H), 0.69 (s, 3H)。
衍生物 7b 产率为30%, 白色固体, 分子式为C61H92O14N16, ESI-MS: m/z 1 273.708 5 [M+H]+, 液相含量99.0%, [α]D25-42.8 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.82 (d, J = 8.5 Hz, 2H), 7.44~7.22 (m, 7H), 7.07 (m, 4H), 4.71 (dd, J = 8.9, 5.3 Hz, 1H), 4.57 (t, J = 8.2 Hz, 1H), 4.49 (s, 2H), 4.40 (d, J = 3.6 Hz, 1H), 4.35~4.13 (m, 7H), 3.41~3.30 (m, 1H), 3.26~2.98 (m, 11H), 2.88 (d, J = 8.1 Hz, 1H), 2.80 (m, 1H), 2.35 (s, 3H), 2.31~1.79 (m, 12H), 1.55~1.35 (m, 2H), 1.20 (m, 6H), 0.76 (s, 4H), 0.69 (d, J = 3.7 Hz, 3H)。
衍生物 7c 产率为27%, 白色固体, 分子式为C54H95O13N17, ESI-MS: m/z 595.871 1 [M+2H]2+, 液相含量95.9%, [α]D25-43.9 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.39 (t, J = 7.2 Hz, 2H), 7.35 (d, J = 7.1 Hz, 1H), 7.26 (d, J = 7.4 Hz, 2H), 4.57 (t, J = 8.3 Hz, 1H), 4.51 (dd, J = 8.5, 4.8 Hz, 3H), 4.36 (d, J = 4.3 Hz, 1H), 4.32~4.17 (m, 7H), 3.33 (m, 1H), 3.21~3.02 (m, 13H), 2.86 (s, 6H), 2.81 (s, 1H), 2.39 (t, J = 7.3 Hz, 2H), 2.30~1.81 (m, 13H), 1.78~1.69 (m, 2H), 1.65 (m, 2H), 1.49 (m, 1H), 1.39 (m, 1H), 1.20 (t, J = 6.2 Hz, 6H), 0.78 (m, 4H), 0.70 (t, J = 8.3 Hz, 3H)。
衍生物 7d 产率为28%, 白色固体, 分子式为C54H94O14N16, ESI-MS: m/z 596.362 9 [M+2H]2+, 液相含量95.2%, [α]D25-46.1 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.38 (d, J = 7.3 Hz, 2H), 7.35 (d, J = 7.2 Hz, 1H), 7.26 (d, J = 7.2 Hz, 2H), 4.57 (t, J = 8.1 Hz, 1H), 4.54~4.47 (m, 3H), 4.36 (d, J = 3.8 Hz, 1H), 4.24 (m, 7H), 3.47 (t, J = 6.5 Hz, 2H), 3.34 (m, 4H), 3.12 (m, 11H), 2.89 (s, 1H), 2.81 (s, 1H), 2.33 (t, J = 7.3 Hz, 2H), 2.30~1.83 (m, 12H), 1.66~1.54 (m, 4H), 1.48 (m, 1H), 1.37 (m, 3H), 1.20 (t, J = 5.9 Hz, 6H), 0.79 (m, 4H), 0.69 (d, J = 5.6 Hz, 3H)。
衍生物 7e 产率为27%, 白色固体, 分子式为C54H92O14N16, ESI-MS: m/z 1 189.706 4 [M+H]+, 液相含量97.1%, [α]D25-51.4 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.40 (t, J = 7.3 Hz, 2H), 7.35 (t, J = 7.2 Hz, 1H), 7.27 (d, J = 7.4 Hz, 2H), 4.61~4.46 (m, 4H), 4.37 (d, J = 4.5 Hz, 1H), 4.33~4.17 (m, 7H), 3.43~3.29 (m, 1H), 3.23~3.03 (m, 11H), 2.95~2.85 (m, 1H), 2.84~2.74 (m, 1H), 2.63 (t, J = 7.4 Hz, 2H), 2.34~1.79 (m, 13H), 1.59~1.46 (m, 3H), 1.40 (m, 1H), 1.30 (m, 2H), 1.25~1.09 (m, 7H), 0.88 (t, J = 7.4 Hz, 3H), 0.77 (s, 4H), 0.70 (d, J = 5.4 Hz, 3H)。
衍生物 7f 产率为30%, 白色固体, 分子式为C56H97O13N16F, ESI-MS: m/z 611.376 1 [M+2H]2+, 液相含量97.0%, [α]D25-48.4 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.25 (s, 2H), 7.12 (s, 2H), 4.50 (s, 4H), 4.36 (s, 1H), 4.33~4.11 (m, 7H), 3.35 (s, 1H), 3.18~2.89 (m, 13H), 2.33 (s, 2H), 2.04 (m, 12H), 1.58 (s, 2H), 1.48 (s, 1H), 1.39 (m, 1H), 1.29 (s, 5H), 1.24~1.16 (m, 6H), 1.12 (s, 2H), 0.86~0.79 (m, 7H), 0.76 (s, 3H), 0.68 (s, 3H)。
衍生物 7g 产率为30%, 白色固体, 分子式为C56H97O13N16Cl, ESI-MS: m/z 619.361 0 [M+2H]2+, 液相含量96.5%, [α]D25-44.6 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.40 (d, J = 7.7 Hz, 2H), 7.22 (d, J = 7.8 Hz, 2H), 4.53 (m, 4H), 4.37 (s, 1H), 4.23 (m, 6H), 4.14 (m, 1H), 3.42~3.32 (m, 1H), 3.04 (m, 13H), 2.32 (d, J = 7.2 Hz, 2H), 2.29~1.83 (m, 12H), 1.58 (d, J = 6.1 Hz, 2H), 1.46 (m, 1H), 1.40~1.24 (m, 6H), 1.20 (t, J = 5.9 Hz, 6H), 1.12 (s, 2H), 0.82 (d, J = 5.5 Hz, 6H), 0.77 (d, J = 5.4 Hz, 3H), 0.64 (m, 4H)。
衍生物 7h 产率为29%, 白色固体, 分子式为C56H97O13N16Br, ESI-MS: m/z 642.335 6 [M+2H]2+, 液相含量95.9%, [α]D25-52.5 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.55 (d, J = 7.6 Hz, 2H), 7.16 (d, J = 7.5 Hz, 2H), 4.51 (m, 4H), 4.37 (s, 1H), 4.23 (m, 6H), 4.13 (m, 1H), 3.42~3.32 (m, 1H), 3.12 (m, 10H), 3.04~2.88 (m, 3H), 2.32 (d, J = 7.0 Hz, 2H), 2.29~1.83 (m, 12H), 1.58 (d, J = 6.0 Hz, 2H), 1.45 (m, 1H), 1.41~1.24 (m, 6H), 1.20 (t, J = 6.2 Hz, 6H), 1.12 (s, 2H), 0.80 (m, 9H), 0.67 (d, J = 4.8 Hz, 3H), 0.60 (s, 1H)。
衍生物 7i 产率为31%, 白色固体, 分子式为C56H97O15N17, ESI-MS: m/z 624.873 2 [M+2H]2+, 液相含量99.0%, [α]D25-59.6 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 8.25 (d, J = 7.5 Hz, 2H), 7.48 (d, J = 7.5 Hz, 2H), 4.62~4.47 (m, 4H), 4.36 (s, 1H), 4.31~4.18 (m, 6H), 4.15 (m, 1H), 3.41~3.24 (m, 2H), 3.21~2.91 (m, 12H), 2.33 (s, 2H), 2.05 (m, 12H), 1.58 (d, J = 4.2 Hz, 2H), 1.50~1.40 (m, 1H), 1.39~1.24 (m, 6H), 1.20 (s, 6H), 1.11 (s, 2H), 0.82 (s, 6H), 0.66 (s, 3H), 0.61 (s, 3H), 0.52 (s, 1H)。
衍生物 7j 产率为29%, 白色固体, 分子式为C56H98O14N16, ESI-MS: m/z 610.378 5 [M+2H]2+, 液相含量99.0%, [α]D25-61.7 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.12 (d, J = 7.1 Hz, 2H), 6.85 (d, J = 7.2 Hz, 2H), 4.50 (m, 4H), 4.36 (s, 1H), 4.18 (m, 7H), 3.42~3.31 (m, 1H), 3.21~2.86 (m, 13H), 2.33 (s, 2H), 2.29~1.85 (m, 12H), 1.59 (s, 2H), 1.45 (m, 1H), 1.30 (s, 6H), 1.20 (s, 6H), 1.12 (s, 2H), 0.82 (s, 6H), 0.74 (s, 3H), 0.65 (s, 4H)。
衍生物 7k 产率为29%, 白色固体, 分子式为C56H99O13N17, ESI-MS: m/z 609.886 1 [M+2H]2+, 液相含量98.4%, [α]D25-55.4 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.40 (s, 4H), 4.58~4.48 (m, 4H), 4.37 (s, 1H), 4.32~4.15 (m, 7H), 3.42~3.31 (m, 1H), 3.23~2.91 (m, 13H), 2.32 (d, J = 6.9 Hz, 2H), 2.11 (m, 12H), 1.58 (d, J = 5.9 Hz, 2H), 1.47 (s, 1H), 1.41~1.24 (m, 6H), 1.20 (s, 6H), 1.12 (s, 2H), 0.82 (d, J = 5.1 Hz, 6H), 0.73 (s, 3H), 0.63 (s, 4H)。
衍生物 7l 产率为32%, 白色固体, 分子式为C57H97O13N17, ESI-MS: m/z 1 228.754 6 [M+H]+, 液相含量99.0%, [α]D25-60.8 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.79 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 8.1 Hz, 2H), 4.53 (m, 4H), 4.37 (d, J = 4.2 Hz, 1H), 4.25 (m, 6H), 4.13 (m, 1H), 3.42~3.33 (m, 1H), 3.25 (m, 1H), 3.21~2.92 (m, 12H), 2.33 (t, J = 7.3 Hz, 2H), 2.29~1.85 (m, 12H), 1.65~1.53 (m, 2H), 1.51~1.41 (m, 1H), 1.40~1.25 (m, 6H), 1.21 (t, J = 7.1 Hz, 6H), 1.18~1.07 (m, 3H), 0.83 (t, J = 6.9 Hz, 6H), 0.76 (d, J = 6.6 Hz, 3H), 0.65 (d, J = 6.4 Hz, 3H)。
衍生物 7m 产率为30%, 白色固体, 分子式为C57H100O13N16, ESI-MS: m/z 609.388 8 [M+2H]2+, 液相含量96.0%, [α]D25-59.2 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.21 (s, 2H), 7.14 (s, 2H), 4.52 (m, 4H), 4.36 (s, 1H), 4.32~4.11 (m, 7H), 3.35 (s, 1H), 3.09 (s, 10H), 2.94 (m, 3H), 2.32 (s, 5H), 2.04 (m, 12H), 1.58 (s, 2H), 1.44 (s, 1H), 1.33 (m, 6H), 1.20 (s, 6H), 1.12 (s, 2H), 0.82 (s, 6H), 0.73 (s, 3H), 0.65 (s, 4H)。
衍生物 7n 产率为29%, 白色固体, 分子式为C57H97O13N16F3, ESI-MS: m/z 636.374 6 [M+2H]2+, 液相含量99.0%, [α]D25-54.1 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.71 (d, J = 7.1 Hz, 2H), 7.42 (d, J = 6.9 Hz, 2H), 4.60~4.46 (m, 4H), 4.36 (s, 1H), 4.19 (m, 7H), 3.35 (s, 1H), 3.26~2.89 (m, 13H), 2.33 (s, 2H), 2.05 (m, 12H), 1.58 (s, 2H), 1.45 (s, 1H), 1.32 (m, 5H), 1.16 (m, 9H), 0.82 (s, 6H), 0.71 (s, 3H), 0.62 (s, 4H)。
衍生物 7o 产率为30%, 白色固体, 分子式为C57H100O14N16, ESI-MS: m/z 617.386 0 [M+2H]2+, 液相含量95.2%, [α]D25-53.5 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.20 (d, J = 7.6 Hz, 2H), 6.98 (d, J = 7.8 Hz, 2H), 4.59~4.42 (m, 4H), 4.37 (s, 1H), 4.33~4.09 (m, 7H), 3.83 (s, 3H), 3.44~3.27 (m, 1H), 3.21~2.90 (m, 13H), 2.32 (d, J = 7.1 Hz, 2H), 2.30~1.82 (m, 12H), 1.59 (s, 2H), 1.44 (m, 1H), 1.30 (s, 6H), 1.20 (d, J = 6.4 Hz, 6H), 1.12 (s, 2H), 0.82 (d, J = 5.2 Hz, 6H), 0.71 (d, J = 5.2 Hz, 3H), 0.61 (m, 4H)。
衍生物 7p 产率为29%, 白色固体, 分子式为C58H102O14N16, ESI-MS: m/z 624.393 7 [M+2H]2+, 液相含量99.0%, [α]D25-54.0 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.19 (d, J = 8.0 Hz, 2H), 6.96 (d, J = 8.2 Hz, 2H), 4.55 (m, 3H), 4.48~4.42 (m, 1H), 4.37 (s, 1H), 4.34~4.05 (m, 9H), 3.43~3.34 (m, 1H), 3.22~2.88 (m, 13H), 2.33 (t, J = 7.1 Hz, 2H), 2.29~1.83 (m, 12H), 1.58 (d, J = 6.6 Hz, 2H), 1.45 (m, 1H), 1.38 (t, J = 6.8 Hz, 3H), 1.30 (d, J = 7.3 Hz, 6H), 1.20 (t, J = 6.5 Hz, 6H), 1.12 (s, 2H), 0.82 (d, J = 5.8 Hz, 6H), 0.71 (d, J = 5.9 Hz, 3H), 0.60 (m, 4H)。
衍生物 7q 产率为29%, 白色固体, 分子式为C60H106O13N16, ESI-MS: m/z 630.411 8 [M+2H]2+, 液相含量98.5%, [α]D25-53.5 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.46 (d, J = 7.0 Hz, 2H), 7.19 (d, J = 7.0 Hz, 2H), 4.51 (s, 4H), 4.36 (s, 1H), 4.31~4.18 (m, 7H), 3.40~3.29 (m, 1H), 3.19~2.91 (m, 13H), 2.33 (s, 2H), 2.29~1.83 (m, 12H), 1.59 (s, 2H), 1.47 (m, 1H), 1.40 (m, 1H), 1.29 (s, 14H), 1.20 (s, 6H), 1.12 (s, 2H), 0.95 (s, 1H), 0.82 (s, 6H), 0.78 (s, 3H), 0.71 (s, 3H)。
衍生物 7r 产率为29%, 白色固体, 分子式为C63H102O14N16, ESI-MS: m/z 654.393 8 [M+2H]2+, 液相含量96.5%, [α]D25-44.4 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.81 (m, 4H), 7.74 (s, 1H), 7.60 (s, 2H), 7.43 (d, J = 7.3 Hz, 2H), 4.57 (s, 2H), 4.51 (s, 2H), 4.37 (s, 1H), 4.23 (m, 6H), 4.14 (m, 1H), 3.36 (d, J = 6.5 Hz, 1H), 3.27 (s, 1H), 3.21~2.92 (m, 12H), 2.33 (s, 2H), 2.05 (m, 12H), 1.58 (s, 2H), 1.43 (s, 1H), 1.29 (s, 6H), 1.20 (s, 6H), 1.11 (s, 2H), 0.82 (s, 6H), 0.66 (s, 3H), 0.57 (s, 4H)。
衍生物 7s 产率为30%, 白色固体, 分子式为C56H97O13N16Cl, ESI-MS: m/z 619.361 5 [M+2H]2+, 液相含量97.1%, [α]D25-50.5 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.36 (s, 2H), 7.28 (s, 1H), 7.19 (s, 1H), 4.53 (m, 4H), 4.36 (s, 1H), 4.32~4.16 (m, 7H), 3.35 (d, J = 5.6 Hz, 1H), 3.21~2.87 (m, 13H), 2.33 (s, 2H), 2.04 (m, 12H), 1.59 (s, 2H), 1.48 (s, 1H), 1.44~1.24 (m, 6H), 1.20 (s, 6H), 1.12 (s, 2H), 0.82 (s, 6H), 0.72 (m, 7H)。
衍生物 7t 产率为31%, 白色固体, 分子式为C56H97O13N16Cl, ESI-MS: m/z 619.361 2 [M+2H]2+, 液相含量99.0%, [α]D25-57.8 (c 0.15, 12% AcOH)。1H NMR (500 MHz, D2O) δ 7.47 (d, J = 3.3 Hz, 1H), 7.33 (s, 2H), 7.26 (s, 1H), 4.67 (s, 1H), 4.54 (m, 3H), 4.36 (s, 1H), 4.31~4.16 (m, 7H), 3.32 (d, J = 6.3 Hz, 1H), 3.16 (m, 11H), 2.95 (m, 2H), 2.33 (s, 2H), 2.29~1.82 (m, 12H), 1.58 (s, 2H), 1.47 (s, 1H), 1.43~1.24 (m, 6H), 1.20 (s, 6H), 1.12 (s, 2H), 0.82 (s, 6H), 0.72 (m, 7H)。
2 生物活性评价 2.1 体外抗菌活性实验参照CLSI推荐方法, 采用平皿二倍稀释法和Denley多点接种器进行最低抑菌浓度(MIC)测定, 阳性药选用硫酸多黏菌素B。化合物用CAMH肉汤二倍稀释成一系列浓度, 分别加适量到平皿中, MH琼脂培养基熔化后定量注入含药液的平皿内混匀, 药物的终浓度分别为0.03、0.06、0.125、0.25……128 μg·mL-1。受试菌用营养肉汤、脑心浸液或HTM肉汤隔夜增菌, 试验时, 菌液适当稀释, 多点接种器接种试验菌(接种量每点为1×104 CFU)于含药琼脂表面, 干燥后, 置35 ℃恒温培养18~24 h后观察结果, 无菌落生长的平皿中所含药物的最小浓度即为MIC。
2.2 肾细胞毒性实验以含10%胎牛血清的DMEM培养液培养Vero细胞株, 于37 ℃、5% CO2培养, 定期换液, 待细胞进入对数生长期, 用胰蛋白酶消化传代。将生长良好的细胞以每毫升2×103个细胞重新接种于96孔板。培养24 h后, 加入药液, 每浓度设立3个平行孔, 另设空白对照, 阳性药选用硫酸多黏菌素B。于37 ℃、5% CO2培养72 h, 每孔加MTT液100 μL, 培养4 h后吸除培养液, 再加150 μL二甲基亚砜并振荡10 min。用Bio-Rad 680型酶标仪在检测波长570 nm下测吸光度(A)值, 计算细胞生存率。细胞生存率= (给药组A值-本底A值/空白对照组A值-本底A值)×100%, 每检测点取3个平行孔的平均值, 绘制抑制曲线, 计算CC50值。实验数据以x±s表示, 数值均为3次实验结果的平均值。使用Graphpad Prism 5.0统计软件计算Vero细胞CC50值, 用SPSS 16.0的方法进行统计学处理。
| [1] | Velkov T, Thompson PE, Nation RL, et al. Structure-activity relationships of polymyxin antibiotic[J]. J Med Chem, 2010, 53: 1898–1916. DOI:10.1021/jm900999h |
| [2] | Benedict RG, Langlykke AF. Antibiotic activity of Bacillus polymyxa[J]. J Bacteriol, 1947, 54: 24–25. |
| [3] | Shoji J, Hinoo H, Wakisaka Y, et al. Isolation of two new polymyxin group antibiotics. (Studies on antibiotics from the genus Bacillus. XX)[J]. J Antibiot, 1977, 30: 1029–1034. DOI:10.7164/antibiotics.30.1029 |
| [4] | Terabe S, Konaka R, Shoji J. Separation of polymyxins and octapeptins by high-performance liquid chromatography[J]. J Chromatogr A, 1979, 173: 313–320. DOI:10.1016/S0021-9673(00)92299-4 |
| [5] | Brown JM, Dorman DC, Roy LP. Acute renal failure due to overdosage of colistin[J]. Med J Aust, 1970, 2: 923–924. DOI:10.5694/j.1326-5377.1970.tb63262.x |
| [6] | Price DJ, Graham DI. Effects of large doses of colistin sulphomethate sodium on renal function[J]. Br Med J, 1970, 4: 525–527. DOI:10.1136/bmj.4.5734.525 |
| [7] | Koch-Weser J, Sidel VW, Federman EB, et al. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy[J]. Ann Intern Med, 1970, 72: 857–868. DOI:10.7326/0003-4819-72-6-857 |
| [8] | Kubin CJ, Ellman TM, Phadke V, et al. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy[J]. J Infect, 2012, 65: 80–87. DOI:10.1016/j.jinf.2012.01.015 |
| [9] | Akajagbor DS, Wilson SL, Shere-Wolfe KD, et al. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center[J]. Clin Infect Dis, 2013, 57: 1300–1303. DOI:10.1093/cid/cit453 |
| [10] | Quale J, Shah N, Kelly P, et al. Activity of polymyxin B and the novel polymyxin analogue CB-182, 804 against contemporary Gram-negative pathogens in New York City[J]. Microb Drug Resist, 2012, 18: 132–136. DOI:10.1089/mdr.2011.0163 |
| [11] | Magee TV, Brown MF, Starr JT, et al. Discovery of Dap-3 polymyxin analogues for the treatment of multidrug-resistant Gram-negative nosocomial infections[J]. J Med Chem, 2013, 56: 5079–5093. DOI:10.1021/jm400416u |
| [12] | Brown P, Dawson MJ. Development of new polymyxin derivatives for multi-drug resistant Gram-negative infections[J]. J Antibiot, 2017, 70: 386–394. DOI:10.1038/ja.2016.146 |
| [13] | Gallardo-Godoy A, Muldoon C, Becker B, et al. Activity and predicted nephrotoxicity of synthetic antibiotics based on polymyxin B[J]. J Med Chem, 2016, 59: 1068–1077. DOI:10.1021/acs.jmedchem.5b01593 |
| [14] | Velkov T, Roberts KD, Nation RL, et al. Teaching 'old' polymyxins new tricks: new-generation lipopeptides targeting gram-negative 'superbugs'[J]. ACS Chem Biol, 2014, 9: 1172–1177. DOI:10.1021/cb500080r |
| [15] | Cui AL, Hu XX, Gao Y, et al. Synthesis and bioactivity investigation of the individual components of cyclic lipopeptide antibiotics[J]. J Med Chem, 2018, 61: 1845–1857. DOI:10.1021/acs.jmedchem.7b01367 |
| [16] | Geske GD, Wezeman RJ, Siegel AP, et al. Small molecule inhibitors of bacterial quorum sensing and biofilm formation[J]. J Am Chem Soc, 2005, 127: 12762–12763. DOI:10.1021/ja0530321 |
 2019, Vol. 54
2019, Vol. 54