2. 解放军第三〇二医院全军中医药研究所, 北京 100039;
3. 北京中医药大学药学院, 北京 100029;
4. 成都中医药大学药学院, 四川 成都 611137
2. China Military Institute of Chinese Medicine, 302 Military Hospital of China, Beijing 100039, China;
3. School of Pharmacy, Beijing University of Traditional Chinese Medicine, Beijing 100029, China;
4. School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China
炎症小体主要是由受体蛋白、凋亡相关斑点样蛋白(apoptosis-associated speck-like protein, ASC)和半胱氨酸天冬氨酸特异蛋白酶1前体(pro-caspase-1)组成的多蛋白复合物[1]。其中, 受体蛋白包括多种NOD样受体蛋白家族如NOD样受体家族3 (NLRP3)、NOD样受体家族半胱天冬酶激活募集结构域4 (NLRC4)、黑色素瘤缺乏因子2 (AIM2)蛋白和pyrin[2]。当细胞受到病原相关分子模式(pathogen- associated molecular pattern, PAMP)或危险相关分子模式(danger-associated molecular pattern, DAMP)刺激时, 会促进炎症小体的组装活化, pro-caspase-1自我剪切产生caspase-1, caspase-1剪切pro-IL-1β和pro-IL-18, 分泌成熟IL-1β和IL-18等促炎因子[3], 从而介导多种疾病的发生。其中NLRP3炎症小体研究最为广泛, 可被ATP、nigericin及病原体等多种因素诱导活化[4]。目前研究发现溃疡性结肠炎[5]、阿尔茨海默病[6]、2型糖尿病[7]、动脉粥样硬化[8]和痛风[9]等都与NLRP3炎症小体的异常激活密切相关。
甘草查尔酮A (licochalcone A, LCA)是一种从胀果甘草(Glycyrrhiza inflata bat.)中提取的类黄酮物质, 是胀果甘草种属特异性成分, 也是胀果甘草的主要黄酮类成分[10], 研究发现LCA具有明显的抗炎作用[11], 在RAW264.7细胞中, 通过阻断NF-κB信号通路抑制LPS诱导的炎症反应[12]。已有研究报道, LCA可以减弱小鼠因LPS引起的急性肺损伤[13]和急性肾损伤[14]。然而, 对于LCA是否能通过抑制NLRP3炎症小体的活性发挥抗炎作用尚未见相关报道。为此, 本研究利用小鼠原代骨髓巨噬细胞(bone marrow- derived macrophages, BMDMs)构建体外研究模型, 探索LCA对NLRP3炎症小体活性的调控作用及初步机制, 以期为NLRP3过度活化诱发的相关疾病治疗提供潜在的先导化合物。
材料与方法材料和试剂 LCA (目录号: HY-N0372)购自美国Med Chem Express公司, 纯度99.44%; ATP和nigericin均购自美国Sigma公司; poly(dA:dT)、ultrapure LPS购自美国Invivogen公司; Lfn-Flic为中国人民解放军军事医学科学院李涛博士赠送; NLRP3、ASC和caspase-1抗体均购自美国Adipogen公司; IL-1β抗体购自美国R & D公司; 辣根过氧化物酶标记羊抗小鼠IgG抗体、辣根过氧化物酶标记羊抗兔IgG抗体购自美国Santa Cruz公司; PVDF膜(孔径0.45 μm)购自美国Millipore公司; opti-MEM无血清培养基及其他所有细胞培养试剂均购自美国Gibco公司; caspase-1荧光底物(Ac-WEHD-AMC)、蛋白酶体抑制剂(Ac-YVAD-CHO)购自美国Promega公司。
主要仪器 SDS-PAGE电泳系统(美国Bio-Rad公司); 控温高速离心机(美国Sigma公司), Promega GloMax 20/20发光检测仪(美国Promega公司)。
BMDMs的分离培养 SPF级雌性C57小鼠(10~12周龄) 20只, 动物合格证号: SCXK (京) 2016-0002, 由北京斯贝福生物技术有限公司提供。本研究在中国人民解放军第302医院伦理委员会审批下进行。取C57BL/6雌鼠, 脱臼处死后在超净台分离小鼠的双侧股骨并用细针头吸取DMEM培养基冲出骨髓细胞, 反复吹散细胞后转移至50 mL离心管, 室温离心后, 去上清, 用10%胎牛血清(fetal bovine serum, FBS)及1%双抗的DMEM培养基重悬细胞, 同时加入巨噬细胞集落刺激因子(macrophage colony-stimulating factor, M-CSF)终质量浓度为25 ng·mL-1, 细胞培养5天后获得BMDMs。
CCK-8法检测细胞毒性 取培养至对数生长期的小鼠骨髓来源巨噬细胞系(immortalized bone marrow- derived macrophages, iBMDMs) (购自中国科学院上海细胞所)和BMDMs, 接种于96孔板。细胞分为正常组、DMSO组、不同浓度的LCA组(1.25、2.5、5、10、20、40、80、120 μmol·L-1), 孵育, 每孔加入CCK-8溶液10 μL, 培养20 min后, 在酶标仪450 nm处测定吸光度值。
刺激方式[15, 16] 取上述分离培养好的BMDMs, 胰酶与EDTA联合消化后接种于24孔板中, 用DMEM高糖培养基细胞培养12 h后, 培养基替换成浓度为50 ng·mL-1 LPS的DMEM培养基, 预处理4 h后, 撤除LPS刺激, 用不同浓度的LCA处理1 h后, 再在实验孔中加入AIM2炎症小体激活剂Poly(dA:dT)、NLRC4炎症小体激活剂Lfn-Flic刺激6 h, 或加入NLRP3炎症小体激活剂ATP和nigericin刺激45 min, 刺激时间结束后收样。
样品处理 细胞培养上清液在3 500 r·min-1离心后, 上清中加入1/4体积三氯乙酸(TCA), 冰箱-20 ℃过夜后, 13 000 r·min-1、4 ℃离心15 min, 弃去上清, 加冰丙酮洗1次, 105 ℃金属浴挥干丙酮, 待丙酮挥发后加1×loading buffer 40 μL振荡混匀, 水浴煮沸, 冷却后作为上清样品。贴壁细胞用PBS洗板2次, 置于冰上, 每孔加入1×loading buffer 200 μL, 10 min后刮下细胞, 吸取细胞裂解液, 水浴煮沸20 min, 冷却后作为细胞裂解样品。
ELISA试剂盒检测细胞培养上清液中成熟IL-1β和TNF-α含量 细胞上清液采用ELISA试剂盒测定IL-1β和TNF-α含量, 操作步骤按照试剂盒说明书进行, 最后用酶标仪上机检测, 通过Excel数据处理和GraphPad Prism 6.0图表制作。
Caspase-Glo® 1 Inflammasome Assay检测细胞培养上清液中caspase-1活性[17] 细胞培养上清液采用caspase-1活性检测试剂盒测定caspase-1活性, 检测按照试剂盒说明书操作进行, 最后用Promega GloMax 20/20发光检测仪检测, 通过Excel处理数据和GraphPad Prism 6.0制作图表。
Western blot测定蛋白的表达 取蛋白样品20 μL进行SDS-聚丙烯酰胺凝胶电泳, 将凝胶中分离的蛋白转移至PVDF膜上, 10%脱脂奶室温封闭0.5 h后, 分别加入pro-IL-1β、caspase-1、IL-1β、ASC、NLRP3、lamin B的一抗溶液4 ℃孵育过夜, 加入辣根过氧化物酶标记的相应二抗溶液室温孵育1 h后, 用Carestream公司的化学发光液显色, X光片显影。
统计学分析 实验数据以x±s表示, 采用SPSS软件对两组间数据进行独立样本t检验, 显著性结果以*P < 0.05、**P < 0.01、***P < 0.001表示。
结果 1 LCA对iBMDMs和BMDMs的细胞毒性作用通过CCK-8法检测LCA (图 1A)对iBMDMs和BMDMs细胞毒性, 确定LCA的给药浓度。如图 1B和C所示, 与对照组相比, LCA在20 μmol·L-1以内不影响iBMDMs和BMDMs细胞生存, 而随着LCA浓度升高表现出对iBMDMs和BMDMs细胞生存的抑制效应。为此, 本研究选择2.5、5、10 μmol·L-1作为给药浓度, 开展LCA调控NLRP3炎症小体活性的研究。
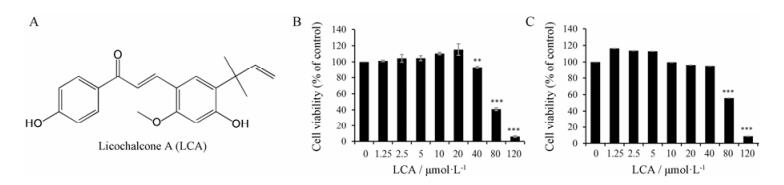
|
Figure 1 The cytotoxicity of licochalcone A (LCA) was analyzed by CCK-8 assay. A: LCA structure; B: Effect of LCA on cell viability in immortalized bone marrow-derived macrophages (iBMDMs). The cells were treated with various of concentrations of LCA for 12 h. n = 5, x±s. C: Effect of LCA on cell viability in bone marrow-derived macrophages (BMDMs). The cells were treated with various of concentrations of LCA for 24 h. n = 3, x±s. **P < 0.01, ***P < 0.001 vs control group |
首先利用LPS预处理BMDMs诱导炎症小体前体蛋白表达后给予LCA, 最后利用内源性刺激因子ATP诱导NLRP3炎症小体活化, 收集细胞培养上清和细胞裂解液。利用免疫印迹法对细胞培养上清中caspase-1 p20和成熟IL-1β及细胞裂解液中的NLRP3炎症小体相关蛋白进行检测, LCA呈剂量依赖性抑制上清液中caspase-1 p20产生和成熟IL-1β分泌, 且对细胞裂解液中NLRP3、ASC等蛋白表达无明显影响(图 2A); 进一步通过Caspase-Glo® 1 Inflammasome Assay和ELISA分别检测细胞上清中caspase-1活性、成熟IL-1β及TNF-α的含量, 结果表明, LCA呈剂量依赖性抑制caspase-1活性(图 2B)和成熟IL-1β分泌(图 2C), 但对TNF-α分泌无影响(图 2D)。因此, 结果表明, LCA通过抑制caspase-1介导的pro-IL-1β剪切活化, 抑制成熟IL-1β分泌, 进而抑制ATP诱导的NLRP3炎症小体激活。
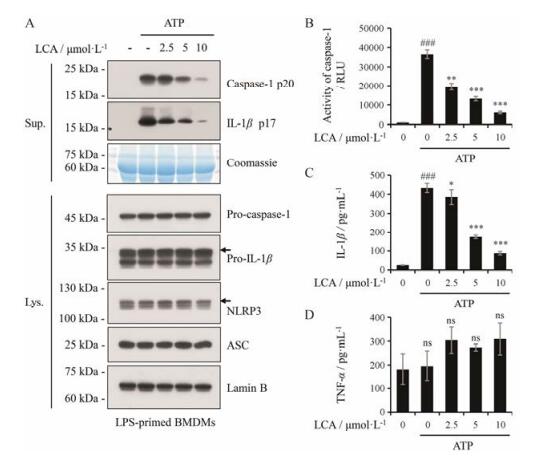
|
Figure 2 LCA inhibits ATP-induced activation of NLRP3 inflammasome. A: Western blot was used to assess the expression levels of indicated proteins in culture supernatants (Sup.) and cell lysates (Lys.). Lamin B was used as a loading control for cell lysates. Coomassie blue staining was provided as the loading control for the supernatants. B: The activity of caspase-1 was analyzed in the supernatant of BMDMs by the Caspase-Glo® 1 Inflammasome Assay. RLU, recombinant luciferase, which is proportional to caspase-1 activity. C, D: The secretion of IL-1β and TNF-α was measured in the supernatant of BMDMs by ELISA. n = 3, x±s. *P < 0.05, **P < 0.01, ***P < 0.001 vs LPS+ATP group; ###P < 0.001 vs control (DMSO) group. ns: Nonsignificant |
为了评价LCA对其他刺激因子诱导的NLRP3炎症小体激活的影响, 本研究在LPS预处理的基础上, 通过外源性刺激因子nigericin构建NLRP3炎症小体活化模型, 免疫印迹法检测细胞培养上清中caspase-1 p20和成熟IL-1β及细胞裂解液中的NLRP3炎症小体相关蛋白, LCA呈剂量依赖性抑制上清液中caspase-1 p20产生和成熟IL-1β分泌, 且对细胞裂解液中NLRP3、ASC等蛋白表达无明显影响(图 3A); 进一步采用Caspase-Glo® 1 Inflammasome Assay和ELISA检测细胞培养上清中caspase-1活性、成熟IL-1β及TNF-α的含量, 本次实验结果表明, LCA呈剂量依赖性抑制caspase-1活性(图 3B)和成熟IL-1β分泌(图 3C), 但对TNF-α分泌无影响(图 3D)。结果表明, LCA能抑制nigericin诱导的NLRP3炎症小体激活, 抑制成熟IL-1β介导的免疫炎症通路。
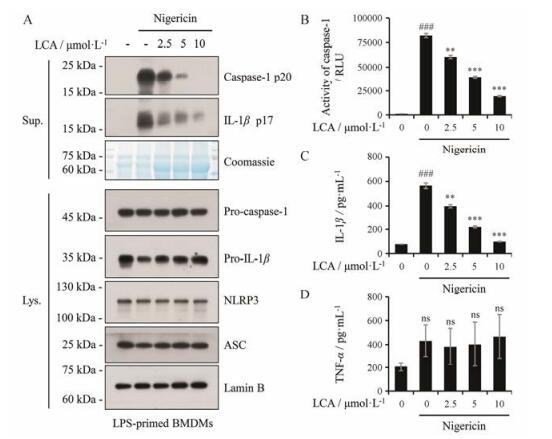
|
Figure 3 LCA inhibits nigericin-induced activation of NLRP3 inflammasome. A: Western blot was used to assess the expression levels of indicated proteins in culture supernatants and cell lysates. Lamin B was used as a loading control for cell lysates. Coomassie blue staining was provided as the loading control for the supernatants. B: The activity of caspase-1 was analyzed in the supernatant of BMDMs by the Caspase-Glo® 1 Inflammasome Assay. RLU, recombinant luciferase, which is proportional to caspase-1 activity. C, D: The secretion of IL-1β and TNF-α was measured in the supernatant of BMDMs by ELISA. n = 3, x±s. **P < 0.01, ***P < 0.001 vs LPS+Nigericin group; ###P < 0.001 vs control (DMSO) group |
为评价LCA是否特异性地抑制NLRP3炎症小体, 本研究评价了LCA对NLRC4炎症小体和AIM2炎症小体的作用。首先利用LPS联合Lfn-Flic构建NLRC4炎症小体活化模型评价LCA的作用。通过免疫印迹法对细胞培养上清及细胞裂解液中的炎症小体相关蛋白进行检测, 结果表明LCA对NLRP3炎症小体及NLRC4炎症小体中caspase-1 p20产生有抑制作用, 其对细胞裂解液中NLRP3、ASC等蛋白表达无明显影响(图 4A); 进一步利用Caspase-Glo® 1 Inflammasome Assay和ELISA检测细胞培养上清液, 结果表明LCA对NLRP3炎症小体及NLRC4炎症小体中caspase-1活性和成熟IL-1β分泌有抑制作用, 而在LPS联合dsDNA类似物poly(dA:dT)诱导的AIM2炎症小体活化模型中, 通过上述检测方法结果表明LCA对caspase-1活性和成熟IL-1β分泌无抑制作用(图 4B、C); 且在上述评价模型中LCA对TNF-α的分泌无影响(图 4D)。由此可见, LCA对NLRP3炎症小体活性具有较强抑制作用, 对NLRC4炎症小体活性有轻微抑制作用, 但对AIM2炎症小体活性无影响。
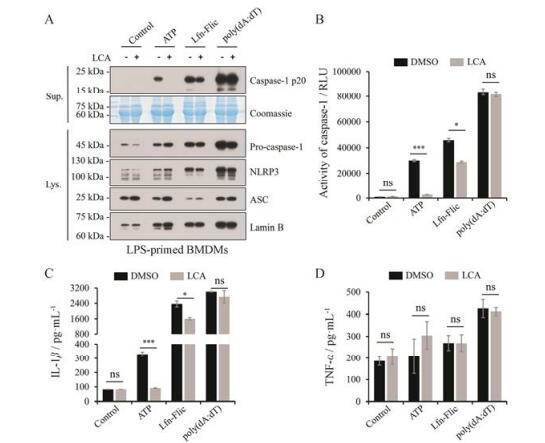
|
Figure 4 Effect of LCA (10 μmol·L-1) on the activation of NLRP3, NLRC4, and AIM2 inflammasome. A: Western blot was used to assess the expression levels of indicated proteins in culture supernatants and cell lysates. Lamin B was used as a loading control for cell lysates. Coomassie blue staining was provided as the loading control for the supernatants. B: The activity of caspase-1 was analyzed in the supernatant of BMDMs by the Caspase-Glo® 1 Inflammasome Assay. RLU, recombinant luciferase, which is proportional to caspase-1 activity. C, D: The secretion of IL-1β and TNF-α was measured in the supernatant of BMDMs by ELISA. n = 3, x±s. *P < 0.05, ***P < 0.001 vs control (DMSO) group. NLRC4: NOD-like receptor containing a caspase activating and recruitment domain 4; AIM2: The absent in melanoma 2 |
目前研究表明, 经典的NLRP3炎症小体组成蛋白NLRP3和pro-IL-1β的表达受NF-κB信号通路调控[18], 为明确LCA是否通过调控NLRP3炎症小体组成蛋白的表达来抑制NLRP3炎症小体激活, 本研究采用LCA预处理BMDMs 30 min后, 联合LPS共同刺激3 h[19], 实验表明, 在2.5~10 μmol·L-1浓度内, LCA对NF-κB介导的NLRP3炎症小体组成蛋白NLRP3和pro-IL-1β的表达无明显影响(图 5)。

|
Figure 5 Effects of LCA on LPS-induced NF-κB signaling pathway in BMDMs. LCA had no effect on LPS-induced NLRP3, pro-IL-1β expression when BMDMs were treated with LCA for 30 min before 3 h LPS stimulation. The results showed that the inhibitory activity of LCA on NLRP3 inflammasome was not dependent on NF-κB pathway |
当宿主细胞受到外来微生物感染或自身释放的损伤信号刺激时, NLRP3、NLRC4和AIM2等多种炎症小体能被激活, 如沙门菌细菌鞭毛蛋白可激活NLRC4炎症小体[20];来自病毒的双链DNA可以激活AIM2炎症小体[21]。其中, 内源性刺激因子ATP能通过与细胞膜上的嘌呤能受体(P2X7R)结合, 诱导细胞内K+外流, 激活NLRP3炎症小体[22, 23];同时, 外源性刺激因子nigericin可能通过线粒体损伤激活NLRP3炎症小体[24]。然而, NLRP3炎症小体过度被激活, 可能导致机体组织损伤及代谢紊乱; 因此, 抑制NLRP3炎症小体的活化有利于防治相关疾病。现代研究表明, 甘草中的黄酮类物质是一类抗炎、抗肿瘤及抗菌活性成分, 广泛用于治疗肝炎、肾炎、溃疡性肠胃炎等[25]。如文献报道甘草素和异甘草素能够治疗NLRP3炎症小体介导的肠胃组织损伤[26], 且甘草酸对NLRP3炎症小体调控的MRL/lpr小鼠狼疮性肾炎具有明显治疗作用[27]。本研究发现, LCA可以剂量依赖性抑制ATP和nigericin诱导的BMDMs中NLRP3炎症小体的激活, 最终抑制caspase-1的活化以及成熟IL-1β等炎症因子的释放, 这表明LCA对NLRP3炎症小体的过度激活引起的免疫相关炎症具有潜在的治疗作用。
经典的NLRP3炎症小体活化需要两种信号通路调控[28]。第一信号包括不同的TLR (toll like receptor)配体如LPS等危险信号, 激活NF-κB通路上调pro-IL-1β、NLRP3蛋白表达[29], 形成第一条通路, 在此基础上, 给予第二信号(如ATP、nigericin)刺激时, NLRP3、ASC、pro-caspase-1组装活化, 促使pro- caspase-1自我剪切成具有活性的caspase-1, 剪切pro-IL-1β并分泌其成熟形式到胞外[30], 发挥促炎作用。其中, NF-κB通路在炎症小体的活化过程中发挥重要作用, 其可通过调控炎症小体组成蛋白的表达, 为炎症小体组装活化提供基础。本研究发现LCA对LPS介导的NLRP3、pro-IL-1β的蛋白表达无明显影响, 提示LCA抑制NLRP3炎症小体的激活并不依赖NF-κB介导的NLRP3、pro-IL-1β蛋白的表达, 而是通过抑制caspase-1活性, 进而阻断pro-IL-1β的剪切活化, 从而降低NLRP3炎性小体下游炎症因子IL-1β分泌水平, 减轻免疫炎症反应, 为LCA防治NLRP3炎性小体相关的疾病提供了参考依据。
| [1] | Rathinam VAK, Fitzgerald KA. Inflammasome complexes:emerging mechanisms and effector functions[J]. Cell, 2016, 165: 792–800. DOI:10.1016/j.cell.2016.03.046 |
| [2] | Broz P, Dixit VM. Inflammasomes:mechanism of assembly, regulation and signalling[J]. Nat Rev Immunol, 2016, 16: 407–420. DOI:10.1038/nri.2016.58 |
| [3] | Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes[J]. Nat Rev Immunol, 2013, 13: 397–411. DOI:10.1038/nri3452 |
| [4] | Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome:from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases[J]. Redox Biol, 2015, 4: 296–307. DOI:10.1016/j.redox.2015.01.008 |
| [5] | Bauer C, Duewell P, Mayer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome[J]. Gut, 2010, 59: 1192–1199. DOI:10.1136/gut.2009.197822 |
| [6] | Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice[J]. Nature, 2013, 493: 674–678. |
| [7] | Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes[J]. Nat Immunol, 2010, 11: 897–904. DOI:10.1038/ni.1935 |
| [8] | Xiao H, Lu M, Lin TY, et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility[J]. Circulation, 2013, 128: 632–642. DOI:10.1161/CIRCULATIONAHA.113.002714 |
| [9] | Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome[J]. Nature, 2006, 440: 237–241. DOI:10.1038/nature04516 |
| [10] | Kondo K, Shiba M, Nakamura R, et al. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. inflata identified by genetic information[J]. Biol Pharm Bull, 2007, 30: 1271–1277. DOI:10.1248/bpb.30.1271 |
| [11] | Furusawa J, Funakoshi-Tago M, Mashino T, et al. Glycyrrhiza inflata-derived chalcones, licochalcone A, licochalcone B and licochalcone D, inhibit phosphorylation of NF-κB p65 in LPS signaling pathway[J]. Int Immunopharmacol, 2009, 9: 499–507. DOI:10.1016/j.intimp.2009.01.031 |
| [12] | Kwon HS, Park JH, Kim DH, et al. Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice[J]. J Mol Med (Berl), 2008, 86: 1287–1295. DOI:10.1007/s00109-008-0395-2 |
| [13] | Chu X, Ci X, Wei M, et al. Licochalcone A inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo[J]. J Agric Food Chem, 2012, 60: 3947–3954. DOI:10.1021/jf2051587 |
| [14] | Hu J, Liu J. Licochalcone A attenuates lipopolysaccharide-induced acute kidney injury by inhibiting NF-κB activation[J]. Inflammation, 2016, 39: 569–574. DOI:10.1007/s10753-015-0281-3 |
| [15] | Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation[J]. Cell, 2013, 2: 1154–1163. |
| [16] | Song N, Liu ZS, Xue W, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation[J]. Mol Cell, 2017, 68: 185–197. DOI:10.1016/j.molcel.2017.08.017 |
| [17] | O'Brien M, Moehring D, Muñoz-Planillo R, et al. A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells[J]. J Immunol Methods, 2017, 447: 1–13. DOI:10.1016/j.jim.2017.03.004 |
| [18] | Si MM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity[J]. Nat Rev Immunol, 2015, 16: 7–22. |
| [19] | Huang Y, Jiang H, Chen Y, et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases[J]. EMBO Mol Med, 2018, 10: 1–15. DOI:10.15252/emmm.201708365 |
| [20] | Franchi L, Amer A, Body-Malapel M, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages[J]. Nat Immunol, 2006, 7: 576–582. DOI:10.1038/ni1346 |
| [21] | Roberts TL, Idris A, Dunn JA, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA[J]. Science, 2009, 323: 1057–1060. DOI:10.1126/science.1169841 |
| [22] | Franceschini A, Capece M, Chiozzi P, et al. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein[J]. FASEB J, 2015, 29: 2450–2461. DOI:10.1096/fj.14-268714 |
| [23] | Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores[J]. Nature, 2016, 535: 153–158. DOI:10.1038/nature18629 |
| [24] | Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis[J]. Immunity, 2012, 36: 401–414. DOI:10.1016/j.immuni.2012.01.009 |
| [25] | Huang YT, Chi ZL, Wang SM, et al. Research progress on licorice flavonoids and their antitumor activities[J]. Chin J New Drugs (中国新药杂志), 2017, 26: 1532–1537. |
| [26] | Tanigericinawa T, Nadatani Y, Shimada S, et al. Isoliquiritigenin, a flavonoid component of licorice, accelerate healing of gastric ulcer through inhibition of NLRP3 inflammasome activation[J]. Gastroenterology, 2017, 152: S886. |
| [27] | Wang YY, Li HT, Lu Y, et al. Protective effects of glycyrrhizic acid against lupus nephritis in MRL/lpr mice[J]. J South Med Univ (南方医科大学学报), 2017, 37: 957–961. |
| [28] | Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation[J]. Ann N Y Acad Sci, 2014, 1319: 82–95. DOI:10.1111/nyas.2014.1319.issue-1 |
| [29] | Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge:NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression[J]. J Immunol, 2009, 183: 787–791. DOI:10.4049/jimmunol.0901363 |
| [30] | Tsutsui H, Imamura M, Fujimoto J, et al. The TLR4/TRIF-mediated activation of NLRP3 inflammasome underlies endotoxin-induced liver injury in mice[J]. Gastroenterol Res Pract, 2010, 2010: 641865–641876. |
 2018, Vol. 53
2018, Vol. 53


