鼻咽癌(nasopharyngeal carcinoma, NPC)是一种主要发生在我国南方地区的鼻咽部恶性肿瘤, 由于鼻咽癌发生的部位比较隐蔽, 早期症状常不明显而容易被忽略, 因此确诊的患者60%以上都处于癌细胞转移的中、晚期, 而侵袭和转移是导致鼻咽癌患者死亡的主要原因之一[1, 2], 因此研究肿瘤细胞的侵袭转移机制, 指导临床用药, 是本课题组研究的主要方向。
甲基莲心碱(neferine, Nef)是从睡莲科植物莲成熟种子的绿色胚芽中提取出的一种双苄基异喹啉类生物碱。它具有扩血管、降压、抗心律失常、抗血小板聚集等药理作用[3-5]。近些年来研究表明, Nef对骨肉瘤、乳腺癌和肺癌等[6-10]具有化疗药物增敏作用, 在肝癌细胞中具有抑制肝癌细胞侵袭转移的作用[11], 而Nef在鼻咽癌的侵袭转移方面暂无研究进展。本文以人鼻咽癌细胞株CNE-1和5-8F为研究对象, 通过观察Nef对CNE-1和5-8F细胞侵袭、转移的影响, 探讨Nef对鼻咽癌细胞侵袭转移的作用及机制, 为Nef在鼻咽癌侵袭、转移的临床应用提供实验基础。
材料与方法实验材料 RPMI-1640培养基(美国Gibco公司); 新生小牛血清(美国Hyclone公司); 胰蛋白酶(美国Sigma公司); 二甲基亚枫(DMSO, 德国BioFroxx公司)。CCK-8试剂盒(中国联科生物技术有限公司); HiPerFect Transfection Reagent (美国Qiagen公司); Nef (纯度99.16%, 中国成都植标化纯生物技术有限公司); Transwell小室(美国Corning公司); Mastercycler Ep Realplex实时荧光定量PCR仪(德国Eppendorf公司); 倒置荧光显微镜(日本Olympus公司)。Total RNA Kit I (中国Omega公司); 微小RNA (microRNA, miRNA)mimic、Bulge-LoopTM miRNA qRT-PCR Starter Kit、RT-PCR primer (中国Ribobio公司); 引物(中国上海生工生物公司)。GAPDH、神经型钙黏蛋白(N-cadherin)、上皮型钙黏蛋白(E-cadherin)、波形蛋白(vimentin)、Twist、Slug和Snail兔抗人单克隆抗体(英国Abcam公司); 荧光二抗(美国GE公司); 蛋白质提取试剂盒(中国江苏凯基生物技术股份有限公司)。
细胞培养及配置Nef溶液 人鼻咽癌细胞系CNE-1、5-8F由中南大学肿瘤研究所赠送。采用含10%新生牛血清的RPMI 1640培养基, 至37 ℃、5% CO2培养箱内培养。用20 μmol·L-1、30 μmol·L-1浓度Nef连续刺激CNE-1、5-8F细胞。20 mg Nef充分溶解于1 mL DMSO溶剂中, 配成32 mmol·L-1母液, -4 ℃贮存备用。
CCK-8检测细胞增殖 取对数生长期的鼻咽癌细胞CNE-1、5-8F, 胰酶消化后制成细胞悬液, 细胞悬液移至96孔板, 每孔100 μL, 调整细胞密度为每孔5×103个, 预培养12 h待细胞贴壁。Nef母液用无胎牛血清的RPMI-1640培养基依次稀释成10、20、30、40 μmol·L-1, 4组实验组培养板分别加入10 μL不同浓度的Nef; 阴性对照组加入等体积的DMSO, 各浓度实验组和对照组均设5个复孔, 培养24 h后, 每孔加入10 μL CCK-8反应液, 孵育1 h, 酶标仪测定各组在450 nm处吸光度值(A)。细胞存活率(%)=(A实验组-A空白组/A对照组-A空白组)×100%。计算CNE-1、5-8F细胞Nef最适用药浓度继续如下实验。
划痕实验 将CNE-1、5-8F细胞以每孔5×105个细胞接种于6孔板中, 培养12 h待细胞单层铺满后, 分别加入DMSO、20 μmol·L-1 Nef、30 μmol·L-1 Nef, 更换无血清培养基培养, 待细胞贴壁后, 以200 μL枪头竖直方向划痕, PBS清洗脱落细胞3次, 观察无血清培养基培养0、24、36 h时细胞迁移距离, 迁移比率=(0 h的边缘距离-N h的边缘距离)/0 h的边缘距离。实验重复3次, 取平均值为最终结果。
Transwell细胞体外侵袭、迁移实验 冰上将融化后的BD Matrigel用冰的1640培养基稀释10倍, 混匀, 往预冷的Transwell板上室加稀释后的Matrigel, 每孔50 μL, 均匀铺平, 并置于细胞培养箱中孵育3~4 h。消化已用无血清培养基RPMI-1640饥饿培养的CNE-1、5-8F细胞, 细胞计数, 调整细胞浓度为每孔5×104个, 离心, 弃上清液后, 实验组分别加入100 μL 20 μmol·L-1、30 μmol·L-1 Nef重悬细胞, 对照组100 μL含等量DMSO无血清的RPMI-1640培养基重悬细胞于Transwell上室中, 下室加入800 μL含10%胎牛血清培养液, 置于培养箱中孵育48 h后取出Transwell板, 移去培养液, PBS清洗Transwell小室, 4%多聚甲醛固定, 无水甲醛透化, 0.1%结晶紫染色, 棉签轻轻擦拭没有侵袭的细胞及基质胶, 干燥后, 倒置显微镜下随机视野观察并拍照, 实验相同条件下重复3次。细胞迁移实验上室未铺Matrigel, 其余步骤与细胞侵袭实验相同。
Western blot实验检测上皮-间质转化(epithelial to mesenchymal transition, EMT)相关蛋白表达 以30 μmol·L-1 Nef、DMSO处理24 h后的CNE-1、5-8F细胞为研究对象, 分别提取细胞总蛋白, BCA法对蛋白进行定量, 蛋白变性处理后每孔加样20 μg, 在6%、12% SDS-PAGE胶上电泳60 min, 电泳完毕后在300 mA恒流下转膜90 min。转膜完毕后, 将电转膜置于5%的脱脂奶粉(PBS配制)中封闭, 4 ℃过夜。封闭的膜用磷酸盐缓冲液加Tween 20配制的PBST漂洗3次, 加入一抗(1:2 000)孵育4 ℃过夜。弃去一抗, PBST洗涤10 min, 反复3次。加入荧光二抗(1:5 000)室温避光下于摇床孵育1 h, 弃去二抗, PBST洗涤10 min, 反复3次, 荧光发光显色。
基因芯片实验及生物信息学分析 以分别用DMSO、30 μmol·L-1 Nef处理的5-8F为实验对象, 分别提取细胞miRNA, 使用Affymetrix的表达谱芯片HG U133Plus 2.0 array进行基因芯片杂交实验。运用miRNA靶向预测软件对芯片结果进行生物信息学分析。
细胞转染及转染效率的检测 实验分2组: ① hsa-let-7c-5p/hsa-miR-423-5p mimic+HiPerFect (mimic组); ② NC+HiPerFect (NC组)。将细胞以每孔2×105接种于6孔板, 培养24 h后换新鲜培养液, 按操作说明以等量无血清培养基分别稀释适当量的miRNA mimic/NC和HiPerFect, 混匀后各自室温静置5 min, 加入培养板中, 轻轻混匀, 放入培养板中继续培养, 转染带荧光的miRNA mimic/NC时, 所有操作均需避光操作, 并以锡箔纸包被培养板放置培养箱中培养, 转染48 h后用PBS冲洗1次, 荧光显微镜下观察转染效率。
Real-time PCR检测各miRNA与下游mRNA的表达 各组5-8F细胞经转染48 h后按照Trizol说明书中步骤抽提细胞miRNA, 取RNA于紫外分光光度计下测A260/A280, 比值处于1.8~2.2之间则符合纯度要求。按Bulge-LoopTM miRNA qRT-PCR试剂盒说明配制逆转录反应液, 逆转录合成cDNA后, 参照试剂盒说明配制PCR反应体系, 每组设置3个复孔。PCR反应步骤: 95 ℃ 10 min, 95 ℃ 2 s, 60 ℃ 20 s, 70 ℃ 10 s, 共40个循环。反应以U6和actin为内参。记录各孔Ct值, 取3孔平均值作为结果, 并采用2-ΔΔCt法对结果进行分析, ΔΔCt = (Ct目标基因-Ct内参基因)处理组-(Ct目标基因-Ct内参基因)对照组。
统计学方法 所有数据以x ± s表示, 用GraphPad Prism 5软件对数据进行分析和作图, 两样本均数比较采用t检验, 多个样本均数比较采用单因素方差分析, 以P < 0.05为具有统计学显著性差异。
结果 1 不同浓度Nef对CNE-1和5-8F细胞毒性的影响Nef为从莲子中提取的双苄基异喹啉生物碱, Nef的分子式C38H44N2O6 (结构式见图 1a, 含酚羟基)。采用CCK8方法, 观察不同浓度Nef作用24 h后对CNE-1和5-8F细胞活性的影响, 结果显示, Nef浓度为30 μmol·L-1时CNE-1、5-8F细胞的存活率均大于80% (图 1b)。因此, 后续的实验选择30 μmol·L-1 Nef处理CNE-1细胞、5-8F细胞。

|
Figure 1 Nef concentration-response (cell viability) curves. a: Chemical structure of Nef; b: Effect of Nef on cell viability of CNE-1 and 5-8F cells. n = 5, x ± s. CNE-1 and 5-8F cells were treated with different doses of Nef for 24 h and cell viability detected by CCK-8 assay. *P < 0.05, ***P < 0.001 vs control group. Nef: Neferine |
Nef抑制CNE-1和5-8F细胞迁移及侵袭作用, 划痕实验结果显示, Nef处理后的CNE-1、5-8F细胞对比对照组细胞, 划痕间距缩小, 速率明显减慢, 且随着Nef浓度增大细胞迁移速率减慢越显著, 差异具有统计学意义(图 2a、b)。Transwell迁移、侵袭实验显示, Nef处理的CNE-1和5-8F细胞相比对照组, 细胞侵袭、迁移能力明显减弱, Nef浓度为30 μmol·L-1时能显著抑制CNE-1和5-8F细胞的迁移、侵袭能力, 差异具有统计学意义(2c、d)。

|
Figure 2 Effects of Nef on the migration and invasion of CNE-1 and 5-8F cells. The migration (a) and relative migration ability analysis (b) of CNE-1 and 5-8F cells with different doses of Nef were examined by scratch assay for 24 h and 36 h. Effects of Nef on migration and invasion in CNE-1 and 5-8F cells (c, d) were analyzed by Transwell assay for 48 h; quantification is expressed as the number of invasive cells in three random microscopic fields per well. *P < 0.05, **P < 0.01, ***P < 0.001 (100×magnification) |
Western blot实验结果显示, 30 μmol·L-1 Nef处理后的CNE-1细胞Twist、Snail、N-cadherin、vimentin表达水平下调, E-cadherin表达上调, 差异具有统计学意义, Slug转录因子无显著差异性表达; 5-8F细胞中Slug、N-cadherin、Vimentin表达水平下调, E-cadherin表达上调, 差异具有统计学意义, Snail、Twist转录因子无显著差异性表达(图 3a、b)。上述实验显示: Nef能调控EMT相关蛋白的表达, 而对Snail、Twist、Slug转录因子无显著调控作用。

|
Figure 3 Effects of Nef on the expression of epithelial-mesenchymal transition (EMT)-associated proteins. CNE-1 and 5-8F cells were cultured with 30 μmol·L-1 Nef for 24 h and EMT-associated proteins analyzed by Western blot assay (a, b). **P < 0.01, ***P < 0.001. E-Ca: E-cadherin; N-Ca: N-cadherin |
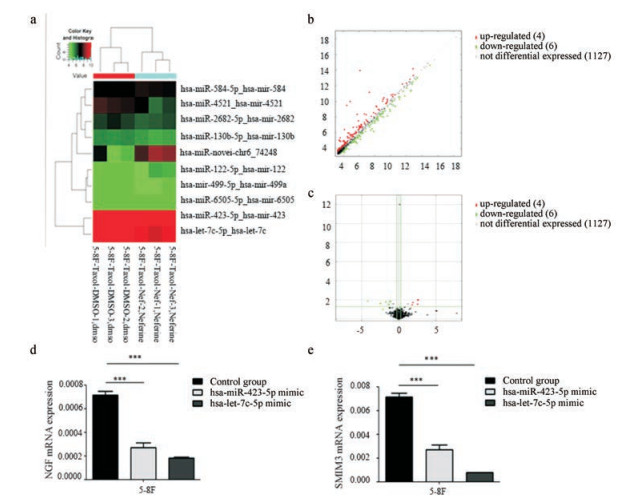
miRNA基因芯片检测分别以DMSO、30 μmol·L-1 Nef处理过的5-8F细胞miRNA表达结果显示, 表达倍数改变 > 2, 且P值< 0.05的有10个miRNA (表 1), 其中表达下调的6个miRNA分别为hsa-miR-130b-5p、hsa-miR-6505-5p、hsa-miR-2682-5p、hsa-let-7c-5p、hsa-miR-423-5p、hsa-miR-4521, 表达上调的4个miRNA分别为hsa-miR-499a-5p、hsa-miR-122-5p、hsa-miR-584-5p、hsa-miR-novel-chr6-74248 (图 4a~c), 其中hsa-let-7c-5p和hsa-miR-423-5p表达变化最明显, 分别下调至30.6%和28.6%, RT-PCR检测30 μmol·L-1 Nef处理后的5-8F细胞中hsa-let-7c-5p、hsa-miR-423-5p表达趋势与芯片一致, 运用miRNA靶向预测软件对基因芯片结果做进一步的生物信息分析, 显示在30 μmol·L-1 Nef处理后的5-8F细胞中下调的hsa-let-7c-5p、hsa-miR-423-5p具有共同的下游靶向负调控基因:小细胞膜蛋白3 (small integral membrane protein 3, SMIM3)、神经生长因子(nerve growth factor, NGF)。Real-time PCR验证转染hsa-let-7c-5p mimic、hsa-miR-423-5p mimic后5-8F细胞中SMIM3、NGF的表达: SMIM3、NGF表达显著下降, 差异具有统计学意义(图 4d、e)。
| Table 1 Differentially expressed gene profiles between the control group and the Nef group (fold change > 2) |
|
Figure 4 Analysis and verification for the results of gene chip. Cluster analysis (a) of microRNAs in 5-8F cells (green: down-regulated, red: up-regulated); 6 microRNAs down-regulated and 4 microRNAs up-regulated after the action of 30 μmol·L-1 Nef. Scatter map (b) and volcanic map (c) of differential gene expression between 5-8F cells treated with 30 μmol·L-1 Nef and the control group. Effect of hsa-let-7c-5p mimic or hsa-miR-423-5p mimic on NGF (d) and SMIM3 (e). mRNA level in 5-8F cells was detected by qPCR. ***P < 0.001. NGF: Nerve growth factor; SMIM3: Small integral membrane protein 3 |
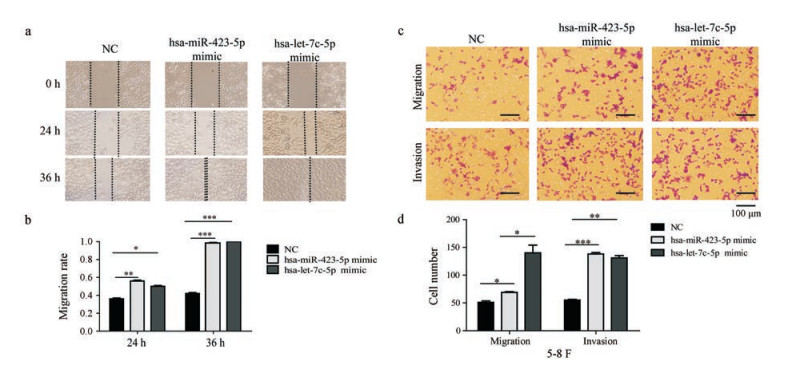
5-8F细胞经hsa-let-7c-5p mimic、hsa-miR-423-5p mimic转染后, 划痕实验显示其细胞迁移能力相对对照组显著增强(图 5a、b), Transwell侵袭、迁移实验显示其迁移、侵袭能力增强(图 5c、d)。hsa-let-7c-5p、hsa-miR-423-5p能增强鼻咽癌5-8F细胞的迁移、侵袭能力。下调hsa-let-7c-5p、hsa-miR-423-5p的表达水平可能抑制鼻咽癌细胞的迁移、侵袭能力。
|
Figure 5 Overexpression of hsa-let-7c-5p and hsa-miR-423-5p promoted the invasion and migration ability of 5-8F cells. Migration (a) and relative migration ability (b) of 5-8F cells with different doses of Nef for 24 h and 36 h. Effects of Nef on migration and invasion in 5-8F cells (c, d) analyzed by Transwell assay after 48 h of transfection. Quantification of the migration and invasion is expressed as the number of invasive and migratory cells in three random microscopic fields per well. *P < 0.05, **P < 0.01, ***P < 0.001 (100×magnification) |
鼻咽癌是头颈部常见恶性肿瘤, 临床治疗以放射治疗为主, 化疗为辅, 鼻咽癌具有较高的侵袭性和淋巴结转移倾向, 没有发生远处转移的鼻咽癌患者的临床治疗5年生存率可达80%以上, 而发生了复发、远处转移的患者的临床治疗5年生存率显著下降[12, 13], 鼻咽癌侵袭、转移是患者治疗预后不佳的主要原因之一。
Nef为睡莲科植物莲的成熟种子绿色胚芽中提取出来的一种双苄基异喹啉生物碱。周雅君等[14]的研究显示Nef能通过抑制肝癌细胞Rho/ROCK信号通路抑制肝癌细胞增殖和侵袭作用; Deng等[15]的研究显示Nef通过抑制肝癌细胞EMT相关蛋白的表达从而抑制其侵袭转移。在本课题组实验中, 通过划痕实验、Transwell细胞体外迁移、侵袭实验显示Nef可显著抑制CNE-1和5-8F细胞的迁移、侵袭能力。Western blot实验结果显示, Nef下调CNE-1、5-8F细胞中N-cadherin、Vimentin的表达, 上调E-cadherin的表达。上述实验表明Nef能显著抑制鼻咽癌细胞的侵袭转移能力。
miRNA是一类短链非编码单链RNA, 可通过结合mRNA非编码区(UTRs)抑制mRNA翻译, 发挥基因转录后水平的负调控作用。miRNA可调控生物生长、发育、繁殖、癌变进程。研究显示, let-7家族是一类功能和碱基序列高度保守的miRNA, 调控癌细胞的增殖、分化, hsa-let-7c-5p为let-7家族的一员[16], 可在肝癌细胞中调控Bcl-xL基因的表达, 进而调节癌细胞的凋亡进程[17], 可作为结肠癌的治疗靶点和预后的生物标志物[18]。hsa-miR-423-5p可在肝癌细胞中增强癌细胞的自噬进程[19, 20], 为了进一步探究Nef抑制鼻咽癌细胞侵袭转移与miRNA的相关性, 本研究对Nef处理过的5-8F细胞与对照组细胞的miRNA的表达进行基因芯片检测, 结果显示, 有10个表达倍数改变 > 2, 且P < 0.05的miRNA, 其中, hsa-let-7c-5p、hsa-miR-423-5p表达下调倍数最显著, Real-time PCR结果与基因芯片一致, hsa-let-7c-5p mimic、hsa-miR-423-5p mimic转染至5-8F细胞后, 细胞划痕实验、Transwell迁移侵袭实验显示鼻咽癌细胞侵袭转移能力增强, 上述实验表明: hsa-let-7c-5p、hsa-miR-423-5p可能增强鼻咽癌细胞的侵袭、转移能力。
通过miRNA靶向预测软件分析miRNA基因芯片结果显示: hsa-let-7c-5p、hsa-miR-423-5p有共同的下游靶向负调控基因: SMIM3、NGF基因, NGF因子是神经营养蛋白家族的一种, 是一种与神经细胞的分化、生长、修复、再生有关的细胞因子。研究表明, NGF在鼻咽癌中诱导癌细胞凋亡[21]。SMIM3是个未知的基因, 可能分泌与细胞膜跨膜结构域的形成和调节离子通道有关蛋白, SMIM3在嗜铬细胞瘤中可由NGF诱导分泌[22]。
综上所述, 本研究通过细胞实验显示Nef可能通过下调鼻咽癌细胞hsa-let-7c-5p、hsa-miR-423-5p的表达水平, 改变下游靶基因SMIM3、NGF表达水平, 抑制鼻咽癌EMT相关蛋白的表达, 从而抑制鼻咽癌的侵袭转移。hsa-let-7c-5p、hsa-miR-423-5p对SMIM3、NGF基因的调控机制以及SMIM3、NGF基因的具体生物学功能尚不清楚, 有待于进一步研究。
| [1] | Chen J. The Initial Investigation of the Relationship between Nasopharngeal Carcinoma-related Gene ID2 and EMT (鼻咽癌相关基因ID2与EMT关系的初步探讨)[D]. Guangzhou: Southern Medical University, 2010. |
| [2] | Ding JH, Su FR, Bo L. Research development of nasopharyngeal carcinoma[J]. Int J Otolaryngol Head Neck Surg (国际耳鼻咽喉头颈外科杂志), 2017, 41: 43–46. |
| [3] | Tang XQ, Cao JG. Review on the pharmacological research of neferine[J]. Chin Pharmacol Bull (中国药理学通报), 2004, 20: 8–10. |
| [4] | Wang JL, Nong Y, Jiang MX. Effects of liensinine on hemodynamics in rats and the physiologic properties of isolated rabbit atria[J]. Acta Pharm Sin (药学学报), 1992, 27: 881–885. |
| [5] | Yu J, Hu WS. Effects of neferine on platelet aggregation in rabbits[J]. Acta Pharm Sin (药学学报), 1997, 32: 1–4. |
| [6] | Zhang X, Liu Z, Xu B, et al. Neferine, an alkaloid ingredient in lotus seed embryo, inhibits proliferation of human osteosarcoma cells by promoting p38 MAPK-mediated p21 stabilization[J]. Eur J Pharmacol, 2012, 677: 47. DOI:10.1016/j.ejphar.2011.12.035 |
| [7] | Cao JG, Tang XQ, Shi SH. Multidrug resistance reversal in human gastric carcinoma cells by neferine[J]. World J Gastroenterol, 2004, 10: 3062–3064. |
| [8] | Sivalingam KS, Paramasivan P, Weng CF, et al. Neferine potentiates the antitumor effect of cisplatin in human lung adenocarcinoma cells via a mitochondria-mediated apoptosis pathway[J]. J Cell Biochem, 2017, 118: 2865–2876. DOI:10.1002/jcb.25937 |
| [9] | Ye ZG, Wang JH, Sun AX, et al. Poteintation of vincristine induced apoptosis by tetrandrine, neferine and dauricine in the human mammary MCF7 multidrug resistant cells[J]. Acta Pharm Sin (药学学报), 2001, 36: 96–99. |
| [10] | Eid W, Abdel-Rehim W. Neferine enhances the antitumor effect of mitomycin-C in HeLa cells through the activation of p38-MAPK pathway[J]. J Cell Biochem, 2017, 118: 3472–3479. DOI:10.1002/jcb.26006 |
| [11] | Yoon JS, Kim HM, Yadunandam AK, et al. Neferine isolated from Nelumbo nucifera enhances anti-cancer activities in Hep3B cells: molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response[J]. Phytomedicine, 2013, 20: 1013–1022. DOI:10.1016/j.phymed.2013.03.024 |
| [12] | Wang ZY, Li GS, Huang HX, et al. Causes of death and prognostic factors of nasopharyngeal carcinoma[J]. Guangdong Med J (广东医学), 2013, 34: 754–757. |
| [13] | Wang X, Zhu N, Li JC, et al. Effect of triptolide on the expression and function of P-gp in nasopharyngeal cancer cells[J]. Acta Pharm Sin (药学学报), 2018, 53: 1107–1112. |
| [14] | Zhou YJ, Shi J, Tian G, et al. Effect of neferine on proliferation and invasion of human hepatocellular carcinoma cell line HepG2 and Bel-7402[J]. Chin Pharmacol Bull (中国药理学通报), 2016, 32: 1539–1542. |
| [15] | Deng G, Zeng S, Ma J, et al. The anti-tumor activities of neferine on cell invasion and oxaliplatin sensitivity regulated by EMT via Snail signaling in hepatocellular carcinoma[J]. Sci Rep, 2017, 7: 41616. DOI:10.1038/srep41616 |
| [16] | Roush S, Slack FJ. The let-7 family of microRNAs[J]. Trends Cell Biol, 2008, 18: 505–516. DOI:10.1016/j.tcb.2008.07.007 |
| [17] | Shimizu S, Takehara T, Hikita H, et al. The let-7 family of microRNAs inhibits Bcl-xL expression and potentiates sorafenib-induced apoptosis in human hepatocellular carcinoma[J]. J Hepatol, 2010, 52: 698–704. DOI:10.1016/j.jhep.2009.12.024 |
| [18] | Zhou XG, Huang XL, Liang SY, et al. Identifying miRNA and gene modules of colon cancer associated with pathological stage by weighted gene co-expression network analysis[J]. Onco Targets Ther, 2018, 11: 2815–2830. DOI:10.2147/OTT.S163891 |
| [19] | Stiuso P, Potenza N, Lombardi A, et al. MicroRNA-423-5p promotes autophagy in cancer cells and is increased in serum from hepatocarcinoma patients treated with sorafenib[J]. Mol Ther Nucleic Acids, 2015, 4: e233. DOI:10.1038/mtna.2015.8 |
| [20] | Lian Y, Xiong F, Yang L, et al. Long noncoding RNA AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway[J]. J Exp Clin Cancer Res, 2018, 37: 253. DOI:10.1186/s13046-018-0918-9 |
| [21] | Wei SX, Liu CJ, Li MY, et al. Study on the apoptosis induction of Guangxi cobra venom NGF in human nasopharyngeal carcinoma CNE-2 cells[J]. Chin J Hosp Pharm (中国医院药学杂志), 2008, 28: 1909–1912. |
| [22] | Vician L, Silver AL, Farias-Eisner R, et al. NID67, a small putative membrane protein, is preferentially induced by NGF in PC12 pheochromocytoma cells[J]. J Neurosci Res, 2001, 64: 108–120. DOI:10.1002/jnr.1058 |
 2019, Vol. 54
2019, Vol. 54


