大量慢性病及年龄相关性疾病都与体内能量代谢障碍有关, 并可以通过生物能量调节疗法(bioenergetic modulation therapy, BIOMET)改善[1]。机体细胞利用葡萄糖、脂肪酸和蛋白质等营养物质, 通过氧化磷酸化和糖酵解途径产生ATP为细胞复杂的生命活动提供能量, 外界干扰或者基因突变等因素导致细胞能量产生-供应和利用平衡打破, 正常活动受到干扰, 将导致或者加重疾病的发生发展。线粒体通过呼吸和氧化磷酸化(OXPHOS)过程产生的ATP是细胞最重要的能量来源。在正常生理和病理条件下, OXPHOS系统的呼吸链也是活性氧(ROS)和自由基的主要细胞内来源。此外, 线粒体在多种细胞过程中发挥关键作用, 包括磷脂生物合成、钙信号传导和细胞凋亡等[2]。
近年来, 包括线粒体功能受损在内的能量代谢障碍对疾病发生发展的影响越来越受到人们的重视[3], 本文综述了神经退行性疾病(阿尔茨海默症、帕金森病、亨廷顿舞蹈病)、癌症、糖尿病和心血管疾病中能量异常及其相关靶点, 为靶向能量代谢的新药创制提供借鉴。
1 神经退行性疾病 1.1 阿尔茨海默病阿尔茨海默病(Alzheimer's disease, AD)是老年痴呆的主要类型, 已成为仅次于心脑血管疾病和恶性肿瘤的重要致死性疾病, 严重危害老年人的健康。葡萄糖是哺乳动物大脑主要的能量来源[4], 虽然人类大脑只占体重的2%, 大脑利用全身葡萄糖的25%[5], 其中大部分用于通过糖酵解和线粒体氧化磷酸化来转导能量以支持突触传递。AD患者在疾病早期尚未出现病理改变和临床症状之前, 就已经出现了大脑对葡萄糖利用的降低和大脑能量缺损现象, 并且随着AD病情的加重而不断恶化[6]。
大脑葡萄糖代谢包括两个主要过程:葡萄糖转运和细胞内氧化分解代谢。正常的生理葡萄糖运输取决于参与组成血脑屏障的星形胶质细胞的功能[7]和分布在大脑中的各种葡萄糖转运蛋白[8]。星形胶质细胞在调节葡萄糖转运和维持脑能量稳态方面发挥着至关重要的作用, AD患者脑内星形胶质细胞退化影响能量代谢, 会损害正常的脑内稳态, 阻碍淀粉样蛋白的清除, 促进细胞因子和其他炎症介质释放, 并且随着时间推移加重神经退行性病变[7]。不同类型的葡萄糖转运蛋白(GLUTs)也参与血液中葡萄糖向神经元的转运。其中, GLUT-1和GLUT-3在大脑葡萄糖转运的调节和AD发病机制中发挥重要作用[8], AD患者脑内GLUT1和GLUT3的水平显著下降, 在大脑皮层中更为明显[9]。
葡萄糖细胞内氧化分解代谢是一个复杂的过程, 包括磷酸戊糖途径、糖酵解、三羧酸循环、氧化磷酸化等[10]。其中三羧酸循环和氧化磷酸化在线粒体中进行, 葡萄糖在细胞质中经糖酵解生成丙酮酸, 通过丙酮酸载体进入线粒体, 在丙酮酸脱氢酶复合体(PDHC)的催化下, 丙酮酸氧化脱羧生成乙酰辅酶A (acetyl-CoA)。三羧酸循环是葡萄糖氧化分解的主要步骤, 其中acetyl-CoA是该循环的入口物质, 研究发现, AD中PDHC、α-酮戊二酸脱氢酶复合体(KGDHC)活性明显降低[11]。AD病理学的线粒体功能缺陷在AD患者的脑、血细胞和成纤维细胞中均有报道[12-14]。除了关键酶PDHC和KGDHC活性降低外, 涉及AD病理生理学的线粒体功能障碍还包括线粒体DNA (mtDNA)缺失、突变或多态性增加, 钙信号受损以及与疾病特异性蛋白质(如Aβ、tau蛋白和突触核蛋白)的相互作用, 都会影响细胞的能量代谢[15] (图 1)。
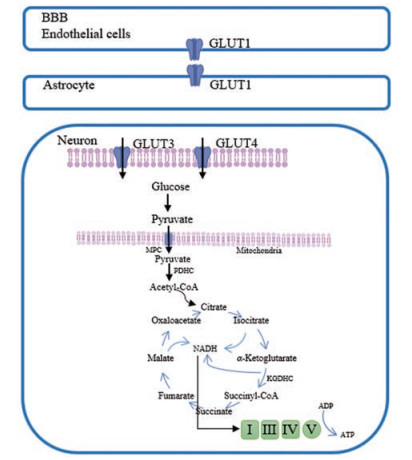
|
Figure 1 Glucose metabolism in the brain. Glucose transporter 1 (GLUT1) is expressed in endothelial cells of the blood-brain barrier (BBB); GLUT1 mediates glucose transport to astrocytes; neurons take up glucose through GLUT3 and GLUT4. Glucose is converted to pyruvate in the cytoplasmic matrix. Pyruvate enters the mitochondria through the mitochondrial pyruvate carrier (MPC), and then is converted to acetyl-CoA, which enters the tricarboxylic acid (TCA) cycle to produce NADH. The energy released from reoxidation of NADH phosphorylate ADP to form ATP |
Aβ可以与细胞内线粒体蛋白质产生相互作用, 如β-淀粉样蛋白结合蛋白(ABAD)和亲环蛋白D (CypD)[16]。ABAD是与Aβ相互作用并促进Aβ介导的线粒体和神经元功能障碍的一种脱氢酶。ABAD通过降低细胞色素氧化酶C (cytochrome C oxidase, COX)活性和加剧神经元中ROS损伤来增强Aβ诱导的细胞应激, 这些变化在来自转基因mAPP/ABAD小鼠的培养神经元中被证实。因此, ABAD调节剂的设计和线粒体ABAD的靶向治疗成为了AD治疗的新策略。合成的ABAD调节剂(ABAD-4a和ABAD-4b)增加了COX活性和ATP水平, 并提示对线粒体有特异性保护作用[17]。Frentizole是一种免疫抑制药物, 通过拮抗Aβ-ABAD相互作用可防止线粒体和神经元功能异常, 并改善阿尔茨海默病模型小鼠的学习记忆功能。目前已开发出其他苯并噻唑基脲衍生物和frentizole类似物[18, 19]。
线粒体Aβ还可以与CypD相互作用并促进线粒体通透性转换孔(mitochondrial permeability transition pore, mPTP)的开放, mPTP是线粒体内膜中的蛋白复合物[20], mPTP的打开诱导线粒体膜电位去极化, 破坏线粒体结构, 导致线粒体功能障碍, 造成神经细胞能量产生受损、细胞死亡途径的开启及神经毒性蛋白的积累[21]。mPTP至少由4种蛋白质组成[22], 包括CypD、电压依赖性阴离子通道蛋白(VDAC)、腺嘌呤核苷酸转运蛋白(ANT)和18 kDa转运蛋白(TSPO)。已有研究表明, mPTP小分子调节剂可以修复Aβ诱导的线粒体功能障碍并恢复细胞活力[23]。TSPO因其参与慢性炎症和神经系统疾病而得到广泛研究, Kim等[24]设计了一系列基于TSPO的配体结构, 其中化合物25能有效改善AD动物模型的认知功能并提高线粒体抵抗Aβ损伤的能力, 从而提高细胞的抗氧化、抗炎水平。
1.2 帕金森病帕金森病(Parkinson's disease, PD)是一种影响中枢神经系统的慢性神经退行性疾病, 早期最明显的症状为颤抖、肢体僵硬、运动功能减退和步态异常。核磁共振波谱(MRS)是直接观察患者大脑中能量代谢的唯一方法, 它为PD等神经退行性疾病的能量缺乏提供了有力证据。MRS观察发现早期和晚期PD患者的中脑和壳核区ATP水平降低[25], 基底神经节和额叶内的高能磷酸盐(ATP和磷酸肌酸)水平降低。并且有临床研究显示, PD患者枕骨和下顶叶中的葡萄糖代谢减少, 基于记忆的行为能力降低与后顶叶和颞区的18F-脱氧葡萄糖(18F-fluorodeoxyglucose, FDG)代谢减少相关, 而注意力相关的行为表现与额叶的FDG代谢水平相关[26]。
遗传因素是PD的已知发病机制之一, 其中常染色体隐性遗传的PINK1和parkin突变是目前公认的发病机制且一直是研究热点, PD许多异常的线粒体功能都归因于PINK1和parkin突变, 包括线粒体自噬[27]、线粒体动力学[28]、线粒体移动[29]和线粒体生物发生[30]。所有这些变化都可能影响线粒体的生物能量代谢, 破坏正常的细胞功能。线粒体能量代谢功能障碍对PD发病影响的更直接证据来自于对PD患者线粒体氧化呼吸复合物Ⅰ (NADH脱氢酶)活性的研究。线粒体氧化呼吸复合物Ⅰ活性降低导致线粒体氧化磷酸化功能发生障碍, ATP产量减少, 线粒体复合物Ⅰ和免疫组织化学复合物-Ⅰ亚基的活性在特发性PD患者的大脑中显著降低[31]。形态计量学和免疫组织化学分析表明, PD患者黑质内线粒体复合体-Ⅰ存在功能缺陷[32]。还有证据表明, 线粒体复合物活性的损伤不仅局限于PD患者脑部, 并且在外周组织如骨骼肌、淋巴细胞和血小板中均有减少[33]。
脑神经元的高能量需求意味着它们特别容易受到胰岛素敏感性改变的影响, 研究表明胰岛素抵抗和2型糖尿病与认知衰退和PD风险增加有关[34]。胰岛素信号传导最终可以促进神经元存活, 这也许并不令人惊讶。此外, 胰岛素可以通过下丘脑葡萄糖感受性神经元来调节脂质和葡萄糖的外周代谢, 维持能量稳态[35], 因此脑胰岛素信号传导的改变可能导致大脑能量代谢的变化。尽管在结构上与胰岛素无关, 但胰高血糖素样肽-1 (glucagon-like peptide-1, GLP-1)类似物可以促进胰岛素信号传导, 增强细胞的能量代谢[36]。但是大多数GLP-1类似物的半衰期都很短, 基于人GLP-1的新型GLP-1类似物, 如liraglutide和lixisenatide等有更长的半衰期, 并且在MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine)诱导的PD动物模型中显示出神经保护作用和运动改善功能[37]。此外, 靶向线粒体丙酮酸载体的胰岛素增敏剂在多种PD相关的细胞、动物模型中都被证明有改善作用[38]。
1.3 亨廷顿病亨廷顿病(Huntington's disease, HD)又称亨廷顿舞蹈病或慢性进行性舞蹈病, 是一种迟发型常染色体显性遗传病, 以舞蹈样不自主运动、精神异常和进行性痴呆为主要临床特点。脑能量代谢障碍一直是HD研究的焦点。有大量证据表明, HD患者大脑中葡萄糖消耗量减少, 尤其在基底节更为明显, 症状前突变携带者中也存在相同的现象[39]。HD患者基底节和枕叶皮层中乳酸浓度增加[40]以及脑脊液中乳酸盐-丙酮酸盐比例升高, 也被认为是脑能量缺乏的表现[41]。HD脑内葡萄糖摄取量减少导致通过糖酵解代谢的葡萄糖比例升高, 而通过磷酸戊糖途径代谢的比例降低, 限制随后抗氧化保护所需的NADPH和GSH产生。结果对糖酵解和三羧酸(TCA)中关键的循环酶产生氧化损伤, 进一步限制了能量产生, 造成恶性循环。
HD患者能量产生也可能受到线粒体生物发生、线粒体钙转运受损[42]、线粒体运输异常[43]以及线粒体生物合成关键因子失调[44]等的影响。外周能量代谢障碍也是一种HD假说, 它表明在大脑外可以检测到发病机制的线索。骨骼肌细胞是一种代谢需求较高的可兴奋性细胞, 在HD患者中表现出了类似于HD纹状体神经元中明显紊乱的能量代谢障碍[45]。另有研究利用31P-核磁共振光谱发现HD患者和症状前个体的静息肌肉中磷酸肌酸/无机磷酸盐比率降低, ATP水平降低[46]。
文献[47]报道, 通过观察纹状体神经元和运动协调性发现过氧化物酶体增殖物激活受体γ辅激活子1α (peroxisome proliferator-activated receptor-γ coactivator 1 alpha, PGC-1α)缺陷可以加快HD进展, 确定PGC-1α在HD中的重要作用。此外, PGC-1α作为参与调节多种途径(如线粒体生物发生和氧化磷酸化)的关键因子在HD中被显著下调[48]。因此, 有人提出, PGC-1α及其下游基因缺陷可能是导致HD肌肉[49]、脂肪组织[50]及脑中的线粒体功能障碍的重要原因。目前有文献[51]报道了PGC-1α在HD线粒体损伤中的作用及其有望成为治疗HD的潜在靶点。PGC-1α通过激活转录因子EB (transcription factor EB, TFEB)促进亨廷顿蛋白(Hungtingtin, Htt)聚集体的去除。另有文献[52]报道天麻(Gastrodia elata)可能通过Adenosine A2A-R receptor (A2A-R)/PKA/CREB/PGC-1α依赖性途径介导线粒体功能和生物发生来减少变异Htt蛋白(mHtt)的聚集。
2 癌症癌症是以细胞异常增殖为特点的一类疾病, 具有侵入或扩散到身体其他部位的潜力, 临床表现和治疗复杂, 预后欠佳。癌症目前已成为发病率和死亡率方面仅次于心血管疾病的第二大疾病, 严重威胁人类的健康。
由于致癌基因激活、抑癌基因功能丧失和PI3K途径的上调, 肿瘤细胞表现出跟正常细胞不同的能量代谢方式[53], 在有氧条件下仍依靠低效生成ATP的糖酵解方式供能并产生大量乳酸, 而不利用高效的氧化磷酸化方法, 这是肿瘤能量代谢的先锋理论——Warburg效应[54]。肿瘤高葡萄糖消耗率的现象已经通过氟代脱氧葡萄糖正电子发射断层扫描成像技术在多种人类癌症中得到验证。通过抑制丙酮酸激酶的活性来限制糖酵解ATP的产生不能阻止肿瘤发生与发展, 这表明糖酵解的主要作用不是为肿瘤细胞提供ATP[55]。肿瘤细胞独特的能量代谢能为其提供大量代谢中间产物, 以利于脂肪酸、膜磷脂、核酸、蛋白质的合成。如肿瘤细胞通过谷氨酰胺还原代谢增加乙酰辅酶A生成, 通过上调脂肪酸合成酶(fatty acid synthas, FASN)表达, 有利于脂肪酸的大量合成, 脂肪酸一方面合成磷脂利于细胞膜构筑, 另一方面合成甘油三酯利于能量的储存和信号的传导, 这都与肿瘤形成及进展密切相关[56]。肿瘤糖酵解产生的大量乳酸可以主动刺激缺氧诱导因子-1 (hypoxia inducible factor-1, HIF-1)表达从而加速血管生成, 协助微环境缺氧性血管新生[57]。
研究认为, 缺氧是引起肿瘤细胞糖酵解能量代谢表型的主要原因。首先, 缺氧刺激HIF-1的表达, HIF-1能上调葡萄糖转运体-1/3 (glucose transporter 1/3, GLUT1/3)的表达以保证大量葡萄糖的摄取[58]; 同时HIF-1上调糖酵解过程中绝大多数酶的表达, 如己糖激酶-1/2 (hexokinase 1/2, HK1/2)、磷酸甘油酸激酶-1 (phosphoglycerate kinase 1, PGK1)、乳酸脱氢酶-A (lactate dehydrogenase A, LDHA)等[59]; 其次, 缺氧和HIF-1抑制线粒体的生物合成, 并引起线粒体自噬, 从而减少线粒体数目, 它们还能通过上调丙酮酸脱氢酶激酶-1 (pyruvate dehydrogenase kinase isozyme 1, PDK1)的表达减少乙酰辅酶A生成, 或抑制线粒体电子传递链成分铁硫簇组装蛋白(iron-sulfur cluster assembly protein, ISCU)及细胞色素C氧化酶组装蛋白-10 (cytochrome C oxidase assembly protein-10, COX-10)的表达, 进而抑制线粒体氧化磷酸化功能[60], 因此, 肿瘤细胞需要通过增强另一种产能方式——糖酵解来补充能量。
溶质载体家族2促进的葡萄糖转运蛋白成员1 (SLC2A1)是细胞能量代谢通路中的重要蛋白, SLC2A1的主要功能是当细胞内葡萄糖浓度低时, 通过促进葡萄糖分子在质膜上的扩散为细胞提供葡萄糖[61]。葡萄糖摄取是有氧糖酵解中的第一个限速步骤, 由于较高的葡萄糖需求, SLC2A1在各种癌症类型中过表达, 包括结直肠癌[62]、肺癌[63]、乳腺癌[64]和子宫内膜癌等。转运至细胞后, 葡萄糖分子经过糖酵解产生ATP和丙酮酸。在癌细胞中, 丙酮酸迅速转化为乳酸, 防止其进入线粒体进行TCA循环, 从而使细胞持续依赖有氧糖酵解产生能量。乳酸脱氢酶A (lactate dehydrogenase A, LDHA)催化丙酮酸转化为乳酸[65], LDHA已被证明在许多类型的癌症中过度表达, 包括胃癌[66]、肺癌、乳腺癌[65]和胰腺癌[67]等。
可以通过靶向抑制SLC2A1和LDHA的活性抑制肿瘤细胞的增殖和迁移。研究证明miR-383在人卵巢癌组织和细胞系中显著下调。miR-383可以直接靶向LDHA降低糖酵解来抑制细胞增殖和侵袭, 并且miR-383表达与卵巢癌中的LDHA表达负相关, 表明miR-383/LDHA轴通过作为葡萄糖代谢的重要调节因子在卵巢癌进展中起着至关重要的作用[68]。miR-148b可以直接靶向SLC2A1介导胃癌细胞糖酵解作用的减少[69]。WZB117可以抑制人红细胞和癌细胞系中的被动糖转运, 并且通过限制糖酵解抑制小鼠中的肿瘤生长[70]; 另有研究表明, WZB117通过特异性抑制GLUT1可部分恢复多柔比星在多柔比星耐药MCF-7细胞系中的抗肿瘤作用[71]; 在乳腺癌MDA-MB-231和MCF-7细胞中发现, GLUT1的抑制使放射抗性癌细胞对辐射敏感, 通过调节葡萄糖代谢为癌细胞对放射治疗的敏感性提供新的机制和策略[72]。
3 2型糖尿病2型糖尿病(T2D)是21世纪危害公共卫生最严重的疾病之一, 其发病机制至今尚不清楚, 目前已知涉及胰岛素抵抗、β细胞功能缺陷、胰岛α细胞功能异常和胰高血糖素样肽-1 (glucagon-like peptide-1, GLP-1)分泌缺陷等[73]。胰岛素抵抗使得胰岛素作用靶器官敏感性下降, 外周组织对葡萄糖利用障碍, 脂肪分解增多, 蛋白质代谢负平衡[74]。有研究报道了2型糖尿病患者能量代谢相关基因的改变, 糖酵解、三羧酸循环、电子传递的相关基因在糖尿病骨骼肌、内脏脂肪组织表达下调, 在糖尿病肝脏组织表达上调; 脂肪酸β-氧化相关基因在糖尿病肝脏和脂肪组织表达下调, 而在糖尿病肌肉组织中表达上调[75]。骨骼肌占胰岛素刺激的葡萄糖摄取的80%以上[76], 在胰岛素抵抗个体中, 胰岛素信号传导被破坏, 胰岛素受体底物1 (IRS1)的丝氨酸(Ser)磷酸化增加, AKT磷酸化降低, 转运至肌纤维膜的葡萄糖转运蛋白GLUT4减少, 从而削弱了骨骼肌对葡萄糖的摄取[77]。在肝脏中, 胰岛素抵抗导致不能抑制糖异生, 促进肝葡萄糖(HGP)生成的增加。胰岛素可以通过AKT和FOXO依赖性机制抑制两种关键的糖异生酶磷酸烯醇丙酮酸羧激酶(PEPCK)和葡萄糖-6磷酸酶(G6Pase)的表达[78]。
T2D在不同组织中已经显示出线粒体数量和(或)功能的改变, 尽管数据丰富, 但仍然不能确定线粒体功能障碍是否是胰岛素抵抗和T2D的风险因素、后果或关键因素[79]。体内核磁共振(NMR)光谱研究显示, T2D患者骨骼肌线粒体ATP产生减少, 磷酸肌酸恢复速度减慢[80]。通过NADH脱氢酶活性测定骨骼肌中线粒体的氧化磷酸化能力, 结果显示T2D患者减少40%[81]。类似地, 在肌肉活检样本[82]或分离线粒体[83]的研究中也证实了T2D肌肉中较低的氧化磷酸化能力。胰岛素抵抗、T2D和严重肥胖患者白色脂肪组织中的线粒体丰度和线粒体基因表达减少[84], 在这些患者中, 白色脂肪组织的功能研究表明脂肪细胞耗氧量和ATP产生减少[85]。这些数据表明线粒体功能障碍与T2D的密切关系, 这种关系对于T2D发病机制是必不可少的, 或者只是一种疾病表象, 仍然不确定。
很多能够清除脂质过氧化自由基和ROS的天然抗氧化化合物已被尝试用于增加线粒体生物发生和改善线粒体功能, 进而改善2型糖尿病症状。研究最多的化合物是白藜芦醇(3, 5, 4'-三羟基二苯乙烯), 一种在红葡萄酒中发现的化合物, 可以通过激活SIRT1和AMPK模拟热量限制的作用[86], 并通过上调PGC1α、NRF1和TFAM增加线粒体生物合成[87]。值得注意的是, 白藜芦醇也被提出具有发挥抗衰老并延长生存期的作用[86]。在啮齿动物中, 白藜芦醇可以预防饮食诱导的肥胖, 激活PGC1α并提升有氧能力[88]。近年来, 成纤维细胞生长因子(fibroblast growth factor, FGF)由于对葡萄糖和脂质代谢的有益作用, 在T2D等代谢性疾病新药发现中受到了重视。在FGF家族所有成员中, FGF1、FGF19、FGF21和FGF23已被证明具有改善葡萄糖、脂质代谢和维持能量稳态的能力[89], 提示FGF可作为代谢性疾病早期诊断的潜在生物标志物等相关用途[90]。基于FGF疗法的开发方面取得了显著进展[91], 还需要进一步的研究来确定它们在T2D的潜在治疗用途。
4 心血管疾病心血管疾病(cardiovascular disease, CVD)指的是关于心脏或血管的疾病, 是全球最常见的死因之一, 成为威胁人类健康的第一大杀手。成人中大约60%~80%的心肌能量来源于游离脂肪酸(FFA)的氧化, 其在维持心肌的正常结构和收缩功能方面起关键作用[92]。在心脏疾病中, 已经检测到能量供应不足和脂肪过度积累[93]。在心肌病中观察到心脏脂质储存动力学改变, 意味着储存的脂质对氧化能量代谢的贡献减少。实验研究表明, 在异丙肾上腺素诱导的心肌梗塞过程中脂质过氧化增加而氧化磷酸化减少[94]。
过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptors, PPAR)是核受体转录因子超家族的成员, 通常被认为是调节脂肪细胞和其他代谢活跃组织脂质和葡萄糖代谢的营养传感器, 在调节参与脂质代谢的基因中起重要作用[95]。与同龄的PPAR-α野生型相比, PPAR-α-/-小鼠的心脏棕榈酸氧化率明显降低[96]。O'Donnell等[97]证实, 在肥厚心脏中PPAR-α的表达下降与脂肪酸氧化减少有关, 并降低脂肪的转运速率。PPAR-β/δ主要在肌肉和脂肪组织中刺激脂肪酸氧化。研究表明, 与PPAR-α一样, PPAR-β/δ也在心脏脂质代谢和能量平衡中发挥关键作用[92]。
PPAR-β/δ选择性配体GW0742可通过激活PPAR-δ来减少自由基, 降低细胞钙离子水平, 从而在H9c2细胞中发挥缓解高血糖诱导的心肌肥厚的作用[98]。PPAR激动剂在糖尿病、胰岛素抵抗和全身炎症加剧等代谢条件下对不同心脏代谢参数均有改善作用, PPAR激动剂不仅可改善脂质谱, 还可改善与致动脉粥样硬化和其他心血管疾病标志物相关的脂蛋白组分[99]。
线粒体损伤会加剧心脏代谢性疾病的发展, 包括心力衰竭、动脉粥样硬化、糖尿病和高血压等[100]。Sirtuins (SIRT1~7)是具有烟酰胺腺嘌呤二核苷酸(NAD+)依赖性的去乙酰化酶、二磷酸腺苷-核糖转移酶家族, 其中SIRT3~5存在于线粒体中, 被称为线粒体Sirtuins[101]。线粒体Sirtuins在脂肪酸氧化中的作用被广泛研究。SIRT3通过使酶去乙酰化来促进脂肪酸氧化, 如长链酰基辅酶A脱氢酶(LCAD)[102]、中链特异性酰基辅酶A脱氢酶(ACADM)和酰基甘油激酶(AGK)[103]。SIRT5靶向烯酰辅酶A水合酶α-亚基(ECHA), 使ECHA脱嘌呤并增加其活性, 促进长链酰基辅酶A的氧化并增加ATP的产生, 提高能量的产生效率[104]。相比之下, SIRT4可作为脂肪酸氧化的抑制剂, 使ECHA脱乙酰化并抑制其活性[105]。SIRT4还使丙二酰辅酶A脱羧酶(MCD)脱乙酰化并抑制脂肪酸氧化, 同时促进脂质合成代谢[106]。间接地, SIRT4还控制PPAR-α以抑制与脂肪酸分解代谢相关的基因[107]。
和厚朴酚是一种天然的双酚类化合物, 新型的SIRT3激活剂。和厚朴酚可以阻断激动剂或压力超负荷介导的心脏肥大并改善已存在的心脏肥大[108]。此外, 葡萄提取物白藜芦醇可以激活SIRT3, 改善心脏纤维化并改善心脏功能[109], 减弱氧化应激诱导的内皮细胞凋亡[110]。SIRT4是心脏肥大和纤维化期间心脏功能的负调节因子[111], 因此特异性SIRT4抑制剂可能是治疗心脏肥大和衰竭的重要候选药物。最近的一项研究表明, ZINC12421989是SIRT4的潜在抑制剂[112], 需要进一步的功能研究来验证ZINC12421989对SIRT4蛋白和随后的生物学表型的抑制作用。miR-497通过特异性靶向SIRT4在体内外抑制心肌肥大而没有其他心脏功能变化[113]。SIRT5在调节心肌能量代谢和心脏功能等许多方面具有关键作用。SIRT5表达下调显著增加了缺血再灌注损伤期间心肌梗死的程度, 并且SIRT5表达上调可能具有逆转线粒体呼吸链紊乱和缺血-再灌注细胞损伤的治疗潜力[114]。因此, 存在于线粒体中的SIRT3、SIRT4和SIRT5均为治疗心血管疾病的潜在靶点。
5 展望能量代谢对疾病发生发展的影响不容小视, 在针对能量代谢障碍相关疾病的治疗中是否可以从能量代谢的调控机制入手, 通过调控细胞生物呼吸和线粒体功能, 进而调控整个细胞和机体的能量代谢, 作为防治能量代谢障碍相关疾病的主要手段值得进一步探索研究。随着蛋白组学、基因组学的深入研究, 细胞能量代谢的过程及其调控机制将会进一步阐明, 对能量代谢性疾病将提供有力的支持, 从整体上调控细胞能量代谢, 为此类疾病的诊疗将提供新的思路。
| [1] | Rossignol R. Energy metabolism disorders in rare and common diseases. Toward bioenergetic modulation therapy and the training of a new generation of European scientists[J]. Int J Biochem Cell Biol, 2015, 63: 2–9. DOI:10.1016/j.biocel.2015.01.003 |
| [2] | Davis RL, Liang C, Sue CM. Mitochondrial diseases[J]. Handb Clin Neurol, 2018, 147: 125–141. DOI:10.1016/B978-0-444-63233-3.00010-5 |
| [3] | Mochel F. Triheptanoin for the treatment of brain energy deficit: a 14-year experience: triheptanoin and treatment of brain energy deficit[J]. J Neurosci Res, 2017, 95: 2236–2243. DOI:10.1002/jnr.24111 |
| [4] | Mergenthaler P, Lindauer U, Dienel GA, et al. Sugar for the brain: the role of glucose in physiological and pathological brain function[J]. Trends Neurosci, 2013, 36: 587–597. DOI:10.1016/j.tins.2013.07.001 |
| [5] | Rossi S, Zanier ER, Mauri I, et al. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage[J]. J Neurol Neurosurg Psychiatry, 2001, 71: 448–454. DOI:10.1136/jnnp.71.4.448 |
| [6] | Cai H, Cong WN, Ji S, et al. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders[J]. Curr Alzheimer Res, 2012, 9: 5–17. DOI:10.2174/156720512799015064 |
| [7] | Fu W, Jhamandas JH. Role of astrocytic glycolytic metabolism in Alzheimer's disease pathogenesis[J]. Biogerontology, 2014, 15: 579–586. DOI:10.1007/s10522-014-9525-0 |
| [8] | Szablewski L. Glucose transporters in brain: in health and in Alzheimer's disease[J]. J Alzheimers Dis, 2016, 55: 1307–1320. DOI:10.3233/JAD-160841 |
| [9] | Winkler EA, Nishida Y, Sagare AP, et al. GLUT1 reductions exacerbate Alzheimer's disease vasculoneuronal dysfunction and degeneration[J]. Nat Neurosci, 2015, 18: 521–530. DOI:10.1038/nn.3966 |
| [10] | Kuzuya T. Outline of glucose metabolism and its regulations[J]. Nihon Rinsho, 1990, 48 Suppl: 51–59. |
| [11] | Velazquez R, Tran A, Ishimwe E, et al. Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease[J]. Neurobiol Aging, 2017, 58: 1–13. DOI:10.1016/j.neurobiolaging.2017.06.003 |
| [12] | Pohland M, Pellowska M, Asseburg H, et al. MH84 improves mitochondrial dysfunction in a mouse model of early Alzheimer's disease[J]. Alzheimers Res Ther, 2018, 10: 18–29. DOI:10.1186/s13195-018-0342-6 |
| [13] | Delbarba A, Abate G, Prandelli C, et al. Mitochondrial alterations in peripheral mononuclear blood cells from Alzheimer's disease and mild cognitive impairment patients[J]. Oxid Med Cell Longev, 2016, 2016: 5923938. |
| [14] | Pérez MJ, Ponce DP, Osorio-Fuentealba C, et al. Mitochondrial bioenergetics is altered in fibroblasts from patients with sporadic Alzheimer's disease[J]. Front Neurosci, 2017, 11: 553–565. DOI:10.3389/fnins.2017.00553 |
| [15] | Hroudová J, Singh N, Fišar Z, et al. Progress in drug development for Alzheimer's disease: an overview in relation to mitochondrial energy metabolism[J]. Eur J Med Chem, 2016, 121: 774–784. DOI:10.1016/j.ejmech.2016.03.084 |
| [16] | Zakaria A, Hamdi N, Abdelkader RM. Methylene blue improves brain mitochondrial ABAD functions and decreases Aβ in a neuroinflammatory Alzheimer's disease mouse model[J]. Mol Neurobiol, 2015, 53: 1220–1228. |
| [17] | Valaasani KR, Sun Q, Hu G, et al. Identification of human ABAD inhibitors for rescuing Aβ-mediated mitochondrial dysfunction[J]. Curr Alzheimer Res, 2014, 11: 128–136. DOI:10.2174/1567205011666140130150108 |
| [18] | Valasani KR, Hu G, Chaney MO, et al. Structure-based design and synthesis of benzothiazole phosphonate analogues with inhibitors of human ABAD-Aβ for treatment of Alzheimer's disease[J]. Chem Biol Drug Des, 2012, 81: 238–249. |
| [19] | Hroch L, Guest P, Benek O, et al. Synthesis and evaluation of frentizole-based indolyl thiourea analogues as MAO/ABAD inhibitors for Alzheimer's disease treatment[J]. Bioorg Med Chem, 2016, 25: 1143–1152. |
| [20] | Alam MM, Lee J, Lee SY. Recent progress in the development of TSPO PET ligands for neuroinflammation imaging in neurological diseases[J]. Nucl Med Mol Imaging, 2017, 51: 283–296. DOI:10.1007/s13139-017-0475-8 |
| [21] | Du H, Guo L, Fang F, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease[J]. Nat Med, 2008, 14: 1097–1105. DOI:10.1038/nm.1868 |
| [22] | Fakharnia F, Khodagholi F, Dargahi L, et al. Prevention of cyclophilin D-mediated mPTP opening using cyclosporine-A alleviates the elevation of necroptosis, autophagy and apoptosis-related markers following global cerebral ischemia-reperfusion[J]. J Mol Neurosci, 2016, 61: 52–60. |
| [23] | Elkamhawy A, Lee J, Park BG, et al. Novel quinazoline-urea analogues as modulators for Aβ-induced mitochondrial dysfunction: design, synthesis, and molecular docking study[J]. Eur J Med Chem, 2014, 84: 466–475. DOI:10.1016/j.ejmech.2014.07.027 |
| [24] | Kim TH, Yang HY, Park BG, et al. Discovery of benzimidazole derivatives as modulators of mitochondrial function: a potential treatment for Alzheimer's disease[J]. Eur J Med Chem, 2017, 125: 1172–1192. DOI:10.1016/j.ejmech.2016.11.017 |
| [25] | Hattingen E, Magerkurth J, Pilatus U, et al. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson's disease[J]. Brain, 2009, 132: 3285–3297. DOI:10.1093/brain/awp293 |
| [26] | Stamelou M, Pilatus U, Reuss A, et al. In vivo evidence for cerebral depletion in high-energy phosphates in progressive supranuclear palsy[J]. J Cereb Blood Flow Metab, 2009, 29: 861–870. DOI:10.1038/jcbfm.2009.2 |
| [27] | Vivesbauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy[J]. Autophagy, 2010, 107: 378–383. |
| [28] | Poole AC, Thomas RE, Andrews LA, et al. The PINK1/Parkin pathway regulates mitochondrial morphology[J]. Proc Natl Acad Sci U S A, 2008, 105: 1638–1643. DOI:10.1073/pnas.0709336105 |
| [29] | Wang X, Winter D, Ashrafi G, et al. PINK1 and parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility[J]. Cell, 2011, 147: 893–906. DOI:10.1016/j.cell.2011.10.018 |
| [30] | Shin JH, Ko HS, Kang H, et al. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson's disease[J]. Cell, 2016, 144: 689–702. |
| [31] | Parker WD Jr, Parks JK, Swerdlow RH. Complex I deficiency in Parkinson's disease frontal cortex[J]. Brain Res, 2008, 1189: 215–218. DOI:10.1016/j.brainres.2007.10.061 |
| [32] | Surmeier DJ, Halliday GM, Simuni T. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson's disease[J]. Exp Neurol, 2017, 298: 202–209. DOI:10.1016/j.expneurol.2017.08.001 |
| [33] | Shinde S, Pasupathy K. Respiratory-chain enzyme activities in isolated mitochondria of lymphocytes from patients with Parkinson's disease: preliminary study[J]. Neurol India, 2006, 54: 390–393. DOI:10.4103/0028-3886.28112 |
| [34] | Takamatsu Y, Ho G, Koike W, et al. Combined immunotherapy with "anti-insulin resistance" therapy as a novel therapeutic strategy against neurodegenerative diseases[J]. NPJ Parkinsons Dis, 2017, 3: 4. DOI:10.1038/s41531-016-0001-1 |
| [35] | Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis[J]. Trends Endocrinol Metab, 2011, 22: 275–285. |
| [36] | Yuan Z, Li D, Feng P, et al. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson's disease[J]. Eur J Pharmacol, 2017, 812: 82–90. DOI:10.1016/j.ejphar.2017.06.029 |
| [37] | Liu W, Jalewa J, Sharma M, et al. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson's disease[J]. Neuroscience, 2015, 303: 42–50. DOI:10.1016/j.neuroscience.2015.06.054 |
| [38] | Quansah E, Peelaerts W, Langston JW, et al. Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration[J]. Mol Neurodegener, 2018, 13: 28–39. DOI:10.1186/s13024-018-0260-x |
| [39] | Roussakis AA, Piccini P. PET imaging in Huntington's disease[J]. J Huntingtons Dis, 2015, 4: 287–296. DOI:10.3233/JHD-150171 |
| [40] | Jenkins BG, Rosas HD, Chen YCI, et al. 1H NMR spectroscopy studies of Huntington's disease: correlations with CAG repeat numbers[J]. Neurology, 1998, 50: 1357–1365. DOI:10.1212/WNL.50.5.1357 |
| [41] | Koroshetz WJ, Jenkins BG, Rosen BR, et al. Energy metabolism defects in Huntington's disease and effects of coenzyme Q10[J]. Ann Neurol, 2010, 41: 160–165. |
| [42] | Lim D, Fedrizzi L, Tartari M, et al. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease[J]. J Biol Chem, 2008, 283: 5780–5789. DOI:10.1074/jbc.M704704200 |
| [43] | Li XJ, Orr AL, Li S. Impaired mitochondrial trafficking in Huntington's disease[J]. Biochim Biophys Acta, 2010, 1802: 62–65. DOI:10.1016/j.bbadis.2009.06.008 |
| [44] | Agarwal S, Yadav A, Chaturvedi RK. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders[J]. Biochem Biophys Res Commun, 2016, 483: 1166–1177. |
| [45] | Gizatullina ZZ, Lindenberg KS, Harjes P, et al. Low stability of Huntington muscle mitochondria against Ca2+, in R6/2 mice[J]. Ann Neurol, 2010, 59: 407–411. |
| [46] | Saft C, Zange J, Andrich J, et al. Mitochondrial impairment in patients and asymptomatic mutation carriers of Huntington's disease[J]. Mov Disord, 2004, 20: 674–679. |
| [47] | Cui L, Jeong H, Borovecki F, et al. Transcriptional repression of PGC-1α by mutant Huntingtin leads to mitochondrial dysfunction and neurodegeneration[J]. Cell, 2006, 127: 59–69. DOI:10.1016/j.cell.2006.09.015 |
| [48] | Chiang MC, Chen CM, Lee MR, et al. Modulation of energy deficiency in Huntington's disease via activation of the peroxisome proliferator-activated receptor gamma[J]. Hum Mol Genet, 2010, 19: 4043–4058. DOI:10.1093/hmg/ddq322 |
| [49] | Chaturvedi RK, Adhihetty P, Shukla S, et al. Impaired PGC-1α function in muscle in Huntington's disease[J]. Hum Mol Genet, 2009, 18: 3048–3065. DOI:10.1093/hmg/ddp243 |
| [50] | Phan J, Hickey MA, Zhang P, et al. Adipose tissue dysfunction tracks disease progression in two Huntington's disease mouse models[J]. Hum Mol Genet, 2009, 18: 1006–1016. DOI:10.1093/hmg/ddn428 |
| [51] | Nierenberg AA, Ghaznavi SA, Mathias IS, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha as a novel target for bipolar disorder and other neuropsychiatric disorders[J]. Biol Psychiatry, 2018, 83: 761–769. DOI:10.1016/j.biopsych.2017.12.014 |
| [52] | Huang CL, Wang KC, Yang YC, et al. Gastrodia elata, alleviates mutant Huntingtin aggregation through mitochondrial function and biogenesis mediation[J]. Phytomedicine, 2018, 39: 75–84. DOI:10.1016/j.phymed.2017.12.017 |
| [53] | Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism[J]. Cell Metab, 2016, 23: 27–47. DOI:10.1016/j.cmet.2015.12.006 |
| [54] | Warburg O. On the origin of cancer cells[J]. Science, 1956, 123: 309–314. DOI:10.1126/science.123.3191.309 |
| [55] | Israelsen WJ, Dayton TL, Davidson SM, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells[J]. Cell, 2013, 155: 397–409. DOI:10.1016/j.cell.2013.09.025 |
| [56] | Jung SY, Jeon HK, Choi JS, et al. Reduced expression of FASN through SREBP-1 down-regulation is responsible for hypoxic cell death in HepG2 cells[J]. J Cell Biochem, 2012, 113: 3730–3739. DOI:10.1002/jcb.24247 |
| [57] | Fukumura D, Xu L, Chen Y, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo[J]. Cancer Res, 2001, 61: 6020–6024. |
| [58] | Lu Y, Wang B, Shi Q, et al. Brusatol inhibits HIF-1 signaling pathway and suppresses glucose uptake under hypoxic conditions in HCT116 cells[J]. Sci Rep, 2016, 6: 39123. DOI:10.1038/srep39123 |
| [59] | Masoud GN, Wei L. HIF-1α pathway: role, regulation and intervention for cancer therapy[J]. Acta Pharm Sin B, 2015, 5: 378–389. DOI:10.1016/j.apsb.2015.05.007 |
| [60] | Zhang H, Gao P, Fukuda R, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity[J]. Cancer Cell, 2007, 11: 407–420. DOI:10.1016/j.ccr.2007.04.001 |
| [61] | Hodeib AA, Neinaa Y, Zakaria SS, et al. Glucose transporter-1 (GLUT-1) expression in psoriasis: correlation with disease severity[J]. Int J Dermatol, 2018, 57: 943–951. DOI:10.1111/ijd.14037 |
| [62] | Wang T, Ning K, Lu TX, et al. Elevated expression of TrpC5 and GLUT1 is associated with chemoresistance in colorectal cancer[J]. Oncol Rep, 2016, 37: 1059–1065. |
| [63] | Koh YW, Lee SJ, Park SY. Differential expression and prognostic significance of GLUT1 according to histologic type of non-small-cell lung cancer and its association with volume-dependent parameters[J]. Lung Cancer, 2017, 104: 31–37. DOI:10.1016/j.lungcan.2016.12.003 |
| [64] | Oh S, Kim H, Nam K, et al. Glut1 promotes cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells[J]. BMB Rep, 2017, 50: 132–137. DOI:10.5483/BMBRep.2017.50.3.189 |
| [65] | Huang X, Li X, Xie X, et al. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer[J]. Breast, 2016, 30: 39–46. DOI:10.1016/j.breast.2016.08.014 |
| [66] | Weihua J, Fei Z, Ning L, et al. FOXM1-LDHA signaling promoted gastric cancer glycolytic phenotype and progression[J]. Int J Clin Exp Pathol, 2015, 8: 6756–6763. |
| [67] | Cui XG, Han ZT, He SH, et al. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells[J]. Oncotarget, 2017, 8: 24840–24852. |
| [68] | Han RL, Wang FP, Zhang PA, et al. miR-383 inhibits ovarian cancer cell proliferation, invasion and aerobic glycolysis by targeting LDHA[J]. Neoplasma, 2017, 64: 244–252. DOI:10.4149/neo_2017_211 |
| [69] | Ding X, Liu J, Liu T, et al. miR-148b inhibits glycolysis in gastric cancer through targeting SLC2A1[J]. Cancer Med, 2017, 6: 1301–1310. DOI:10.1002/cam4.1008 |
| [70] | Ojelabi OA, Lloyd KP, Simon AH, et al. WZB117 (2-fluoro-6-(m-hydroxybenzoyloxy) phenyl m-hydroxybenzoate) inhibits GLUT1-mediated sugar transport by binding reversibly at the exofacial sugar binding site[J]. J Biol Chem, 2011, 291: 26762–26772. |
| [71] | Chen Q, Meng YQ, Xu XF, et al. Blockade of GLUT1 by WZB117 resensitizes breast cancer cells to adriamycin[J]. Anticancer Drugs, 2017, 28: 880–887. DOI:10.1097/CAD.0000000000000529 |
| [72] | Zhao F, Ming J, Zhou Y, et al. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation[J]. Cancer Chemother Pharmacol, 2016, 77: 963–972. DOI:10.1007/s00280-016-3007-9 |
| [73] | Zhou K, Pedersen HK, Dawed AY, et al. Pharmacogenomics in diabetes mellitus: insights into drug action and drug discovery[J]. Nat Rev Endocrinol, 2016, 12: 337–346. DOI:10.1038/nrendo.2016.51 |
| [74] | Koliaki C, Roden M. Alterations of mitochondrial function and insulin sensitivity in human obesity and diabetes mellitus[J]. Annu Rev Nutr, 2016, 36: 337–367. DOI:10.1146/annurev-nutr-071715-050656 |
| [75] | Wang M, Wang XC, Zhao L, et al. Oligonucleotide microarray analysis reveals dysregulation of energy-related metabolism in insulin-sensitive tissues of type 2 diabetes patients[J]. Genet Mol Res, 2014, 13: 4494–4504. DOI:10.4238/2014.June.17.1 |
| [76] | Gonzalez-Franquesa A, Patti ME. Insulin resistance and mitochondrial dysfunction[J]. Adv Exp Med Biol, 2017, 982: 465–520. |
| [77] | Victor EJ, Javier EF, Fabres MU. MicroRNAs-mediated regulation of skeletal muscle GLUT4 expression and translocation in insulin resistance[J]. J Diabetes Res, 2017, 2017: 7267910. |
| [78] | Hall RK, Yamasaki T, Kucera T, et al. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin: the role of winged helix/forkhead proteins[J]. J Biol Chem, 2000, 275: 30169–30175. DOI:10.1074/jbc.M004898200 |
| [79] | Rovirallopis S, Ba uls C, Diazmorales N, et al. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications[J]. Redox Biol, 2017, 11: 637–645. DOI:10.1016/j.redox.2017.01.013 |
| [80] | Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects[J]. Diabetologia, 2007, 50: 113–120. DOI:10.1007/s00125-006-0475-1 |
| [81] | Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes[J]. Diabetes, 2002, 51: 2944–2950. DOI:10.2337/diabetes.51.10.2944 |
| [82] | Ritov VB, Menshikova EV, Azuma K, et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity[J]. Am J Physiol Endocrinol Metab, 2010, 298: E49–E58. DOI:10.1152/ajpendo.00317.2009 |
| [83] | Mogensen M, Sahlin K, Fernstr m M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes[J]. Diabetes, 2007, 56: 1592–1599. DOI:10.2337/db06-0981 |
| [84] | Krishnan J, Danzer C, Simka T, et al. Dietary obesity-associated HIF1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the SIRT2-NAD+ system[J]. Genes Dev, 2012, 26: 259–270. DOI:10.1101/gad.180406.111 |
| [85] | Bogacka I, Ukropcova B, Mcneil M, et al. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro[J]. J Clin Endocrinol Metab, 2005, 90: 6650–6656. DOI:10.1210/jc.2005-1024 |
| [86] | Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet[J]. Nature, 2016, 444: 337–342. |
| [87] | Biala A, Tauriainen E, Siltanen A, et al. Resveratrol induces mitochondrial biogenesis and ameliorates Ang Ⅱ-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes[J]. Blood Press, 2010, 19: 196–205. DOI:10.3109/08037051.2010.481808 |
| [88] | Lagouge M, Argmann C, Gerharthines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha[J]. Cell, 2006, 127: 1109–1122. DOI:10.1016/j.cell.2006.11.013 |
| [89] | Izaguirre M, Gil MJ, Monreal I, et al. The role and potential therapeutic implications of the fibroblast growth factors in energy balance and type 2 diabetes[J]. Curr Diab Rep, 2017, 17: 43–56. DOI:10.1007/s11892-017-0866-3 |
| [90] | Hanks LJ, Casazza K, Judd SE, et al. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults[J]. PLoS One, 2015, 10: e0122885. DOI:10.1371/journal.pone.0122885 |
| [91] | Gaich G, Chien JY, Fu H, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes[J]. Cell Metab, 2013, 18: 333–340. DOI:10.1016/j.cmet.2013.08.005 |
| [92] | Ajith TA, Jayakumar TG. Peroxisome proliferator-activated receptors in cardiac energy metabolism and cardiovascular disease[J]. Clin Exp Pharmacol Physiol, 2016, 43: 649–658. DOI:10.1111/1440-1681.12579 |
| [93] | Rydén M, Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism[J]. J Intern Med, 2017, 282: 220–228. DOI:10.1111/joim.12641 |
| [94] | Sudheesh NP, Ajith TA, Janardhanan KK. Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes[J]. Int J Cardiol, 2013, 165: 117–125. DOI:10.1016/j.ijcard.2011.07.103 |
| [95] | Nikolic D, Castellino G, Banach M, et al. PPAR agonists, atherogenic dyslipidemia and cardiovascular risk[J]. Curr Pharm Des, 2016, 23: 894–902. |
| [96] | Campbell FM, Kozak R, Wagner A, et al. A role for peroxisome proliferator-activated receptor alpha (PPARalpha) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase[J]. J Biol Chem, 2002, 277: 4098–4103. DOI:10.1074/jbc.M106054200 |
| [97] | O'Donnell JM, Fields AD, Sorokina N, et al. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover[J]. J Mol Cell Cardiol, 2008, 44: 315–322. DOI:10.1016/j.yjmcc.2007.11.006 |
| [98] | Cheng KC, Chang WT, Li YX, et al. GW0742 activates peroxisome proliferator-activated receptor δ to reduce free radicals and alleviate cardiac hypertrophy induced by hyperglycemia in cultured H9c2 cells[J]. J Cell Biochem, 2018, 119: 9532–9542. DOI:10.1002/jcb.27270 |
| [99] | Han L, Shen WJ, Bittner S, et al. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part Ⅱ: PPAR-β/δ and PPAR-γ[J]. Future Cardiol, 2017, 13: 279–296. DOI:10.2217/fca-2017-0019 |
| [100] | Tang X, Chen XF, Chen HZ, et al. Mitochondrial sirtuins in cardiometabolic diseases[J]. Clin Sci, 2017, 131: 2063–2078. DOI:10.1042/CS20160685 |
| [101] | Singh CK, Chhabra G, Ndiaye M, et al. The role of sirtuins in antioxidant and redox signaling[J]. Antioxid Redox Signal, 2017, 28: 643–661. |
| [102] | Bedi KC, Snyder NW, Brandimarto J, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure[J]. Circulation, 2011, 133: 706–716. |
| [103] | Yang W, Nagasawa K, Münch C, et al. Mitochondrial sirtuin network reveals dynamic SIRT3-dependent deacetylation in response to membrane depolarization[J]. Cell, 2016, 167: 985–1000. DOI:10.1016/j.cell.2016.10.016 |
| [104] | Sadhukhan S, Liu X, Ryu D, et al. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function[J]. Proc Natl Acad Sci U S A, 2016, 113: 4320–4325. DOI:10.1073/pnas.1519858113 |
| [105] | Guo L, Zhou SR, Wei XB, et al. Acetylation of mitochondrial trifunctional protein α-subunit enhances its stability to promote fatty acid oxidation and is decreased in NAFLD[J]. Mol Cell Biol, 2016, 36: 2553–2567. DOI:10.1128/MCB.00227-16 |
| [106] | Laurent G, German NJ, Saha AK, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase[J]. Mol Cell, 2013, 50: 686–698. DOI:10.1016/j.molcel.2013.05.012 |
| [107] | Laurent G, De Boer VCJ, Finley LWS, et al. SIRT4 represses peroxisome proliferator-activated receptor α activity to suppress hepatic fat oxidation[J]. Mol Cell Biol, 2013, 33: 4552–4561. DOI:10.1128/MCB.00087-13 |
| [108] | Pillai VB, Samant S, Sundaresan NR, et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial SIRT3[J]. Nat Commun, 2015, 6: 6656. DOI:10.1038/ncomms7656 |
| [109] | Chen T, Li J, Liu J, et al. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway[J]. Am J Physiol Heart Circ Physiol, 2015, 308: H424–H434. DOI:10.1152/ajpheart.00454.2014 |
| [110] | Shimada T, Furuta H, Doi A, et al. Des-acyl ghrelin protects microvascular endothelial cells from oxidative stress-induced apoptosis through sirtuin 1 signaling pathway[J]. Metabolism, 2014, 63: 469–474. DOI:10.1016/j.metabol.2013.12.011 |
| [111] | Luo YX, Tang X, An XZ. SIRT4 accelerates Ang Ⅱ-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity[J]. Eur Heart J, 2016, 38: 1389–1398. |
| [112] | Choubey SK, Prabhu D, Nachiappan M, et al. Molecular modeling, dynamics studies and density functional theory approaches to identify potential inhibitors of SIRT4 protein from Homo sapiens: a novel target for the treatment of type 2 diabetes[J]. J Biomol Struct Dyn, 2017, 35: 3316–3329. DOI:10.1080/07391102.2016.1254117 |
| [113] | Xiao Y, Zhang X, Fan S, et al. MicroRNA-497 inhibits cardiac hypertrophy by targeting Sirt4[J]. PLoS One, 2016, 11: e0168078. DOI:10.1371/journal.pone.0168078 |
| [114] | Zou R, Shi W, Tao J, et al. SIRT5 and post-translational protein modifications: a potential therapeutic target for myocardial ischemia-reperfusion injury with regard to mitochondrial dynamics and oxidative metabolism[J]. Eur J Pharmacol, 2017, 818: 410–418. |
 2019, Vol. 54
2019, Vol. 54


