瓜蒌为葫芦科植物栝楼(Trichosanthes kirilowii Maxim.)或双边栝楼(Trichosanthes rosthornii Harms.)的干燥成熟果实, 具宽胸散结之功效, 用于胸痹心痛等[1]。
心肌缺血再灌注损伤(myocardial ischemia-reperfusion injury, MIRI)是指心脏的血液供应由缺少至恢复所致的心肌损伤加重。课题组通过瓜蒌及瓜蒌滴丸预处理抗大鼠MIRI的药效学研究发现, 与模型组比较, 瓜蒌及瓜蒌滴丸均可有效抑制MIRI大鼠心电图ST段的抬高(P < 0.01), 降低MIRI大鼠血浆肌酸激酶同工酶MB (creatine kinase-MB, CK-MB)、肌红蛋白(myoglobin, MYO)、心肌肌钙蛋白-T (cardiac troponin-T, cTnT)含量(P < 0.01), 有显著的抗大鼠MIRI作用[2]。
新药研发中常遵循的“一个药物、一个基因、一种疾病”模式可能是导致许多新药临床试验失败的主要原因之一。临床上的多种疾病, 如MIRI是多基因、多因素作用的疾病[3], 仅针对单一作用靶点治疗难以达到预期效果。网络药理学强调信号通路的多靶点多途径调节, 在新药研发特别是在中药的创新药物研发中可发挥重要作用。为综合分析瓜蒌抗MIRI的作用机制, 本课题采用网络药理学方法[4, 5], 预测瓜蒌可能的靶点蛋白及信号通路, 并结合Western blot法测定MIRI大鼠ERK1/2、JNK1、p38MAPK蛋白及其磷酸化的表达加以验证, 以期为瓜蒌抗MIRI作用机制的深入研究提供参考。
材料与方法实验动物 SPF级Sprague Dawley (SD)大鼠, 雄性, 体重180~220 g, 由南京青龙山动物养殖场提供, 合格证号: SCXK (苏) 2017-0001, 本实验所用动物经皖南医学院医学伦理委员会批准同意。
药品与试剂 瓜蒌滴丸(批号: 20170801, 中国发明专利申请号: 201710792082.1。平均丸重0.03 g, 每丸含瓜蒌提取物0.01 g, 相当于瓜蒌饮片0.2 g); 放射免疫沉淀法(radio-immunoprecipitation assay, RIPA)细胞裂解液(directory number: 13A10A05)、十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate-polyacrylamide gel electrophoresis, SDS-PAGE)蛋白上样缓冲液(变性) (1×) (directory number: 13D23C38)、三羟甲基氨基甲烷-缓冲生理盐水(tris-buffered saline, TBS)漂洗缓冲液(干粉, NO.13B11B44)、封闭蛋白TBS缓冲系统封闭液(干粉, NO.13E07A43)、甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH, NO.2P9378BP78)、ERK1/2 (NO.BST17874326)、JNK1 (NO.12CM609)、p38MAPK (NO.12C63)、一抗二抗稀释液(Directory number: 13D19B17)均购自美国博士德生物工程有限公司; p-ERK1/2 (NO.AH07137723)、p-JNK1 (NO.AH08079092)、p-p38MAPK (NO.AH06044692)均购自北京博奥森生物技术有限公司, Tris (NO.117W0710)、SDS (NO. 7141031)、glycine (NO.418Q0614)购自北京索莱宝科技有限公司。
仪器 HX-300小动物呼吸机、BL-420生物机能采集系统(成都泰盟软件有限公司); MINI PROTEAN电泳系统、MINI PROTEAN转膜系统(美国Bio-Rad公司); Alpha荧光化学凝胶成像系统Fluor Chem FC3 (美国Protein Simple公司)。
化合物的筛选 依据口服生物利用度(oral bioavailability, OB) ≥30%、类药性(drug like, DL) ≥0.18, 通过TCM Database@Taiwan (http://tcm.cmu.edu.tw/)、TCMSP数据库(http://lsp.nwsuaf.edu.cn/tcmsp.php)筛选到11个化合物, 腺苷(adenosine)为文献[6]报道的瓜蒌中药效成分。采用ChemBioDrawUltta 14.0软件绘制12个化合物分子结构式, 以MDL SDfile (*.sdf)文件格式保存备用, 见图 1。
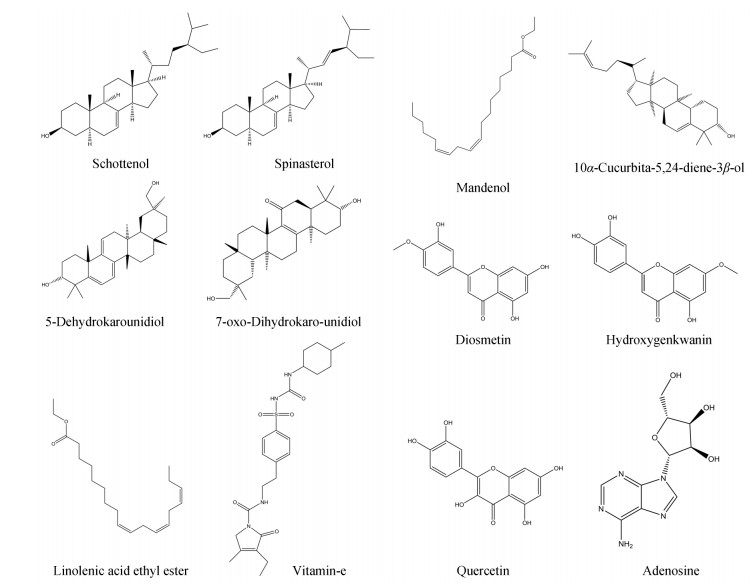
|
Figure 1 Information on main compounds in Trichosanthes |
成分-蛋白靶点-信号通路网络分析和构建 利用DRAR-CPI数据库(http://cpi.bio-x.cn/drar)获取图 1中12种成分的PDB ID值(Z'-score < -0.5), 并由UniProt数据库(http://www.uniprot.org/)转换成靶点蛋白。通过CooLGeN数据库(http://ci.smu.edu.cn/CooLGeN/Home.php), 以“myocardial ischemia reperfusion injury”为关键词, 收集人类基因靶点蛋白。利用DAVID数据库(https://david.ncifcrf.gov/tools.jsp)进行靶点蛋白的GOTERM_BP_DIRECT富集分析和Kyoto Encyclopedia of Genes and Genomes (KEGG)_PATHWAY通路注释分析(标示符为official_gene_symbol, 物种注释为Homo sapiens)。根据预测结果, 使用Gephi0.9.2软件构建成分-靶点蛋白-信号通路网络。
动物分组及给药 SPF级SD大鼠随机分为6组, 每组6只, 假手术组、模型组、瓜蒌滴丸组(0.2、1.0和2.0 g·kg-1)、复方丹参滴丸组85.05 mg·kg-1, 每日1次, 连续7天, 10 mL·kg-1灌胃。
大鼠MIRI模型的制备 大鼠末次给药后1 h, 经腹腔注射10%水合氯醛(3 mL·kg-1)麻醉, 气管插管, 监测心电图, 结扎左冠状动脉30 min后解结扎120 min, MIRI造模结束, 取心脏, -80 ℃冰箱保存, 备用。
心肌组织蛋白提取及Western blot法分析 按心脏重量(g):裂解液(mL) = 1:8加入RIPA裂解液, 加入蛋白酶抑制剂(磷酸化蛋白还需加入磷酸化酶抑制剂)匀浆, 冰上孵育2 h后, 12 000 r·min-1, 离心15 min, 取上清液, 煮沸15 min, 加入SDS-PAGE蛋白上样缓冲液, 制备上样样品。用PAGE分离蛋白样品, 根据说明书提供的分子量条带选定切胶范围, 湿法转膜, 5%脱脂奶粉TBST (Tris-buffered saline and tween 20)溶液封闭2 h, 一抗、二抗孵育, Alpha荧光化学凝胶成像系统观察ERK1/2、JNK1、p38MAPK及其磷酸化蛋白的表达情况。
统计学分析 应用SPSS13.0软件进行统计分析, 各统计指标以均数±标准差(x ± s)表示, 两组间比较用t检验。P < 0.05为差异有统计学意义, P < 0.01为差异有显著统计学意义。
结果 1 靶点的预测通过DRAR-CPI数据库得到瓜蒌中12个成分的262个靶点蛋白, 结合CooLGeN数据库搜索结果, 发现有39个靶点蛋白与MIRI相关。利用DAVID数据库对39个靶点蛋白进行KEGG_PATHWAY通路注释分析, 取P < 0.05, 结果见表 1。
| Table 1 Main potential target information related to myocardial ischemia reperfusion injury in Trichosanthes (P < 0.05) |
利用DAVID数据库进行GOTERM_BP_DIRECT富集分析, 结果见图 2。结果显示, 生物学过程与信号转导、蛋白激酶级联、细胞凋亡调控、刺激反应、磷酸化等相关性较大, 表明瓜蒌通过改善这些生物学过程可能是其发挥抗MIRI的机制之一。

|
Figure 2 Enriched gene ontology terms for biological processes from main active ingredients of Trichosanthes |
利用DAVID数据库进行KEGG_PATHWAY富集分析, P < 0.05的信号通路结果见表 2。选取通路P值较小且靶点蛋白富集的10条通路, 见图 3, 主要为癌症的途径、神经营养因子信号通路、黏着斑、MAPK信号通路、NOD样受体信号通路、结直肠癌、孕酮介导卵母细胞成熟、前列腺癌、Toll样受体信号通路、T细胞受体信号通路。
| Table 2 KEGG signaling pathway enrichment analysis of potential anti-myocardial ischemia reperfusion injury targets in Trichosanthes |
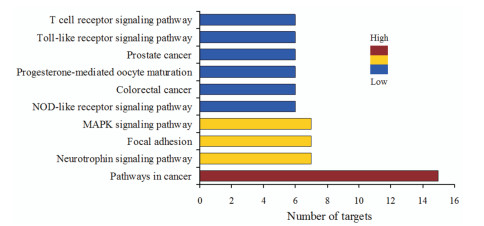
|
Figure 3 Enriched KEGG pathways of potential targets from main active ingredients of Trichosanthes |
使用Gephi 0.9.2软件构建活性成分-靶点蛋白-信号通路网络, 见图 4。由图 4可知, 瓜蒌中的12个活性成分和23个靶点蛋白、28条作用通路间存在复杂的网络关系, 瓜蒌中活性成分可通过多靶点蛋白、多信号通路发挥作用。其中, 槲皮素(quercetin)、维生素-E (vitamin-E)、7-氧代二氢栝楼仁二醇(7-oxo-dihydrokaro-unidiol)、5-脱氢栝楼仁二醇(5-dehydrokarounidiol)、葫芦二烯醇(10α-cucurbita-5, 24-diene-3β-ol)、α-菠甾醇(spinasterol)、24α/R-豆甾-7-烯醇(schottenol)、腺苷(adenosine)等8种成分通过调控MAPK信号通路的ERK2、JNK1、p38MAPK、AKT1、FGFR1、CASP3和RAC1等7个靶点蛋白发挥抗MIRI作用。
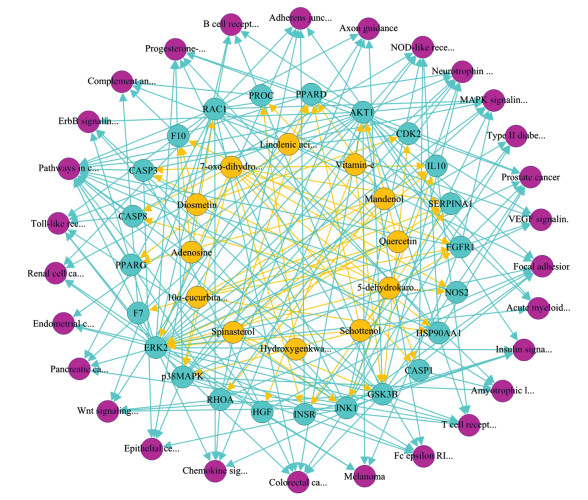
|
Figure 4 Compounds (   |
鉴于MAPK级联是细胞内重要的跨膜信号转导系统, 主要由ERK、JNK、p38MAPK组成, 并构成基本的信号转导途径。为此, 本课题以复方丹参滴丸(85.05 mg·kg-1)为阳性对照, 瓜蒌滴丸(0.2、1.0和2.0 g·kg-1)预处理MIRI大鼠, 采用Western blot法对MAPK信号通路中相关的ERK1/2、JNK1、p38MAPK蛋白及其磷酸化表达进行分析, 以验证瓜蒌是否可作用于ERK1/2、JNK1和p38MAPK蛋白并影响MAPK信号通路。
各组MIRI大鼠ERK1/2、JNK1、p38MAPK及其磷酸化的表达结果, 见图 5。由图 5可知, 与假手术组比较, 模型组ERK1/2、JNK1及p38MAPK的磷酸化水平呈剂量依赖性上调; 与模型组比较, 瓜蒌滴丸低、中、高剂量组的ERK1/2磷酸化水平呈剂量依赖性上调, 且中、高剂量组有显著性差异(P < 0.01);瓜蒌滴丸低、中、高剂量组的JNK1、p38MAPK磷酸化水平呈剂量依赖性下调, 且中、高剂量组有显著性差异(P < 0.01)。

|
Figure 5 The protein expressions of MAPK pathway were determined by Western blot analysis (A) and quantitative graphs (B: p-ERK1/ERK1; C: p-ERK2/ERK2; D: p-JNK1/JNK1; E: p-p38MAPK/p38MAPK). 1: Normal group; 2: Sham operation group; 3: Model group; 4: Compound Danshen dropping pills group; 5: Trichosanthes dropping pills group (0.2 g·kg-1); 6: Trichosanthes dropping pills group (1.0 g·kg-1); 7: Trichosanthes dropping pills group (2.0 g·kg-1). n = 6, x ± s. *P < 0.05, **P < 0.01 vs model group; ##P < 0.01 vs Sham operation group |
本文通过对瓜蒌中12个化合物及其涉及到的MIRI靶点蛋白和作用通路进行网络药理学分析, 发现24α/R-豆甾-7-烯醇等12个化合物通过多靶点、多生物途径及多通路方式协同发挥作用, 涉及癌症、MAPK等信号通路, 部分功能及信号通路已有文献报道, 如瓜蒌皮通过降低细胞内c-fos、c-myc mRNA原癌基因高表达, 抑制血小板源生长因子BB (platelet-derived growth factor-BB, PDGF-BB)所致的增殖[7], 改善血管钙化和血管L-精氨酸/NO途径紊乱, 抑制氧化应激和减少炎症因子释放, 降低caspase-3活性和下调NF-κB及血管内皮细胞黏附分子-1表达等途径, 可改善血管内皮功能障碍[8, 9]、抑制体外高糖诱导的人脐静脉内皮细胞凋亡[10]及动脉粥样硬化的形成[11]。此外, 瓜蒌皮中的低聚糖具有血管紧张素转化酶抑制作用[12]; 瓜蒌皮注射液可促进血管内皮生长因子的表达, 提高缺血缺氧内皮祖细胞的生存能力[13]。
MAPK通路包括MAPK激酶激酶(MAP kinase kinase kinase, MKKK)、MAPK激酶(MAP kinase kinase, MKK)和MAPK三级激酶模式。MAPK级联是细胞内重要的跨膜信号转导系统, 是多种信号通路的中心, 通过依次磷酸化激活3种激酶, 将上游信号传递至下游应答分子, 在基因表达调控和细胞质功能活动中发挥关键作用。
MAPK信号转导通路主要通过ERK1/2、JNK、p38MAPK等3条主要通路参与调节细胞的分化、增殖和凋亡等生命过程[14]。ERK1/2通路一般由细胞外促有丝分裂原刺激激活[14], 参与细胞的增殖分化的调控; JNK和p38MAPK通路一般由各种应激刺激如细胞因子激活[15], 参与应激反应, 介导炎症和凋亡等。在未受刺激的细胞内, MAPK处于静止状态。心肌缺血再灌注时, MAPK可通过级联反应激活下游的ERK1/2、JNK和p38MAPK通路, 使ERK1/2、JNK和p38MAPK磷酸化水平上调。磷酸化的ERK1/2能够抑制内质网应激, 使心肌细胞凋亡减少, 产生心肌保护作用[16]; 磷酸化的JNK、p38MAPK可加剧炎症反应, 诱导凋亡, 增大心肌梗死面积[17]。本研究结果显示, 瓜蒌滴丸可呈剂量依赖性上调ERK1/2磷酸化表达, 下调JNK1、p38MAPK磷酸化表达, 通过调控MAPK信号转导通路ERK1/2、JNK、p38MAPK靶点蛋白及其磷酸化发挥抗MIRI作用, 验证了网络药理学的预测结果, 为深入阐明瓜蒌抗MIRI作用机制提供了科学依据。
| [1] | China Pharmacopoeia Committee. Chinese Pharmacopoeia Vol Ⅰ (中国药典.一部) [S]. Beijing: China Medical Science Press, 2015: 112. |
| [2] | Zou CC, Zong QN, Yan HY. Study on the spectrum-activity relationship of Trichosanthis Fructus and Trichosanthes Strip Pieces on rat anti-myocardial ischemia-reperfusion injury[J]. China J Chin Mater Med (中国中药杂志), 2018, 43: 92–99. |
| [3] | Zou CC, Yan HY, Wei ML. Study of the anti-heart failure mechanisms of compatibility of Gualou with Xiebai in basis of network pharmacology[J]. Acta Pharm Sin (药学学报), 2018, 53: 1406–1413. |
| [4] | Wu D, Gao Y, Xiang H, et al. Exploration into mechanism of antidepressant of Bupleuri radix based on network pharmacology[J]. Acta Pharm Sin (药学学报), 2018, 53: 210–219. |
| [5] | Sun LM, Liu LF, Zhu HX, et al. Network pharmacology-based study on intervention mechanism of Huanglian Jiedu decoction in the treatment of Alzheimer's disease[J]. Acta Pharm Sin (药学学报), 2017, 52: 1268–1275. |
| [6] | Liu DL, Qu GX, Wang NL, et al. Antiplatelet aggregation constituents from Trichosanthes kirilowii[J]. Chin Tradit Herb Drugs (中草药), 2004, 35: 1334–1336. |
| [7] | Yan Z, Qiu M, Guo XH, et al. Effect of extractive of Pericarpium Trichosanthis on cell cycle of rat vascular smooth cell proliferation induced by PDGF-BB[J]. Chin J Arterioscler (中国动脉硬化杂志), 2012, 20: 899–902. |
| [8] | Liu Y. EPT Protects against Low-density Lipoprotein-induced Endothelial Dysfunction by DDAH/ADMA Pathway (瓜蒌皮提取物对低密度脂蛋白所致血管内皮功能障碍的保护作用与DDAH/ADMA途径的关系)[D]. Hengyang: University of South China, 2010. |
| [9] | Tan B, Liu Y, Gu B, et al. Protective effect of EPT against damages of the endothelium induced by low-density lipoprotein in rats[J]. Mod Med J Chin (中国现代医药杂志), 2010, 12: 9–11. |
| [10] | Liu SY, Gu B, Lu XH, et al. Effects of extractive Pericarpium Trichosanthes on apoptosis of HUVECs induced by high glucose[J]. Chin Pharmacol Bull (中国药理学通报), 2015, 31: 988–993. |
| [11] | Wang DM. Experimental Studies on Effects and Mechanisms of Extract of Trichosanthes Kirilowii Pericarp in Anti-atherosclerosis (瓜蒌皮提取液的抗动脉粥样硬化作用及机制的实验研究)[D]. Beijing: Peking University, 2008. |
| [12] | Wang HJ, Ke Y, Ye G. Bioactivity-guided isolation of anti-angiotensin converting enzyme constituents from Trichosanthis Pericarpium[J]. China J Chin Mater Med (中国中药杂志), 2017, 42: 3131–3135. |
| [13] | Zhao QT. Protective effect of Trichosanthes pericarpium injection on hypoxic-ischemic endothelial progenitor cells[J]. Chin Tradit Pat Med (中成药), 2015, 37: 247–251. |
| [14] | Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades[J]. Adv Cancer Res, 1998, 74: 49–139. DOI:10.1016/S0065-230X(08)60765-4 |
| [15] | Irving EA, Bamford M. Role of mitogen-and stress activated kinases in ischemic injury[J]. J Cereb Blood Flow Metab, 2002, 22: 631–647. DOI:10.1097/00004647-200206000-00001 |
| [16] | Tao JP, Zhu W, Li YP, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion[J]. Am J Physiol Heart Circ Physiol, 2011, 301: H1471–H1486. DOI:10.1152/ajpheart.00097.2011 |
| [17] | Bogoyevitch MA, Ngoei KR, Zhao TT, et al. C-Jun N-terminal kinase (JNK) signaling: recent advances and challenges[J]. Biochim Biophys Acta, 2010, 1804: 463–475. DOI:10.1016/j.bbapap.2009.11.002 |
 2019, Vol. 54
2019, Vol. 54


