山香圆叶(Turpinia arguta (Lindl.) Seem)为省沽油科(Staphyleaceae)山香圆属植物, 又名千打捶、七寸钉、蛾子药, 主要分布于我国西南至台湾地区, 植物资源十分丰富[1]。山香圆叶作为民间药的用药历史久远, 传统中医以其干燥叶入药, 性味苦寒, 有清热解毒、利咽消肿、活血止痛之功效, 对乳蛾喉痹、咽喉肿痛、疮疡肿毒、跌扑伤痛有一定的疗效。民间用于治疗咽喉炎、扁桃体炎、扁桃体脓肿、上呼吸道感染、气管炎等具有速效高效的特点[2]。根据文献报道从该植物分离得到黄酮及其苷类成分、三萜及其苷类成分和一些单萜吲哚类生物碱, 并且具有良好的生物活性。药理研究表明, 山香圆叶具有抗炎、抗菌、镇痛和免疫调节等药理作用[3-5]。为更有效的开发利用这一植物资源, 本课题组进一步对山香圆正丁醇部位的化学成分进行了系统的研究, 通过大孔树脂、聚酰胺、正向硅胶、反向硅胶、制备液相和现代波谱学等方法共分离并鉴定了15个黄酮类化合物: argutoside F (1)、木犀草素-7-O-[α-L-吡喃鼠李糖基-(1→6)-α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷(2)、女贞苷(3)、刺槐素-7-O-[α-L-吡喃鼠李糖基-(1→6)-α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷(4)、芹菜素(5)、槲皮素(6)、槲皮素-3-O-α-L-吡喃鼠李糖基-(1→6)-β-D-吡喃葡萄糖苷(7)、野漆树苷(8)、木犀草素-7-O-α-L-吡喃鼠李糖基-(1→2)-β-D-吡喃葡萄糖苷(9)、刺槐素-7-O-α-L-吡喃鼠李糖基-(1→2)-β-D-吡喃葡萄糖苷(10)、木犀草素-7-O-β-D-吡喃葡萄糖苷(11)、木犀草素(12)、neodiosmin (13)、芹菜素-7-O-芸香糖苷(14)和槲皮素-3-O-α-L-吡喃阿拉伯糖苷(15)。其中化合物1为新化合物, 化合物2、7、9、13~15为首次从该植物中获得。
结果与讨论化合物1 黄色粉末, 易溶于甲醇, [α]D20 -114.4 (c 0.086, MeOH), UV (MeOH) λmax (log ε): 266 (4.15)、338 (4.11) nm, IR显示有羟基(3 385 cm-1)、羰基(1 715 cm-1)、共轭双键(1 610 cm-1)以及C-O-C (1 015 cm-1)特征吸收, HR-TOF-MS m/z 777.220 1 [M+Na]+ (Calcd. for C34H42NaO19 777.232 0), 根据高分辨质谱给出的准确分子量结合1H NMR和13C NMR推断分子式为C34H42O19, 不饱和度为14。在甲醇溶液中, 与盐酸-镁粉反应呈现阳性, 其硅胶薄层斑点在紫外灯下为黄色, 喷洒10%硫酸-乙醇显色呈黄褐色, 判断该化合物可能为黄酮类化合物。与Molish试剂反应呈阳性, 判断该化合物可能为黄酮苷。
在1H NMR (600 MHz, DMSO-d6)数据(表 1)上观察到δ 12.75 (1H, s)、8.58 (1H, s)两个活泼质子信号, 其中δ 12.75为与羰基形成分子内氢键的活泼质子信号, 即5-OH; δ 8.04~6.90为芳香区质子信号, 其中δ 8.04 (2H, d, J = 8.7 Hz)和δ 7.17 (2H, d, J = 8.7 Hz)两组质子构成AA'BB'偶合系统, 由此确定该化合物B环为1, 4-二取代, 并可将前者化学位移归属于H-2'、H-6', 后者化学位移归属于H-3'、H-5'; δ 6.97 (1H, s)为A环质子信号, 根据其峰型可知A环只有1个质子, 其他位置均被取代; δ 6.90 (1H, s)为黄酮类化合物C-3位的特征信号; δ 5.34 (1H, d, J = 6.6 Hz)、5.17 (1H, br s)和4.55 (1H, br s)为3个糖端基质子信号; δ 3.88 (3H, br s)为连在芳环上的甲氧基质子信号, δ 1.08 (6H, br s)为两个甲基质子信号。
| Table 1 1H NMR and 13C NMR spectroscopic data for compound 1 (in DMSO-d6) |
在13C NMR (150 MHz, DMSO-d6)和HSQC谱中, 给出34个碳信号, 由δ 182.8 (C=O)以及δ 164.3、147.4、151.9、149.6的4个sp2杂化的醚氧键碳信号确定该化合物为黄酮类化合物。还观察到3个单糖的信号, δ 98.4 (C-1'')、77.4 (C-2'')、77.7 (C-3'')、70.1 (C-4'')、76.0 (C-5'')、66.4 (C-6'')与文献[6]的β-D-吡喃葡萄糖的数据基本一致(推测该化合物含有1个葡萄糖); δ 101.0 (C-1''')、71.0 (C-2''')、70.9 (C-3''')、72.5 (C-4''')、68.8 (C-5''')、18.3 (C-6''')、101.0 (C-1'''')、71.2 (C-2'''')、70.9 (C-3'''')、72.5 (C-4'''')、69.2 (C-5'''')、18.4 (C-6'''')与文献[6]的α-L-吡喃鼠李糖的数据基本一致, 推测该化合物可能含有两个吡喃鼠李糖。将该化合物进行酸水解, TLC检查, 与标准品共TLC, 证实有葡萄糖和鼠李糖存在。
以上1H NMR、13C NMR和HMQC信息可得到结构:
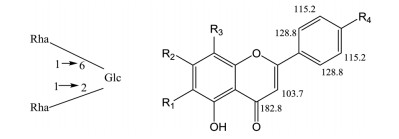
|
此外, 在HMBC谱中, δ 8.04 (128.8)的质子与δ 162.7、164.3的碳有远程相关, δ 7.17 (115.2)的质子与δ 123.5的碳有远程相关, δ 6.90 (103.7)的质子与δ 106.1、123.5、164.3、182.8的碳有远程相关, 以上信息可得到C-2δ 164.3, C-10δ 106.1, C-4'δ 162.7; δ 3.88 (56.0)的质子与δ 162.7的碳有远程相关, 说明为R4为OCH3; δ 6.97 (94.2)的质子与δ 106.1、131.2、149.6、151.9、182.8的碳有远程相关, δ 12.75的活泼质子与δ 106.1、131.2、147.4的碳有远程相关, 以上信息可得到C-5δ 147.4, C-6δ 131.2, C-7的碳信号δ 151.9, C-8δ 94.2, C-9δ 149.6, R1为OH, R3为H; 两个鼠李糖端基质子δ 5.17 (101.0)、δ 4.55 (101.0)与δ 77.4、66.4的碳远程相关, 说明两个鼠李糖分别与葡萄糖的C-2位和C-6位相连, 葡萄糖端基质子δ 5.34 (98.4)与δ 151.9的碳有远程相关, 说明葡萄糖与苷元的C-7位相连。
综合以上信息确定该化合物为5, 6-二羟基-4'-甲氧基黄酮-7-O-[α-L-吡喃鼠李糖基-(1→6)-α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷[5, 6-dihydroxy-4'-methoxyflavone-7-O-α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside], 查阅相关文献及Sci Finfer数据库无该化合物, 确定为新化合物, 因前人从山香圆叶中分离得到argutoside A~E五个新化合物, 且结构类似, 故新化合物命名为argutoside F (图 1)。
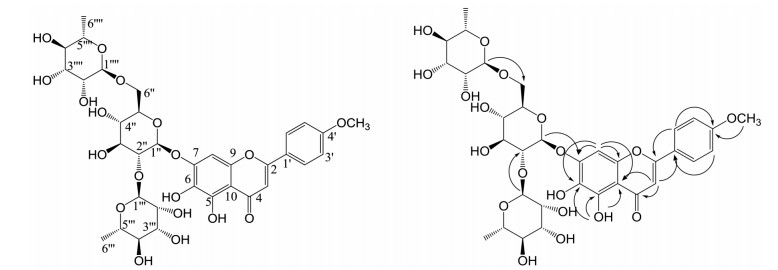
|
Figure 1 Chemical structure and key HMBC correlations of compound 1 |
化合物2 为黄色粉末, ESI-MS m/z 741 [M+H]+, 763 [M+Na]+, 分子式为C33H40O19, 不饱和度为14。1H NMR (600 MHz, DMSO-d6) δ 12.99 (1H, s, 5-OH), 10.02 (1H, s, 4'-OH), 9.50 (1H, s, 3'-OH), 7.44 (1H, dd, J = 2.0, 8.2 Hz, H-6'), 7.42 (1H, d, J = 2.0 Hz, H-2'), 6.94 (1H, d, J = 8.2 Hz, H-5'), 6.75 (1H, s, H-3), 6.68 (1H, m, H-8), 6.41 (1H, s, H-6), 5.25 (1H, d, J = 7.1 Hz, H-1"), 5.13 (1H, br s, H-1"'), 4.55 (1H, br s, H-1"''), 3.85 (1H, d, J = 11.7 Hz, Hb-6''), 1.22 (3H, d, J = 6.1 Hz, 6"'-CH3), 1.08 (3H, d, J = 6.2 Hz, 6"''-CH3)。13C NMR (150 MHz, DMSO-d6) δ 165.1 (C-2), 103.7 (C-3), 182.3 (C-4), 161.7 (C-5), 99.7 (C-6), 162.9 (C-7), 94.8 (C-8), 157.4 (C-9), 105.9 (C-10), 121.8 (C-1'), 114.0 (C-2'), 146.2 (C-3'), 150.4 (C-4'), 116.6 (C-5'), 119.7 (C-6'), 98.2 (C-1"), 76.8 (C-2"), 77.5 (C-3"), 70.1 (C-4"), 75.8 (C-5"), 66.4 (C-6"), 101.0 (C-1"'), 70.9 (C-2"'), 70.7 (C-3"'), 72.3 (C-4"'), 68.8 (C-5"'), 18.6 (C-6"'), 100.0 (C-1"''), 71.2 (C-2"''), 70.8 (C-3"''), 72.5 (C-4"''), 68.8 (C-5"''), 18.2 (C-6"'')。以上数据与文献[6]报道基本一致, 故鉴定该化合物为木犀草素-7-O-[α-L-吡喃鼠李糖基-(1→6)-α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷。
化合物3 黄色粉末, ESI-MS m/z 747 [M+Na]+, 723 [M-H]-, 分子式为C33H40O18, 不饱和度为14。1H NMR (600 MHz, DMSO-d6) δ 12.94 (1H, s, 5-OH), 10.44 (1H, s, 4'-OH), 7.88 (2H, d, J = 8.3 Hz, H-2', H-6'), 6.95 (2H, d, J = 8.3 Hz, H-3', H-5'), 6.80 (1H, s, H-3), 6.70 (1H, d, J = 2.0 Hz, H-8), 6.37 (1H, d, J = 2.0 Hz, H-6), 5.23 (1H, d, J = 6.9 Hz, H-1"), 5.16 (1H, br s, H-1"'), 4.58 (1H, s, H-1"''), 3.88 (1H, d, J = 10.8 Hz, Hb-6''), 1.24 (3H, d, J = 6.0 Hz, 6"'-CH3), 1.08 (3H, d, J = 6.1 Hz, 6'"'-CH3)。13C NMR (150 MHz, DMSO-d6) δ 164.9 (C-2), 103.6 (C-3), 182.4 (C-4), 161.7 (C-5), 99.8 (C-6), 162.8 (C-7), 94.9 (C-8), 157.4 (C-9), 105.9 (C-10), 121.4 (C-1'), 129.0 (C-2', C-6'), 116.6 (C-3', C-5'), 161.7 (C-4'), 101.0 (C-1"), 77.5 (C-2"), 76.9 (C-3"), 70.1 (C-4"), 75.9 (C- 5"), 66.4 (C-6"), 98.2 (C-1"'), 70.8 (C-2"'), 71.0 (C-3"'), 72.4 (C-4"'), 68.8 (C-5"'), 18.5 (C-6"'), 101.0 (C-1"''), 70.8 (C-2"''), 71.2 (C-3"''), 72.5 (C-4"''), 68.8 (C-5"''), 18.2 (C-6"'')。以上数据与文献[7]报道对照基本一致, 故鉴定该化合物为女贞苷。
化合物4 黄色粉末, ESI-MS m/z 761 [M+Na]+, 739 [M+H]+, 分子式为C34H42O18, 不饱和度为14。1H NMR (600 MHz, DMSO-d6) δ 12.93 (1H, s, 5-OH), 8.01 (2H, d, J = 8.4 Hz, H-2', H-6'), 7.14 (2H, d, J = 8.4 Hz, H-3', H-5'), 6.92 (1H, s, H-3), 6.73 (1H, d, J = 2.1 Hz, H-8), 6.40 (1H, d, J = 2.1 Hz, H-6), 5.25 (1H, d, J = 7.3 Hz, H-1"), 5.16 (1H, br s, H-1"'), 4.56 (1H, s, H-1"''), 3.89 (1H, d, J = 11.0 Hz, Hb-6''), 3.85 (3H, s, 4'-OCH3), 1.24 (3H, d, J = 6.2 Hz, 6"'-CH3), 1.10 (3H, d, J = 6.2 Hz, 6'"'-CH3)。13C NMR (150 MHz, DMSO-d6) δ 164.4 (C-2), 104.3 (C-3), 182.5 (C-4), 161.6 (C-5), 99.9 (C-6), 163.0 (C-7), 94.9 (C-8), 157.5 (C-9), 106.0 (C-10), 123.1 (C-1'), 128.9 (C-2', C-6'), 115.2 (C-3', C-5'), 162.9 (C-4'), 56.0 (4'-OCH3), 98.3 (C-1"), 76.8 (C-2"), 77.5 (C-3"), 71.2 (C-4"), 76.0 (C-5"), 66.5 (C-6"), 101.0 (C-1"'), 70.8 (C-2"'), 70.2 (C-3"'), 72.3 (C-4"'), 68.8 (C-5"'), 18.6 (C-6"'), 101.0 (C-1"''), 71.0 (C-2"''), 70.8 (C-3"''), 72.5 (C-4"''), 68.8 (C-5"''), 18.3 (C-6"'')。以上数据与文献[8]报道对照基本一致, 故鉴定该化合物为刺槐素-7-O-[α-L-吡喃鼠李糖基-(1→6)-α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷。
化合物5 淡黄色粉末, ESI-MS m/z 271 [M+H]+, 分子式为C15H10O5, 不饱和度为11。1H NMR (600 MHz, DMSO-d6) δ 12.97 (1H, s, 5-OH), 10.92 (1H, s, 7-OH), 10.42 (1H, s, 4'-OH), 7.93 (2H, d, J = 8.3 Hz, H-2', H-6'), 6.94 (2H, d, J = 8.4 Hz, H-3', H-5'), 6.79 (1H, s, H-3), 6.50 (1H, d, J = 2.0 Hz, H-8), 6.20 (1H, d, J = 2.2 Hz, H-6)。13C NMR (150 MHz, DMSO-d6) δ 164.6 (C-2), 103.3 (C-3), 182.2 (C-4), 157.8 (C-5), 99.3 (C-6), 164.2 (C-7), 94.4 (C-8), 161.9 (C-9), 104.1 (C-10), 121.6 (C-1'), 128.9 (C-2', C-6'), 116.4 (C-3', C-5'), 161.7 (C-4')。以上数据与文献[9]报道对照基本一致, 故鉴定该化合物为芹菜素
化合物6 淡黄色粉末, ESI-MS m/z 303 [M+H]+, 分子式为C15H10O7, 不饱和度为11。1H NMR (600 MHz, DMSO-d6) δ 12.51 (1H, s, 5-OH), 10.79 (1H, s, 7-OH), 9.60 (1H, s, 3-OH), 9.38 (1H, s, 4'-OH), 9.34 (1H, s, 3'-OH), 7.69 (1H, d, J = 2.2 Hz, H-2'), 7.55 (1H, q, J = 8.4, 2.2 Hz, H-6'), 6.90 (1H, d, J = 8.4 Hz, H-5'), 6.42 (1H, d, J = 2.2 Hz, H-8), 6.20 (1H, d, J = 2.2 Hz, H-6)。13C NMR (150 MHz, DMSO-d6) δ 147.2 (C-2), 136.2 (C-3), 176.3 (C-4), 156.6 (C-5), 98.6 (C-6), 164.3 (C-7), 93.8 (C-8), 161.2 (C-9), 103.5 (C-10), 122.4 (C-1'), 115.5 (C-2'), 145.5 (C-3'), 148.1 (C-4'), 116.1 (C-5'), 120.4 (C-6')。以上数据与文献[10]报道对照基本一致, 故鉴定该化合物为槲皮素。
化合物7 黄色粉末, ESI-MS m/z 611 [M+H]+, 分子式为C27H30O16, 不饱和度为13。1H NMR (600 MHz, DMSO-d6) δ 12.61 (1H, br s, 5-OH), 9.60 (1H, s, 7-OH), 9.38 (1H, s, 4'-OH), 9.34 (1H, s, 3'-OH), 7.54 (2H, br s, H-2', H-6'), 6.85 (1H, d, J = 8.2 Hz, H- 5'), 6.39 (1H, br s, H-8), 6.20 (1H, br s, H-6), 5.35 (1H, d, J = 7.3 Hz, H-1"), 5.13 (1H, br s, H-1"'), 1.00 (3H, d, J = 6.2 Hz, H-6"')。13C NMR (150 MHz, DMSO-d6) δ 156.9 (C-2), 133.7 (C-3), 177.8 (C-4), 161.7 (C-5), 99.1 (C-6), 164.5 (C-7), 94.0 (C-8), 157.1 (C-9), 104.4 (C-10), 121.6 (C-1'), 115.7 (C-2'), 145.2 (C-3'), 148.9 (C-4'), 116.7 (C-5'), 122.0 (C-6'), 101.2 (C-1''), 74.5 (C-2''), 76.9 (C-3''), 71.0 (C-4''), 76.3 (C-5''), 67.4 (C-6''), 101.6 (C-1'''), 70.8 (C-2'''), 70.4 (C-3'''), 72.3 (C-4'''), 68.7 (C-5'''), 18.2 (C-6''')。以上数据与文献[11]报道对照基本一致, 故鉴定该化合物为槲皮素-3-O-α-L-吡喃鼠李糖基- (1→6)-β-D-吡喃葡萄糖苷。
化合物8 黄色粉末, ESI-MS m/z 601 [M+Na]+, 579 [M+H]+, 分子式为C27H30O14, 不饱和度为13。1H NMR (600 MHz, DMSO-d6) δ 12.98 (1H, s, 5-OH), 10.41 (1H, s, 4'-OH), 7.95 (2H, d, J = 8.5 Hz, H-2', H-6'), 6.95 (2H, d, J = 8.5 Hz, H-3', H-5'), 6.88 (1H, s, H-3), 6.80 (1H, d, J = 2.2 Hz, H-8), 6.38 (1H, d, J = 2.2 Hz, H-6), 5.24 (1H, d, J = 7.4 Hz, H-1"), 5.19 (1H, br s, H-1"'), 3.77 (1H, d, J = 9.4 Hz, Hb-6''), 1.21 (3H, d, J = 6.2 Hz, 6"'-CH3)。13C NMR (150 MHz, DMSO-d6) δ 164.7 (C-2), 103.6 (C-3), 182.4 (C-4), 161.6 (C-5), 99.8 (C-6), 163.0 (C-7), 95.0 (C-8), 157.4 (C-9), 105.9 (C-10), 121.4 (C-1'), 129.0 (C-2', C-6'), 116.5 (C-3', C-5'), 161.9 (C-4'), 100.9 (C-1"), 77.7 (C-2"), 77.5 (C-3"), 70.1 (C-4"), 76.7 (C-5"), 60.9 (C-6"), 98.3 (C-1"'), 70.9 (C-2"'), 70.9 (C-3"'), 72.3 (C-4"'), 68.8 (C-5"'), 18.5 (C- 6"')。以上数据与文献[7]报道对照基本一致, 故鉴定该化合物为野漆树苷。
化合物9 黄色粉末, ESI-MS m/z 595 [M+H]+, 617 [M+Na]+, 分子式为C27H30O15, 不饱和度为13。1H NMR (600 MHz, DMSO-d6) δ 13.01 (1H, s, 5-OH), 10.04 (1H, s, 4'-OH), 9.47 (1H, s, 3'-OH), 7.44 (1H, dd, J = 8.2, 2.3 Hz, H-6'), 7.42 (1H, d, J = 2.3 Hz, H-2'), 6.92 (1H, d, J = 8.3 Hz, H-5'), 6.76 (1H, s, H-3), 6.75 (1H, dd, J = 2.2 Hz, H-8), 6.38 (1H, d, J = 2.2 Hz, H-6), 5.26 (1H, d, J = 7.3 Hz, H-1"), 5.14 (1H, br s, H-1"'), 1.21 (3H, d, J = 6.2 Hz, 6"'-CH3)。13C NMR (150 MHz, DMSO-d6) δ 164.9 (C-2), 103.7 (C-3), 182.3 (C-4), 161.6 (C-5), 99.7 (C-6), 163.0 (C-7), 94.8 (C-8), 157.4 (C-9), 105.9 (C-10), 121.8 (C-1'), 114.0 (C-2'), 146.3 (C-3'), 150.4 (C-4'), 116.5 (C-5'), 119.6 (C-6'), 98.2 (C-1"), 77.4 (C-2"), 77.6 (C-3"), 70.9 (C-4"), 76.7 (C-5"), 60.9 (C-6"), 100.9 (C-1"'), 70.9 (C-2"'), 70.1 (C-3"'), 72.3 (C-4"'), 68.8 (C-5"'), 18.6 (C-6"')。以上数据与文献[12]报道对照基本一致, 故鉴定该化合物为木犀草素-7-O-α-L-吡喃鼠李糖基- (1→2)-β-D-吡喃葡萄糖苷。
化合物10 黄色粉末, ESI-MS m/z 615 [M+Na]+, 593 [M+H]+分子式为C28H32O14, 不饱和度为13。1H NMR (600 MHz, DMSO-d6) δ 12.94 (1H, s, 5-OH), 8.06 (2H, d, J = 8.5 Hz, H-2', H-6'), 7.15 (2H, d, J = 8.5 Hz, H-3', H-5'), 6.97 (1H, s, H-3), 6.83 (1H, d, J = 2.1 Hz, H-8), 6.40 (1H, d, J = 2.1 Hz, H-6), 5.25 (1H, d, J = 7.4 Hz, H-1"), 5.14 (1H, br s, H-1"'), 3.88 (3H, s, 4'-OCH3), 1.22 (3H, d, J = 6.2 Hz, 6"'-CH3)。13C NMR (150 MHz, DMSO-d6) δ 164.3 (C-2), 104.4 (C-3), 182.5 (C-4), 157.5 (C-5), 99.8 (C-6), 162.9 (C-7), 95.0 (C-8), 161.6 (C-9), 106.0 (C-10), 123.1 (C-1'), 128.9 (C-2', C-6'), 115.1 (C-3', C-5'), 163.1 (C-4'), 56.1 (4'-OCH3), 98.3 (C-1"), 76.8 (C-2"), 77.5 (C-3"), 71.0 (C-4"), 77.7 (C-5"), 60.9 (C-6"), 101.0 (C-1"'), 70.9 (C-2"'), 70.1 (C-3"'), 72.3 (C-4"'), 68.8 (C-5"'), 18.5 (C-6"')。以上数据与文献[7]报道对照基本一致, 故鉴定该化合物为刺槐素-7-O-α-L-吡喃鼠李糖基-(1→2)-β-D-吡喃葡萄糖苷。
化合物11 黄色粉末, ESI-MS m/z 471 [M+Na]+, 449 [M+H]+, 分子式为C21H20O11, 不饱和度为12。1H NMR (600 MHz, DMSO-d6) δ 13.00 (1H, s, 5-OH), 9.91 (1H, s, 4'-OH), 9.50 (1H, s, 3'-OH), 7.46 (1H, dd, J = 8.3, 2.3 Hz, H-6'), 7.43 (1H, d, J = 2.3 Hz, H-2'), 6.92 (1H, d, J = 8.3 Hz, H-5'), 6.80 (1H, d, J = 2.2 Hz, H-8), 6.77 (1H, s, H-3), 6.46 (1H, d, J = 2.2 Hz, H-6), 5.10 (1H, d, J = 7.5 Hz, H-1"), 3.72~3.17 (5H, m, H-2"~H-6")。13C NMR (150 MHz, DMSO-d6) δ 164.9 (C-2), 105.8 (C-3), 182.4 (C-4), 161.6 (C-5), 100.0 (C-6), 163.4 (C-7), 95.2 (C-8), 157.4 (C-9), 103.6 (C-10), 121.8 (C-1'), 114.0 (C-2'), 146.2 (C-3'), 150.4 (C-4'), 116.4 (C-5'), 119.6 (C-6'), 100.3 (C-1''), 73.6 (C-2''), 76.8 (C-3''), 77.6 (C-4''), 70.0 (C-5''), 61.1 (C-6'')。以上数据与文献[9]报道对照基本一致, 故鉴定该化合物为木犀草素-7-O-β-D-吡喃葡萄糖苷。
化合物12 淡黄色粉末, ESI-MS m/z 299 [M+Na]+, 287 [M+H]+, 分子式为C15H10O6, 不饱和度为11。1H NMR (600 MHz, DMSO-d6) δ 12.99 (1H, s, 5-OH), 10.82 (1H, s, 7-OH), 9.91 (1H, s, 4'-OH), 9.50 (1H, s, 3'-OH), 7.43 (1H, d, J = 2.3 Hz, 8.3 Hz, H-6'), 7.42 (1H, d, J = 2.3 Hz, H-2'), 6.90 (1H, d, J = 8.3 Hz, H-5'), 6.68 (1H, d, J = 2.2 Hz, H-8), 6.45 (1H, s, H-3), 6.20 (1H, d, J = 2.2 Hz, H-6)。13C NMR (151 MHz, DMSO-d6) δ 163.3 (C-2), 104.2 (C-3), 182.1 (C-4), 161.9 (C-5), 99.3 (C-6), 164.6 (C-7), 94.3 (C-8), 157.7 (C-9), 103.3 (C-10), 122.0 (C-1'), 113.8 (C-2'), 146.2 (C-3'), 150.1 (C-4'), 116.5 (C-5'), 119.4 (C-6')。以上数据与文献[9]报道对照基本一致, 故鉴定该化合物为木犀草素。
化合物13 黄色粉末, ESI-MS m/z 607 [M-H]-, 631 [M+Na]+, 分子式为C28H32O15, 不饱和度为13。1H NMR (600 MHz, DMSO-d6) δ 12.96 (1H, s, 5-OH), 9.53 (1H, s, 3'-OH), 7.57 (1H, dd, J = 8.5, 2.3 Hz, H-6'), 7.45 (1H, d, J = 2.3 Hz, H-2'), 7.12 (1H, d, J = 8.7 Hz, H-5'), 6.85 (1H, s, H-3), 6.78 (1H, d, J = 2.2 Hz, H-8), 6.40 (1H, d, J = 2.2 Hz, H-6), 5.27 (1H, d, J = 7.4 Hz, H-1"), 5.14 (1H, s, H-1"'), 3.88 (3H, s, 3'-OCH3), 1.22 (3H, d, J = 6.2 Hz, CH3)。13C NMR (150 MHz, DMSO-d6) δ 164.6 (C-2), 104.3 (C-3), 182.4 (C-4), 161.6 (C-5), 99.8 (C-6), 163.0 (C-7), 94.9 (C-8), 157.5 (C-9), 105.9 (C-10), 123.3 (C-1'), 119.4 (C-2'), 147.3 (C-3'), 151.8 (C-4'), 112.6 (C-5'), 113.5 (C-6'), 56.2 (4'-OCH3), 98.2 (C-1''), 76.8 (C-2''), 77.4 (C-3''), 70.9 (C-4''), 77.6 (C-5''), 60.9 (C-6''), 100.9 (C-1'''), 70.9 (C-2'''), 70.1 (C-3'''), 72.3 (C-4'''), 68.8 (C-5'''), 18.5 (C-6''')。以上数据与文献[13]报道对照基本一致, 故鉴定该化合物为neodiosmin。
化合物14 黄色粉末, ESI-MS m/z 601 [M+Na]+, 分子式为C27H30O14, 不饱和度为13。1H NMR (600 MHz, DMSO-d6) δ 7.92 (2H, d, J = 9.0 Hz, H-2', H-6'), 6.92 (2H, d, J = 9.0 Hz, H-3', H-5'), 6.83 (1H, s), 6.76 (1H, d, J = 2.4 Hz, 8-H), 6.44 (1H, d, J = 2.4 Hz, 6-H)。13C NMR (150 MHz, DMSO-d6) δ 181.9 (C-4), 162.8 (C-5), 99.4 (C-6), 161.1 (C-7), 94.7 (C-8), 156.8 (C-9), 105.3 (C-10), 128.6 (C-2', C-6'), 116.2 (C-3', C-5'), 100.5 (C-1''), 72.0 (C-2''), 75.5 (C-3''), 70.7 (C-4''), 76.2 (C-5''), 66.0 (C-6''), 99.8 (C-1'''), 69.5 (C-2'''), 70.3 (C-3'''), 673.0 (C-4'''), 8.2 (C-5'''), 17.7 (C-6''')。以上数据与文献[14]报道对照基本一致, 故鉴定该化合物为芹菜素-7-O-芸香糖苷。
化合物15 黄色粉末, ESI-MS m/z 457 [M+Na]+, 分子式为C20H18O11, 不饱和度为12。1H NMR (600 MHz, DMSO-d6) δ 7.64 (1H, d, J = 9.2 Hz, H-6'), 7.50 (1H, d, J = 1.8 Hz, H-2'), 6.83 (1H, d, J = 8.4 Hz, H-5'), 6.38 (1H, d, J = 1.8 Hz, H-8), 6.18 (1H, d, J = 1.2 Hz, H-6), 5.26 (1H, d, J = 5.4 Hz, H-1'')。13C NMR (150 MHz, DMSO-d6) δ 56.1 (C-2), 133.6 (C-3), 177.4 (C-4), 161.1 (C-5), 98.7 (C-6), 164.6 (C-7), 93.5 (C-8), 156.3 (C-9), 103.7 (C-10), 120.8 (C-1'), 115.3 (C-2'), 145.0 (C-3'), 148.6 (C-4'), 115.7 (C-5'), 122.0 (C-6'), 101.4 (C-1''), 71.6 (C-2''), 70.7 (C-3''), 60.0 (C-4''), 64.2 (C-5'')。以上数据与文献[15]报道对照基本一致, 故鉴定该化合物为槲皮素-3-O-α-L-吡喃阿拉伯糖苷。
实验部分核磁共振仪Bruker AVANCEIII HD (瑞士Bruker公司, 600 MHz); 高分辨飞行时间质谱联用仪AB SCIEX Triple TOF (美国AB SCIEX公司, 5600+); 薄层/柱色谱硅胶GF254 (青岛海洋化工厂); 大孔吸附树脂D101型(天津海光化工有限公司); 葡聚糖凝胶Sephadex LH-20 (Amersham Pharmacia Biotech); 旋转蒸发仪(瑞士Buchi公司); 高效液相色谱仪(美国Waters公司); 半制备型液相色谱仪(美国Waters公司); 制备型反相色谱柱(日本YMC-PackODS-A C18, 20 mm×250 mm, 5 μm), 分析型反相色谱柱(日本Inertsil ODS-3, 4.6 mm×250 mm, 5 μm); 所用试剂均为分析纯或色谱纯。
药材于2016年9月采自江西省赣州市, 经江西中医药大学药学院胡生福教授鉴定为省沽油科山香圆属植物山香圆Turpinia arguta叶。凭证标本保存在江西中医药大学实验楼D504研究室。
取山香圆干燥叶30 kg, 用95%乙醇加热提取3次, 每次2 h, 减压浓缩得到山香圆总浸膏。山香圆总浸膏(2 238 g)用水混悬, 分别用氯仿、乙酸乙酯、正丁醇萃取4次, 每次2.5 L, 减压回收溶剂得到氯仿部位(39 g)、乙酸乙酯部位(58 g)、正丁醇部位(202 g)。
取正丁醇部位(64.2 g)经大孔树脂, 以乙醇-水(30%、50%、75%、95%)为洗脱剂洗脱, 共得到Fr.A~Fr.D。Fr.A (25.3 g)经聚酰胺以乙醇-水(30%、50%、75%、95%)为洗脱剂洗脱, 共得到Fr.A1~Fr.A4, Fr.A2组分经硅胶柱色谱, CH2Cl2-MeOH (10:1→1:1)梯度洗脱得到Fr.A2-1~Fr.A2-5, Fr.A2-2经甲醇溶解经ODS柱色谱, 甲醇-水(30:70)洗脱得到Fr.A2-2-2和Fr.A2-2-3, Fr.A2-2-2经制备液相25%甲醇得到化合物4 (7 mg)和6 (9 mg), Fr.A2-2-3经制备液相45%甲醇得到化合物11 (5 mg), Fr.A2-3经甲醇溶解经ODS柱色谱, 甲醇-水(35:65)洗脱得到Fr.A2-3-4, Fr.A2-3-4经制备液相40%甲醇得到化合物3 (25 mg)。Fr.A3经硅胶柱色谱, CH2Cl2-MeOH (20-1→1-1)梯度洗脱得到Fr.A3-1~Fr.A3-6, Fr.A3-4经甲醇溶解经ODS柱色谱, 甲醇-水(30:70)洗脱得到Fr.A3-4-2, Fr.A3-4-2经制备液相35%甲醇得到化合物1 (8 mg)。
Fr.B (11.2 g)经硅胶柱色谱, CH2Cl2-MeOH (20:1→1:1)为洗脱剂洗脱, 共得到Fr.B1~Fr.B2, Fr.B1经硅胶柱色谱, CH2Cl2-MeOH (30:1→1:1)梯度洗脱得到Fr.B1-1~Fr.B1-4, Fr.B1-2经硅胶柱色谱, CH2Cl2-MeOH (10:1)等度洗脱得到Fr.B1-2-1~Fr.B1-2-5, Fr.B1-2-2经制备液相75%甲醇得到化合物15 (17 mg), Fr.B1-2-4经制备液相60%甲醇得到化合物10 (14 mg)和14 (11 mg), Fr.B1-3经硅胶柱色谱, CH2Cl2-MeOH (8:1)等度洗脱得到Fr.B1-3-1~Fr.B1-3-4, Fr.B1-3-3经制备液相35%甲醇得到化合物8 (35 mg)。Fr.B2经硅胶柱色谱CH2Cl2-MeOH (10:1→1:1)梯度洗脱得到Fr.B2-1~Fr.B2-5, Fr.B2-2经硅胶柱色谱, CH2Cl2-MeOH (8:1)等度洗脱得到Fr.B2-2-1~Fr.B2-2-4, Fr.B2-2-1经制备液相45%甲醇得到化合物9 (8 mg), Fr.B2-2-3经制备液相36%甲醇得到化合物5 (9 mg)和7 (12 mg)。
Fr.C (5.6 g)经硅胶柱色谱, CH2Cl2-MeOH (15:1→1:1)梯度洗脱, 得到Fr.C1~Fr.C3。Fr.C2经硅胶柱色谱, CH2Cl2-MeOH (10:1)等度洗脱, 得到Fr.C2-1~Fr.C2-3, Fr.C2-2经制备液相45%甲醇得到化合物12 (13 mg)。Fr.C3经硅胶柱色谱, CH2Cl2-MeOH (6:1)等度洗脱, 得到Fr.C3-1~Fr.C3-4, Fr.C3-2制备液相30%甲醇得到化合物2 (6 mg)和13 (18 mg)。
| [1] | Editorial Committee of Flora of China, Chinese Academy of Science. Flora of China (中国植物志(第46卷))[M]. Beijing: Science Press, 1981: 27-37. |
| [2] | Zhang WG, Zhou GP, Yang XJ, et al. Determination of nuezhenide and apigenin 7-O-neohesperidoside in Shanxiangyuan tablets by HPLC[J]. Chin J Pharm Anal (药物分析杂志), 2009, 29: 912–914. |
| [3] | Huang ZY. Preliminary report of Shanxiangyuan tablet in the treatment of pharyngitis and other disease[J]. (中草药), 1978(3): 36–38. |
| [4] | Yang YF, Wu ZR. Study on the antimicrobial activities of different processing products of Shanxiangyua[J]. China J Chin Mater Med (中国中药杂志), 1986, 11: 30–32. |
| [5] | Zhan YF, Zhang JJ, Chen JT, et al. Studies on the analgesia effect of Shanxiangyuan baccal tablet[J]. Lishizhen Med Mater Med Res (时珍国医国药), 2005, 16: 389–390. |
| [6] | Ma SG, Yuan SP, Liu YB, et al. 3-Hydroxy-3-methylglutaryl flavone glycosides from the leaves of Turpinia Arguta[J]. Fitoterapia, 2018, 124: 80–85. DOI:10.1016/j.fitote.2017.10.017 |
| [7] | Ma SG, Yuan SP, Hou Q, et al. Flavonoid glycosides from leaves of Turpinia arguta and their anti-inflammatory activity[J]. China J Chin Mater Med (中国中药杂志), 2013, 38: 1747–1750. |
| [8] | Li JS, Zhao YY, Wang B, et al. Separation and identification of the flavonoids from Buddleia officinalis Maxim[J]. Acta Pharm Sin (药学学报), 1996, 31: 849–854. |
| [9] | Li YQ. Studies on the Chemical Constituents of Capparis himalayensis and Turpinia arguta (爪瓣山柑及锐尖山香圆叶化学成分的研究)[D]. Beijing: Peking Union Medical College & Chinese Academy Medical Sciences, 2007. |
| [10] | Zhang WG, Zhou GP, Xie EL, et al. Study of the flavonoids from leaves of Turpinia arguta[J]. China J Chin Mater Med (中国中药杂志), 2009, 34: 1603–1604. |
| [11] | Cheng YX, Zhou J, Tan NH. The chemical constituents of Parakmeria yunnanensis[J]. Acta Bot Yunnan (云南植物研究), 2001, 23: 335–356. |
| [12] | Tian Y, Tang HF, Wang XJ, et al. Studies on antibacterial constituents of Discocleidion rufescens (2)[J]. China J Chin Mater Med (中国中药杂志), 2009, 34: 1377–1380. |
| [13] | Jose ADR, Obdulio B, Julian C, et al. Neodiosmin, a flavone glycoside of Citrus aurantium[J]. Phytochemistry, 1992, 31: 712–724. |
| [14] | Tian Y, Liu X, Dong J. Apigenin glycosides from Euphorbia humifusa wild.[J]. Acta Pharm Sin (药学学报), 2009, 44: 496–499. |
| [15] | Shi S, Zou H, Zhang Y, et al. Chemical constituents from Neo-Taraxacum siphonathum[J]. China J Chin Mater Med (中国中药杂志), 2009, 34: 1002–1004. |
 2019, Vol. 54
2019, Vol. 54


