由于早期诊断技术的进步和治疗方案的优化, 多数恶性肿瘤的临床预后有所改善。但抗肿瘤药物, 尤其是化疗药物长期应用所致的耐药问题仍然是目前肿瘤治疗的一大障碍。因此, 阐述肿瘤耐药机制, 寻找克服或逆转肿瘤耐药的策略一直是肿瘤治疗的热门课题。外泌体是机体细胞分泌的直径40~100 nm的双层膜性囊泡, 可参与细胞间的信息传递及微环境调节, 在肿瘤细胞的多种恶性生物学行为的调控过程中发挥重要作用[1]。近年来研究发现, 肿瘤来源或与肿瘤相关的外泌体是调控肿瘤耐药的重要机制, 可以通过传递RNA、蛋白质等分子赋予敏感细胞耐药性[2]。本文将对近年来外泌体介导肿瘤耐药发生及机制的研究进展作一综述。
1 外泌体细胞外囊泡(extracellular vesicles, EVs)是指从细胞膜上脱落或者由细胞分泌的双层膜结构的囊泡状小体, 根据直径大小大致分为3种:外泌体(40~100 nm)、微囊泡(microvesicles, 100~1 000 nm)和凋亡小体(apoptotic body, 1~4 μm)[3]。外泌体是在细胞多泡体中形成的微囊泡, 通过与细胞膜融合将外泌体释放到细胞外环境中[4, 5]。外泌体可以由各种类型的细胞产生, 囊泡内含有蛋白质、脂质、核酸(DNA、lncRNA、miRNAs、mRNAs), 此外也有研究发现外泌体中含有环状RNA (circRNA), 外泌体作为细胞间信号转导的媒介, 参与体内多种生理和病理活动[6-8]。外泌体最早由Pan等[9]研究者在研究网织红细胞向成熟红细胞转变过程时发现。随着研究的逐渐深入, 科学家发现除了网织红细胞以外, T细胞、B细胞、树突细胞、肥大细胞等血细胞以及肿瘤细胞、上皮细胞、间充质干细胞、神经细胞都会分泌外泌体, 随后外泌体会进入血液、唾液及尿液等体液中, 通过循环系统到达其他细胞与组织, 产生远程调控作用[10-13]。当将外泌体与细胞膜进行比较时, 一些研究观察到外泌体具有鞘磷脂、胆固醇、饱和脂肪酸和磷脂酰丝氨酸的更高表达。通过电子显微镜分析外泌体通常显示“碟状”或“杯形”形态[14]。另外, 外泌体表面存在多种蛋白质, 如四跨膜蛋白(CD9、CD63、CD81)、溶酶体蛋白(Lamp2b)、热休克蛋白(Hsc70)和融合蛋白(CD9、flotillin、膜联蛋白), 四跨膜蛋白已被用作外泌体标记物区分它们与微囊泡、凋亡小体和其他囊泡[15, 16]。外泌体最早被认为是细胞的垃圾袋, 来清除一些不需要的蛋白质, 但在近几年研究中表明, 外泌体所携带的货物具有重要的生物学意义, 尤其外泌体所包含的许多RNA、蛋白质已被证实会参与到肿瘤发生等重要疾病发生过程[17]。
2 外泌体介导的肿瘤耐药 2.1 外泌体参与肿瘤微环境调节肿瘤微环境是由肿瘤细胞、内皮细胞、炎症细胞、免疫细胞、成纤维细胞及细胞外基质等共同构成的利于肿瘤发生、发展和转移的局部病理环境[18, 19]。肿瘤微环境中的外泌体主要通过3种途径完成肿瘤细胞间的信息交流和物质运输。一是通过抗原递呈和受体-配体的相互作用激活特定的信号转导通路进而将外泌体中的生物活性分子如ncRNA和蛋白质等内容物释放到靶细胞中。二是外泌体自身的膜蛋白与靶细胞的胞膜直接融合后将内容物释放到靶细胞中。三是靶细胞通过胞饮、吞噬作用或受体介导的内吞作用摄取外泌体及其内容物从而实现细胞间的物质运输和信息交流。外泌体作为细胞间通讯的介质穿梭于肿瘤微环境中, 被周围的癌细胞或基质细胞吸收, 并可通过释放内容物传递信息从而引起肿瘤细胞的增殖、侵袭和转移及耐药[20, 21]。
2.2 外泌体参与肿瘤局部免疫微环境调节肿瘤微环境包含大量的免疫细胞如巨噬细胞、T淋巴细胞、B淋巴细胞、肥大细胞、中性粒细胞、树突状细胞、自然杀伤细胞等。其中肿瘤相关巨噬细胞和T淋巴细胞在肿瘤发生、发展及耐药中发挥着重要的作用。肿瘤微环境中的外泌体在肿瘤细胞与免疫细胞之间的互相作用中发挥着关键作用[22]。已有大量研究表明, 肿瘤细胞来源的外泌体具有抗肿瘤免疫和促进肿瘤免疫逃逸的双重作用[23, 24]。有研究表明, 免疫细胞来源的外泌体被肿瘤细胞摄取后通过释放内容物诱导肿瘤细胞产生耐药性[25]。
2.3 外泌体在肿瘤耐药发生中的作用及机制目前肿瘤化疗耐药是肿瘤治疗的主要挑战。研究表明, 肿瘤细胞及基质细胞分泌的外泌体可以将其内容物(DNA、mRNA、miRNA、lncRNA、蛋白质等)转运到受体细胞中从而改变受体细胞的表型进而增强或诱导受体细胞产生耐药性(图 1、表 1[26-39])。
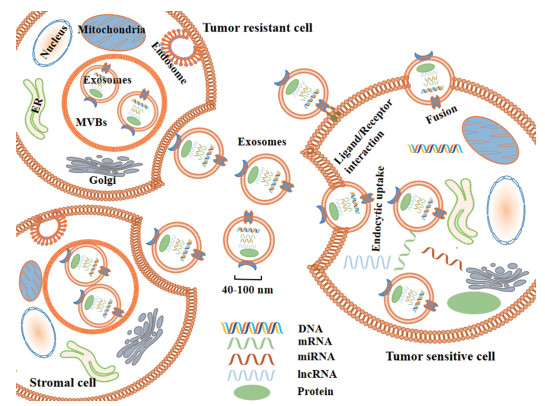
|
Figure 1 Schematic diagram of exosome-mediated tumor resistance. ER: Endoplasmic reticulum; MVBs: Multivesicular bodies; Golgi: Golgi apparatus |
| Table 1 Exosomes of different tumor cells are involved in tumor resistance |
肿瘤的异质性是恶性肿瘤的特征之一, 通常表现在同一瘤体内的肿瘤细胞存在不同的特性和差异, 如不同肿瘤细胞对化疗药物的敏感性不一致[40]。根据这一特性, 可以将肿瘤细胞分为耐药细胞和敏感细胞两类, 这种存在于肿瘤细胞之间对化疗药物敏感性的不一致性, 可以在细胞之间传递, 如耐药细胞可以传递其耐药性给敏感细胞从而使其获得耐药性[41]。肿瘤来源的外泌体, 在肿瘤化疗耐药、放疗耐受、侵袭转移及免疫逃逸等方面发挥重要作用[42, 43]。
非编码RNA (non-coding RNA, ncRNA)指的是不被翻译成蛋白质的一类小RNA, 虽然它们没有编码蛋白质的功能, 但参与蛋白质翻译过程。ncRNA依据核苷酸长度可分为不同的ncRNA:长度小于200个核苷酸的被称为短链非编码RNA (small-non-coding RNA, sncRNA), 如miRNA和tRNA; 长度大于200个核苷酸的被称之为长链非编码RNA (long-non-coding RNA, lncRNA)[44]。已有大量研究表明, ncRNA尤其是miRNA和lncRNA在化疗耐药中起着重要作用[45, 46]。耐药肿瘤细胞源性外泌体可以在细胞外环境中通过释放ncRNA和蛋白质等从而将耐药性传递给药物敏感的细胞[47, 48]。
Mikamori等[26]研究表明miR-155在吉西他滨耐药的胰腺导管腺癌(PDAC)细胞和致吉西他滨耐药的外泌体中过度表达, 并在临床样本中发现在吉西他滨治疗后, 患者的PDAC上皮细胞表现出高miRNA-155表达以及不良的预后情况, 进一步研究证实了以下重要发现: ①吉西他滨的长期用药增加了PDAC细胞中的miR-155表达; ② miR-155表达水平与致吉西他滨耐药的外泌体分泌呈正相关; ③外泌体将miR-155传递给其他PDAC细胞并诱导其耐药性。因此对miR-155或外泌体分泌的靶向治疗能够有效地减弱耐药性。有研究发现, 外泌体转移多种miRNA使乳腺癌敏感细胞对多种药物产生耐药性, 如外泌体分泌及转移miR-17、miR-30a、miR-100、miR-222进而增强敏感细胞对多西他赛和多柔比星的耐药性[27]。在卵巢癌中, 外泌体通过转移miR-433促进敏感细胞紫杉醇耐药[32]。Liu等[33]研究团队建立了裸鼠皮下异种移植模型, 将顺铂与来源于顺铂耐药的口腔鳞状癌细胞(OSCC) HSC-3-R和亲本OSCC细胞HSC-3的外泌体注射入小鼠体内。结果表明, 源于HSC-3-R细胞的外泌体促进肿瘤生长, 增强顺铂耐药性, 此研究进一步证实了含有miR-21的外泌体通过靶向口腔鳞状细胞癌中的PTEN和PDCD4转移顺铂耐药。
此外, lncRNA对肿瘤耐药中也有一定的影响。Kang等[34]建立了吉非替尼抗性细胞系并发现耐药细胞的外泌体能够传递lncRNA PART1到敏感细胞并通过调节miR-129/Bcl-2途径促进晚期食管鳞状细胞癌(ESCC)对吉非替尼耐药, 该团队在临床样本研究中发现吉非替尼耐药患者血清外泌体中的lncRNA PART1的含量高于非耐药患者, 外泌体中高水平的血清lncRNA PART1与ESCC患者对吉非替尼治疗的反应差有关。在晚期肾细胞癌(RCC)中, 耐药细胞分泌的外泌体传递lncARSR并竞争性结合miR-34/miR-449以促进AXL和c-Met表达, 从而赋予细胞舒尼替尼抗性。该研究证实靶向关闭lncARSR或用AXL/c-MET抑制剂治疗舒尼替尼耐药RCC可以有效缓解肿瘤细胞对舒尼替尼抵抗[35]。Huang等[28]研究表明, lncRNA AX747207及其潜在的靶向基因RUNX3与乳腺癌的化疗耐药有关。据报道, 外泌体介导的细胞间通路为肝细胞癌(HCC)对化疗应激耐受提供了新的见解。lncVLDLR参与化疗耐药, 并使其耐药性转移至HCC中的受体细胞, 但其机制尚不清楚[49]。此外, 有研究表明, TGFβ可降低HCC细胞对索拉非尼或多柔比星的敏感性, 并且外泌体中的lncRNA ROR通过激活TGFβ信号通路使HCC受体细胞获得耐药性[36]。在雌激素受体(ER)阳性乳腺癌细胞中, lncRNA UCA1被载入外泌体中, 导致他莫昔芬抗性[29]。在非小细胞肺癌中, 外泌体介导转移的lncRNA RP11838N2.4促进埃罗替尼耐药[37]。在膀胱癌中, 外泌体中的lncRNA UCA1可以通过活化Wnt信号通路增加膀胱癌细胞的化疗耐药性[38]。
2.3.2 肿瘤细胞来源的外泌体通过转运活性蛋白质参与耐药肿瘤来源的外泌体可以将蛋白质转运至受体细胞中, 从而使肿瘤细胞获得耐药性。研究人员发现, 乳腺癌耐药细胞株MCF-7/DOC外泌体中的P-糖蛋白(P-glycoprotein, P-gp)明显高于乳腺癌细胞MCF-7/S, 富含P-gp的外泌体可以向MCF-7/S细胞传递多西他赛耐药性[30]。在结肠癌的研究中发现, 结肠癌敏感细胞株Coca-2与耐药细胞株RKO分泌的外泌体共培养后耐药性升高, 进一步研究发现耐药细胞RKO的外泌体可通过下调PTEN蛋白和增加磷酸化的Akt水平诱导Coca-2细胞的西妥珠单抗耐药性[39]。Ning等[31]发现乳腺癌耐药细胞高表达UCH-L1蛋白并通过外泌体将这些蛋白释放到肿瘤微环境中, 从而将化疗耐药性转移至受体细胞。随后研究人员进一步证实高表达的UCH-L1可通过激活MAPK/ERK信号途径上调P-gp的表达, 从而增强乳腺癌耐药性[50]。
2.3.3 基质细胞来源的外泌体参与耐药除了肿瘤细胞之间的相互影响之外, 一些基质细胞的外泌体也可以对肿瘤细胞的耐药发挥作用。在胰腺导管腺癌(PDAC)中, 巨噬细胞来源的外泌体(MDE)转移miR-365诱导PDAC小鼠对吉西他滨的抗药性, 该研究发现有外泌体分泌缺陷的小鼠对吉西他滨的敏感性显著高于野生型小鼠[25]。Lobb等[51]研究表明, 源自间充质、癌基因转化的肺细胞的外泌体可能通过ZEB1 mRNA的转移将化学抗性和间充质表型转移到受体细胞中。Au Yeung等[52]使用下一代测序技术, 鉴定出肿瘤相关的脂肪细胞(CAAs)和成纤维细胞(CAFs)来源的外泌体比卵巢癌细胞来源的外泌体含有更高水平的miR-21, 功能研究表明, miR-21从CAAs或CAFs转移至卵巢癌细胞, 抑制卵巢癌细胞凋亡并直接结合它的新靶点APAF1从而使癌细胞耐药, 这些数据表明, 在网膜肿瘤微环境中相邻基质细胞来源的外泌体通过传递miR-21改变转移性卵巢癌细胞的恶性表型, 而抑制基质来源的miR-21的转移是治疗转移性和复发性卵巢癌的另一种方式。此外, 通过PGE2/EP4拮抗可诱导外泌体介导的肿瘤干细胞清除, 从而来增强肿瘤化疗敏感性以克服间充质干细胞对化疗药物的耐药性[53]。Ji等[54]发现人间充质干细胞来源的外泌体通过激活钙/钙调蛋白依赖性蛋白激酶(CaMK)和Raf/MEK/ERK通路来拮抗5-氟尿嘧啶诱导的细胞凋亡并增强多药耐药(multi-drug resistance, MDR)蛋白质的表达, 从而诱导胃癌细胞对5-氟尿嘧啶耐药。
2.3.4 外泌体直接介导药物分子的外排肿瘤来源的外泌体可以包裹肿瘤细胞内药物, 介导药物分子的外排从而降低药效。Shedden等[55]使用荧光抗肿瘤药物多柔比星进行脉冲追踪实验, 证实肿瘤细胞通过脱落囊泡(外泌体)将药物外排。随后的研究发现在多种恶性肿瘤中, 细胞内体和多囊泡小体(MVBs)的界膜上富含大量的转运蛋白, 例如P-gp、多药耐药相关蛋白1 (MRP-1)、乳腺癌耐药蛋白(BCRP)和ATP转运蛋白(ABCA3)等蛋白, 这类蛋白具有相同的跨膜结构域, 又称为MDR-ABC转运体蛋白, 当此种转运体系被激活后, 胞内的化疗药物分子及其代谢产物可被MDR-ABC转运至内体, 内体进一步聚集形成MVBs, MVBs与细胞膜融合后释放外泌体, 药物便以外泌体为载体, 从胞内排至胞外, 致使肿瘤细胞产生耐药性[56-59]。
3 外泌体在肿瘤耐药治疗中的作用外泌体在肿瘤耐药中发挥着重要作用。不但肿瘤细胞来源的外泌体可以传递耐药, 基质细胞来源的外泌体也可以传递耐药, 以至于降低了化疗疗效。因此可以使外泌体充当纳米粒子传递抗miRNA或通过抑制外泌体释放、改变外泌体成分来逆转肿瘤耐药性从而提高化疗疗效[60]。有研究表明, 外泌体可以充当纳米粒递送抗miRNA-214来逆转胃癌对顺铂的耐药性[61]。雷帕霉素和U18666A可以分别通过干扰MVBs的合成和干扰胆固醇参与细胞膜的形成来抑制外泌体的释放从而提高B淋巴瘤对利妥昔单抗的敏感性[62]。也有研究人员发现, β-榄香烯(β-elemene)能作用于乳腺癌细胞株的靶基因来影响外泌体内耐药相关的miRNA表达, 从而降低外泌体的耐药传递, 增强化疗敏感性[63]。
4 前景与挑战外泌体是细胞间物质和信息的交流工具, 在肿瘤微环境中发挥重要作用。外泌体及其所含的miRNAs、lncRNAs和蛋白质等都与肿瘤耐药的发生密切相关。它们通过与包括肿瘤细胞在内的靶细胞融合或相作用介导特定的细胞与细胞之间相互作用, 激活细胞中的信号通路。已经有新的证据表明, 外泌体内容物在调节肿瘤微环境和肿瘤发展中发挥重要作用。然而, 外泌体和外泌体内容物(RNA和蛋白质等)在肿瘤微环境中的生理和病理作用还需待进一步探索。同时, 体液中外泌体的数量和异质性可能导致肿瘤诊断的假阴性或假阳性, 因此外泌体作为生物学标志物有一定的缺陷。为了克服这些障碍, 研究者需要更多地了解外泌体在肿瘤进展中精确的调控机制。预计在不久的将来, 外泌体可能用作液体活组织检查和无创生物标志物, 用于肿瘤的早期检测, 同时外泌体作为药物载体治疗肿瘤也将成为一种有效治疗策略。对外泌体介导的肿瘤化疗耐药中的深入研究, 将有助于揭示肿瘤耐药的分子机制, 同时寻找耐药标志物为肿瘤新的治疗方式提供了可能。
| [1] | Raposo G, Stoorvogel W. Extracellular vesicles:exosomes, microvesicles, and friends[J]. J Cell Biol, 2013, 200: 373–383. DOI:10.1083/jcb.201211138 |
| [2] | Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance:a comprehensive review[J]. Cancer Metastasis Rev, 2013, 32: 623–642. DOI:10.1007/s10555-013-9441-9 |
| [3] | Yuan P, Guo XC, Zhang JP, et al. Research progress of the exosomes as drug delivery vehicles of Chinese herbal drugs[J]. Acta Pharm Sin (药学学报), 2017, 52: 1667–1672. |
| [4] | Zhuo X, Chang A, Huang C, et al. Expression and clinical significance of microvessel density and its association with TWIST in nasopharyngeal carcinoma[J]. Int J Clin Exp Med, 2015, 8: 1265–1270. |
| [5] | Yu DD, Wu Y, Shen HY, et al. Exosomes in development, metastasis and drug resistance of breast cancer[J]. Cancer Sci, 2015, 106: 959–964. DOI:10.1111/cas.2015.106.issue-8 |
| [6] | Sheridan C. Exosome cancer diagnostic reaches market[J]. Nat Biotechnol, 2016, 34: 359–360. DOI:10.1038/nbt0416-359 |
| [7] | Taylor DD, Lyons KS, Gercel-Taylor C. Shed membrane fragment-associated markers for endometrial and ovarian cancers[J]. Gynecol Oncol, 2002, 84: 443–448. DOI:10.1006/gyno.2001.6551 |
| [8] | Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences[J]. Nat Commun, 2011, 2: 180. DOI:10.1038/ncomms1180 |
| [9] | Pan BT, Teng K, Wu C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes[J]. J Cell Biol, 1985, 101: 942–948. DOI:10.1083/jcb.101.3.942 |
| [10] | Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes[J]. Proc Natl Acad Sci U S A, 2014, 111: 14888–14893. DOI:10.1073/pnas.1408301111 |
| [11] | Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma[J]. Int Immunol, 2005, 17: 879–887. DOI:10.1093/intimm/dxh267 |
| [12] | Shi R, Wang PY, Li XY, et al. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients[J]. Oncotarget, 2015, 6: 26971–26981. |
| [13] | Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk[J]. J Immunol, 2007, 179: 1969–1978. DOI:10.4049/jimmunol.179.3.1969 |
| [14] | Stranford DM, Leonard JN. Delivery of biomolecules via extracellular vesicles:a budding therapeutic strategy[J]. Adv Genet, 2017, 98: 155–175. DOI:10.1016/bs.adgen.2017.08.002 |
| [15] | Poliakov A, Spilman M, Dokland T, et al. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen[J]. Prostate, 2009, 69: 159–167. DOI:10.1002/pros.v69:2 |
| [16] | Blanchard N, Lankar D, Faure F, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex[J]. J Immunol, 2002, 168: 3235–3241. DOI:10.4049/jimmunol.168.7.3235 |
| [17] | Vyas N, Dhawan J. Exosomes:mobile platforms for targeted and synergistic signaling across cell boundaries[J]. Cell Mol Life Sci, 2017, 74: 1567–1576. DOI:10.1007/s00018-016-2413-9 |
| [18] | Hede K. Environmental protection:studies highlight importance of tumor microenvironment[J]. J Natl Cancer Inst, 2004, 96: 1120–1121. DOI:10.1093/jnci/96.15.1120 |
| [19] | Hanahan D, Weinberg RA. Hallmarks of cancer:the next generation[J]. Cell, 2011, 144: 646–674. DOI:10.1016/j.cell.2011.02.013 |
| [20] | Cho JA, Park H, Lim EH, et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts[J]. Gynecol Oncol, 2011, 123: 379–386. DOI:10.1016/j.ygyno.2011.08.005 |
| [21] | Chowdhury R, Webber JP, Gurney M, et al. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts[J]. Oncotarget, 2015, 6: 715–731. |
| [22] | Shalapour S, Karin M. Immunity, inflammation, and cancer:an eternal fight between good and evil[J]. J Clin Invest, 2015, 125: 3347–3355. DOI:10.1172/JCI80007 |
| [23] | Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells[J]. J Natl Cancer Inst, 2015, 107: 363. |
| [24] | Lundholm M, Schroder M, Nagaeva O, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells:mechanism of immune evasion[J]. PLoS One, 2014, 9: e108925. DOI:10.1371/journal.pone.0108925 |
| [25] | Binenbaum Y, Fridman E, Yaari Z, et al. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma[J]. Cancer Res, 2018, 78: 5287–5299. DOI:10.1158/0008-5472.CAN-18-0124 |
| [26] | Mikamori M, Yamada D, Eguchi H, et al. MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma[J]. Sci Rep, 2017, 7: 42339. DOI:10.1038/srep42339 |
| [27] | Chen WX, Liu XM, Lv MM, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs[J]. PLoS One, 2014, 9: e95240. DOI:10.1371/journal.pone.0095240 |
| [28] | Huang L, Zeng L, Chu J, et al. Chemoresistance related long noncoding RNA expression profiles in human breast cancer cells[J]. Mol Med Rep, 2018, 18: 243–253. |
| [29] | Xu CG, Yang MF, Ren YQ, et al. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells[J]. Eur Rev Med Pharmacol Sci, 2016, 20: 4362–4368. |
| [30] | Lv MM, Zhu XY, Chen WX, et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein[J]. Tumour Biol, 2014, 35: 10773–10779. DOI:10.1007/s13277-014-2377-z |
| [31] | Ning K, Wang T, Sun X, et al. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer[J]. J Surg Oncol, 2017, 115: 932–940. DOI:10.1002/jso.v115.8 |
| [32] | Weiner-Gorzel K, Dempsey E, Milewska M, et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells[J]. Cancer Med, 2015, 4: 745–758. DOI:10.1002/cam4.409 |
| [33] | Liu T, Chen G, Sun D, et al. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma[J]. Acta Biochim Biophys Sin, 2017, 49: 808–816. DOI:10.1093/abbs/gmx078 |
| [34] | Kang M, Ren M, Li Y, et al. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA[J]. J Exp Clin Cancer Res, 2018, 37: 171. DOI:10.1186/s13046-018-0845-9 |
| [35] | Qu L, Ding J, Chen C, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA[J]. Cancer Cell, 2016, 29: 653–668. DOI:10.1016/j.ccell.2016.03.004 |
| [36] | Takahashi K, Yan IK, Kogure T, et al. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer[J]. FEBS Open Bio, 2014, 4: 458–467. DOI:10.1016/j.fob.2014.04.007 |
| [37] | Zhang W, Cai X, Yu J, et al. Exosome-mediated transfer of lncRNA RP11838N2.4 promotes erlotinib resistance in non-small cell lung cancer[J]. Int J Oncol, 2018, 53: 527–538. |
| [38] | Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling[J]. FEBS J, 2014, 281: 1750–1758. DOI:10.1111/febs.12737 |
| [39] | Zhang S, Zhang Y, Qu J, et al. Exosomes promote cetuximab resistance via the PTEN/Akt pathway in colon cancer cells[J]. Braz J Med Biol Res, 2017, 51: e6472. |
| [40] | He QY. Tumor heterogeneity and drug resistance of targeted antitumor agents[J]. Acta Pharm Sin (药学学报), 2016, 51: 197–201. |
| [41] | Kibria G, Hatakeyama H, Harashima H. Cancer multidrug resistance:mechanisms involved and strategies for circumvention using a drug delivery system[J]. Arch Pharm Res, 2014, 37: 4–15. DOI:10.1007/s12272-013-0276-2 |
| [42] | Deng H, Zhang J, Shi J, et al. Role of long non-coding RNA in tumor drug resistance[J]. Tumour Biol, 2016, 37: 11623–11631. DOI:10.1007/s13277-016-5125-8 |
| [43] | Fan Q, Yang L, Zhang X, et al. The emerging role of exosome-derived non-coding RNAs in cancer biology[J]. Cancer Lett, 2018, 414: 107–115. DOI:10.1016/j.canlet.2017.10.040 |
| [44] | Liu H, Li Z, Wang C, et al. Expression of long non-coding RNA-HOTAIR in oral squamous cell carcinoma Tca8113 cells and its associated biological behavior[J]. Am J Transl Res, 2016, 8: 4726–4734. |
| [45] | Wang Y, Zhang L, Zheng X, et al. Long non-coding RNA LINC00161 sensitises osteosarcoma cells to cisplatin-induced apoptosis by regulating the miR-645-IFIT2 axis[J]. Cancer Lett, 2016, 382: 137–146. DOI:10.1016/j.canlet.2016.08.024 |
| [46] | Yan J, Cheng Y, Chen J. Chemoresistance and non-coding RNA[J]. Chin J Pathol (中华病理学杂志), 2016, 45: 498–500. |
| [47] | Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression[J]. Cell Mol Life Sci, 2015, 72: 1–10. DOI:10.1007/s00018-014-1710-4 |
| [48] | Hu W, Tan C, He Y, et al. Functional miRNAs in breast cancer drug resistance[J]. Onco Targets Ther, 2018, 11: 1529–1541. DOI:10.2147/OTT |
| [49] | Kunej T, Obsteter J, Pogacar Z, et al. The decalog of long non-coding RNA involvement in cancer diagnosis and monitoring[J]. Crit Rev Clin Lab Sci, 2014, 51: 344–357. DOI:10.3109/10408363.2014.944299 |
| [50] | Wang W, Zou L, Zhou D, et al. Overexpression of ubiquitin carboxyl terminal hydrolase-L1 enhances multidrug resistance and invasion / metastasis in breast cancer by activating the MAPK / Erk signaling pathway[J]. Mol Carcinog, 2016, 55: 1329–1342. DOI:10.1002/mc.v55.9 |
| [51] | Lobb RJ, van Amerongen R, Wiegmans A, et al. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance[J]. Int J Cancer, 2017, 141: 614–620. DOI:10.1002/ijc.30752 |
| [52] | Au Yeung CL, Co NN, Tsuruga T, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1[J]. Nat Commun, 2016, 7: 11150. DOI:10.1038/ncomms11150 |
| [53] | Wang J, Zhang L, Kang D, et al. Activation of PGE2/EP2 and PGE2/EP4 signaling pathways positively regulate the level of PD-1 in infiltrating CD8(+) T cells in patients with lung cancer[J]. Oncol Lett, 2018, 15: 552–558. |
| [54] | Ji R, Zhang B, Zhang X, et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer[J]. Cell Cycle, 2015, 14: 2473–2483. DOI:10.1080/15384101.2015.1005530 |
| [55] | Shedden K, Xie XT, Chandaroy P, et al. Expulsion of small molecules in vesicles shed by cancer cells:association with gene expression and chemosensitivity profiles[J]. Cancer Res, 2003, 63: 4331–4337. |
| [56] | Sharma A. Chemoresistance in cancer cells:exosomes as potential regulators of therapeutic tumor heterogeneity[J]. Nanomedicine (Lond), 2017, 12: 2137–2148. DOI:10.2217/nnm-2017-0184 |
| [57] | Chen F, Zhang W, Song S, et al. The influence of exosomes derived from tumor cells and stromal cells on tumor drug resistance[J]. Chin J Cancer Biother (中国肿瘤生物治疗杂志), 2016, 23: 432–436. |
| [58] | Koch R, Aung T, Vogel D, et al. Nuclear trapping through inhibition of exosomal export by indomethacin increases cytostatic efficacy of doxorubicin and pixantrone[J]. Clin Cancer Res, 2016, 22: 395–404. DOI:10.1158/1078-0432.CCR-15-0577 |
| [59] | Chapuy B, Koch R, Radunski U, et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration[J]. Leukemia, 2008, 22: 1576–1586. DOI:10.1038/leu.2008.103 |
| [60] | Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes:current perspectives and future challenges[J]. Acta Pharm Sin B, 2016, 6: 287–296. DOI:10.1016/j.apsb.2016.02.001 |
| [61] | Wang X, Zhang H, Bai M, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer[J]. Mol Ther, 2018, 26: 774–783. DOI:10.1016/j.ymthe.2018.01.001 |
| [62] | Aung T, Chapuy B, Vogel D, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3[J]. Proc Natl Acad Sci U S A, 2011, 108: 15336–15341. DOI:10.1073/pnas.1102855108 |
| [63] | Zhang J, Zhang HD, Yao YF, et al. β-Elemene reverses chemoresistance of breast cancer cells by reducing resistance transmission via exosomes[J]. Cell Physiol Biochem, 2015, 36: 2274–2286. DOI:10.1159/000430191 |
 2019, Vol. 54
2019, Vol. 54


