恶性肿瘤是目前影响人类健康的最主要疾病之一, 化疗是目前肿瘤治疗的主要方式, 但大多数化疗药物水溶性较差、生物利用度不高, 且基于单一抗肿瘤机制的单药治疗作用较弱, 长时间使用可能激发肿瘤其他相关恶性增殖机制, 导致药物疗效降低、肿瘤多药耐药(multidrug resistance, MDR)、肿瘤复发及机体不良反应等[1]。联合用药已成为肿瘤治疗的标准策略, 联合用药能够通过调节异常细胞的多个信号通路来获得协同治疗效果, 同时减少MDR现象的发生, 预后良好且不良反应较少[2-4]。目前基于小分子化疗药物的联合用药策略常包括以下几种类型[5]:小分子化疗药物之间的联合、基因药物与小分子药物联合、单克隆抗体与小分子药物联合、中药(天然)活性成分与小分子化疗药物的联合。其中, 中药(天然)活性成分来源广泛, 目前已发现多种成分具有抗肿瘤的作用, 在抗肿瘤新药的研究中具有重要意义[6], 大多数毒性较小、安全性高[7, 8], 具有可作用于肿瘤相关机制的多靶点、多通路的特性[9, 10]。因此, 近年来随着天然药物高通量筛选技术的进步, 越来越多研究者将化疗药物的联合应用对象聚焦于中药活性成分, 以联合进行肿瘤治疗。
尽管许多联合用药方案在体外和动物实验研究中展现出显著治疗优势, 但在临床实践中往往收效甚微, 甚至造成药物毒副反应叠加。究其原因在于缺乏高效的体内药物传递。随着纳米技术在药物传递领域的积极探索, 为联合用药抗肿瘤带来了新思路。纳米粒通过粒径控制、结构改造和表面修饰等途径, 可具有较强的载药能力、体内长循环、肿瘤组织被动/主动靶向等特点, 联合药物经纳米粒递送, 可有效改善药物自身性质(如水溶性差、体内循环时间短等), 改善药物间不同药代动力学行为导致的差异化传递, 提高肿瘤组织的药物专属性分布, 从而增强药物的治疗效果并减小毒性等。目前基于纳米载体传递的药物联合抗肿瘤常包括3种方式:所有药物经纳米粒共载; 将不同药物采用纳米粒分别包载后再联合; 某一药物经纳米包载后与其他游离药物联合。其中, 第1种方式有利于精准控制联用药物到达肿瘤组织中的药物比例, 实现最佳比例起效。作者对中药活性成分与化疗药物联合用药的纳米递送策略, 进行了研究现状和应用特色的综述和分析, 旨在揭示基于纳米载体药物共递送联合抗肿瘤的研究意义。
1 中药活性成分与化疗药物联合抗肿瘤的机制中药活性成分具有多靶点抗肿瘤作用特点, 可从诱导肿瘤细胞凋亡、抑制肿瘤血管的生成、诱导肿瘤细胞自噬和调节肿瘤微环境等多方面促进药物联合抗肿瘤(图 1)。
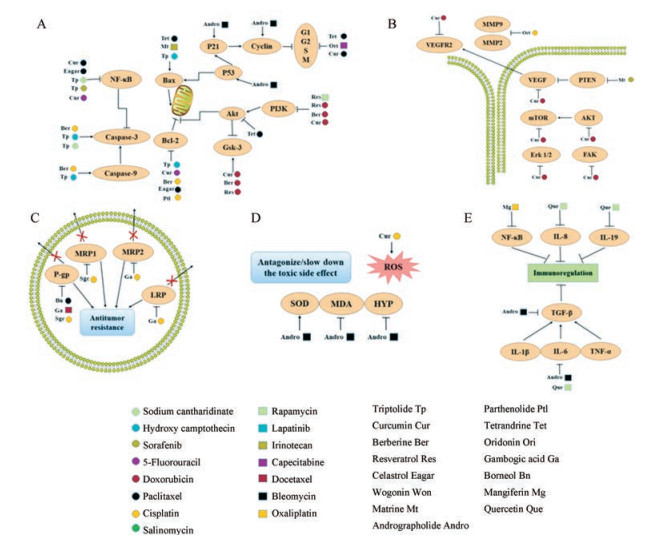
|
Figure 1 The advantages of combining active ingredients of Chinese herbs with small-molecule anticancer drugs. A: Proliferation inhibition; B: Metastasis inhibition; C: Antitumor resistance; D: Antagonize/slow down the toxic side effect; E: Immunoregulation |
肿瘤细胞具有无限增殖的特性, 中药(天然药物)小分子与化疗药物联合给药可从促进肿瘤细胞凋亡、诱导细胞自噬、增强氧化应激、促进增敏和阻滞细胞周期等方面抑制肿瘤细胞增殖, 从而达到治疗肿瘤的作用。Meng等[11]研究报道了雷公藤甲素和羟基喜树碱的联合给药, 其抗肿瘤机制为增强细胞凋亡, 通过调控PP2A控制ERK、p38、MAPKs和Akt信号通路。该研究以A549细胞为实验对象, 探究雷公藤甲素和羟基喜树碱联合用药的分子机制, 从参与PP2A控制的MAPKs和Akt信号通路进行了实验研究。结果表明, 联合给药使其抗肿瘤的毒性增强, 具体作用靶点为增强caspase-3和caspase-9蛋白活性、增强Bax/Bcl-2比率和释放cytochrome C等, 表明抑制了Akt信号通路, 促进了ERK和p38、MAPKs信号通路。该研究提供了一种雷公藤甲素和羟基喜树碱联合用药的机制基础, 从PP2A和PP2A控制的信号通路为出发点进行抗肿瘤研究。
1.2 抑制肿瘤细胞转移肿瘤细胞转移是恶性肿瘤的主要特征, 是引起癌症患者死亡的首要因素。肿瘤细胞释放各种蛋白水解酶, 破坏其黏附部位的组织, 即破坏细胞外基质和血管壁的基底膜, 实现浸润转移和新生血管的生成[12]。而在这一过程中, 基质金属蛋白酶家族(matrix metalloproteinase, MMP)及其抑制剂发挥了重要的作用。Zhang等[13]前期研究了从促进细胞凋亡和抗血管新生两方面实现姜黄素和多柔比星联合治疗肝癌的作用, 表现出更高效地诱导细胞凋亡, 良好的抑制肿瘤血管生成的作用, 包括抑制人脐静脉血管内皮细胞(human umbilical vein endothelial ceils, HUVEC)增殖、转移、侵袭和血管内皮生长因子(vascular endothelial growth factor, VEGF)通路。Zhang等[14]研究报道了冬凌草甲素和顺铂联合给药逆转肿瘤MDR, 该联合给药不仅可以抑制肿瘤移行和侵袭, 并且从MMP2和MMP9蛋白的表达方面阐述了抑制肿瘤MDR的机制。
1.3 抗肿瘤多药耐药肿瘤MDR是指肿瘤细胞在对1种化疗药产生耐药的情况下同时对一系列不同结构和不同机制的化疗药产生耐药的现象, 是临床上导致化疗失败的重要原因[15, 16]。MDR发生机制复杂, 包括细胞内因和肿瘤微环境改变等, 其发生机制的复杂性为克服肿瘤耐药带来挑战[17, 18]。采用纳米载体共递送化疗药物和中药活性成分的一大优势就体现在能够逆转MDR。Zhang等[19]研究表明藤黄酸和顺铂联合用药可下调多药耐药相关蛋白2 (multidrug resistance- associated protein 2, MRP2)和肺耐药蛋白(lung resistance protein, LRP)的表达, 达到治疗肿瘤的作用。其中藤黄酸可阻滞细胞周期G0/G1, 并且上调caspase-3和Bax蛋白的表达, 下调pro-caspase-9和Bcl-2蛋白表达; 当两药合用后, 其细胞凋亡作用增强, 减小抗肿瘤药物顺铂耐药指数。Zou等[20]研究了将冰片与紫杉醇联合用于逆转MDR, 体外细胞实验发现, 联合用药可提高紫杉醇在A2780/PTX细胞内的浓度、细胞摄取,细胞毒性增强,减少MMP蛋白和增强细胞凋亡。
1.4 拮抗/减缓毒副作用化疗药物在治疗肿瘤的同时也会损伤机体正常组织或细胞, 采用中药(天然药)联合化疗药物使用, 可以从多靶点、多途径作用于肿瘤组织, 减少化疗药物的用量从而降低其毒性,并且有些中药(天然药)能够减缓化疗药物所造成的毒副作用, 联合使用能提高临床用药安全性。Guo等[21]利用中药穿心莲的有效成分穿心莲内酯与博来霉素联合用药, 不仅增强抗肿瘤的作用, 还可减缓博来霉素长期使用对机体产生的毒性。实验结果表明, 将穿心莲内酯与博来霉素联合给药后, 使博来霉素单独用药造成的肺纤维化得到缓解, 具体表现在激活超氧化物歧化酶(superoxide dismutase, SOD)、抑制丙二醛(malondialdehyde, MDA)和羟脯氨酸(hydroxyproline, HYP)的产生, 同时衰减一些炎症因子的蛋白表达。Cheng等[22]利用姜黄素能够增敏化疗药物对HCC细胞的作用, 研究了姜黄素和顺铂两种药物的联合使用, 姜黄素能够减轻顺铂药物单独使用时所造成的毒副作用(肾毒性、耳毒性和神经毒性), 提高了临床上合理用药的可能性。
1.5 免疫调节机体的免疫功能状态对肿瘤的发生和发展影响很大, 肿瘤免疫治疗能通过调节患者自身的免疫能力达到识别肿瘤细胞、杀伤肿瘤细胞的目的, 具有能将毒性降至最小的特点。Quagliariello等[23]研究雷帕霉素和槲皮素的联合用药, 发现可明显降低IL-8、IL-6和IL-19细胞因子水平, 提示该联合用药可调节机体免疫状态, 增强肿瘤免疫; 并且可下调VEGF、MMP2和MMP9, 表明该联合用药可抑制肿瘤细胞转移。Guo等[21]研究发现穿心莲内酯和博来霉素联用不仅可以增强博来霉素抗肿瘤的疗效, 而且可以减轻毒副作用, 还可衰减IL-1β、TNF-α、IL-6和TGF-β1细胞因子水平, 调节肿瘤细胞的免疫状态, 表明穿心莲内酯可成为博来霉素的辅助治疗药物。
目前, 多种中药活性成分联合化疗药物的抗肿瘤的研究报道见表 1[11, 13, 14, 19, 21-44]。
| Table 1 Antitumor of active ingredients of Chinese herbs combined with chemotherapeutic drugs. IGF: Insulin-like growth factor; EGFR: Epidermal growth factor receptor; COX: Cyclooxygenase; VEGF: Vascular endothelial growth factor; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; MMP: Matrix metalloproteinase; LRP: Lung resistance protein; SOD: Superoxide dismutase; MDA: Malondialdehyde; HYP: Hydroxyproline; ROS: Reactive oxygen species |
具有合适粒径的纳米药物载体可通过渗透滞留效应(enhanced permeability and retention effect, EPR)增加化疗药物在肿瘤部位的蓄积。因此, 与游离药物比较, 采用载体递送药物可达到更好地逆转MDR的作用[45]。Hu等[46]将槲皮素和替莫唑胺共载于脂质体中, 发现该纳米体系可显著增强药物进入脑组织。再者, 纳米载体还可通过表面修饰, 利用靶头分子与肿瘤细胞表面特异性高表达的受体结合, 达到靶向递送药物[47]。常见的靶向修饰分子包括叶酸[48]、透明质酸[49]、细胞穿膜肽[50]、转铁蛋白[51]和生物素[52]等。Baek等[53]采用多功能脂质纳米粒共递送姜黄素和紫杉醇以逆转肿瘤MDR, 通过结构外侧接有叶酸分子, 可靶向到肿瘤部位的叶酸受体, 该主动靶向可显著地逆转肿瘤MDR。
2.2 保证联合药物最佳比例联合给药的药物比例对抗肿瘤效果的发挥起关键作用, 不合适的药物比例甚至可能会使联合作用的药物产生拮抗作用, 降低治疗效果。将不同药物共载于同一纳米载体, 可改变药物原有药动学特征, 从而确保联用药物以恒定比例进入肿瘤细胞, 有利于发挥药物间协同作用[54]。Houdaihed等[55]研究了一种共递送紫杉醇和依维莫司最优比例的聚合物纳米粒, 由于这两种药物具有显著不同的药代动力学行为, 临床联合用药效果不理想, 因此制备了一种纳米体系, 使紫杉醇和依维莫司能够在体内维持最优比率1:0.5, 从而改善了由于体内药代动力学行为不同造成的联用疗效弱, 精准地控制联合用药在肿瘤部位的比率。
2.3 增强药物瘤内/细胞内多层次作用纳米给药系统可以通过调节联用药物在不同刺激下的释放响应机制和释放速率, 从而控制药物联合治疗的顺序和时程, 以实现更为精准的给药过程, 提高联合作用的效果和特异性[56]。Ruttala等[28]制备了一种能够按顺序释放姜黄素和紫杉醇的脂质体, 先将紫杉醇-白蛋白制成纳米粒, 再利用姜黄素包封此纳米粒, 形成内外两层结构, 从而使姜黄素能够下调NF-κB和Akt信号通路, 增加紫杉醇的治疗作用。在生理条件下带正电荷的物质容易被机体消除, 因此Yang等[57]制备了一种可以多级pH响应的纳米胶束, 该胶束能够实现电荷翻转, 当胶束在机体循环的时候呈电中性, 到达肿瘤组织时电荷翻转为正电荷, 通过静电相互作用促进肿瘤细胞对载体的吞噬。
3 联合抗肿瘤的常用纳米载体类型及特点 3.1 脂质体脂质体的水相和脂质双分子层可以包载多种药物, 如亲水性的药物可以包封于亲水性的核心, 疏水性的药物可以包载于脂质膜层中, 两性药物可定位于水相和膜内部的磷脂上, 蛋白质类抗体还可修饰于脂质体表面赋予其靶向性[58, 59]。Hu等[46]利用二硬脂酰基磷脂酰乙醇胺-聚乙二醇2000 (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000], DSPE-PEG2000)材料制备了一种新型脂质体, 共载替莫唑胺和槲皮素用于耐药细胞株神经胶质瘤U87。透射电子显微镜法揭示, 替莫唑胺-槲皮素共载脂质体纳米粒具有较小的粒径; 体外细胞实验表明, 该脂质体有利于药物细胞摄取, 从而实现替莫唑胺的给药量减少而疗效不减的效果。
3.2 纳米粒脂质纳米粒的性质稳定、制备较简便, 具有一定的缓释作用, 主要适合于难溶性药物的包裹, 被用作静脉注射或局部给药达到靶向定位和控释作用的载体。Xu等[42]制备了一种聚乳酸-羟基乙酸共聚物[poly(lactic-co-glycolic acid), PLGA]脂质纳米粒, 用于多西他赛和藤黄酸的共递送, 首先筛选多西他赛和藤黄酸的最优比率将其包封到PLGA脂质纳米粒, 细胞凋亡实验和免疫印迹分析结果表明, 该共载纳米粒可通过下调P-gp的表达而增强细胞的凋亡, 有效地抑制了肿瘤细胞的MDR。
此外, 为了改善纳米载体的靶向性及稳定性, 近年来国内外学者不断尝试对其表面进行功能化修饰, 以更好地达到主动靶向的目的。Cui等[60]制备了一种具有双重靶向性的纳米粒(图 2A), 该体系同时具有磁性导向和T7转铁蛋白受体。首先合成NH2-PEG3500-T7材料[图 2B(a)], 再制备负载药物的磁性PLGA-PEG-T7纳米粒[图 2B(b)]。实验结果表明, 该系统提高了神经胶质瘤的治疗效果。另外, 也研究了具有叶酸受体[61]、表皮生长因子受体[62]主动靶向性的纳米粒。
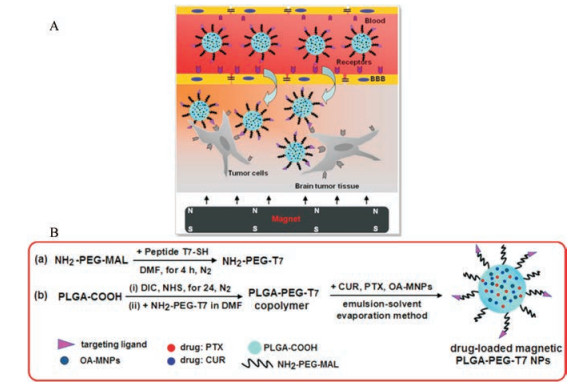
|
图 2 Schematic illustration of blood-brain barrier (BBB)-penetrating andtumor-targeting delivery via the T7-mediated and magnetic-guided, dual-targeting MNP/T7-PLGA NPs (A). Synthesis of PLGA-PEG-T7 polymer (a) and drug-loaded MNP/T7-PLGA NPs (b) (B)[60]. PTX: Paclitaxel; CUR: Curcumin |
利用肿瘤组织和肿瘤细胞内涵体/溶酶体内的酸性微环境, 研发了pH敏感的聚合物纳米载体以实现抗癌药物在肿瘤处的高效快速释放。Peng等[63]研究采用一步制成聚丙烯酸-碳酸钙(polyacrylic acid-calcium carbonate, PAA-CaCO3)纳米粒包封多柔比星和姜黄素两种药物, 当此纳米体系到达肿瘤部位时, 在肿瘤部位的微酸性条件下, pH敏感键断裂, 结构破坏,从而释放出所载药物。
3.3 聚合物胶束聚合物胶束具有疏水性的核心, 常用于包载水溶性较差或疏水性药物, 并且能够提高包载药物的生物利用度, 避免药物在体内被快速降解[59]。Yao等[64]设计了一种能够共载紫杉醇和姜黄素的胶束(图 3), 结果表明该胶束对于疏水性药物的递送具有很大的应用价值。为了更好地控制药物在肿瘤部位的释放, Yang等[57]研制了一种多级pH响应的胶束, 此胶束共递送了紫杉醇和姜黄素用于乳腺癌干细胞的治疗, 具有良好的效果。此胶束所用的材料为聚乙二醇-苯亚胺-聚γ-苄基-L-天冬氨酸-聚乙烯基咪唑[poly(ethylene glycol)-benzoic imine-poly(γ-benzyl-L-aspartate)-b-poly(1-vinylimidazole), mPEG-PBLA-PVIm], 可实现表面电荷由中性到阳性的智能转换, 并且粒径的减小有利于长时间的血液循环和从肿瘤血管的溢出, 促进细胞的摄取和更好的肿瘤渗透率。Sarisozen等[65]为了实现纳米载体对肿瘤部位具有更好的靶向性, 利用转铁蛋白-聚乙二醇-聚乙烯(transferrin-polyethylene glycol-polyethylene, TF-PEG-PE)等材料制备了一种具有转铁蛋白主动靶向的混合胶束。Fang等[66]制备了一种磁性胶束用于共递送多柔比星和姜黄素, 此共载体系具有乳铁蛋白靶向和磁性响应的性质, 此体系相比单一递送任何一种药物, 不仅延长了药物在肿瘤部位的停留时间, 并且更有效地抑制了肿瘤。

|
Figure 3 Scheme of the bottlebrush PEG-PNB-TC polymericmicelles. The combination of PTX and CUR showed synergistic anticancer effect in both the drug mixture and drug coloaded micelles[64]. PTX: Paclitaxel |
聚合物-药物结合物在机体内转运过程中保持稳定, 通过对连接基团进行合理设计, 获得生理环境如pH值、酶、温度及磁等敏感性质, 从而实现肿瘤靶向部位有效释放药物[67]。Xue等[68]制备了一种能够维持体外细胞毒性和具有多功能纳米医学特征的自组装前药纳米粒, 采用还原敏感键将香茅醇和卡巴他塞两种药物连接, 制得的纳米粒能响应肿瘤细胞的高生物还原剂谷胱甘肽(glutathione, GSH)浓度(图 4)。Zhang等[69]设计了PEG-DOX-CUR前体药物纳米粒, 用于同时递送多柔比星(DOX)和姜黄素(CUR), 通过希夫碱反应将多柔比星连接到PEG上制得多柔比星前体药物, 通过自组装将姜黄素包封在纳米粒内部形成PEG-DOX-CUR NPs。实验结果表明, 此系统具有酸敏感性, 到达肿瘤内部使结构破坏从而释放出多柔比星和姜黄素两种药物, 能够使其到达肿瘤细胞的细胞核和细胞质中, 发挥抗肿瘤的作用。Cui等[70]采用转铁蛋白修饰纳米粒用于共递送多柔比星和姜黄素两种药物, 首先合成pH敏感的Tf-PEG-CUR前体药物, 再将多柔比星包在Tf-PEG-CUR NPs中制得Tf-PEG-CUR/DOX NPs体系。此体系具有主动靶向性, 并能够响应肿瘤部位微酸性而实现药物的释放。
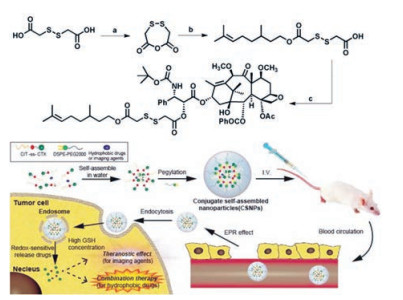
|
Figure 4 Synthesis of citronellol-cabazitaxel (CIT-ss-CTX) conjugate self-assembled noparticles (CSNPs). It has a promising perspective as a multifunctional nanomedicine for combination therapy and theranostics attribute to its long circulation property, redox-sensitive mechanism and high drug co-loading capability[68] |
纳米笼和纳米水凝胶也可用于共递送化疗药物。Zhang等[71]利用生物素聚乙二醇巯基(biotin PEG thiol, biotin-PEG-SH)制备了一种具有近红外响应的金纳米笼, 共递送多柔比星和槲皮素治疗乳腺癌。该体系应用生物素修饰金纳米笼使其具有近红外响应性, 再用十四醇填补中空的载体使其能在39 ℃融化从而控制药物释放。Quagliariello等[23]制备了一种纳米水凝胶用于共载雷帕霉素和槲皮素, 该研究是基于CD44主动靶向策略将雷帕霉素和槲皮素有效递送至乳腺癌组织。
目前, 中药活性成分联合化疗药物抗肿瘤的常用纳米载体类型及特点的研究报道见表 2[13, 23, 28, 42, 46, 53, 57, 61, 63, 65, 66, 69-88]。
| 表 2 The researches of several nanoparticles used to co-deliver two different drugs. EPR: Enhanced permeability and retention effect; DSPE-PEG: 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)]; PLGA: Poly(lactic-co-glycolic acid); PEI-IPDI-PEA: Branched polyethylenimine-isophorone diisocyanate-poly(L-lactide)-PEI; PAA: Polyacrylic acid; GMS-TPGS-SA-FA: Glyceryl monostearate-D-alpha tocopherol acid polyethylene glycol succinate-stearic acid and folate; PVDF: Poly(vinylidene fluoride); PAE: Polyamide epichiorobydrin; PVA: Polyvinyl alcohol; PLMS: PEGylated lipid bilayer coated mesoporous silica; ETP-CUR-NLC: Etoposide and curcumin loaded nanostructured lipid carriers; mPEG-PBLA-PVIm: Poly(ethylene glycol)-benzoic imine-poly (γ-benzyl-L-aspartate)-b-poly(1-vinylimidazole); Tf: Transferrin; MePEG/PCL: Methoxy poly(ethylene glycol)-ε-poly(caprolactone) diblock copolymers |
纳米载药体系递送药物的原理可以是简单的物理包封, 如脂质体通常是将两种水溶性的药物载入其亲水性的内核, 聚合纳米粒倾向于在其疏水内核中同时包裹两种脂溶性药物。Lin等[79]制备了一种具备双分子层结构的介孔硅纳米粒用于共递送姜黄素和紫杉醇, 该结构采用物理包载的方式将姜黄素和紫杉醇包封在双分子层结构内, 从而提高其对肿瘤细胞的细胞毒性。与脂质体或胶束等主要依靠物理作用包封药物不同, 聚合物-药物结合物是通过化学键共价作用实现载体与药物的连接, 由高分子聚合物与药物键合形成的化合物受到广泛关注, 这类化合物被称为“聚合物前药”。与传统纳米药物输送系统相比具有合成方法灵活、性质及构成广泛多样性、载药率确定、稳定性高和爆释现象小等优点。Cui等[70]设计了一种具有pH敏感的姜黄素前药, 同时将转铁蛋白修饰在纳米载体的表面以帮助制剂实现转铁蛋白介导的肿瘤主动靶向; 并且依靠聚乙二醇-姜黄素前药两亲性结构实现自组装, 将多柔比星包裹在其中。
4.2 共载形式采用纳米粒共载药物具有多种形式, 可以将两种药物均包封在纳米核内。Li等[76]制备了两种不同材料的纳米粒用于递送顺铂和姜黄素, 一种为脂质-聚合物混合纳米粒, 另一种为聚合物纳米粒, 两种纳米粒均是通过物理包载的方式将两种药物包封在纳米材料内部, 对HeLa细胞均具有较高的细胞毒性。结果表明, 采用纳米载体将药物包载在核内能较好地提高抗肿瘤作用。也可利用核壳形式将药物分层包载, Guo等[75]制备了一种具有核壳结构的共载多柔比星和姜黄素的纳米粒, 姜黄素包载于聚乳酸(poly(L-lactide), PLLA)疏水内核, 将多柔比星吸附于亲水的纳米粒壳表面, 从而实现了分层载药。结果表明, 该设计可增强联合给药的抗肿瘤疗效, 并且降低了多柔比星对心脏组织的病理损伤。Wu等[89]制备了一种具有核壳的PLGA纳米粒, 该纳米粒的外层为透明质酸, 可主动靶向到肿瘤部位的CD44细胞, 结构的内层为PLGA包载的紫杉醇, 采用核壳形式达到了主动靶向到肿瘤部位和治疗肿瘤的作用。因此, 纳米共载的形式是多种多样的, 根据纳米材料的性质结合药物的物理性质和作用机制, 设计更有效的纳米载体形式是科学工作者的使命。
5 结语与展望随着肿瘤发生、发展机制研究的不断深入, 药物联合治疗方案在肿瘤治疗中展现出显著优势, 而纳米技术在药剂学领域的发展更是为其带来了广阔的应用前景。但目前联合用药纳米体系在制剂设计、制备工艺和评价等方面依然面临诸多挑战, 如依靠纳米粒尺寸效应带来的被动靶向或受体介导的主动靶向特性, 尽管在细胞或动物模型上可观察到肿瘤趋向性, 但仍难以取得真正的临床效果; 如何在共载纳米制备过程实现预设载药量和两种(多种)药物配比不变, 并稳定传递入肿瘤组织; 联合用药方案中不同药物可能存在不同作用位点, 如何控制共载体系在肿瘤组织具有良好的释放特性; 随着对纳米技术生物安全性的逐渐关注, 纳米材料的毒理性质也逐渐被人们重视, 但对于在体内纳米粒是否会导致机体损伤仍不明确等。目前国内外学者不断尝试对纳米载体表面进行功能化修饰, 以更好地达到主动靶向的目的; 利用肿瘤复杂微环境中低氧、低pH、间质高压和免疫抑制等生物学特征, 进行纳米结构的改造以实现药物的多步骤多空间的肿瘤组织内释放特性, 均表现出更好的抗肿瘤效果。相信随着中药活性成分作用机制的不断揭示和纳米技术的不断发展, 纳米载体共载中药活性成分和化疗药物以联合抗肿瘤将为肿瘤临床治疗带来惊喜和希望。
| [1] | Shen S, Liu M, Li T, et al. Recent progress in nanomedicine-based combination cancer therapy using a site-specific co-delivery strategy[J]. Biomater Sci, 2017, 5: 1367–1381. DOI:10.1039/C7BM00297A |
| [2] | Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer[J]. Biochem Pharmacol, 2012, 83: 1104–1111. DOI:10.1016/j.bcp.2012.01.008 |
| [3] | Chen H, Zhao Y, Wang H, et al. Co-delivery strategies based on multifunctional nanocarriers for cancer therapy[J]. Curr Drug Metab, 2012, 13: 1087–1096. |
| [4] | Kemp JA, Shim MS, Heo CY, et al. "Combo" nanomedicine:co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy[J]. Adv Drug Deliv Rev, 2016, 98: 3–18. DOI:10.1016/j.addr.2015.10.019 |
| [5] | Mujokoro B, Adabi M, Sadroddiny E, et al. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment:a review[J]. Mat Sci Eng C, 2016, 69: 1092–1102. DOI:10.1016/j.msec.2016.07.080 |
| [6] | Huang MY, Zhang LL, Ding J, et al. Anticancer drug discovery from Chinese medicinal herbs[J]. Chin Med, 2018, 13: 35. DOI:10.1186/s13020-018-0192-y |
| [7] | Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor[J]. J Integr Med, 2014, 12: 331–335. DOI:10.1016/S2095-4964(14)60038-8 |
| [8] | Wang X, Feng Y, Wang N, et al. Chinese medicines induce cell death:the molecular and cellular mechanisms for cancer therapy[J]. Biomed Res Int, 2014, 2014: 530342. |
| [9] | Xu Y, Li XJ. Multi-target therapeutics and new drug discovery[J]. Acta Pharm Sin (药学学报), 2009, 44: 226–230. |
| [10] | Chen SZ. Research progress in anticancer effects and molecular targets of honokiol in experimental therapy[J]. Acta Pharm Sin (药学学报), 2016, 51: 202–207. |
| [11] | Meng G, Wang W, Chai K, et al. Combination treatment with triptolide and hydroxycamptothecin synergistically enhances apoptosis in A549 lung adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt signaling pathways[J]. Int J Oncol, 2015, 46: 1007–1017. DOI:10.3892/ijo.2015.2814 |
| [12] | Li Z, Cheng LF. Advances in the molecular mechanism of invasion and metastasis of hepatoma cells[J]. J Liaoning Med Univ (辽宁医学院学报), 2007, 28: 87–89. |
| [13] | Zhang J, Li J, Shi Z, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities[J]. Acta Biomater, 2017, 58: 349–364. DOI:10.1016/j.actbio.2017.04.029 |
| [14] | Zhang Y, Wang L, Zi Y, et al. Oridonin effectively reverses the drug resistance of cisplatin involving induction of cell apoptosis and inhibition of MMP expression in human acute myeloid leukemia cells[J]. Saudi J Biol Sci, 2017, 24: 678–686. DOI:10.1016/j.sjbs.2017.01.042 |
| [15] | Kunjachan S, Rychlik B, Storm G, et al. Multidrug resistance:physiological principles and nanomedical solutions[J]. Adv Drug Deliv Rev, 2013, 65: 1852–1865. DOI:10.1016/j.addr.2013.09.018 |
| [16] | Cao XF, Meng LH, Liu Z, et al. Research progress on reversal of multidrug resistance by combined drug delivery mediated by nano drug carriers[J]. China Pharm (中国药房), 2018, 29: 716–720. |
| [17] | Wu Q, Yang Z, Nie Y, et al. Multi-drug resistance in cancer chemotherapeutics mechanisms and lab approaches[J]. Cancer Lett, 2014, 347: 159–166. DOI:10.1016/j.canlet.2014.03.013 |
| [18] | Patel NR, Pattni BS, Abouzeid AH, et al. Nanopreparations to overcome multidrug resistance in cancer[J]. Adv Drug Deliv Rev, 2013, 65: 1748–1762. DOI:10.1016/j.addr.2013.08.004 |
| [19] | Zhang W, Zhou H, Yu Y, et al. Combination of gambogic acid with cisplatin enhances the antitumor effects on cisplatin-resistant lung cancer cells by downregulating MRP2 and LRP expression[J]. Oncotargets Ther, 2016, 9: 3359–3368. |
| [20] | Zou L, Wang D, Hu Y, et al. Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles[J]. Oncotarget, 2017, 8: 60453–60468. |
| [21] | Guo H, Zhang Z, Su Z, et al. Enhanced anti-tumor activity and reduced toxicity by combination andrographolide and bleomycin in ascitic tumor-bearing mice[J]. Eur J Pharmacol, 2016, 776: 52–63. DOI:10.1016/j.ejphar.2016.02.032 |
| [22] | Cheng Y, Zhao P, Wu S, et al. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma[J]. Int J Pharm, 2018, 545: 261–273. DOI:10.1016/j.ijpharm.2018.05.007 |
| [23] | Quagliariello V, Iaffaioli RV, Armenia E, et al. Hyaluronic acid nanohydrogel loaded with quercetin alone or in combination to a macrolide derivative of rapamycin RAD001(everolimus) as a new treatment for hormone-responsive human breast cancer[J]. J Cell Physiol, 2016, 232: 2063–2074. |
| [24] | Zhou Y, Wang M, Pan X, et al. Combination of triptolide with sodium cantharidinate synergistically enhances apoptosis on hepatoma cell line 7721[J]. J Cent South Univ T (Med Sci) (中南大学学报(医学版)), 2016, 41: 911–917. |
| [25] | Alsaied OA, Sangwan V, Banerjee S, et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma[J]. Surgery, 2014, 156: 270–279. DOI:10.1016/j.surg.2014.04.055 |
| [26] | Wei Y, Yang P, Cao S, et al. The combination of curcumin and 5-fluorouracil in cancer therapy[J]. Arch Pharmacol Res, 2018, 41: 1–13. DOI:10.1007/s12272-017-0979-x |
| [27] | McCubrey JA, Abrams SL, Lertpiriyapong K, et al. Effects of berberine, curcumin, resveratrol alone and in combination with chemotherapeutic drugs and signal transduction inhibitors on cancer cells-power of nutraceuticals[J]. Adv Biol Regul, 2018, 67: 190–211. DOI:10.1016/j.jbior.2017.09.012 |
| [28] | Ruttala HB, Ko YT. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy[J]. Colloids Surf B Biointerfaces, 2015, 128: 419–426. DOI:10.1016/j.colsurfb.2015.02.040 |
| [29] | Zhao Y, Jing Z, Li Y, et al. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis[J]. Oncol Rep, 2016, 36: 567–572. DOI:10.3892/or.2016.4785 |
| [30] | Dewangan J, Tandon D, Srivastava S, et al. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells[J]. Apoptosis, 2017, 22: 1246–1259. DOI:10.1007/s10495-017-1394-y |
| [31] | Alayev A, Salamon RS, Schwartz NS, et al. Combination of rapamycin and resveratrol for treatment of bladder cancer[J]. J Cell Physiol, 2017, 232: 436–446. DOI:10.1002/jcp.v232.2 |
| [32] | Yan YY, Bi H, Zhang W, et al. Downregulation and subcellular distribution of HER2 involved in MDA-MB-453 breast cancer cell apoptosis induced by lapatinib/celastrol combination[J]. J Buon, 2017, 22: 644–651. |
| [33] | Kim SH, Kang JG, Kim CS, et al. Cytotoxic effect of celastrol alone or in combination with paclitaxel on anaplastic thyroid carcinoma cells[J]. Tumour Biol, 2017, 39: 1010428317698369. |
| [34] | Rong LW, Wang RX, Zheng XL, et al. Combination of wogonin and sorafenib effectively kills human hepatocellular carcinoma cells through apoptosis potentiation and autophagy inhibition[J]. Oncol Lett, 2017, 13: 5028–5034. DOI:10.3892/ol.2017.6059 |
| [35] | Duan L, Deng L, Wang D, et al. Treatment mechanism of matrine in combination with irinotecan for colon cancer[J]. Oncol Lett, 2017, 14: 2300–2304. DOI:10.3892/ol.2017.6407 |
| [36] | Kim SL, Kim SH, Trang KT, et al. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer[J]. Cancer Lett, 2013, 335: 479–486. DOI:10.1016/j.canlet.2013.03.007 |
| [37] | Jia L, Li Z, Shen J, et al. Multifunctional mesoporous silica nanoparticles mediated co-delivery of paclitaxel and tetrandrine for overcoming multidrug resistance[J]. Int J Pharm, 2015, 489: 318–330. |
| [38] | Zhang H, Tian Y, Zhu Z, et al. Efficient antitumor effect of co-drug-loaded nanoparticles with gelatin hydrogel by local implantation[J]. Sci Rep, 2016, 6: 26546. DOI:10.1038/srep26546 |
| [39] | Zhang J, Wang L, Chan HF, et al. Co-delivery of paclitaxel and tetrandrine via iRGD peptide conjugated lipid-polymer hybrid nanoparticles overcome multidrug resistance in cancer cells[J]. Sci Rep, 2017, 7: 46057. DOI:10.1038/srep46057 |
| [40] | Lu HP, Ma FF, Gong JR, et al. Effects of oridonin combined with capecitabine on the proliferaction of MDA-MB-231 human breast cancer cells[J]. Chin Med J (中华医学杂志), 2017, 97: 3647–3651. |
| [41] | Lin Y, Lin L, Jin Y, et al. Combination of matrine and sorafenib decreases the aggressive phenotypes of hepatocellular carcinoma cells[J]. Chemotherapy, 2014, 60: 112–118. DOI:10.1159/000371736 |
| [42] | Xu Y, Wang C, Ding Y, et al. Nanoparticles with optimal ratiometric co-delivery of docetaxel with gambogic acid for treatment of multidrug-resistant breast cancer[J]. J Biomed Nanotechnol, 2016, 12: 1774–1781. DOI:10.1166/jbn.2016.2282 |
| [43] | He Z, Xiao X, Cai F, et al. Oridonin induces apoptosis and reverses drug resistance in cisplatin resistant human gastric cancer cells[J]. J Hubei Univ Med (湖北医药学院学报), 2016, 35: 261–265. |
| [44] | Du Plessis-Stoman D, Du Preez J, Van Der Venter M. Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines[J]. Afr J Tradit Complement Altern Med, 2011, 8: 177. |
| [45] | Yang X, Yi C, Luo N, et al. Nanomedicine to overcome cancer multidrug resistance[J]. Curr Drug Metab, 2014, 15: 632–649. DOI:10.2174/1389200215666140926154443 |
| [46] | Hu J, Wang J, Wang G, et al. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide:analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells[J]. Int J Mol Med, 2016, 37: 690–702. DOI:10.3892/ijmm.2016.2458 |
| [47] | Wang B, Rosano JM, Cheheltani R, et al. Towards a targeted multi-drug delivery approach to improve therapeutic efficacy in breast cancer[J]. Expert Opin Drug Deliv, 2010, 7: 1159–1173. DOI:10.1517/17425247.2010.513968 |
| [48] | Zeng Y, Yang Z, Li H, et al. Multifunctional nanographene oxide for targeted gene-mediated thermochemotherapy of drug-resistant tumour[J]. Sci Rep, 2017, 7: 43506. DOI:10.1038/srep43506 |
| [49] | Wang H, Agarwal P, Zhao S, et al. Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells[J]. Biomaterials, 2015, 72: 74–89. DOI:10.1016/j.biomaterials.2015.08.048 |
| [50] | Liu B, Han L, Liu J, et al. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer[J]. Int J Nanomedicine, 2017, 12: 955–968. DOI:10.2147/IJN |
| [51] | Shao Z, Shao J, Tan B, et al. Targeted lung cancer therapy:preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA[J]. Int J Nanomedicine, 2015, 10: 1223–1233. DOI:10.2217/nnm.14.202 |
| [52] | Lv L, Liu C, Chen C, et al. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer[J]. Oncotarget, 2016, 7: 32184–32199. |
| [53] | Baek JS, Cho CW. A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells[J]. Oncotarget, 2017, 8: 30369–30382. |
| [54] | Cao P, Bae Y. Polymer nanoparticulate drug delivery and combination cancer therapy[J]. Future Oncol, 2012, 8: 1471–1480. DOI:10.2217/fon.12.139 |
| [55] | Houdaihed L, Evans J, Allen C. Co-delivery of paclitaxel and everolimus at the optimal synergistic ratio:a promising solution for the treatment of breast cancer[J]. Mol Pharm, 2018. DOI:10.1021/acs.molpharmaceut.8b00217 |
| [56] | Pacardo DB, Ligler FS, Gu Z. Programmable nanomedicine:synergistic and sequential drug delivery systems[J]. Nanoscale, 2015, 7: 3381–3391. DOI:10.1039/C4NR07677J |
| [57] | Yang Z, Sun N, Cheng R, et al. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells[J]. Biomaterials, 2017, 147: 53–67. DOI:10.1016/j.biomaterials.2017.09.013 |
| [58] | Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems:an update review[J]. Curr Drug Deliv, 2007, 4: 297–305. DOI:10.2174/156720107782151269 |
| [59] | Li M, Yang YT, He Q, et al. Recent advances of nanocarriers in tumor immunotherapy[J]. Acta Pharm Sin (药学学报), 2017, 52: 1839–1848. |
| [60] | Cui Y, Zhang M, Zeng F, et al. Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy[J]. ACS Appl Mater Interfaces, 2016, 8: 32159–32169. DOI:10.1021/acsami.6b10175 |
| [61] | Lv L, Qiu K, Yu X, et al. Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer[J]. J Biomed Nanotechnol, 2016, 12: 973–985. DOI:10.1166/jbn.2016.2231 |
| [62] | Yan J, Wang Y, Jia Y, et al. Co-delivery of docetaxel and curcumin prodrug via dual-targeted nanoparticles with synergistic antitumor activity against prostate cancer[J]. Biomed Pharmacother, 2017, 88: 374–383. DOI:10.1016/j.biopha.2016.12.138 |
| [63] | Peng J, Fumoto S, Miyamoto H, et al. One-step formation of lipid-polyacrylic acid-calcium carbonate nanoparticles for co-delivery of doxorubicin and curcumin[J]. J Drug Target, 2017, 25: 704–714. DOI:10.1080/1061186X.2017.1315687 |
| [64] | Yao Q, Gutierrez DC, Hoang NH, et al. Efficient codelivery of paclitaxel and curcumin by novel bottlebrush copolymer-based micelles[J]. Mol Pharm, 2017, 14: 2378–2389. DOI:10.1021/acs.molpharmaceut.7b00278 |
| [65] | Sarisozen C, Abouzeid AH, Torchilin VP. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors[J]. Eur J Pharm Biopharm, 2014, 88: 539–550. DOI:10.1016/j.ejpb.2014.07.001 |
| [66] | Fang JH, Lai YH, Chiu TL, et al. Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas[J]. Adv Healthc Mater, 2014, 3: 1250–1260. DOI:10.1002/adhm.v3.8 |
| [67] | Delplace V, Couvreur P, Nicolas J. Recent trends in the design of anticancer polymer prodrug nanocarriers[J]. Polym Chem, 2014, 5: 1529–1544. DOI:10.1039/C3PY01384G |
| [68] | Xue P, Liu D, Wang J, et al. Redox-sensitive citronellol-cabazitaxel conjugate:maintained in vitro cytotoxicity and self-assembled as multifunctional nanomedicine[J]. Bioconjug Chem, 2016, 27: 1360–1372. DOI:10.1021/acs.bioconjchem.6b00155 |
| [69] | Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer[J]. Sci Rep, 2016, 6: 21225. DOI:10.1038/srep21225 |
| [70] | Cui T, Zhang S, Sun H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment[J]. Oncol Rep, 2017, 37: 1253–1260. DOI:10.3892/or.2017.5345 |
| [71] | Zhang Z, Xu S, Wang Y, et al. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells[J]. J Colloid Interface Sci, 2018, 509: 47–57. DOI:10.1016/j.jcis.2017.08.097 |
| [72] | Dash TK, Konkimalla VB. Formulation and optimization of doxorubicin and biochanin A combinational liposomes for reversal of chemoresistance[J]. AAPS Pharm Sci Tech, 2017, 18: 1116–1124. |
| [73] | Minaei A, Sabzichi M, Ramezani F, et al. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells[J]. Mol Biol Rep, 2016, 43: 99–105. DOI:10.1007/s11033-016-3942-x |
| [74] | Zhao Y, Huan ML, Liu M, et al. Doxorubicin and resveratrol co-delivery nanoparticle to overcome doxorubicin resistance[J]. Sci Rep, 2016, 6: 35267. DOI:10.1038/srep35267 |
| [75] | Guo Q, Li X, Yang Y, et al. Enhanced 4T1 breast carcinoma anticancer activity by co-delivery of doxorubicin and curcumin with core-shell drug-carrier based on heparin modified poly(L-lactide) grafted polyethylenimine cationic nanoparticles[J]. J Biomed Nanotechnol, 2014, 10: 227–237. DOI:10.1166/jbn.2014.1785 |
| [76] | Li C, Ge X, Wang L. Construction and comparison of different nanocarriers for co-delivery of cisplatin and curcumin:a synergistic combination nanotherapy for cervical cancer[J]. Biomed Pharmacother, 2017, 86: 628–636. DOI:10.1016/j.biopha.2016.12.042 |
| [77] | Zhao X, Chen Q, Li Y, et al. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice[J]. Eur J Pharm Biopharm, 2015, 93: 27–36. DOI:10.1016/j.ejpb.2015.03.003 |
| [78] | Xiao B, Si X, Han MK, et al. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy[J]. J Mater Chem B, 2015, 3: 7724–7733. DOI:10.1039/C5TB01245G |
| [79] | Lin J, Cai Q, Tang Y, et al. PEGylated Lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin:design, characterization and its cytotoxic effect[J]. Int J Pharm, 2018, 536: 272–282. DOI:10.1016/j.ijpharm.2017.10.043 |
| [80] | Dilnawaz F, Sahoo SK. Enhanced accumulation of curcumin and temozolomide loaded magnetic nanoparticles executes profound cytotoxic effect in glioblastoma spheroid model[J]. Eur J Pharm Biopharm, 2013, 85: 452–462. DOI:10.1016/j.ejpb.2013.07.013 |
| [81] | Jiang H, Geng D, Liu H, et al. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors[J]. Drug Deliv, 2016, 23: 3665–3673. DOI:10.1080/10717544.2016.1217954 |
| [82] | Abouzeid AH, Patel NR, Torchilin VP. Polyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin E micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancer[J]. Int J Pharm, 2014, 464: 178–184. DOI:10.1016/j.ijpharm.2014.01.009 |
| [83] | Wang J, Ma W, Tu P. Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles[J]. Macromol Biosci, 2015, 15: 1252–1261. DOI:10.1002/mabi.201500043 |
| [84] | Mohanty AK, Mohanta GP. Micelle-assisted combination therapies for effective glioblastoma treatment:an in-vitro assessment[J]. Anticancer Drug, 2015, 26: 312–322. DOI:10.1097/CAD.0000000000000186 |
| [85] | Li S, Wang L, Li N, et al. Combination lung cancer chemotherapy:design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin[J]. Biomed Pharmacother, 2017, 95: 548–555. DOI:10.1016/j.biopha.2017.08.090 |
| [86] | Qureshi WA, Zhao R, Wang H, et al. Co-delivery of doxorubicin and quercetin via mPEG-PLGA copolymer assembly for synergistic anti-tumor efficacy and reducing cardio-toxicity[J]. Sci Bull, 2016, 61: 1689–1698. DOI:10.1007/s11434-016-1182-z |
| [87] | Zhu B, Yu L, Yue Q. Co-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapy[J]. Biomed Pharmacother, 2017, 91: 287. DOI:10.1016/j.biopha.2017.02.112 |
| [88] | Liu Q, Li J, Pu G, et al. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy[J]. Drug Deliv, 2016, 23: 1364–1368. |
| [89] | Wu J, Zhang J, Deng C, et al. Vitamin E-oligo(methyl diglycol l-glutamate) as a biocompatible and functional surfactant for facile preparation of active tumor-targeting PLGA nanoparticles[J]. Biomacromolecules, 2016, 17: 2367–2374. DOI:10.1021/acs.biomac.6b00380 |
 2019, Vol. 54
2019, Vol. 54


