抑郁症是一种慢性、易复发的精神疾病, 患者常表现为兴趣缺失、无助感、强烈的负罪感和学习记忆能力的下降, 更为严重的是, 抑郁患者有较高的自杀倾向。部分抑郁患者的症状并不能通过传统的抗抑郁药物治疗得到缓解, 并且抗抑郁药物药效的滞后和潜在的不良反应限制了抑郁症的治疗, 因此阐明抑郁症的发病机制、研究新型的抗抑郁药物成为当务之急[1, 2]。
可靠的动物模型能够为人类疾病的研究提供有力支撑。嗅球切除(olfactory bulbectomy, OBX)模型是较早建立并应用于抑郁症研究的动物模型, OBX后动物表现出空场活性增加、强迫游泳和悬尾不动时间增加和糖水偏爱下降等行为学特征。同时OBX也引起神经和内分泌系统的异常, 包括:皮层和海马结构的改变、神经可塑性的下降、单胺类递质水平的降低、神经炎症的产生和血清皮质酮增加。这些改变与临床上抑郁症的表现极为相似, 慢性给予抗抑郁药能够逆转OBX诱发的行为学和生化改变, 而急性给药无效[3], 这与临床上抗抑郁药需服用一定时间才能够发挥作用的特点相类似, 因此OBX模型被广泛应用于抑郁症发病机制的研究和抗抑郁药物的筛选。此外, OBX模型也表现出认知功能障碍, OBX动物脑中β-淀粉样蛋白(amyloid-β protein, Aβ)沉积[4], 这些特征与阿尔茨海默症(Alzheimer's disease, AD)的临床表现有一些共同之处, 因此近年来研究者将OBX模型应用于AD的研究。本文从嗅觉与抑郁和AD的关系、OBX模型的建立方法和行为学特点、OBX后皮层和海马的改变及该模型的应用等几个方面进行介绍, 以期更好地理解该模型的行为学和生化特点, 并为该模型在抑郁和AD中的应用提供参考。
1 嗅觉与抑郁和AD的关系嗅球及其中枢连接对情绪行为起重要作用, 嗅球向边缘系统结构(如杏仁核、海马、脑岛、前扣带皮层和眶额叶皮层)发送投射[5], 同时也收到来自腹侧脑区、中脑和脑干区域胆碱能、γ-氨基丁酸能、5-羟色胺(5-hydroxytryptamine, 5-HT)能和去甲肾上腺素(noradrenaline, NE)能的神经输入[6] (图 1)。一项针对抑郁人群和嗅觉功能紊乱人群的系统性研究表明, 抑郁患者嗅觉功能降低, 而嗅觉功能障碍的患者伴随有抑郁症状, 这些症状的严重程度和嗅觉缺失程度相关[7]。神经成像技术显示, 抑郁患者和幼年时期遭受虐待者嗅球体积减小[8, 9]。对24个重症抑郁患者的研究发现, 在急性抑郁期, 患者的嗅觉敏感性显著下降, 抗抑郁药物能够改善嗅觉敏感性[10]。心理疗法在缓解抑郁症状的同时也能改善嗅觉功能[11]。慢性应激诱导的抑郁模型大鼠嗅觉受损, 嗅球体积减少21.3%, 同时嗅球神经发生减少, 伴有突触前膜功能障碍[12]。在AD早期阶段患者嗅觉已经受损, 嗅觉功能的改变甚至早于其他临床症状的出现[13]。AD患者嗅球中僧帽细胞减少甚至完全缺失[14], 皮层和皮层下与嗅球相关的脑区形态发生改变[15]。AD患者也会有情感障碍的症状, 嗅觉缺失和改变的嗅觉信息过程可能是AD与抑郁症表现出部分共同症状的原因[16]。在抑郁患者、非OBX抑郁动物模型和AD患者中均发现了嗅觉功能的减退, 这表明嗅觉系统在抑郁症及AD的病理中起重要作用。
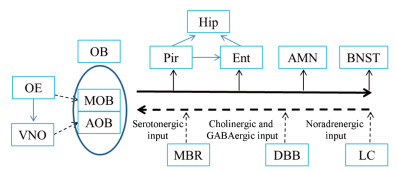
|
Figure 1 The major output (black solid lines) and input (black dotted lines) connections of olfactory system. AMN: Amygdala nucleus; AOB: Accessory olfactory bulb; BNST: Bed nucleus of the stria terminals; DBB: Diagonal band of Broca; Ent: Entorhinal cortex; Hip: Hippocampus; LC: Locus coeruleus; MBR: Midbrain raphe; MOB: Main olfactory bulb; OB: Olfactory bulb; OE: Olfactory epithelium; Pir: Pyriform cortex; VNO: Vomeronasal organ |
1907年, Watson[17]首先报道了OBX后大鼠好斗并且易怒的行为学表现, 随后研究者发现, OBX大鼠表现出高活性、残杀行为、攻击行为、母性行为丧失、性行为改变和被动回避反应缺失。1976年van Riezen等[18]推测OBX引起的行为学改变能够用于检测新型的抗抑郁药物, 一年后OBX大鼠用于评价药物的抗抑郁活性[19]。1981年, Leonard等[20]首次详细介绍了OBX后动物神经解剖学、生理学和行为学的改变, 并指出OBX模型优于当时已有的利血平模型和行为绝望模型。之后, 研究者报道了该模型的其他行为学和多种生化改变, 并将此模型广泛应用于抗抑郁药物的筛选。
OBX模型所选用的动物主要是大鼠和小鼠, 普遍采用的方法为嗅球吸除。对于大鼠而言, 购入后适应1周[3]或2周[21], 手术时大鼠体重250~350 g, 经麻醉后剪开头皮, 暴露颅骨, 在前囟前7~8 mm, 中线左右旁开2 mm处, 各钻一个直径约2 mm的钻孔[22, 23]。用连着真空泵的针头将嗅球吸出, 以明胶海绵堵塞钻孔止血, 缝合后连续3天经腹腔或肌肉注射一定剂量的抗生素, 以防止术后感染。手术后每笼饲养4只大鼠, 包含2只假手术和2只OBX大鼠[3, 21], 或者手术后单笼饲养[22, 24], 以避免或减少OBX大鼠之间的攻击性, 假手术组大鼠除不移除嗅球外其他操作相同。对于小鼠, 采用类似的方法将嗅球吸除[24]。手术后每天抚摸动物, 消除其他因素引起的攻击性[25]。恢复2周后给药或者进行行为学检测, 这个时间段可能是边缘系统和下丘脑发生改变所必需的, 这些脑区在情感调节和记忆中起重要作用[26]。实验结束后, 解剖大脑, 嗅球残留大于30%或前额叶皮层受到损伤的动物数据被排除[27, 28]。Kang等[29]采用尾静脉注射玫瑰红并结合卤素灯照射的方法建立小鼠OBX模型, 术后2周, 小鼠表现出抑郁样行为, 但这种方法并没有被其他研究者广泛采用。
3 OBX模型动物的行为学特点 3.1 高活性空场中探索活性增加是OBX动物最早和最广为人知行为学改变之一[30], 利多卡因、硫酸锌和传入神经阻滞引起的外周嗅觉缺失并不影响动物的空场行为[31], 这表明OBX后动物的行为改变不仅仅是嗅觉缺失引起的。OBX后3天, 大鼠在饲养笼中的活性增加[32], 而此时大鼠的空场活性并未改变; 手术后1周, OBX大鼠和小鼠的空场活性均增加[33]。在手术后2周进行的24 h动态监测中发现, OBX大鼠在饲养笼中的夜活性高于假手术大鼠; 在光照期, 两组动物在饲养笼中活性相似[34]。OBX大鼠在空场中的高活性在1天的光-暗循环中是相似的[30], 这种高活性可以持续至术后20周[35]。
空场的设计对OBX大鼠的高活性检测尤为重要。在昏暗环境下的方形空场中, OBX动物活动性反而降低[36]。Kelly等[37]用于检测OBX大鼠活性的空场直径为90 cm, 空场壁是75 cm铝制墙壁, 一个60 W的灯泡悬挂于空场正中, 距空场底部90 cm, 并且此灯是检测房间唯一的灯光来源。检测时将动物放于空场中心, 检测3 min或5 min内动物的运动情况[23, 25, 28]。动物的高活性依赖于空场中较高的光强和空场壁的反光, 以此来增加对于动物的应激。Primeaux等[38]在手术后13天对比了不同光照强度(15 W或150 W灯泡照明)下OBX大鼠的运动活性, 发现在两个条件下OBX大鼠活性均显著高于假手术组, 故认为OBX大鼠的高活性不依赖与空场中的光强。但笔者认为, 可能15 W灯泡的光强对OBX大鼠也能产生足够的应激。多数研究者按照Kelly等[37]报道的光照条件或者在较强的光照[3, 23]下进行OBX大鼠空场的检测。高光强的空场装置诱发的高活性并不能模拟动物的基础活性, 因此只能在较短的检测时间内评价动物的运动情况, 当OBX大鼠继续暴露于空场中, 将会出现适应性反应[30]。
对于较强光照下OBX动物表现出的高活性, 不同的研究者有不同的解释。Leonard等[20]认为, 嗅球切除后, 动物缺少完整的感觉输入, 需要较长时间来适应新环境。也有报道认为OBX引起的高活性并不是一般性的活动程度增加, 而是动物防御性反应降低的表现[38]。另外, 动物的自发活动依赖于多巴胺(dopamine, DA)能神经传递[39], 微透析技术显示OBX大鼠背侧和腹侧纹状体中DA基础水平增加, 表明纹状体DA的增加可能与OBX动物的高活性相关[40]。Eisenstein等[41]证明内源性大麻素系统的紊乱(尤其是2-花生酰基甘油的降低)与OBX诱导的高活性有关。
OBX动物的高活性模拟激越行为, 不同于应激造成的抑郁动物模型(模拟情绪行为低落的表现), 因此有研究者认为, OBX模型能够模拟精神运动激越的抑郁患者亚群的特征[42, 43]。
3.2 高情绪性或易怒性高情绪性(hyperemotionality)或易怒性(irritability)是OBX模型区别于其他抑郁模型的另一个重要特征。通常以大鼠对4种轻微刺激的反应来评价其高情绪性, ①挣扎反应(struggle response):以带着手套的手抚摸大鼠; ②攻击反应(attack response):将一根木棒伸至大鼠鼻子前4~5 cm; ③打斗反应(fight response):以医用镊子温和地夹持大鼠尾巴; ④惊吓反应(startle response):以5 mL或10 mL注射器向大鼠背部吹气。大鼠反应的评判标准为:无反应(0分)、轻微反应(1分)、中等反应(2分)、显著反应(3分)和极强烈反应(4分)。同时也对大鼠在刺激过程中的发声进行评分:不发声(0分)、偶尔发声(1分)和强烈发声(2分)[22, 44, 45]。根据评分总和来判断大鼠的高情绪性, OBX大鼠通常会分别表现出剧烈的逃避反应、对刺激物的攻击性和僵滞状态[46]。
不同品系和性别的大鼠经历OBX后, 高情绪反应有所差异。在性别上, 雄性的反应强于雌性; 在品系上, OBX大鼠的反应性依次为Sprague-Dawley (SD)大鼠 > Wistar大鼠 > Hooded大鼠[20]。Song等[47]认为, 大鼠的易怒性取决于OBX后动物得到的抚摸程度、每笼大鼠的数量和手术损伤的精确度, 额叶皮层和嗅球茎受到的损伤越严重, 大鼠的易怒性越强。高情绪反应大多用来评价OBX大鼠的行为学表现, 仅有少数研究对OBX小鼠进行高情绪反应评价[48]。嗅球切除后, 嗅球到杏仁核的抑制性输入被解除, 从而导致OBX大鼠对应激产生的急性惊吓非常敏感[47]。
3.3 认知功能下降OBX后3天, 大鼠在被动回避测试中入暗潜伏期下降, 表现出认知功能的缺失, 而在此时OBX大鼠的空场活性并未改变, 这表明OBX引起的认知障碍早于抑郁样行为的出现[49]。OBX大鼠空间记忆能力降低, 在Morris水迷宫中逃避潜伏期增加, 撤台后在目标象限的穿越次数和停留时间均减少; 在跳台和穿梭被动回避中, OBX大鼠潜伏期减少, 错误次数增加[50, 51]; OBX大鼠对新物体和新位置的识别能力降低[52]。OBX小鼠也表现出空间记忆的降低和新物体辨别指数的降低[53, 54]。OBX模型在表现出抑郁样行为的同时也出现认知功能的缺失, 认知障碍甚至先于抑郁行为的出现, 表明该模型也能够用于认知功能减退相关疾病的研究。
3.4 抗焦虑样反应在应激等因素诱导的抑郁模型中, 动物会表现出焦虑样行为, 能够模拟抑郁患者伴随焦虑症状的特点。与应激模型相反, OBX模型大鼠在高架十字迷宫呈现抗焦虑反应, 抗焦虑药能够增强这种作用[21]。Mar等[55]发现OBX大鼠在高架十字迷宫中运动评分增加, 进入开臂的时间增加, 在开臂的运动距离比率增加, 劳拉西泮能进一步增加OBX大鼠的开臂时间, 以及开臂运动距离比率。快速抗抑郁药物氯胺酮也能增加OBX大鼠的开臂时间[56], 而依他唑酯、咯利普兰和氟西汀能够降低OBX大鼠进入开臂的次数比率和时间比率[57, 58]。OBX小鼠空场检测中中央区运动时间增加, 这同样是抗焦虑样行为的表现[59]。Jindal等[58]认为在高架十字迷宫中运动距离增加、进入开臂的时间和次数增加, 反映了OBX大鼠精神运动的激越行为, 也显示出OBX大鼠在陌生环境中防御行为减少。
3.5 其他抑郁样行为嗅球切除动物还表现出与应激抑郁模型相似的行为学特征, 如快感缺失和绝望状态, 这些特点能够模拟抑郁症患者兴趣缺失和无助感的临床表现。在手术后4周均观察到OBX大鼠和小鼠对糖水的偏爱程度降低[60, 61], 在悬尾和强迫游泳检测中观察到OBX动物不动时间增加[60, 62], 而经典的抗抑郁药阿米替林和丙咪嗪能够提高OBX动物的糖水偏爱指数, 减少强迫游泳不动时间[53, 60]。
4 OBX引起皮层和海马的改变嗅球是端脑的双侧延伸, 约占成年大鼠脑体积的4%[63], 同时嗅球也是一个可塑性很强的脑区, 嗅球从室管膜下区得到新神经元, 而室管膜下区在动物的整个生命过程中保持着神经发生[64]。嗅球和皮层以及其他脑区存在广泛的直接或非直接的联系, OBX引起嗅觉丧失, 手术引起的损伤导致脑部水肿、局部血流供应改变、嗅球和其他脑区神经元联系破坏, 投射至嗅球的神经元和接受嗅球投射的神经元发生顺行性、逆行性和跨神经元的退化[65], 这种退化导致动物出现抑郁样和认知障碍等行为, 并伴随神经传递、内分泌系统和免疫反应的改变, Duman等[66]已经在抑郁患者中发现类似的神经退化。
4.1 皮层和海马结构可塑性的变化大鼠鼻腔内有嗅上皮和犁鼻器两个化学感受器, 嗅上皮的轴索传递至主嗅球和犁鼻器, 犁鼻器向副嗅球发送投射。嗅球中的小球细胞、簇状细胞和僧帽细胞通过神经投射和神经递质与梨状皮层、杏仁核和终纹核相互连通[47] (图 1)。OBX引起梨状皮层神经元发生持久的改变, 梨状皮层近55 000个神经元出现跨突触死亡, 这些细胞死亡在OBX手术后22 h达到峰值[67]。OBX一个月后观察到梨状皮层锥体神经元树突萎缩[68]。梨状皮层向内嗅皮层和海马传递神经输入。内嗅皮层在嗅觉记忆中扮演重要角色, 能够直接或间接(通过梨状皮层)收到来自嗅球的神经输入[69, 70]。OBX后, 由于来自嗅球和梨状皮层的输入缺失, 内嗅皮层神经元重新排列, 神经元树突分支和树突长度减少[71]。海马对机体的生理和行为调节至关重要, 包括:学习记忆、空间定位、下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal, HPA)轴调控和情感过程。海马能够直接或间接(通过梨状皮层和内嗅皮层)收到嗅球的神经输入, OBX后海马重量减轻[52], CA1/2区、CA3区和齿状回(dentate gyrus, DG)区体积减少[72]; CA1区神经元生长不足、棘密度减少[73], DG区细胞增殖减少和新生细胞存活率降低[74]; CA1区和DG区的长时程增强减弱[75]。
4.2 皮层和海马神经递质的改变抑郁症的单胺假说认为中枢单胺类递质5-HT、NE和DA功能不足与抑郁症关系密切, 尤其是5-HT能信号减弱被公认是抑郁症的生物学基础。利用微透析技术发现, OBX大鼠基底外侧杏仁核和背侧海马细胞外液中5-HT显著降低, 而5-羟基吲哚乙酸(5-hydroxyindole acetic acid, 5-HIAA)、DA和NE并无明显改变。基础条件下OBX动物基底外侧杏仁核和背侧海马5-HT合成率降低[35], 内侧前额叶皮质中5-HT降低、谷氨酸升高[23]。在OBX大鼠海马和额叶皮层匀浆液中检测到5-HT、DA、NE和5-HIAA均减少[51, 76], OBX小鼠海马中5-HT、NE、3-甲氧基-4-羟基苯乙醇酸和DA降低, 5-HIAA升高, 5-HIAA/5-HT比值和二羟基苯乙酸/DA的比值升高[48, 77]。
4.3 皮层和海马神经炎症的产生小胶质细胞是脑源性免疫细胞, 激活后产生大量炎症因子和自由基。OBX小鼠海马小胶质细胞标志物Iba 1和星形胶质细胞标志物胶质纤维酸性蛋白显著增加, 这表明海马中炎症相关细胞被激活, 同时海马中白介素-6、肿瘤坏死因子-α、p-NF-kBp65和p-IkB-α蛋白表达量显著增加[48], 海马和额叶皮层中白介素-1含量增加, 而白介素-10减少[78]。在OBX大鼠皮层和海马中促炎因子水平升高, 抗炎因子水平降低[24, 76]。
5 OBX模型的应用 5.1 在抗抑郁药物研究中的应用OBX模型应用于抗抑郁药物的研发已有超过40年的历史, 经典的抗抑郁药物(如氟西汀、丙咪嗪、阿米替林、地昔帕明、文拉法辛和西酞普兰等)均能够逆转OBX动物行为学、内分泌和神经生化的改变, 表明该模型具有良好的预测效度。表 1[24, 45, 51, 53, 60-62, 79-84]汇总了近年来在OBX模型上表现出抗抑郁作用的药物, 包括植物活性单体和新型抗抑郁药物。
| Table 1 Behavioral effects of plant active components and novel antidepressants in olfactory bulbectomy animals. ♀: Female; ♂: Male; ↑: Increased; ↓: Decreased; p.o.: Per os; i.p.: Intraperitoneal injection; s.c.: Subcutaneous injection; FST: Forced swimming test; MWM: Morris water maze; OFT: Open field test; ORT: Object recognition test; ST: Splash test; TST: Tail suspension test |
水飞蓟素[24]、姜黄素[51]、橙皮素[53]、知母皂苷元[60]、红景天苷[62, 76]、槲皮素[79]等均能够逆转OBX引起的行为学改变, 降低OBX动物的空场活性, 减少强迫游泳或/和悬尾不动时间, 表现出抗抑郁效果。除了逆转抑郁样行为, 姜黄素能够逆转OBX大鼠被动回避的缺失[51]; 橙皮素还能够增加水迷宫中OBX小鼠的空间记忆, 提高新物体辨别指数[53]。另外, 这些活性单体还能调节胆碱能的异常[60]、抑制神经炎症的产生和提高海马和皮层中单胺类递质(如5-HT、DA和NE)水平[24, 76]。这表明, 植物中的活性单体是抗抑郁药物筛选的重要来源, 鉴于OBX模型良好的预测效度, 应该对这些活性单体进行深入研究, 确定可应用于临床抑郁症治疗的潜在活性分子。
应用于OBX模型的新型抗抑郁药物有5-HT3受体拮抗剂QCF-21[80]、4i[81]和7a[82], 5-HT1A受体激动剂8-OH-DPAT[83], 5-HT再摄取抑制剂和5-HT1A受体部分激动剂DSP-1053[84]等, 这些新型化合物均能够逆转OBX动物的行为学异常。另外, δ阿片受体激动剂KNT-127在给药后3天即能够降低OBX大鼠的高情绪反应, 表现出快速抗抑郁作用, 起效时间早于阳性药氟西汀[45]; 选择性雌激素受体调节剂BE360还能够逆转小鼠在Y迷宫中短期记忆的缺失[61]。以上表明OBX模型不仅可以用于慢性抗抑郁药物的筛选, 也能够用于快速起效抗抑郁药物的评价。
5.2 在抗AD药物研究中的应用嗅球切除导致多个脑区发生退行性改变, 该模型也能够模拟AD的一些重要特点, 包括海马依赖的学习记忆损伤、海马长时程增强的损害和突触密度的破坏[75]。OBX小鼠海马和新皮层中能量代谢紊乱、线粒体功能受损, 表现为较低的还原型烟酰胺腺嘌呤二核苷酸氧化率、线粒体膜电位下降、细胞色素C氧化酶活性降低和线粒体内可溶性Aβ1-40增加。在转基因AD模型动物和散发性AD患者中也观察到脑内线粒体相似的变化[4, 85]。AD患者的胆碱能神经受损, 类似的表现也出现于OBX动物中。OBX小鼠前脑胆碱乙酰转移酶免疫阳性细胞减少[86], 大鼠海马乙酰胆碱酯酶(acetylcholine esterase, AChE)表达升高, α4-和α7-乙酰胆碱受体表达减少[60]。
用于治疗AD的药物能够逆转OBX引起的认知功能下降。AChE抑制剂毒扁豆碱和NIK-247能够改善OBX大鼠的记忆损伤[87]。美金刚是一种特异性的、非竞争性N-甲基-D-天冬氨酸受体拮抗剂, 是第一个经美国食品药品监督管理局批准用于治疗重症AD的药物。在手术前2天开始给予大鼠美金刚, 持续给药28天能够阻止OBX诱导的高活性和部分恐惧记忆的丧失, 这种行为学影响在停止给药后3周依然存在[88]。奈非西坦是一种益智药, 能够恢复OBX小鼠海马长时程增强, 提高在Y迷宫和新物体识别任务中的记忆能力[54]。AD和抑郁共患率超过60%, 已经发现抗抑郁药西酞普兰能够减少转基因AD模型动物脑内Aβ水平[89]。这表明抑郁症和AD存在一些共同的病理改变, 然而OBX后部分脑区的神经退化、空间记忆的损伤、胆碱能系统紊乱和Aβ寡聚体的聚集等是AD的特异性表现。OBX诱导的行为学和生化改变也符合AD的部分特征, 并且临床上用于治疗AD的药物能够逆转这些改变, 因此OBX模型是散发型AD可靠的动物模型。
6 结语OBX模型表现出的行为学改变能够模拟临床抑郁症的特点, OBX动物脑中神经递质的改变、皮层和海马的炎症反应、神经可塑下降和HPA轴功能亢进符合抑郁症发病机制的多个假说(单胺假说、神经炎症假说、神经可塑性假说和HPA轴假说)。已有多篇综述从不同角度阐述OBX模型与抑郁患者表现的相似性, 如Morales-Medina等[2]讨论了OBX大鼠海马结构和生化的改变、海马依赖的行为学异常和药物治疗对海马异常的调节作用; Rajkumar等[90]讨论了OBX模型啮齿类动物和抑郁患者额叶皮层中共同的异常改变, 认为此模型能够为研究抑郁患者额叶皮层的紊乱提供有力支撑。近年来的研究还支持将OBX模型应用于AD和抑郁症伴发疾病(慢性疼痛和药物成瘾)的研究。
尽管有很多强有力的证据支持OBX作为抑郁症的动物模型, 但是这个模型在研究中的使用频率明显降低[90]。手术操作的一致性、术后恢复时间较长(2周)、与临床抑郁症在病因上的差异和应激相关动物模型的发展等可能是OBX模型使用减少的原因。与OBX动物空场高活性、高情绪反应和抗焦虑样行为相对应的神经生化机制并不明确, 少有文献进行深入的研究。另外, 已有研究者对OBX模型提出质疑, Yurttas等[72]对OBX大鼠海马形态学和体视学的研究发现, 虽然氟西汀(10 mg·kg-1)能够逆转OBX大鼠海马CA1/2、CA3和DG区体积的减少, 但不能逆转这些区域神经元的丢失, 因此认为该模型不适合于检测药物的抗抑郁活性。因此, 有必要利用当前发展的成像手段、蛋白质组学、脂质组学和代谢组学对OBX模型进行再评价, 寻找与抑郁症或AD相关的新靶点和生物标志物, 从而更好地将OBX模型应用于抑郁症和AD的研究。
| [1] | Zhang X, Long Q, Chu SF, et al. Inhibitory effect of extratable petroleum ether of Polyrhachis vicina Roger on neuroinflammatory response in depressed rats[J]. Acta Pharm Sin (药学学报), 2018, 53: 1042–1047. |
| [2] | Morales-Medina JC, Iannitti T, Freeman A, et al. The olfactory bulbectomized rat as a model of depression: the hippocampal pathway[J]. Behav Brain Res, 2017, 317: 562–575. DOI:10.1016/j.bbr.2016.09.029 |
| [3] | Breuer ME, Groenink L, Oosting RS, et al. Long-term behavioral changes after cessation of chronic antidepressant treatment in olfactory bulbectomized rats[J]. Biol Psychiatry, 2007, 61: 990–995. DOI:10.1016/j.biopsych.2006.08.032 |
| [4] | Avetisyan AV, Samokhin AN, Alexandrova IY, et al. Mitochondrial dysfunction in neocortex and hippocampus of olfactory bulbectomized mice, a model of Alzheimer's disease[J]. Biochemistry, 2016, 81: 615–623. |
| [5] | Heimer L. The legacy of the silver methods and the new anatomy of the basal forebrain: implications for neuropsychiatry and drug abuse[J]. Scand J Psychol, 2003, 44: 189–201. DOI:10.1111/sjop.2003.44.issue-3 |
| [6] | Shipley MT, Ennis M. Functional organization of olfactory system[J]. J Neurobiol, 1996, 30: 123–176. DOI:10.1002/(ISSN)1097-4695 |
| [7] | Kohli P, Soler ZM, Nguyen SA, et al. The association between olfaction and depression: a systematic review[J]. Chem Senses, 2016, 41: 479–486. DOI:10.1093/chemse/bjw061 |
| [8] | Negoias S, Croy I, Gerber J, et al. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression[J]. Neuroscience, 2010, 169: 415–421. DOI:10.1016/j.neuroscience.2010.05.012 |
| [9] | Croy I, Negoias S, Symmank A, et al. Reduced olfactory bulb volume in adults with a history of childhood maltreatment[J]. Chem Senses, 2013, 38: 679–684. DOI:10.1093/chemse/bjt037 |
| [10] | Pause BM, Miranda A, Goder R, et al. Reduced olfactory performance in patients with major depression[J]. J Psychiatr Res, 2001, 35: 271–277. DOI:10.1016/S0022-3956(01)00029-2 |
| [11] | Croy I, Symmank A, Schellong J, et al. Olfaction as a marker for depression in humans[J]. J Affect Disord, 2014, 160: 80–86. DOI:10.1016/j.jad.2013.12.026 |
| [12] | Yang D, Li Q, Fang L, et al. Reduced neurogenesis and pre-synaptic dysfunction in the olfactory bulb of a rat model of depression[J]. Neuroscience, 2011, 192: 609–618. DOI:10.1016/j.neuroscience.2011.06.043 |
| [13] | Ferreyra-Moyano H, Barragan E. The olfactory system and Alzheimer's disease[J]. Int J Neurosci, 1989, 49: 157–197. DOI:10.3109/00207458909084824 |
| [14] | Loopuijt LD, Sebens JB. Loss of dopamine receptors in the olfactory bulb of patients with Alzheimer's disease[J]. Brain Res, 1990, 529: 239–244. DOI:10.1016/0006-8993(90)90833-W |
| [15] | Pearson RC, Esiri MM, Hiorns RW, et al. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease[J]. Proc Natl Acad Sci U S A, 1985, 82: 4531–4534. DOI:10.1073/pnas.82.13.4531 |
| [16] | Korczyn AD. Neuropsychiatric manifestations in Parkinson's disease[J]. Adv Neurol, 2001, 86: 395–398. |
| [17] | Watson JB. Kinasthetic and organic sensations: their role in the reactions of the white rat to the maze[J]. Psychol Rev, 1907. DOI:10.1037/h0093040 |
| [18] | van Riezen H, Schnieden H, Wren A. Behavioural changes following olfactory bulbectomy in rats: a possible model for the detection of antidepressant drugs[J]. Br J Pharmacol, 1976, 57: 426P–427P. |
| [19] | van Riezen H, Schnieden H, Wren AF. Olfactory bulb ablation in the rat: behavioural changes and their reversal by antidepressant drugs[J]. Br J Pharmacol, 1977, 60: 521–528. DOI:10.1111/bph.1977.60.issue-4 |
| [20] | Leonard BE, Tuite M. Anatomical, physiological, andbehavioral aspects of olfactory bulbectomy in the rat[J]. Int Rev Neurobiol, 1981, 22: 251–286. DOI:10.1016/S0074-7742(08)60295-0 |
| [21] | Wieronska JM, Papp M, Pilc A. Effects of anxiolytic drugs on some behavioral consequences in olfactory bulbectomized rats[J]. Pol J Pharmacol, 2001, 53: 517–525. |
| [22] | Yu HY, Yin ZJ, Yang SJ, et al. Baicalin reverses depressive-like behaviours and regulates apoptotic signalling induced by olfactory bulbectomy[J]. Phytother Res, 2016, 30: 469–475. DOI:10.1002/ptr.v30.3 |
| [23] | Jimenez-Sanchez L, Linge R, Campa L, et al. Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex[J]. Neuropharmacology, 2016, 108: 91–102. DOI:10.1016/j.neuropharm.2016.04.020 |
| [24] | Thakare VN, Aswar MK, Kulkarni YP, et al. Silymarin ameliorates experimentally induced depressive like behavior in rats: involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response[J]. Physiol Behav, 2017, 179: 401–410. DOI:10.1016/j.physbeh.2017.07.010 |
| [25] | Uriguen L, Arteta D, Diez-Alarcia R, et al. Gene expression patterns in brain cortex of three different animal models of depression[J]. Genes Brain Behav, 2008, 7: 649–658. DOI:10.1111/gbb.2008.7.issue-6 |
| [26] | Yuan TF, Slotnick BM. Roles of olfactory system dysfunction in depression[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2014, 54: 26–30. DOI:10.1016/j.pnpbp.2014.05.013 |
| [27] | Watanabe A, Tohyama Y, Nguyen KQ, et al. Regional brain serotonin synthesis is increased in the olfactory bulbectomy rat model of depression: an autoradiographic study[J]. J Neurochem, 2003, 85: 469–475. DOI:10.1046/j.1471-4159.2003.01702.x |
| [28] | Norman TR, Cranston I, Irons JA, et al. Agomelatine suppresses locomotor hyperactivity in olfactory bulbectomised rats: a comparison to melatonin and to the 5-HT(2c) antagonist, S32006[J]. Eur J Pharmacol, 2012, 674: 27–32. DOI:10.1016/j.ejphar.2011.10.010 |
| [29] | Kang HM, Jin J, Lee S, et al. A novel method for olfactory bulbectomy using photochemically induced lesion[J]. Neuroreport, 2010, 21: 179–184. DOI:10.1097/WNR.0b013e328334884c |
| [30] | Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update[J]. Pharmacol Ther, 1997, 74: 299–316. DOI:10.1016/S0163-7258(97)00004-1 |
| [31] | Sieck MH, Baumbach HD. Differential effects of peripheral and central anosmia producing techniques on spontaneous behavior patterns[J]. Physiol Behav, 1974, 13: 407–425. DOI:10.1016/0031-9384(74)90096-1 |
| [32] | Vinkers CH, Breuer ME, Westphal KG, et al. Olfactory bulbectomy induces rapid and stable changes in basal and stress-induced locomotor activity, heart rate and body temperature responses in the home cage[J]. Neuroscience, 2009, 159: 39–46. DOI:10.1016/j.neuroscience.2008.12.009 |
| [33] | Hendriksen H, Korte SM, Olivier B, et al. The olfactory bulbectomy model in mice and rat: one story or two tails?[J]. Eur J Pharmacol, 2015, 753: 105–113. DOI:10.1016/j.ejphar.2014.10.033 |
| [34] | Giardina WJ, Radek RJ. Effects of imipramine on the nocturnal behavior of bilateral olfactory bulbectomized rats[J]. Biol Psychiatry, 1991, 29: 1200–1208. DOI:10.1016/0006-3223(91)90327-I |
| [35] | van der Stelt HM, Breuer ME, Olivier B, et al. Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: an in vivo microdialysis study[J]. Biol Psychiatry, 2005, 57: 1061–1067. DOI:10.1016/j.biopsych.2004.12.040 |
| [36] | Stockert M, Serra J, De Robertis E. Effect of olfactory bulbectomy and chronic amitryptiline treatment in rats. 3H-imipramine binding and behavioral analysis by swimming and open field tests[J]. Pharmacol Biochem Behav, 1988, 29: 681–686. DOI:10.1016/0091-3057(88)90187-6 |
| [37] | Kelly JP, Leonard BE. The effect of tianeptine and sertraline in three animal models of depression[J]. Neuropharmacology, 1994, 33: 1011–1016. DOI:10.1016/0028-3908(94)90160-0 |
| [38] | Primeaux SD, Holmes PV. Role of aversively motivated behavior in the olfactory bulbectomy syndrome[J]. Physiol Behav, 1999, 67: 41–47. DOI:10.1016/S0031-9384(99)00027-X |
| [39] | Kuczenski R, Leith NJ, Applegate CD. Striatal dopamine metabolism in response to apomorphine: the effects of repeated amphetamine pretreatment[J]. Brain Res, 1983, 258: 333–337. DOI:10.1016/0006-8993(83)91161-7 |
| [40] | Masini CV, Holmes PV, Freeman KG, et al. Dopamine overflow is increased in olfactory bulbectomized rats: an in vivo microdialysis study[J]. Physiol Behav, 2004, 81: 111–119. DOI:10.1016/j.physbeh.2004.01.003 |
| [41] | Eisenstein SA, Clapper JR, Holmes PV, et al. A role for 2-arachidonoylglycerol and endocannabinoid signaling in the locomotor response to novelty induced by olfactory bulbectomy[J]. Pharmacol Res, 2010, 61: 419–429. DOI:10.1016/j.phrs.2009.12.013 |
| [42] | Willner P. Animal models of depression: an overview[J]. Pharmacol Ther, 1990, 45: 425–455. DOI:10.1016/0163-7258(90)90076-E |
| [43] | Lumia AR, Teicher MH, Salchli F, et al. Olfactory bulbectomy as a model for agitated hyposerotonergic depression[J]. Brain Res, 1992, 587: 181–185. DOI:10.1016/0006-8993(92)90995-L |
| [44] | Takahashi K, Murasawa H, Yamaguchi K, et al. Riluzole rapidly attenuates hyperemotional responses in olfactory bulbectomized rats, an animal model of depression[J]. Behav Brain Res, 2011, 216: 46–52. DOI:10.1016/j.bbr.2010.07.002 |
| [45] | Gotoh L, Saitoh A, Yamada M, et al. Effects of repeated treatment with a delta opioid receptor agonist KNT-127 on hyperemotionality in olfactory-bulbectomized rats[J]. Behav Brain Res, 2017, 323: 11–14. DOI:10.1016/j.bbr.2016.11.008 |
| [46] | Devadoss T, Pandey DK, Mahesh R, et al. Effect of acute and chronic treatment with QCF-3 (4-benzylpiperazin-1-yl) (quinoxalin-2-yl) methanone, a novel 5-HT3 receptor antagonist, in animal models of depression[J]. Pharmacol Rep, 2010, 62: 245–257. DOI:10.1016/S1734-1140(10)70263-2 |
| [47] | Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression[J]. Neurosci Biobehav Rev, 2005, 29: 627–647. DOI:10.1016/j.neubiorev.2005.03.010 |
| [48] | Takahashi K, Nakagawasai O, Nemoto W, et al. Memantine ameliorates depressive-like behaviors by regulating hippocampal cell proliferation and neuroprotection in olfactory bulbectomized mice[J]. Neuropharmacology, 2018, 137: 141–155. DOI:10.1016/j.neuropharm.2018.04.013 |
| [49] | Borre Y, Lemstra S, Westphal KG, et al. Celecoxib delays cognitive decline in an animal model of neurodegeneration[J]. Behav Brain Res, 2012, 234: 285–291. DOI:10.1016/j.bbr.2012.07.007 |
| [50] | Hu J, Huang HZ, Wang X, et al. Activation of glycogen synthase kinase-3 mediates the olfactory deficit-induced hippocampal impairments[J]. Mol Neurobiol, 2015, 52: 1601–1617. DOI:10.1007/s12035-014-8953-9 |
| [51] | Chang XR, Wang L, Li J, et al. Analysis of anti-depressant potential of curcumin against depression induced male albino Wistar rats[J]. Brain Res, 2016, 1642: 219–225. DOI:10.1016/j.brainres.2016.03.010 |
| [52] | Hendriksen H, Meulendijks D, Douma TN, et al. Environmental enrichment has antidepressant-like action without improving learning and memory deficits in olfactory bulbectomized rats[J]. Neuropharmacology, 2012, 62: 270–277. DOI:10.1016/j.neuropharm.2011.07.018 |
| [53] | Antunes MS, Jesse CR, Ruff JR, et al. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity[J]. Eur J Pharmacol, 2016, 789: 411–420. DOI:10.1016/j.ejphar.2016.07.042 |
| [54] | Moriguchi S, Han F, Shioda N, et al. Nefiracetam activation of CaM kinase Ⅱ and protein kinase C mediated by NMDA and metabotropic glutamate receptors in olfactory bulbectomized mice[J]. J Neurochem, 2009, 110: 170–181. DOI:10.1111/jnc.2009.110.issue-1 |
| [55] | Mar A, Spreekmeester E, Rochford J. Fluoxetine-induced increases in open-field habituation in the olfactory bulbectomized rat depend on test aversiveness but not on anxiety[J]. Pharmacol Biochem Behav, 2002, 73: 703–712. DOI:10.1016/S0091-3057(02)00881-X |
| [56] | Holubova K, Kleteckova L, Skurlova M, et al. Rapamycin blocks the antidepressant effect of ketamine in task-dependent manner[J]. Psychopharmacology, 2016, 233: 2077–2097. DOI:10.1007/s00213-016-4256-3 |
| [57] | Jindal A, Mahesh R, Bhatt S. Etazolate, a phosphodiesterase-4 enzyme inhibitor produces antidepressant-like effects by blocking the behavioral, biochemical, neurobiological deficits and histological abnormalities in hippocampus region caused by olfactory bulbectomy[J]. Psychopharmacology, 2015, 232: 623–637. DOI:10.1007/s00213-014-3705-0 |
| [58] | Jindal A, Mahesh R, Bhatt S. Type 4 phosphodiesterase enzyme inhibitor, rolipram rescues behavioral deficits in olfactory bulbectomy models of depression: involvement of hypothalamic-pituitary-adrenal axis, cAMP signaling aspects and antioxidant defense system[J]. Pharmacol Biochem Behav, 2015, 132: 20–32. DOI:10.1016/j.pbb.2015.02.017 |
| [59] | Roche M, Kerr DM, Hunt SP, et al. Neurokinin-1 receptor deletion modulates behavioural and neurochemical alterations in an animal model of depression[J]. Behav Brain Res, 2012, 228: 91–98. DOI:10.1016/j.bbr.2011.11.035 |
| [60] | Feng B, Zhao XY, Song YZ, et al. Sarsasapogenin reverses depressive-like behaviors and nicotinic acetylcholine receptors induced by olfactory bulbectomy[J]. Neurosci Lett, 2017, 639: 173–178. DOI:10.1016/j.neulet.2016.12.025 |
| [61] | Nakagawasai O, Nemoto W, Onogi H, et al. BE360, a new selective estrogen receptor modulator, produces antidepressant and antidementia effects through the enhancement of hippocampal cell proliferation in olfactory bulbectomized mice[J]. Behav Brain Res, 2016, 297: 315–322. DOI:10.1016/j.bbr.2015.10.033 |
| [62] | Yang SJ, Yu HY, Kang DY, et al. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats[J]. Pharmacol Biochem Behav, 2014, 124: 451–457. DOI:10.1016/j.pbb.2014.07.015 |
| [63] | Cain DP. The role of the olfactory bulb in limbic mechanisms[J]. Psychol Bull, 1974, 81: 654–671. DOI:10.1037/h0036954 |
| [64] | Altman J. Autoradiographic and histological studies of postnatal neurogenesis. Ⅳ. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb[J]. J Comp Neurol, 1969, 137: 433–457. DOI:10.1002/(ISSN)1096-9861 |
| [65] | Leonard BE. The olfactory bulbectomized rat as a model of depression[J]. Pol J Pharmacol Pharm, 1984, 36: 561–569. |
| [66] | Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment[J]. Neuropsychopharmacology, 2001, 25: 836–844. DOI:10.1016/S0893-133X(01)00358-X |
| [67] | Capurso SA, Calhoun ME, Sukhov RR, et al. Deafferentation causes apoptosis in cortical sensory neurons in the adult rat[J]. J Neurosci, 1997, 17: 7372–7384. DOI:10.1523/JNEUROSCI.17-19-07372.1997 |
| [68] | Morales-Medina JC, Juarez I, Venancio-Garcia E, et al. Impaired structural hippocampal plasticity is associated with emotional and memory deficits in the olfactory bulbectomized rat[J]. Neuroscience, 2013, 236: 233–243. DOI:10.1016/j.neuroscience.2013.01.037 |
| [69] | Mouly AM, Di Scala G. Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat[J]. Neuroscience, 2006, 137: 1131–1141. DOI:10.1016/j.neuroscience.2005.10.024 |
| [70] | Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat[J]. Hippocampus, 2009, 19: 1159–1186. DOI:10.1002/hipo.v19:12 |
| [71] | Morales-Medina JC, Juarez I, Iannitti T, et al. Olfactory bulbectomy induces neuronal rearrangement in the entorhinal cortex in the rat[J]. J Chem Neuroanat, 2013, 52: 80–86. DOI:10.1016/j.jchemneu.2013.07.001 |
| [72] | Yurttas C, Schmitz C, Turgut M, et al. The olfactory bulbectomized rat model is not an appropriate model for studying depression based on morphological/stereological studies of the hippocampus[J]. Brain Res Bull, 2017, 134: 128–135. DOI:10.1016/j.brainresbull.2017.07.010 |
| [73] | Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment[J]. Synapse, 2001, 42: 151–163. DOI:10.1002/(ISSN)1098-2396 |
| [74] | Jaako-Movits K, Zharkovsky A. Impaired fear memory and decreased hippocampal neurogenesis following olfactory bulbectomy in rats[J]. Eur J Neurosci, 2005, 22: 2871–2878. DOI:10.1111/ejn.2005.22.issue-11 |
| [75] | Moriguchi S, Shinoda Y, Yamamoto Y, et al. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice[J]. PLoS One, 2013, 8: e60863. DOI:10.1371/journal.pone.0060863 |
| [76] | Zhang X, Du Q, Liu C, et al. Rhodioloside ameliorates depressive behavior via up-regulation of monoaminergic system activity and anti-inflammatory effect in olfactory bulbectomized rats[J]. Int Immunopharmacol, 2016, 36: 300–304. DOI:10.1016/j.intimp.2016.05.008 |
| [77] | Filho CB, Jesse CR, Donato F, et al. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice[J]. Chem Biol Interact, 2016, 260: 154–162. DOI:10.1016/j.cbi.2016.11.005 |
| [78] | Almeida RF, Ganzella M, Machado DG, et al. Olfactory bulbectomy in mice triggers transient and long-lasting behavioral impairments and biochemical hippocampal disturbances[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2017, 76: 1–11. DOI:10.1016/j.pnpbp.2017.02.013 |
| [79] | Holzmann I, da Silva LM, Correa da Silva JA, et al. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways[J]. Pharmacol Biochem Behav, 2015, 136: 55–63. DOI:10.1016/j.pbb.2015.07.003 |
| [80] | Pandey DK, Devadoss T, Modak N, et al. Antidepressant and anxiolytic activities of N-(pyridin-3-yl) quinoxalin-2-carboxamide: a novel serotonin type 3 receptor antagonist in behavioural animal models[J]. Indian J Med Res, 2016, 144: 614–621. |
| [81] | Gupta D, Radhakrishnan M, Thangaraj D, et al. Antidepressant and anti-anxiety like effects of 4i (N-(3-chloro-2-methylphenyl) quinoxalin-2-carboxamide), a novel 5-HT3 receptor antagonist in acute and chronic neurobehavioral rodent models[J]. Eur J Pharmacol, 2014, 735: 59–67. DOI:10.1016/j.ejphar.2014.04.008 |
| [82] | Gautam BK, Jindal A, Dhar AK, et al. Antidepressant-like activity of 2-(4-phenylpiperazin-1-yl)-1, 8-naphthyridine-3-carboxylic acid (7a), a 5-HT3 receptor antagonist in behaviour based rodent models: evidence for the involvement of serotonergic system[J]. Pharmacol Biochem Behav, 2013, 109: 91–97. DOI:10.1016/j.pbb.2013.05.006 |
| [83] | Jiang ZC, Qi WJ, Wang JY, et al. Chronic administration of 5-HT1A receptor agonist relieves depression and depression-induced hypoalgesia[J]. ScientificWorldJournal, 2014, 2014: 405736. |
| [84] | Kato T, Matsumoto Y, Yamamoto M, et al. DSP-1053, a novel serotonin reuptake inhibitor with 5-HT1A partial agonistic activity, displays fast antidepressant effect with minimal undesirable effects in juvenile rats[J]. Pharmacol Res Perspect, 2015, 3: e00142. DOI:10.1002/prp2.142 |
| [85] | Wang LS, Liu XM, Tao X, et al. Application of the animal model of intracerebral injection of amyloid-β oligomers to the study of Alzheimer's disease[J]. Acta Pharm Sin (药学学报), 2018, 53: 1060–1067. |
| [86] | Bobkova NV, Nesterova IV, Nesterov VV. The state of cholinergic structures in forebrain of bulbectomized mice[J]. Bull Exp Biol Med, 2001, 131: 427–431. DOI:10.1023/A:1017907511482 |
| [87] | Yamamoto T, Jin J, Watanabe S. Characteristics of memory dysfunction in olfactory bulbectomized rats and the effects of cholinergic drugs[J]. Behav Brain Res, 1997, 83: 57–62. DOI:10.1016/S0166-4328(97)86046-9 |
| [88] | Borre Y, Bosman E, Lemstra S, et al. Memantine partly rescues behavioral and cognitive deficits in an animal model of neurodegeneration[J]. Neuropharmacology, 2012, 62: 2010–2017. DOI:10.1016/j.neuropharm.2011.12.034 |
| [89] | Sheline YI, West T, Yarasheski K, et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice[J]. Sci Transl Med, 2014, 6: 236re234. |
| [90] | Rajkumar R, Dawe GS. OBscure but not OBsolete: perturbations of the frontal cortex in common between rodent olfactory bulbectomy model and major depression[J]. J Chem Neuroanat, 2018, 91: 63–100. DOI:10.1016/j.jchemneu.2018.04.001 |
 2019, Vol. 54
2019, Vol. 54


