脊椎动物肠道微生物群是地球上最复杂的生态系统之一, 微生物细胞的数量超过人体细胞数量的10倍, 其拥有2百万~4百万个基因的宏基因组, 大概是宿主基因数量的100倍[1, 2]。肠道微生物主要由厌氧菌组成, 其数量约为好氧菌的100~1 000倍, 且这些微生物中至少有一半不能被体外人工培养[3]。人类肠道微生物群与宿主健康密切相关, 1983年, Wostmann和他的同事[4]观察到, 与常规环境饲养的啮齿动物相比, 无菌动物在维持相同的体重情况下卡路里的摄取量要高出30%。之后Bäckhed和Hooper等[5, 6]研究发现肠道菌群有助于碳水化合物和脂质的吸收, 才使这一现象得以解释。事实上, 益生微生物早已成为人类营养的重要组成部分, 含有大量活菌的某些发酵食品, 特别是乳制品和发酵蔬菜, 是全球数百万人日常食用的一部分。
肠道微生物可分成黏液层微生物和肠腔内微生物, 两类微生物菌群组成有较大不同, 且在营养物质交换、信息交流、免疫系统发育和抵抗病原微生物入侵等方面均发挥重要作用。整个胃肠道被黏液层覆盖, 人类每天由杯状细胞分泌产生的黏液高达10 L[7]。黏液层主要由黏蛋白(一类大分子量糖蛋白)和水(约95%)组成, 另外还包括少量的盐、脂质、肠蛋白(溶菌酶和防御素)、免疫球蛋白、生长因子、三叶因子以及部分脱落的上皮细胞[8, 9]。黏液层在小肠中为单层结构, 利于机体对食物营养成分的摄取; 在胃和结肠中为双层结构, 起保护润滑的作用。在较松散的外层黏液层内能够降解黏蛋白的细菌定殖, 而致密的内层上基本没有细菌定殖[10]。
在哺乳动物胃肠道内定殖的微生物主要分为厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、变形菌门(Proteobacteria)和疣微菌门(Verrucomicrobia)等。A. muciniphila作为疣微菌门的代表菌种, 占整个疣微菌门数量的80%以上, 该菌主要定殖于回肠末端、结肠和盲肠外层黏液层, 是人类肠道微生物群中较丰富的物种之一, 占肠道细菌总数的1%~4%[2, 11]。该菌是近年来有关肠道微生物的研究热点, 大量研究表明该菌与宿主健康存在密切联系。
1 A. muciniphila的生物学特征与定殖环境A. muciniphila在2004年首次从人类粪便中分离得到。瓦赫宁恩大学的微生物学家通过在含有胃黏蛋白作为唯一碳源和氮源的厌氧培养基中稀释粪便, 鉴定出了新的黏液降解细菌A. muciniphila[12], 并进一步通过使用厌氧软琼脂技术获得了该细菌的纯培养物。该菌以一位备受尊敬的荷兰微生物学家Antoon Akkermans博士的名字命名, 为了纪念他对微生物生态学领域做出的杰出贡献[2, 12]。A. muciniphila属于疣微菌门(Verrucomicrobia), 它进一步属于疣微菌纲(Verrucomicrobiae), 疣微菌目(Verrucomicrobiales), 疣微菌科(Verrucomicrobiaceae), Akkermansia属(Akkermansia spp.)。作为人类肠道中的疣微菌门的唯一肠道代表, A. muciniphila相对容易与其他肠道微生物(包括厚壁菌门、拟杆菌门、放线菌门和变形菌门等)区分开来[13, 14]。
A. muciniphila呈椭圆形, 视野下常以单个菌或成对菌存在, 属于革兰阴性菌, 无运动性, A. muciniphila MucT (ATCC BAA-835)的完整基因组由一个2 664 102 bp的环状染色体组成, 共包含2 176个预测的蛋白编码序列[15]。Guo等[16]基于来自人和小鼠粪便的39个分离株的全基因组测序、组装和注释表征了A. muciniphila的基因组结构, 对该菌的种群结构、进化和功能特异性提供了见解。A. muciniphila最初被分类为严格的厌氧菌, 但与其他厌氧肠道定居者如脆弱拟杆菌(Bacteroidesfragilis)和青春双歧杆菌(Bifidobacteriumadolescentis)一样, A. muciniphila可以耐受少量氧气, 当氧气以纳摩尔浓度存在时, A. muciniphila可以利用氧气, 并且与在严格的厌氧条件下观察到的相比, 其生长速率和产量均有所增加[17]。有研究表明, 将细菌暴露于空气中1 h, 仍有超过90%的A. muciniphila菌体存活[18], 该特征使其与一些产丁酸盐的严格厌氧菌区分开来。
A. muciniphila可以产生60多种可以降解寡糖链的酶, 如糖苷酶、硫酸酯酶以及唾液酸苷酶等, 以适应黏液层中富含黏蛋白和内源性糖蛋白的生存环境。A. muciniphila主要的生长代谢基质为黏蛋白, 由宿主胃肠道组织中杯状细胞持续分泌, 所以该菌群的定殖并不严格依赖饮食, 具有其独特的生存优势。A. muciniphila在降解宿主黏蛋白同时, 释放单糖或氨基酸, 为其他常驻细菌提供营养物质[12]。A. muciniphila在生命早期定殖于肠道中, 1年内其丰度能达到接近健康成年人所观察到的水平, 但在老年人肠道内该种群的定殖明显降低[19]。除人类外, A. muciniphila也存在于许多哺乳动物肠道中, 如小鼠[20]、仓鼠[21]、松鼠[22]、豚鼠[23]、兔子[24]、驴[25]以及非哺乳动物如蟒蛇[26]、鸡[2]和斑马鱼[27]。
2 A. muciniphila与宿主代谢性疾病的关系肠道微生物群目前被认为是在几种非传染性疾病中干扰能量代谢和宿主易感性的关键因素。自A. muciniphila被发现以来, 研究者发现该菌与诸多代谢性疾病密切相关, 其中与肥胖及糖尿病等代谢紊乱的关系研究最为广泛。大部分研究主要将A. muciniphila的丰度与其对机体的有益效应联系起来, 不少于25项研究表明, 在饮食诱导或基因缺陷诱导的肥胖或其他代谢紊乱的小鼠模型中, A. muciniphila的丰度降低。目前, A. muciniphila被广泛认为是改善肥胖、糖尿病、肝脏疾病和心脏代谢紊乱等代谢疾病的新型潜在靶点[28]。
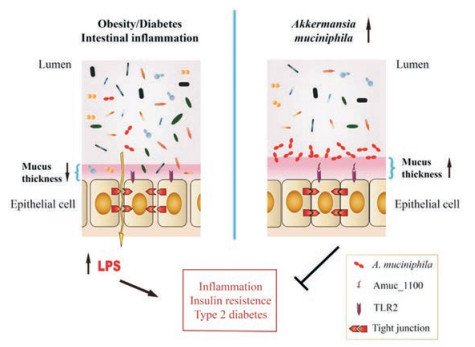
2.1 A. muciniphila与代谢综合征Everard等[29-31]在2013年发现菊粉型果聚糖作为一种益生元, 能影响肠道中超过100种不同的分类群。而A. muciniphila的相对丰度在摄入该益生元后增加了100倍以上, 并且在遗传和饮食诱导的肥胖及糖尿病小鼠的肠道微生物群中该菌丰度降低, 提示该菌可能作为一种益生菌改善代谢紊乱。随后, Everard等[29]近一步发现对高脂饲料喂养的小鼠进行A. muciniphila活菌灌胃(1×108 CFUs·day-1)后, 小鼠体重增加降低50%, 内脏和皮下脂肪量减少了一半, 上皮细胞恢复完整, 代谢紊乱症状减轻。而使用高压蒸汽灭菌, 破坏细菌和孢子的所有成分后, A. muciniphila的保护作用消失。Plovier等[32]意外发现, 采用一种比高压灭菌更温和的热灭活方法, 即巴氏消毒法(70 ℃、30 min)处理菌液后, 并没有消除A. muciniphila的有益作用, 甚至提升了该菌对小鼠代谢紊乱症状的改善作用, 并且这些效应与食物摄入量无关。巴氏消毒的A. muciniphila可有效减少小鼠体重及脂肪含量的增加, 改善糖耐量, 提高胰岛素敏感性, 并可完全阻断饮食诱导的代谢性内毒素血症。这种改变可能与提高肠道中内源性大麻素的水平, 从而抑制炎症反应、改善肠道屏障以及促进肠肽分泌有关。同时, 巴氏消毒的A. muciniphila灌胃增加了小鼠粪便中的能量, 表明体内能量吸收减少, 可能有助于解释体重增加减少。此外, A. muciniphila在体外模型中也显示出免疫刺激能力, 包括诱导细胞因子的产生, 激活Toll样受体2和4 (TLR2和TLR4)等。研究发现, 由A. muciniphila活菌诱导的IL-10水平与由Faecalibacterium prausnitzii A2-165和Lactobacillus plantarum WCFS1诱导的水平相似[33]。尽管该菌影响宿主代谢的作用机制还没有被完全阐明, 但研究发现A. muciniphila在其外膜上表达许多高度丰富的蛋白质, 在这些蛋白质中, Amuc_1100涉及A. muciniphila菌毛的形成, 是其中含量最为丰富的蛋白[34]。该蛋白能激活TLR2, 通过维持免疫系统和改善肠道屏障功能, 促进肠黏膜中的免疫稳态[32, 33]。该膜蛋白在巴氏杀菌的温度下稳定, 能部分重现A. muciniphila活菌的有益效果(图 1)[32]。另外, Lukovac等[35]使用小鼠回肠类器官的新型离体模型来表征微生物群对宿主上皮的影响, 实验中观察到A. muciniphila代谢物能影响类器官中各种转录因子和参与细胞脂质代谢和生长的基因, 支持以前的体内研究结果, 同时也为宿主-微生物组相互作用研究提供了一种新颖的离体模型。同时也有研究表明, A. muciniphila可以介导γ-干扰素(interferon-γ, IFNγ)对葡萄糖耐受的负面影响。在IFNγ缺陷型小鼠中, A. muciniphila显着增加, 并且恢复IFNγ水平可以减少A. muciniphila丰度。该研究进一步显示, 微生物群不含有A. muciniphila的IFNγ基因敲除小鼠并没有显示出糖耐量的改善, 而给予A. muciniphila后改善了其糖耐量[36]。
|
Figure 1 Effects of A. muciniphila on host metabolism. A. muciniphila has been found to be lower in conditions such as obesity, diabetes, intestinal inflammation, which is associated with an altered gut barrier function leading to an increased plasma lipopolysaccharide (LPS) levels and further triggering low grade inflammation and metabolic disorders. A. muciniphila as well as it's membrane protein Amuc_1100 have been shown to restore gut barrier function by activating toll-like receptor 2 (TLR2) and restoring tight junction proteins expression |
在临床研究中, 代谢障碍患者(如肥胖儿童和成人)肠道菌中Akkermansia属的丰度普遍下降[37, 38], 也有其他研究结果表明, Akkermansia属与代谢紊乱标志物之间呈负相关性[39]。在最近的一项包括肥胖和瘦个体的大型观察性研究中, 研究人员发现肠道菌宏基因组丰富度高的受试者比宏基因组丰富度低的个体更健康, 此外, A. muciniphila在前者中存在的数量更多[40]。另外, Dao等[41]对肥胖和超重人群进行了为期6周的卡路里限制实验, 分析干预前后其肠道微生物群、代谢综合征和饮食摄入量之间的关系。结果发现A. muciniphila丰度与空腹血糖、腰臀比和皮下脂肪细胞直径呈负相关。有趣的是, A. muciniphila基础携带量更多的个体表现出更好的代谢特征, 包括改善胰岛素敏感性等。此外, 斯坦福大学医学院的研究团队在一项临床研究中发现, 在胰岛素敏感的受试者中, 高脂饮食使体重增加后, A. muciniphila的丰度显著提高, 也就是说, 在体重增加的情况下, A. muciniphila会帮助机体应对胰岛素抵抗, 起到一种保护作用。而胰岛素抵抗的受试者中该菌群却没有增加, 或者说其本身的含量就很低[42]。
2.2 A. muciniphila与糖尿病Hänninen等[43]通过比较分别来自Jackson和Taconic实验室的NOD小鼠糖尿病发生率, 发现Jackson实验室NOD小鼠的高发病率也与A. muciniphila有关, 口服移植A. muciniphila给Jackson NOD小鼠后, 能降低其糖尿病发病率, 具体机制与促进黏液分泌、增加抗菌肽Reg3γ表达、降低血浆内毒素水平和胰岛样受体表达并增加胰岛中的Foxp3+ Treg细胞有关。然而, 在对345例中国2型糖尿病(type 2 diabetes, T2D)患者及健康人群的粪便分析发现, 与健康对照相比, Akkermansia属在T2D患者中更为丰富[44], 这可能是由于基因和饮食差异所致。
上述内容中已有多项研究表明, A. muciniphila可减少肠道屏障破坏和胰岛素抵抗。Chelakkot等[45]评估了A. muciniphila衍生的细胞外囊泡(Akkermansia muciniphila-derived extracellular vesicles, AmEVs)对肠道通透性的调节作用。作者发现, 与T2D患者相比, 健康对照人的粪便样本中存在更多的AmEVs。此外, AmEVs给药增强了高脂饮食诱导的糖尿病小鼠的肠道紧密连接功能, 降低体重增加并改善了葡萄糖耐受。另外, 用这些囊泡体外处理培养的结肠腺癌细胞(Caco-2细胞), 发现AmEVs能降低脂多糖处理的Caco-2细胞的通透性, 而源自大肠杆菌的细胞外囊泡(extracellular vesicles, EVs)没有显著影响。这些结果提示AmEVs可以调节肠道通透性, 从而进一步改善高脂饮食诱导的小鼠代谢功能紊乱。
2.3 A. muciniphila与其他疾病在一项对酒精性肝病的研究中发现, 在酒精性脂肪型肝炎(alcoholic steatohepatitis, ASH)患者及酒精性肝炎(alcoholic liver disease, ALD)小鼠模型粪便中A. muciniphila丰度降低。灌胃给予A. muciniphila能增加黏液层厚度和紧密连接蛋白表达, 促进ALD小鼠肠道屏障修复, 改善脂肪肝和嗜中性粒细胞浸润, 减少肝损伤[46]。此外, 在饮食诱导的载脂蛋白E基因敲除(Apoe-/-)小鼠动脉粥样硬化模型中, A. muciniphila活菌灌胃, 可修复肠道屏障, 减轻代谢性内毒素血症诱导的炎症反应, 改善动脉粥样硬化病变[47]。
已有多项研究表明, A. muciniphila与炎症性肠病(inflammatory bowel disease, IBD)相关[48-50], Png等[50]发现在IBD患者中, 黏液降解菌总菌量增加, 而菌A. muciniphila的量减少, 提示该菌可能有潜在的抗炎作用。在葡聚糖硫酸钠(dextran sulfate sodium, DSS)诱导的结肠炎模型小鼠中, 粪便中A. muciniphila和Bacteroides acidifaciens的EVs降低。A. muciniphila衍生的EVs的体外预处理减少了结肠上皮细胞由大肠杆菌EVs诱导的促炎细胞因子IL-6的产生。另外, 口服给予A. muciniphila EVs还能改善DSS诱导的肠炎表型, 如体重减轻、结肠缩短和结肠壁的炎性细胞浸润等[51]。也有临床报道在急性阑尾炎患者中, 粪便中A. muciniphila的含量与疾病的严重程度成负相关[52]。
此外, 多项研究结果表明, 结肠外科手术后的伤口愈合与肠道微生物密切相关[53]。数据显示, 特定活性氧的产生和特定甲酰肽受体的活化能调节肠道伤口愈合。有证据表明, 一些特定的肠道微生物, 如乳杆菌属(Lactobacillus spp.)和A. muciniphila, 可通过以上两种机制调节伤口愈合过程[53]。另外, Zhang等[54]分析了分别来自于正常体重、病态肥胖和胃旁路手术后人的粪便样本, 发现胃旁路手术后A. muciniphila的丰度增加。
许多研究表明, 癌症的发生发展也与微生物菌群有关, 一些促癌物质(如砷)可通过干扰肠道菌诱发癌症[55]。胃肠道细菌及产生的促炎物质被认为能够促进消化道肿瘤生长, Dingemanse等[56]观察到肠道特异性Apc突变小鼠在常规环境下比在低病原体环境下产生更多的结肠直肠肿瘤。通过宏基因组测序以及粪便DNA定量PCR分析发现, H. typhlonius和A. muciniphila这两种菌在常规环境饲养小鼠中存在, 而在低病原体环境饲养小鼠中缺失。在Apc突变小鼠中单独定殖这两种菌能增加肠道肿瘤数目, 而同时定殖这两种菌则会减少肿瘤数目, 提示这两种菌可以调节小鼠的肠道微生物群组成, 影响肠道肿瘤的发展。Routy等[57]发现肿瘤患者对靶向PD-1/PD-L1轴的免疫检查点抑制剂(immune check point, ICI)抗性的产生也与肠道微生物组成有关。抗生素能抑制ICI对晚期癌症患者的临床疗效。将对ICI敏感的癌症患者的粪便微生物群移植于无菌或者抗生素给药小鼠, 可以改善PD-1阻断剂的抗肿瘤作用。粪便的宏基因组学分析显示, 临床上对ICI的反应性与A. muciniphila的相对丰度相关。移植对ICI不敏感肿瘤患者粪便微生物群的同时口服灌胃给予A. muciniphila, 能促进CCR9+ CXCR3+ CD4+ T淋巴细胞向肿瘤床募集, 从而恢复PD-1阻断剂的抗肿瘤作用。
另外, Wang等[58]发现在孤独症儿童中, 肠道微生物群结构也发生了改变, 其中双歧杆菌和A. muciniphila的相对丰度降低。特应性儿童的肠道微生物群显示出IV型梭菌群, Faecalibacterium prausnitzii、A. muciniphila的丰度显著减少, 并且肠杆菌的相对丰度增加[59]。Olson等[60]的最新研究揭示了生酮饮食治疗难治性癫痫的作用机制也与A. muciniphila有关, 生酮饮食使肠道中A. muciniphila和Parabacteroides这两种菌群富集, 直接口服给予A. muciniphila和Parabacteroides对正常饮食小鼠的癫痫发作起保护作用。硫酸软骨素在临床试验中常常会改善或加重对骨关节炎, Wang等[61]发现这两个相反的作用结果也可能与A. muciniphila有关:在硫酸酯酶分泌菌(sulfatase-secreting bacteria, SSB)和硫酸盐还原菌(sulfate-reducing bacteria, SRB)没有过度生长, A. muciniphila正常存在的条件下, 硫酸软骨素将改善骨关节炎; 而在SSB和SRB过度生长, 导致A. muciniphila的生长受到竞争性抑制的条件下, 硫酸软骨素将加重骨关节炎。
3 影响和调节肠道内A. muciniphila丰度的影响因素近年来大量研究证实抗生素、食物来源的益生元、药物等均对A. muciniphlia的肠道定殖产生显著影响, 提示通过改变膳食结构或药物干预提高肠道菌群中A. muciniphlia的丰度将对宿主代谢产生有利影响。本节对相关研究内容进行了总结, 具体内容见表 1[29, 62-78]。
| Table 1 Recent studies about interventions that affect and regulate the abundance of Akkermansia in vivo. FODMAP: Fermentable oligosaccharides, disaccharides, monosaccharides and polyols; FISH: Fluorescent in situ hybridisation; qPCR: Real-time quantitative PCR; IBS: Irritable bowel syndrome |
抗生素作为抗菌的主要手段, 然而在一部分动物和人体实验中, 给予特定抗生素会导致Akkermansia属菌群的大量爆发。但是大部分的研究结果基于16S RNA测序, 表示的是该菌在整个肠道菌群中的相对含量, 不能定量单位粪便中该菌的绝对含量, 因此很多情况下研究者并不清楚在给予抗生素后, 其他菌群也受到影响的情况下, A. muciniphila的绝对含量是否是增加的。在体外药敏实验中, A. muciniphila菌株对亚胺培南(最低抑菌浓度minimum inhibitory concentration, MIC = 0.7 mg·L-1)、哌拉西林/他唑巴坦(MIC = 0.7 mg·L-1)和强力霉素(MIC = 0.38 mg·L-1)敏感, 但对万古霉素(MIC > 64 mg·L-1)、甲硝唑(MIC > 64 mg·L-1)和青霉素G (MIC = 2.8 mg·L-1)有耐药性[63]。这也解释了为何尽管万古霉素是多数微生物, 包括耐药菌的终极杀手, 但NOD小鼠在万古霉素给药8周之后, 反而能促进A. muciniphila在胃肠道黏液层中的定殖, 使其成为优势种群[62]。在Dubourg等[63]进行的一项临床研究中, 两名受试者在经过广谱抗生素治疗后, Akkermansia属菌群大量增殖, 相对丰度高达80%, 且两位受试者均未出现明显的胃肠道疾病, 这一极端的数据也表明该菌对人体无明显有害作用。最近的一项研究表明, 使用泰乐菌素可促进小鼠胃肠道中Akkermansia属的定殖[64]。另外, 一位接受β-内酰胺治疗的患者, 6天后Akkermansia属的相对丰度增加[65]。
3.2 食物来源的益生元菊粉型低聚果糖作为一种益生元, 能影响超过100种不同的分类群。Everard等[29-31]发现A. muciniphila在遗传和饮食诱导的肥胖和糖尿病小鼠的肠道微生物群中丰度较低, 而A. muciniphila的相对丰度在摄入该益生元后增加了100倍以上。
多酚也是影响A. muciniphila菌群丰度的一类重要物质, Anhê等[66]发现富含多酚的蔓越莓提取物能直接增加肠道Akkermansia属丰度, 这种效应在改善高脂肪/高蔗糖饮食诱导的代谢综合征中起着关键作用。与此结果一致的是, 之前的一项体外研究表明, 在体外肠道微生物生态系统中, 从红茶或红葡萄酒葡萄中提取的多酚混合物也能增加A. muciniphila的丰度[79]。有趣的是, 给予高脂喂养小鼠绿茶多酚也与A. muciniphila所占肠道菌比例增加相关[80]。Roopchand等[81]的另一项研究表明, 葡萄多酚也显著促进了A. muciniphila的生长, 并降低了菌群中厚壁菌对拟杆菌的比例, 这与之前有关微生物群落结构变化可以改善饮食诱导的肥胖和代谢疾病的报道一致。
其他一些特定膳食成分也可以增加A. muciniphila的数量, Reid等[82]发现饮食中加入益生元纤维能增加早期营养过剩的大鼠肠道A. muciniphila的丰度, 可能有助于改善其代谢表征。然而也有研究结果表明, 小鼠饮食中若缺乏膳食纤维也会增加肠道中A. muciniphila的丰度[83]。另外共轭亚油酸[67]、燕麦麸[68]、人体难吸收的一些短链碳水化合物, 包括可发酵低聚糖、二糖、单糖和多元醇[69]、全谷物大麦[70]和多胺[71]、红色火龙果[72]、动物双歧杆菌乳酸亚种LMG P-28149[73]、玉米衍生的非可消化的铁化寡糖和多糖[74]等也能增加肠道A. muciniphila的数量。
3.3 药物及其他无热量人造甜味剂(non-caloric artificial sweeteners, NAS)是世界上使用最广泛的食品添加剂之一, 由于其卡路里含量低, 通常被认为对人体安全无害, 但Suez等[75]研究发现, NAS能通过改变肠道微生物结构诱导葡萄糖不耐受, 16S RNA测序检测到NAS能使小鼠肠道菌中A. muciniphila丰度降低。
二甲双胍作为用于治疗2型糖尿病患者的最常用药物之一, 也能影响肠道菌结构。Shin等[76]研究发现, 口服给药二甲双胍在显著改善高脂喂养小鼠糖耐受的同时, 显著增加了菌群中A. muciniphila的丰度。另外, Lee等[84]也在体外证明了二甲双胍能富集混菌中的A. muciniphia。另外, 也有文献报导金银花、炮制过的白术以及灵芝均可增加肠道菌A. muciniphila的比例, 同时对宿主代谢有益[77, 78, 85]。
4 前景及展望尽管少部分研究报道了该菌的一些负面效应, 目前在大部分临床前和临床研究中一致发现代谢紊乱疾病中A. muciniphila丰度降低, 部分干预研究的报道表明A. muciniphila直接参与改善宿主代谢。A. muciniphila是疣微菌门里唯一的代表菌种, 也是疣微菌门里唯一能被体外培养的菌种, 虽然A. muciniphila可在微氧条件下呼吸, 但作为厌氧菌的一种, 对氧气相对敏感[17], 因此它的培养需要特定的培养条件, 并且在其常规培养基中需要加入动物来源的黏蛋白作为其能量来源, 这些条件阻碍了A. muciniphila的临床应用。为了解决这些问题, Plovier等[32]成功开发了一种合成培养基以允许高产率地培养A. muciniphila, 并且该合成培基不含有与人体给药不兼容的物质, 解决了A. muciniphila在临床应用道路上的一大障碍。此外, A. muciniphila具有仅由该微生物表达的特有蛋白, 并且与其他微生物没有同源性。Cani团队和Willem M. de Vos团队[32]先后验证该菌表面膜蛋白Amuc_1100在与宿主相互作用中发挥关键作用, 更重要的是验证了该菌在人体用药上的安全性[41], 为临床新药的开发提供了新的靶标。另外, Marcial-Coba等[86]开发了一种用于在黄原胶/结冷胶基质中微囊化A. muciniphila的方案, 并且将微囊化的菌体嵌入黑巧克力中能增强其在储存和体外胃通道期间的存活率[87]。同时基于细菌成分的鉴定和分离, 打开了基于A. muciniphila相关产品的药物开发的大门。
| [1] | Qin JJ, Li RQ, Raes J, et al. A human gut microbial gene cata logue established by metagenomic sequencing[J]. Nature, 2010, 464: 59–65. DOI:10.1038/nature08821 |
| [2] | Belzer C, de Vos WM. Microbes inside——from diversity to func tion:the case of Akkermansia[J]. ISME J, 2012, 6: 1449–1458. DOI:10.1038/ismej.2012.6 |
| [3] | Sears CL. A dynamic partnership:celebrating our gut flora[J]. Anaerobe, 2005, 11: 247–251. DOI:10.1016/j.anaerobe.2005.05.001 |
| [4] | Wostmann BS, Larkin C, Moriarty A, et al. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats[J]. Lab Anim Sci, 1983, 33: 46–50. |
| [5] | Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine[J]. Science, 2005, 307: 1915–1920. DOI:10.1126/science.1104816 |
| [6] | Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine[J]. Science, 2001, 291: 881–884. DOI:10.1126/science.291.5505.881 |
| [7] | Cone RA. Barrier properties of mucus[J]. Adv Drug Delivery Rev, 2009, 61: 75–85. DOI:10.1016/j.addr.2008.09.008 |
| [8] | Hamer HM, Jonkers DM, Loof A, et al. Analyses of human colonic mucus obtained by an in vivo sampling technique[J]. Dig Liver Dis, 2009, 41: 559–564. DOI:10.1016/j.dld.2008.12.100 |
| [9] | Clamp JR, Ene D. The gastric mucosal barrier[J]. Methods Find Exp Clin Pharmacol, 1989, 11(Suppl 1): 19–25. |
| [10] | Johansson MEV, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria[J]. Proc Natl Acad Sci U S A, 2008, 105: 15064–15069. DOI:10.1073/pnas.0803124105 |
| [11] | Derrien M, Collado MC, Ben-Amor K, et al. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract[J]. Appl Environ Microbiol, 2008, 74: 1646–1648. DOI:10.1128/AEM.01226-07 |
| [12] | Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucindegrading bacterium[J]. Int J Syst Evol Microbiol, 2004, 54: 1469–1476. DOI:10.1099/ijs.0.02873-0 |
| [13] | Gómez-Gallego C, Pohl S, Salminen S, et al. Akkermansia muciniphila:a novel functional microbe with probiotic properties[J]. Benef Microbes, 2016, 7: 571–584. DOI:10.3920/BM2016.0009 |
| [14] | Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota[J]. FEMS Microbiol Rev, 2014, 38: 996–1047. DOI:10.1111/1574-6976.12075 |
| [15] | van Passel MW, Kant R, Zoetendal EG, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes[J]. PLoS One, 2011, 6: e16876. DOI:10.1371/journal.pone.0016876 |
| [16] | Guo XF, Li SH, Zhang JC, et al. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas[J]. BMC Genomics, 2017, 18: 800. DOI:10.1186/s12864-017-4195-3 |
| [17] | Ouwerkerk JP, van der Ark KCH, Davids M, et al. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer[J]. Appl Environ Microbiol, 2016, 82: 6983–6993. DOI:10.1128/AEM.01641-16 |
| [18] | Reunanen J, Kainulainen V, Huuskonen L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer[J]. Appl Environ Microbiol, 2015, 81: 3655–3662. DOI:10.1128/AEM.04050-14 |
| [19] | Collado MC, Derrien M, Isolauri E, et al. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly[J]. Appl Environ Microbiol, 2007, 73: 7767–7770. DOI:10.1128/AEM.01477-07 |
| [20] | Sonoyama K, Ogasawara T, Goto H, et al. Comparison of gut microbiota and allergic reactions in BALB/c mice fed different cultivars of rice[J]. Br J Nutr, 2010, 103: 218–226. DOI:10.1017/S0007114509991589 |
| [21] | Sonoyama K, Fujiwara R, Takemura N, et al. Response of gut microbiota to fasting and hibernation in Syrian hamsters[J]. Appl Environ Microbiol, 2009, 75: 6451–6456. DOI:10.1128/AEM.00692-09 |
| [22] | Carey HV, Walters WA, Knight R. Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle[J]. Am J Physiol Regul Integr Comp Physiol, 2013, 304: R33–R42. DOI:10.1152/ajpregu.00387.2012 |
| [23] | Hildebrand F, Ebersbach T, Nielsen HB, et al. A comparative analysis of the intestinal metagenomes present in guinea pigs (Cavia porcellus) and humans (Homo sapiens)[J]. BMC Genomics, 2012, 13: 514. DOI:10.1186/1471-2164-13-514 |
| [24] | Zeng B, Han SS, Wang P, et al. The bacterial communities as sociated with fecal types and body weight of rex rabbits[J]. Sci Rep, 2015, 5: 9342. DOI:10.1038/srep09342 |
| [25] | Liu XF, Fan HL, Ding XB, et al. Analysis of the gut microbiota by high-throughput sequencing of the V5-V6 regions of the 16S rRNA gene in donkey[J]. Curr Microbiol, 2014, 68: 657–662. DOI:10.1007/s00284-014-0528-5 |
| [26] | Costello EK, Gordon JI, Secor SM, et al. Postprandial remodeling of the gut microbiota in Burmese pythons[J]. ISME J, 2010, 4: 1375–1385. DOI:10.1038/ismej.2010.71 |
| [27] | Roeselers G, Mittge EK, Stephens WZ, et al. Evidence for a core gut microbiota in the zebrafish[J]. ISME J, 2011, 5: 1595–1608. DOI:10.1038/ismej.2011.38 |
| [28] | Neef A, Sanz Y. Future for probiotic science in functional food and dietary supplement development[J]. Curr Opin Clin Nutr Metab Care, 2013, 16: 679–687. DOI:10.1097/MCO.0b013e328365c258 |
| [29] | Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls dietinduced obesity[J]. Proc Natl Acad Sci U S A, 2013, 110: 9066–9071. DOI:10.1073/pnas.1219451110 |
| [30] | Everard A, Lazarevic V, Gaïa N, et al. Microbiome of prebiotictreated mice reveals novel targets involved in host response during obesity[J]. ISME J, 2014, 8: 2116–2130. DOI:10.1038/ismej.2014.45 |
| [31] | Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice[J]. Diabetes, 2011, 60: 2775–2786. DOI:10.2337/db11-0227 |
| [32] | Plovier H, Everard A, Druart C, et al. A purified membrane pro tein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice[J]. Nat Med, 2017, 23: 107–113. DOI:10.1038/nm.4236 |
| [33] | Ottman N. Host Immunostimulation and Substrate Utilization of The Gut Symbiont Akkermansia muciniphila[D]. Wageningen: Wageningen University, 2015. http://library.wur.nl/WebQuery/wurpubs/490930 |
| [34] | Ottman N, Reunanen J, Meijerink M, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function[J]. PLoS One, 2017, 12: e0173004. DOI:10.1371/journal.pone.0173004 |
| [35] | Lukovac S, Belzer C, Pellis L, et al. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids[J]. mBio, 2014, 5: e01438–14. |
| [36] | Greer RL, Dong XX, Carolina A, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism[J]. Nat Commun, 2016, 7: 13329. DOI:10.1038/ncomms13329 |
| [37] | Teixeira TFS, Grześkowiak ŁM, Salminen S, et al. Faecal levels of Bifidobacterium and Clostridium coccoides but not plasma lipopolysaccharide are inversely related to insulin and HOMA index in women[J]. Clin Nutr, 2013, 32: 1017–1022. DOI:10.1016/j.clnu.2013.02.008 |
| [38] | Karlsson CLJ, Önnerfalt J, Xu J, et al. The microbiota of the gut in preschool children with normal and excessive body weight[J]. Obesity, 2012, 20: 2257–2261. DOI:10.1038/oby.2012.110 |
| [39] | Remely M, Tesar I, Hippe B, et al. Gut microbiota composition correlates with changes in body fat content due to weight loss[J]. Benef Microbes, 2015, 6: 431–439. DOI:10.3920/BM2014.0104 |
| [40] | Le Chatelier E, Nielsen T, Qin JJ, et al. Richness of human gut microbiome correlates with metabolic markers[J]. Nature, 2013, 500: 541–546. DOI:10.1038/nature12506 |
| [41] | Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity:relationship with gut microbiome richness and ecology[J]. Gut, 2016, 65: 426–436. DOI:10.1136/gutjnl-2014-308778 |
| [42] | Piening BD, Zhou WY, Contrepois K, et al. Integrative personal omics profiles during periods of weight gain and loss[J]. Cell Syst, 2018, 6: 157–170.e8. DOI:10.1016/j.cels.2017.12.013 |
| [43] | Hänninen A, Toivonen R, Pöysti S, et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoim munity in NOD mice[J]. Gut, 2018, 67: 1445–1453. DOI:10.1136/gutjnl-2017-314508 |
| [44] | Qin JJ, Li YR, Cai ZM, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes[J]. Nature, 2012, 490: 55–60. DOI:10.1038/nature11450 |
| [45] | Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphiladerived extracellular vesicles influence gut permeability through the regulation of tight junctions[J]. Exp Mol Med, 2018, 50: e450. DOI:10.1038/emm.2017.282 |
| [46] | Grander C, Adolph TE, Wieser V, et al. Recovery of ethanolinduced Akkermansia muciniphila depletion ameliorates alcoholic liver disease[J]. Gut, 2017, 67: 891–901. |
| [47] | Li J, Lin SQ, Vanhoutte PM, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endo toxemia-induced inflammation in Apoe-/- mice[J]. Circulation, 2016, 133: 2434–2446. DOI:10.1161/CIRCULATIONAHA.115.019645 |
| [48] | James SL, Christophersen CT, Bird AR, et al. Abnormal fibre usage in UC in remission[J]. Gut, 2015, 64: 562–570. DOI:10.1136/gutjnl-2014-307198 |
| [49] | Vigsnæs LK, Brynskov J, Steenholdt C, et al. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls[J]. Benef Microbes, 2012, 3: 287–297. DOI:10.3920/BM2012.0018 |
| [50] | Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utili zation of mucin by other bacteria[J]. Am J Gastroenterol, 2010, 105: 2420–2428. DOI:10.1038/ajg.2010.281 |
| [51] | Kang CS, Ban M, Choi EJ, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis[J]. PLoS One, 2013, 8: e76520. DOI:10.1371/journal.pone.0076520 |
| [52] | Swidsinski A, Dörffel Y, Loening-Baucke V, et al. Acute appendi citis is characterised by local invasion with Fusobacterium nucleatum/necrophorum[J]. Gut, 2011, 60: 34–40. DOI:10.1136/gut.2009.191320 |
| [53] | Bachmann R, Leonard D, Delzenne N, et al. Novel insight into the role of microbiota in colorectal surgery[J]. Gut, 2017, 66: 738–749. DOI:10.1136/gutjnl-2016-312569 |
| [54] | Zhang HS, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass[J]. Proc Natl Acad Sci U S A, 2009, 106: 2365–2370. DOI:10.1073/pnas.0812600106 |
| [55] | Choiniere J, Wang L. Exposure to inorganic arsenic can lead to gut microbe perturbations and hepatocellular carcinoma[J]. Acta Pharm Sin B, 2016, 6: 426–429. DOI:10.1016/j.apsb.2016.07.011 |
| [56] | Dingemanse C, Belzer C, van Hijum SA, et al. Akkermansia muciniphila and Helicobacter typhlonius modulate intestinal tumor development in mice[J]. Carcinogenesis, 2015, 36: 1388–1396. DOI:10.1093/carcin/bgv120 |
| [57] | Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influ ences efficacy of PD-1-based immunotherapy against epithelial tumors[J]. Science, 2018, 359: 91–97. DOI:10.1126/science.aan3706 |
| [58] | Wang L, Christophersen CT, Sorich MJ, et al. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism[J]. Appl Environ Microbiol, 2011, 77: 6718–6721. DOI:10.1128/AEM.05212-11 |
| [59] | Candela M, Rampelli S, Turroni S, et al. Unbalance of intestinal microbiota in atopic children[J]. BMC Microbiol, 2012, 12: 95. DOI:10.1186/1471-2180-12-95 |
| [60] | Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet[J]. Cell, 2018, 173: 1728–1741.e13. DOI:10.1016/j.cell.2018.04.027 |
| [61] | Wang Q, Huang SQ, Li CQ, et al. Akkermansia muciniphila may determine chondroitin sulfate ameliorating or aggravating osteoarthritis[J]. Front Microbiol, 2017, 8: 1955. DOI:10.3389/fmicb.2017.01955 |
| [62] | Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse[J]. Diabetologia, 2012, 55: 2285–2294. DOI:10.1007/s00125-012-2564-7 |
| [63] | Dubourg G, Lagier JC, Armougom F, et al. High-level colonisa tion of the human gut by Verrucomicrobia following broad-spec trum antibiotic treatment[J]. Int J Antimicrob Agents, 2013, 41: 149–155. DOI:10.1016/j.ijantimicag.2012.10.012 |
| [64] | Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metage nomic outcomes from early-life pulsed antibiotic treatment[J]. Nat Commun, 2015, 6: 7486. DOI:10.1038/ncomms8486 |
| [65] | Ferrer M, Martins dos Santos VA, Ott SJ, et al. Gut microbiota disturbance during antibiotic therapy:a multi-omic approach[J]. Gut Microbes, 2014, 5: 64–70. DOI:10.4161/gmic.27128 |
| [66] | Anhê FF, Roy D, Pilon G, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice[J]. Gut, 2015, 64: 872–883. DOI:10.1136/gutjnl-2014-307142 |
| [67] | Chaplin A, Parra P, Serra F, et al. Conjugated linoleic acid supplementation under a high-fat diet modulates stomach protein expression and intestinal microbiota in adult mice[J]. PLoS One, 2015, 10: e0125091. DOI:10.1371/journal.pone.0125091 |
| [68] | Andersson KE, Axling U, Xu J, et al. Diverse effects of oats on cholesterol metabolism in C57BL/6 mice correlate with expression of hepatic bile acid-producing enzymes[J]. Eur J Nutr, 2013, 52: 1755–1769. DOI:10.1007/s00394-012-0479-1 |
| [69] | Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvi ronment[J]. Gut, 2015, 64: 93–100. DOI:10.1136/gutjnl-2014-307264 |
| [70] | Zhong YD, Nyman M, Fåk F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt[J]. Mol Nutr Food Res, 2015, 59: 2066–2076. DOI:10.1002/mnfr.201500187 |
| [71] | Gómez-Gallego C, Collado MC, Ilo T, et al. Infant formula supplemented with polyamines alters the intestinal microbiota in neonatal BALB/cOlaHsd mice[J]. J Nutr Biochem, 2012, 23: 1508–1513. DOI:10.1016/j.jnutbio.2011.10.003 |
| [72] | Song HZ, Chu Q, Yan FJ, et al. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice[J]. J Gastroenterol Hepatol, 2016, 31: 1462–1469. DOI:10.1111/jgh.2016.31.issue-8 |
| [73] | Alard J, Lehrter V, Rhimi M, et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resis tance in mice are associated with improvement of dysbiotic gut microbiota[J]. Environ Microbiol, 2016, 18: 1484–1497. DOI:10.1111/1462-2920.13181 |
| [74] | Yang JY, Bindels LB, Segura Munoz RR, et al. Disparate metabolic responses in mice fed a high-fat diet supplemented with maize-derived non-digestible feruloylated oligo-and poly saccharides are linked to changes in the gut microbiota[J]. PLoS One, 2016, 11: e0146144. DOI:10.1371/journal.pone.0146144 |
| [75] | Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota[J]. Nature, 2014, 514: 181–186. DOI:10.1038/nature13793 |
| [76] | Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice[J]. Gut, 2014, 63: 727–735. DOI:10.1136/gutjnl-2012-303839 |
| [77] | Wang JH, Bose S, Kim GC, et al. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota[J]. PLoS One, 2014, 9: e86117. DOI:10.1371/journal.pone.0086117 |
| [78] | Wang JH, Bose S, Kim HG, et al. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in associ ation with regulation of intestinal permeability and microbiota in rats[J]. Sci Rep, 2015, 5: 8391. DOI:10.1038/srep08391 |
| [79] | Kemperman RA, Gross G, Mondot S, et al. Impact of poly-phenols from black tea and red wine/grape juice on a gut model microbiome[J]. Food Res Int, 2013, 53: 659–669. DOI:10.1016/j.foodres.2013.01.034 |
| [80] | Axling U, Olsson C, Xu J, et al. Green tea powder and Lactoba-cillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice[J]. Nutr Metab, 2012, 9: 105. DOI:10.1186/1743-7075-9-105 |
| [81] | Roopchand DE, Carmody RN, Kuhn P, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome[J]. Diabetes, 2015, 64: 2847–2858. DOI:10.2337/db14-1916 |
| [82] | Reid DT, Eller LK, Nettleton JE, et al. Postnatal prebiotic fibre intake mitigates some detrimental metabolic outcomes of early overnutrition in rats[J]. Eur J Nutr, 2016, 55: 2399–2409. DOI:10.1007/s00394-015-1047-2 |
| [83] | Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility[J]. Cell, 2016, 167: 1339–1353. DOI:10.1016/j.cell.2016.10.043 |
| [84] | Lee H, Ko GP. Effect of metformin on metabolic improvement and gut microbiota[J]. Appl Environ Microbiol, 2014, 80: 5935–5943. DOI:10.1128/AEM.01357-14 |
| [85] | Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut micro-biota[J]. Nat Commun, 2015, 6: 7489. DOI:10.1038/ncomms8489 |
| [86] | Marcial-Coba MS, Cieplak T, Cahú TB, et al. Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage[J]. Food Funct, 2018, 9: 5868–5879. DOI:10.1039/C8FO01331D |
| [87] | Marcial-Coba MS, Saaby L, Knøchel S, et al. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei[J]. FEMS Microbiol Lett, 2019. DOI:10.1093/femsle/fny290 |
 2019, Vol. 54
2019, Vol. 54


