长期以来, 恶性肿瘤治疗一直是临床上面临的最大难题。分子靶向治疗凭借其针对性、特异性和有效性强且毒副作用低等优点, 已成为国内外肿瘤治疗领域的研究热点[1]。天然产物凭借其结构的多样性, 较高的生物活性和较小的毒副作用成为靶向药物开发的重点[2]。齐墩果酸(oleanolic acid, OA)是一种重要的五环三萜类天然产物, 具有多种生物活性, 尤其是抗肿瘤活性, 包括抑制人肺癌细胞、人宫颈癌HeLa细胞、人乳腺癌MCF细胞以及肝癌细胞株HepG2等, 但分子作用机制尚不完全明确[3, 4]。以血管内皮生长因子(vascular endothelial growth factor, VEGF)及其受体VEGFR为靶点的药物可以减少血管内皮生长因子和血管内皮生长因子受体的表达, 消耗肿瘤细胞产生的血管内皮生长因子, 从而抑制肿瘤血管的生成, 遏制肿瘤生长[5]。已有研究表明OA能够抑制肿瘤细胞中VEGFR的表达, 因此VEGFR可能是齐墩果酸抗肿瘤作用的重要靶点[6-9]。
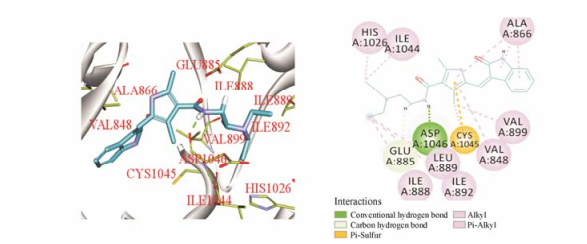
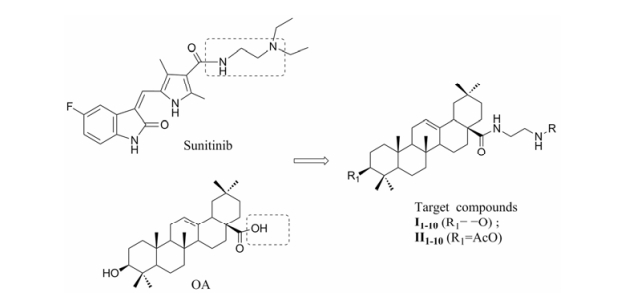
舒尼替尼(sunitinib)是由辉瑞公司研制的VEGFR抑制剂(VEGFR-2, IC50 = 9 nmol·L-1)[10]。运用分子模拟对接方法研究舒尼替尼与VEGFR靶蛋白(PDB编号: 5ew3)的相互作用(图 1), 结果表明舒尼替尼结构中的乙二胺片段可以和VEGFR蛋白的氨基酸残基(Asp1046)形成牢固的氢键, 与靶蛋白产生较大结合力, 说明乙二胺基团可能是其抗肿瘤作用的重要药效基团。此外, 文献报道多个小分子VEGFR-2抑制剂能与靶蛋白狭长空腔的氨基酸残基Asp1046产生氢键作用[11, 12], 进一步说明Asp1046氨基酸残基可能是小分子与VEGFR蛋白作用的重要结合位点。本文将乙二胺基团引入到OA-28位, 并以乙二胺作为连接臂, 利用拼合法引入多种生物活性基团, 设计并合成了20个衍生物Ⅰ1~Ⅰ10和Ⅱ1~Ⅱ10 (图 2), 目标化合物的合成见合成路线1。同时对目标化合物进行了体外抗肿瘤活性测试和分子模拟对接研究。
|
Figure 1 Binding of sunitinib to the active site of vascular endothelial growth factor receptor (VEGFR) |
|
Figure 2 The design of oleanolic acid (OA) derivatives |
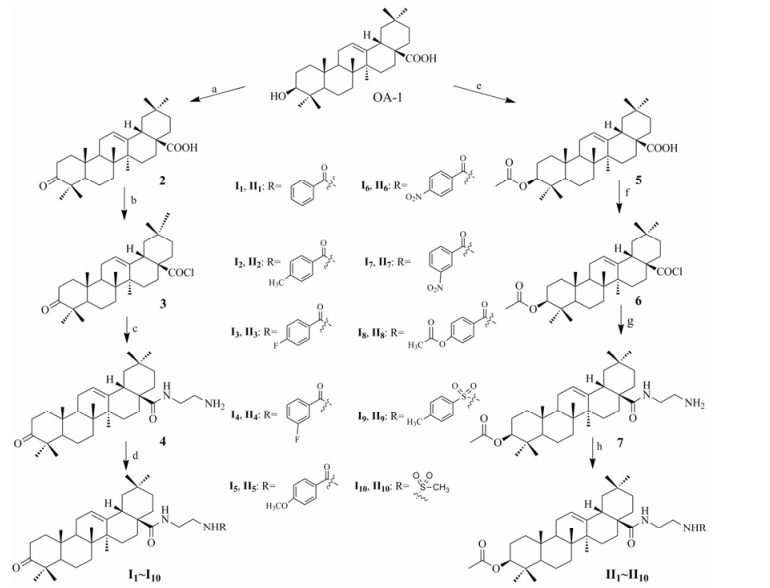
以齐墩果酸为起始原料, 经过C-3位氧化或C-3位乙酰化反应、C-28位酰氯化反应、取代反应和酰胺化反应共4步反应合成了20个齐墩果酸衍生物。目标化合物经1H NMR、13C NMR和HR-MS确证其结构, 数据见实验部分。
2 生物活性评价 2.1 目标化合物对人癌细胞株的体外增殖抑制实验采用MTT法测试目标化合物对人肝癌细胞(HepG2)和人胃癌细胞(SGC7901)的体外细胞毒活性, 以舒尼替尼(sunitinib)为阳性对照物, 结果见表 1。结果表明, 目标化合物对两种肿瘤细胞的抑制活性均明显强于OA, 其中Ⅰ6、Ⅰ8和Ⅰ9对HepG2细胞显示出较强的活性, IC50值分别是16.7、9.8和6.3 μmol·L-1。
| Table 1 Anti-tumor activity of the target compounds on HepG2 and SGC7901 cell lines. aInhibitory percentage of cells treated with each compound at a concentration of 10 μmol·L-1 for 72 h; bThe agent concentration that inhibited SGC7901 and HepG2 cells growth by 50% |
以PDB数据库中VEGFR为靶点(PDB编号: 5ew3), 作为分子对接受体模型。将5ew3导入计算机辅助药物设计软件Molegro Virtual Docker (MVD 6.0), 根据cavity的探测选择最佳对接区域(center x: 18.57 y: 8.63 z: 12.57, radius: 15)。将受体蛋白中原有配体替换为目标配体分子, 利用Docking wizard向导设定配体的对接运算次数后, 选择打分函数MolDock Score [GRID]与构象搜寻MolDock SE[13], 分别对化合物(Ⅰ1~Ⅰ10和Ⅱ1~Ⅱ10)与受体对接的结合能进行评分, 评分数值的绝对值越大表明配体与受体亲和力越好[14]。选取MD score绝对值最高的分子构象作为与靶点的模拟对接模型, 利用Discovery studio 4.0分析得到配体与氨基酸残基作用的图像。利用打分函数评价受体与配体的结合情况, 同时考虑氢键作用以及靶点关键氨基酸的结合情况[14-16], 化合物Ⅰ1~Ⅰ10和Ⅱ1~Ⅱ10的对接结果见表 2。结果表明, 目标化合物均能较好地插入到靶点的活性口袋中, 利用氢键、疏水键、π-σ键、π-alkyl等与靶蛋白的多个氨基酸残基相互作用(Asp1046、Glu885、Cys1045、Val899、Val848、Ala866、Ile892、Ile1044、His1026、Leu889等)。Ⅰ系列化合物结合能明显高于Ⅱ系列, Ⅰ2~Ⅰ9有较高的分值, Ⅰ5~Ⅰ9具有氢键作用, 其中化合物Ⅰ6、Ⅰ7、Ⅰ9结构中的乙二胺片段与VEGFR靶蛋白的关键氨基酸Asp1046通过氢键紧密结合。通过对比化合物Ⅰ9和sunitini与靶蛋白的对接图(图 3和图 1)发现, sunitinib和化合物Ⅰ9与靶蛋白连有相同的疏水氨基酸残基(Cys1045、Ala866、Ile892、Ile1044、His1026、Leu889等), 乙二胺片段均与Asp1046产生氢键作用, 初步判断化合物Ⅰ9和sunitinib与靶蛋白具有相同的结合位点[11, 17]。
| Table 2 Energy scores for different compounds with VEGFR (PDB: 5ew3) |

|
Figure 3 Binding of Ⅰ9 to the active site of VEGFR |
|
Reagent and conditions: (a) Jone's reagent, 0 ℃, 2 h; (b) (COCl)2, CH2Cl2, 40 ℃, 2 h; (c) NH2CH2CH2NH2, CHCl3, Py, DMAP, rt, 3 h; (d) RCOCl, CHCl3, Py, DMAP, 60 ℃, 3 h; (e) (CH3CO)2O, Py, DMAP, rt, overnight; (f) (COCl)2, CH2Cl2, 40 ℃, 2 h; (g) NH2CH2CH2NH2, CHCl3, Py, DMAP, 60 ℃, 3 h; (h) RCOCl, CHCl3, Py, DMAP, 60 ℃, 3 h Scheme1 Synthetic route of target compounds |
选取化合物Ⅰ6~Ⅰ9进行VEGFR-2抑制实验, 实验由Invitrogen公司完成。Ⅰ6~Ⅰ9对VEGFR-2的IC50值为10.15、12.56、4.83和0.56 μmol·L-1, 结果表明化合物Ⅰ9对VEGFR-2蛋白具有较好的抑制作用。
3 小结本文以OA为起始原料, 通过在OA-28位引入乙二胺基团, 并以此为连接臂, 进一步通过拼合的方法引入多个生物活性片段, 设计并合成了20个新的OA衍生物, 目标化合物结构经1H NMR、13C NMR和HR-MS确证。采用MTT法考察目标化合物的体外抗肿瘤活性, 结果表明化合物对人肝癌细胞(HepG2)和人胃癌细胞(SGC7901)具有抑制活性。初步的构效关系研究表明OA-3位羰基化合物活性优于OA-3位乙酰基(Ⅰn > Ⅱn), OA-28位末端N上引入苯磺酰基(Ⅰ9)活性优于取代苯甲酰基(Ⅰ1~Ⅰ8)和磺酰基衍生物(Ⅰ10), 苯环上取代基R的电性效应及体积大小均可能影响化合物活性(CH3OCO > NO2 > F > CH3O > CH3), 苯环上对位取代优于邻位取代(Ⅰ3 > Ⅰ4, Ⅰ6 > Ⅰ7)。其中化合物Ⅰ6、Ⅰ8和Ⅰ9对HepG2细胞表现出显著的活性, IC50 = 16.7、9.8和6.3 μmol·L-1。通过计算机辅助设计分子对接方法预测目标化合物和VEGFR靶点的结合能, 结果表明OA-3位羰基化合物的结合能均高于OA-3位乙酰基化合物。选取可以通过氢键与VEGFR蛋白氨基酸残基(Asp1046)相互作用的化合物Ⅰ6~Ⅰ9进行VEGFR抑制活性测试, 结果表明其具有较强的VEGFR抑制活性。实测活性与分子模拟对接预测的结果相关性较好, 本研究结果对进一步优化设计齐墩果酸衍生物作为VEGFR抑制剂的研究具有参考价值。
实验部分Büchi B-540熔点测定仪(温度计未经校正); ARX-400型核磁共振仪(TMS为内标); Autospec Ultima-TOF质谱测定仪; WZZ-1S (2s)自动旋光仪; 齐墩果酸(质量分数 > 98%)购于陕西慈缘生物技术有限公司; 薄层色谱硅胶GF254 (青岛海洋化工厂); 显色剂为10%硫酸乙醇溶液; RPMI-1640培养基(含10%胎牛血清, 100 μg·mL-1青霉素, 100 μg·mL-1链霉素)、溴化四氮唑盐(MTT)、胰蛋白酶(Trypsin)和标准胎牛血清(FBS)、SGC7901细胞和HepG2细胞由沈阳药科大学药理教研室提供; 实验所用试剂均为市售分析纯。
1 化学合成 1.1 3-羰基齐墩果酸(2)的制备将化合物1 (OA, 2.5 g, 5.47 mmol)溶于25 mL丙酮中, 冰盐浴冷却至-10 ℃, 缓慢滴加28.9 mmol Jones试剂, 直至反应液橘红色不再消失, 滴毕, 室温下继续反应2 h, TLC监测反应进程。反应完毕, 加入75 mL异丙醇, 淬灭反应30 min。减压旋蒸, 除去部分溶剂, 加入适量水稀释, 乙酸乙酯萃取, 水洗, 合并有机相, 无水Na2SO4干燥过夜, 抽滤, 洗涤, 减压旋蒸, 得淡绿色固体, 50 ℃真空干燥。甲醇/二氯甲烷(1:10)重结晶, 得白色晶体化合物2 1.92 g, 收率97%, mp 218.1~220.7 ℃ (文献值mp 220.3~222.1 ℃)[18]。
1.2 3-羰基齐墩果酸酰氯(3)的制备将化合物2 (3.50 g, 7.69 mmol)溶于40 mL无水二氯甲烷中, 加热至40 ℃, 缓慢滴加草酰氯(4.2 mL, 49.63 mmol), 滴毕, 回流反应2 h, TLC监测反应, 反应完全后, 减压蒸干溶剂得浅棕色固体粉末, 密闭备用。
1.3 化合物(4)的制备将溶于25 mL无水三氯甲烷中的化合物3 (1.5 g, 3.17 mmol)缓慢滴加到乙二胺(2.12 mL, 31.7 mmol)溶液中, 加入DMAP (0.019 g, 0.16 mmol)和无水吡啶(1.27 mL, 15.85 mmol)。室温反应3 h, TLC监测反应, 反应结束后, 用10 mL稀盐酸(5%)洗涤, 二氯甲烷萃取, 水洗, 合并有机相, 无水Na2SO4干燥过夜, 抽滤, 洗涤, 旋蒸除去溶剂, 得棕色固体, 50 ℃真空干燥。硅胶柱色谱分离, 甲醇/二氯甲烷(1:150)洗脱, 得白色粉末化合物4 1.26 g, 收率80%。mp 149.5~150.3 ℃; HR-MS m/z: 497.402 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 6.60 (1H, d, J = 5.1 Hz, CONH), 5.40 (1H, d, J = 15.1 Hz, H-12), 3.51 (1H, dd, J = 13.7, 5.8 Hz, CONHCH2CH2), 3.22 (1H, dd, J = 13.9, 5.6 Hz, CONHCH2CH2), 3.07 (2H, s, CONHCH2CH2), 2.94 (2H, t, J = 5.4 Hz, NH2), 2.63 (1H, d, J = 9.4 Hz, H-18), 2.55 (1H, m, H-2), 2.37 (1H, m, H-12), 2.55 (1H, ddd, J = 15.9, 11.1, 7.3 Hz, H-2), 2.37 (1H, ddd, J = 15.7, 6.5, 3.5 Hz, H-2), 1.17, 1.09, 1.05, 0.92, 0.91, 0.81 (3H, s, CH3)。
1.4 化合物Ⅰ1~Ⅰ10的制备化合物Ⅰ1的制备 将化合物4 (0.497 g, 1.0 mmol)溶于10 mL无水三氯甲烷中, 加入苯甲酰氯(0.71 g, 5.0 mmol)、无水吡啶(0.41 mL, 5.0 mmol)和DMAP (0.006 g, 0.05 mmol), 升温至60 ℃, 持续回流反应3 h。TLC监测反应, 反应结束后, 用10 mL稀盐酸(5%)洗涤, 加入适量水稀释, 二氯甲烷萃取, 水洗, 合并有机相, 无水Na2SO4干燥, 抽滤, 洗涤, 旋蒸除去溶剂, 得淡黄色油状物质, 50 ℃真空干燥。硅胶柱色谱分离, 甲醇/二氯甲烷(0~10%)洗脱, 得白色固体化合物Ⅰ1 0.42 g, 收率70%。mp110.2~112.0 ℃; [α]D25 +36.5 (c 0.40, CHCl3); HR-MS m/z: 601.429 0 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.86~7.80 (2H, m, ph-H-2', H-6'), 7.60 (1H, dd, J = 15.1, 7.6 Hz, CONHCH2CH2NH), 7.45~7.40 (2H, m, ph-H-3', H-5'), 6.48 (1H, d, J = 5.7 Hz, CONH), 5.42 (1H, t, J = 3.5 Hz, H-12), 3.66~3.35 (4H, m, CONHCH2CH2), 2.63~2.57 (1H, m, H-18), 2.54 (1H, ddd, J = 15.9, 11.3, 7.Hz, H-2), 2.35 (1H, ddd, J = 15.8, 6.6, 3.Hz, H-2), 1.17, 1.07, 1.02, 0.97, 0.89, 0.87, 0.75 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.63, 180.55, 166.46, 165.92, 163.52, 144.45, 132.61, 130.29, 129.40, 115.67, 115.58, 115.12, 115.36, 55.24, 47.46, 46.73, 46.59, 46.42, 42.28, 41.90, 39.54, 39.20, 39.23, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.65, 27.25, 26.36, 25.60, 23.68, 23.37, 21.48, 19.39, 16.68, 14.97。
同法合成Ⅰ2~Ⅰ10
Ⅰ2, 白色固体, 收率70%。mp 99.5~101.7 ℃; [α]D25 +42.3 (c 0.55, CHCl3); HR-MS m/z: 615.444 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.72 (2H, d, J = 8.1 Hz, ph-H-2', H-6'), 7.46 (1H, s, CONHCH2CH2NH), 7.22 (2H, d, J = 8.0 Hz, ph-H-3', H-5'), 6.50 (1H, t, J = 5.3 Hz, CONH), 5.42 (1H, t, J = 3.5 Hz, H-12), 3.65~3.37 (4H, m, CONHCH2CH2NH), 2.62~2.58 (1H, m, H-18), 2.57~2.49 (1H, m, H-2), 2.39 (3H, s, ph-CH3), 2.37~2.32 (1H, m, H-2), 1.15, 1.09, 1.03, 0.97, 0.89, 0.87, 0.75 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.56, 180.68, 166.68, 166.12, 163.45, 144.28, 141.15, 132.65, 130.03, 129.38, 115.58, 115.36, 55.64, 48.46, 47.83, 46.59, 45.37, 42.08, 41.95, 39.44, 39.29, 39.11, 36.66, 34.08, 32.92, 32.67, 31.81, 30.69, 29.70, 27.20, 26.46, 25.65, 23.72, 23.28, 21.58, 20.93, 19.41, 16.70, 14.95。
Ⅰ3, 白色固体, 收率61%。mp 102.3~103.5 ℃; [α]D25 +40.6 (c 0.45, CHCl3); HR-MS m/z: 619.419 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.13 (2H, dd, J = 8.3, 5.5 Hz, ph-H-2', H-6'), 7.89 (1H, dd, J = 8.2, 5.7 Hz, CONHCH2CH2NH), 7.13 (2H, dt, J = 16.7, 8.5 Hz, ph-H-3', H-5'), 6.56 (1H, d, J = 5.3 Hz, CONH), 5.43 (1H, s, H-12), 3.54 (4H, m, CONHCH2CH2NH), 2.60 (1H, dd, J = 15.9, 8.7 Hz, H-18), 2.37 (1H, m, H-2), 1.16, 1.09, 1.04, 0.99, 0.91, 0.87, 0.76 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.68, 180.99, 166.77, 166.02, 163.52, 144.40, 132.69, 130.00, 129.48, 115.70, 115.58, 115.48, 115.36, 55.24, 47.46, 46.73, 46.59, 46.47, 42.08, 41.95, 39.44, 39.29, 39.11, 36.66, 34.08, 32.92, 32.67, 31.81, 30.69, 29.70, 27.22, 26.36, 25.64, 23.73, 23.49, 21.48, 19.47, 16.72, 14.97。
Ⅰ4, 白色粉末状固体, 收率56%。mp 98.5~ 102.2 ℃; [α]D25 +45.6 (c 0.55, CHCl3); HR-MS m/z: 619.419 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.20 (1H, ph-H-2'), 7.60 (1H, dd, J = 15.1, 7.6 Hz, CONHCH2CH2NH), 7.38~7.12 (3H, m, ph-H-4', 5', 6'), 6.56 (1H, d, J = 5.3 Hz, CONH), 5.43 (1H, s, H-12), 3.54 (4H, m, CONHCH2CH2NH), 2.60 (1H, dd, J = 15.9, 8.7 Hz, H-18), 2.37 (1H, ddd, J = 15.7, 6.2, 3.3 Hz, H-2), 1.16, 1.09, 1.04, 0.99, 0.89, 0.85, 0.75 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl) δ 217.65, 180.86, 166.67, 166.01, 163.50, 144.42, 132.67, 130.05, 129.46, 115.72, 115.58, 115.48, 115.38, 55.25, 47.46, 46.74, 46.60, 46.47, 42.10, 41.93, 39.45, 39.29, 39.11, 37.66, 34.10, 32.93, 32.68, 31.81, 30.65, 29.72, 27.23, 26.35, 25.64, 23.74, 23.47, 21.46, 19.45, 16.74, 14.95。
Ⅰ5, 白色粉末固体, 收率91%。mp 159.6~162.8 ℃; [α]D25 +41.8 (c 0.50, CHCl3); HR-MS m/z: 631.439 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.82 (2H, d, J = 8.7 Hz, ph-H-2', H-6'), 7.63 (1H, d, J = 12.0 Hz, CONHCH2CH2NH), 6.91 (2H, dd, J = 8.5, 2.9 Hz, ph-H-3', H-5'), 6.63 (1H, s, CONH), 5.41 (1H, s, H-12), 3.85 (3H, dd, J = 10.8, 3.1 Hz, ph-OCH3), 3.56~3.11 (4H, m, CONHCH2CH2NH), 2.70~2.59 (1H, m, H-18), 2.53 (1H, dt, J = 11.8, 7.8Hz, H-2), 2.40~2.28 (1H, m, H-2), 1.14, 1.07, 1.02, 0.96, 0.92, 0.83, 0.74 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.69, 180.43, 167.50, 162.15, 144.35, 128.94, 126.23, 122.81, 113.63, 55.38, 55.22, 47.43, 46.75, 46.59, 46.37, 45.91, 42.03, 41.82, 41.46, 39.82, 39.27, 39.10, 36.64, 34.10, 32.96, 32.74, 31.80, 30.69, 29.69, 27.26, 26.38, 25.66, 23.64, 23.52, 21.47, 19.49, 16.71, 14.96。
Ⅰ6, 白色粉末固体, 收率91%。mp 167.2~ 170.5 ℃; [α]D25 +38.6 (c 0.45, CHCl3); HR-MS m/z: 646.414 2 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.40 (1H, s, CONHCH2CH2NH), 8.06 (2H, d, J = 8.4 Hz, ph-H-2', H-6'), 7.82 (2H, t, J = 7.5 Hz, ph-H-3', H-5'), 6.56 (1H, s, CONH), 5.45 (1H, s, H-12), 3.7~3.41 (4H, m, CONHCH2CH2NH), 2.61 (1H, d, J = 9.7 Hz, H-18), 2.42~2.31 (1H, m, H-2), 1.18, 1.09, 1.04, 0.99, 0.93, 0.89, 0.76 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.50, 181.77, 165.33, 148.45, 144.47, 144.41, 137.33, 130.91, 128.32, 124.29, 123.71, 123.43, 123.03, 55.21, 47.45, 46.69, 46.57, 43.15, 42.16, 42.09, 39.30, 39.20, 39.10, 36.65, 34.09, 34.00, 32.87, 32.66, 31.80, 30.69, 27.18, 26.35, 25.61, 23.83, 23.53, 23.46, 21.48, 19.45, 16.75, 14.97。
Ⅰ7, 白色粉末状固体, 收率88%。mp 158.5~160.4 ℃。[α]D25 +32.8 (c 0.35, CHCl3); HR-MS m/z: 646.414 2 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.42 (1H, s, CONHCH2CH2NH), 8.25 (1H, m, ph-H-2'), 7.82~7.39 (3H, m, ph-H-3', 4', 5'), 6.76 (1H, s, CONH), 5.39 (1H, s, H-12), 3.60~3.28 (4H, m, CONHCH2CH2NH), 2.76 (1H, d, J = 9.7 Hz, H-18), 2.39~2.18 (1H, m, H-2), 1.18, 1.09, 1.04, 0.99, 0.94, 0.90, 0.76 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl) δ 217.49, 181.76, 165.31, 147.45, 145.46, 143.42, 137.34, 130.95, 128.35, 124.25, 123.73, 123.45, 123.05, 55.23, 47.43, 46.68, 46.57, 43.14, 42.16, 41.95, 39.32, 39.20, 39.08, 36.64, 34.09, 34.02, 32.85, 32.65, 31.80, 30.65, 27.18, 26.34, 25.62, 23.82, 23.54, 23.45, 21.48, 19.46, 16.76, 14.96。
Ⅰ8, 白色粉末固体, 收率73%。mp 180.2~182.1 ℃; [α]D25 +39.8 (c 0.45, CHCl3); HR-MS m/z: 659.434 6 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.81 (2H, d, J = 8.5 Hz, ph-H-2', H-6'), 7.72 (1H, s, -CONHCH2CH2NH), 7.10 (2H, dd, J = 16.5, 8.4 Hz, ph-H-3', H-5'), 6.52 (1H, s, CONH), 5.34 (1H, s, H-12), 3.58~3.29 (4H, m, -CONHCH2CH2NH), 2.58~2.50 (1H, m, H-18), 2.25 (3H, d, J = 7.0 Hz, ph-OAc), 1.07 (3H, s, CH3), 1.00 (3H, s, CH3), 0.95 (3H, s, CH3), 0.89 (3H, s, CH3), 0.83 (3H, s, CH3), 0.79 (3H, s, CH3), 0.67 (3H, s, CH3); 13C NMR (101 MHz, CDCl) δ 217.85, 180.90, 179.58, 169.00, 153.19, 144.35, 131.72, 131.28, 128.60, 122.97, 121.68, 55.25, 47.47, 46.75, 46.60, 46.47, 42.08, 41.95, 41.89~41.85, 39.60, 39.30, 39.10, 36.66, 34.14, 34.03, 32.93, 32.68, 31.80, 30.69, 29.70, 27.24, 26.37, 25.66, 23.69, 23.49, 21.49, 21.16, 19.48, 16.73, 14.96。
Ⅰ9, 白色粉末固体, 收率69%。mp 124.2~125.9 ℃; [α]D25 +46.8 (c 0.55, CHCl3); HR-MS m/z: 651.411 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.74 (2H, d, J = 8.3 Hz, ph-H-2', H-6'), 7.30 (2H, d, J = 8.0 Hz, ph-H-3', H-5'), 6.33 (1H, t, J = 5.4 Hz, CONH), 5.44 (1H, t, J = 3.5 Hz, H-12), 5.31 (1H, d, J = 8.6 Hz, CONHCH2CH2NH), 3.51~3.00 (4H, m, CONHCH2CH2NH), 2.55 (1H, m, H-18), 2.55 (1H, m, H-2), 2.42 (1H, s, ph-CH3), 2.37 (1H, m, H-2), 1.17 (3H, s, CH3), 1.09 (3H, s, CH3), 1.05 (6H, s, 2CH3), 0.93 (3H, s, CH3), 0.89 (3H, s, CH3), 0.76 (3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.75, 180.63, 166.58, 166.42, 163.39, 142.38, 140.19, 132.63, 130.83, 129.35, 115.53, 115.38, 55.64, 48.46, 47.83, 46.59, 45.37, 42.08, 41.95, 39.44, 39.29, 39.11, 36.66, 34.08, 32.92, 32.67, 31.81, 30.69, 29.70, 27.20, 26.46, 25.65, 23.72, 23.28, 21.58, 20.67, 19.41, 16.70, 14.95。
Ⅰ10, 白色粉末固体, 收率64%。mp 123.4~125.2 ℃; [α]D25 +34.5 (c 0.35, CHCl3); HR-MS m/z: 575.380 4 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 6.35 (1H, d, J = 5.3 Hz, CONH), 5.44 (1H, t, J = 3.5 Hz, H-12), 5.05 (1H, s, 1H, CONHCH2CH2NH), 3.60~3.16 (4H, m, CONHCH2CH2), 2.98~2.95 (3H, m, S-CH3), 2.67 (1H, m, H-18), 2.56 (1H, m, H-2), 2.36 (1H, m, H-2), 1.24, 1.17, 1.13, 1.09, 1.01, 0.91, 0.82 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 217.65, 180.65, 166.56, 163.39, 129.35, 115.53, 55.64, 48.46, 47.83, 46.59, 45.37, 42.08, 41.95, 39.44, 39.29, 39.11, 36.66, 34.08, 32.92, 32.67, 31.81, 30.69, 29.70, 27.20, 26.46, 25.65, 23.72, 23.28, 21.58, 20.67, 19.41, 16.70, 14.95。
1.5 3-乙酰氧基齐墩果酸的制备(5)的制备将化合物1 OA (4.16 g, 11.12 mmol)溶于30 mL无水吡啶中, 冰浴冷却至0 ℃, 缓慢滴加乙酸酐(10.52 mL, 111.2 mmol), 滴毕, 加入DMAP (0.052 g, 0.42 mmol), 室温反应过夜, TLC监测反应。反应结束后, 将反应液倾入200 mL冰水中, 不断搅拌直至固体全部析出, 二氯甲烷萃取, 稀盐酸(5%)洗, 水洗, 无水Na2SO4干燥, 抽滤, 洗涤, 蒸干溶剂, 得深黄色油状物, 50 ℃真空干燥。用甲醇/二氯甲烷重结晶得白色针状晶体化合物5 4.9 g, 收率88%, mp 268.1~270.8 ℃。(文献值: mp 269~271 ℃)[19]。
1.6 3-乙酰氧基齐墩果酸酰氯(6)的制备将化合物5 (2.5 g, 5.02 mmol)溶于25 mL无水二氯甲烷中, 加热至40 ℃, 搅拌下缓慢滴加草酰氯(2.5 mL, 29.25 mmol), 回流反应2 h, TLC监测反应, 反应完全后, 减压蒸干溶剂, 减压蒸干得浅棕色固体粉末, 密闭备用。
1.7 化合物(7)的制备将溶于30 mL无水三氯甲烷中的化合物6 (2.5 g, 4.84 mmol)缓慢滴加到乙二胺(2.5 mL, 37.4 mmol)溶液中, 加入DMAP (0.02 g, 0.16 mmol)和无水吡啶(1.5 mL, 18.63 mmol), 升温至60 ℃, 持续回流反应3 h。室温反应3 h, TLC监测反应, 反应结束后, 用10 mL稀盐酸(5%)洗涤, 二氯甲烷萃取, 水洗, 合并有机相, 无水Na2SO4干燥过夜, 抽滤, 洗涤, 旋蒸除去溶剂, 得棕色固体粉末, 50 ℃真空干燥。硅胶柱色谱分离, 甲醇/二氯甲烷(1:50)洗脱, 得白色粉末化合物7 (2.35 g, 4.35 mmol), 收率90%。mp 198.7~201.3 ℃; HR-MS m/z: 541.429 0 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 6.38 (1H, t, J = 5.4 Hz, CONH), 5.39 (1H, t, J = 3.4 Hz, H-12), 4.48 (1H, dd, J = 10.0, 5.9 Hz, H-3), 3.45 (1H, dq, J = 12.0, 6.0 Hz, NHCH2), 3.15~3.04 (1H, m, NHCH2), 3.02 (2H, s, NH2), 2.85~2.78 (2H, m, NHCH2CH2), 2.56 (1H, dd, J = 12.8, 3.3 Hz, H-18), 2.05 (3H, s, 3-COCH3), 0.91 (6H, s, 2CH3), 1.16, 0.93, 0.87, 0.85, 0.78 (each 3H, s, CH3)。
1.8 化合物Ⅱ1~Ⅱ10的制备化合物Ⅱ1的制备 将化合物7 (0.50 g, 0.925 mmol)溶于10 mL无水三氯甲烷中, 加入苯甲酰氯(0.63 g, 4.65 mmol)、无水吡啶(0.37 mL, 4.65 mmol)和DMAP (0.011 g, 0.093 mmol), 升温至60 ℃, 持续回流反应3 h。TLC监测反应, 反应结束后, 用10 mL稀盐酸(5%)洗涤, 加入适量水稀释, 二氯甲烷萃取, 水洗, 合并有机相, 无水Na2SO4干燥, 抽滤, 洗涤, 旋蒸除去溶剂, 得淡黄色油状物质, 50 ℃真空干燥。硅胶柱色谱分离, 甲醇/二氯甲烷(0~10%)洗脱, 得白色粉末固体化合物Ⅱ1 0.423 g, 收率71%。mp 167.1~168.9 ℃; [α]D25 +62.8 (c 0.95, CHCl3); HR-MS m/z: 645.455 4 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.88~7.78 (1H, m, 1H, ph-H-2', H-6'), 7.61 (1H, d, J = 14.8 Hz, CONHCH2CH2NH), 7.51~7.40 (3H, m, ph-H-3', H-4', H-5'), 6.47 (1H, d, J = 5.7 Hz, CONH), 5.40 (1H, t, J = 3.4 Hz, H-12), 4.47 (1H, dd, J = 10.3, 5.9 Hz, H-3), 3.75~3.21 (4H, m, CONHCH2CH2), 2.56 (1H, dd, J = 12.8, 3.3 Hz, H-18), 2.04 (3H, s, 3-OCOCH3), 1.14, 0.89, 0.87, 0.86, 0.85, 0.84, 0.70 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.55, 169.25, 165.92, 163.52, 162.59, 147.45, 133.63, 131.23, 129.40, 115.67, 115.58, 115.12, 114.59, 55.24, 47.46, 46.73, 46.59, 46.42, 42.28, 41.90, 39.54, 39.20, 37.23, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.65, 27.25, 26.36, 25.60, 23.68, 23.37, 22.32, 21.48, 19.39, 17.38, 16.68, 14.97。
Ⅱ2, 白色粉末固体, 收率70%。mp 161.1~163.7 ℃; [α]D25 +40.6 (c 0.52, CHCl3); HR-MS m/z: 659.471 2 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.72 (2H, d, J = 8.2 Hz, ph-H-2', H-6'), 7.49 (1H, d, J = 8.1 Hz, CONHCH2CH2NH), 7.23 (2H, dd, J = 14.3, 7.9 Hz, ph-H-3', H-5'), 6.26 (1H, t, J = 5.4 Hz, CONH), 5.38 (1H, dt, J = 12.6, 3.3 Hz, H-12), 4.48 (1H, dt, J = 8.1, 6.8 Hz, H-3), 3.71~3.16 (4H, m, CONHCH2CH2), 2.54 (1H, dd, J = 10.1, 3.4 Hz, H-18), 2.46 (3H, s, ph-CH3), 2.05 (3H, s, 3-OCOCH3), 1.15, 0.92, 0.90, 0.86, 0.84, 0.73, 0.70 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.54, 169.26, 165.93, 163.48, 162.59, 148.35, 132.96, 131.35, 129.45, 115.61, 115.25, 115.22, 114.59, 57.14, 49.46, 47.78, 46.59, 45.42, 42.38, 41.95, 39.56, 39.25, 38.25, 36.83, 33.15, 32.75, 32.53, 31.85, 30.44, 29.65, 28.15, 26.56, 25.36, 23.65, 23.35, 22.34, 21.56, 21.02, 19.16, 17.37, 16.57, 14.65。
Ⅱ3, 白色粉末状固体, 收率75%, mp 170.8~173.2 ℃; [α]D25 +58.6 (c 0.85, CHCl3); HR-MS m/z: 663.445 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.91~7.84 (4H, m, ph-H-2', 3', 5', 6'), 6.48 (1H, t, J = 5.8 Hz, CONH), 5.40 (1H, t, J = 3.3 Hz, H-12), 4.47 (1H, dd, J = 10.8, 5.3 Hz, H-3), 3.39 (4H, m, CONHCH2CH2), 2.63~2.51 (1H, m, H-18), 2.05 (3H, s, 3-OCOCH3), 1.14, 0.91, 0.88, 0.87, 0.85, 0.76, 0.65 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.56, 169.45, 165.76, 163.52, 147.37, 134.58, 131.23, 129.40, 119.05, 116.59, 115.12, 55.24, 47.46, 46.73, 46.59, 46.42, 42.28, 41.90, 39.54, 39.20, 38.13, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.65, 27.25, 26.36, 25.60, 23.68, 23.37, 19.11, 17.45, 16.65, 14.95。
Ⅱ4, 白色粉末状固体, 收率65%。mp 162.1~164.2 ℃; [α]D25 +30.6 (c 0.43, CHCl3); HR-MS m/z: 663.445 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.03~7.64 (4H, m, ph-H-2', 4', 5', 6'), 6.40 (1H, t, J = 5.8 Hz, CONH), 5.36 (1H, t, J = 3.3 Hz, H-12), 4.51 (1H, dd, J = 10.8, 5.3 Hz, H-3), 3.45 (4H, m, CONHCH2CH2), 2.76~2.41 (1H, m, H-18), 2.15 (3H, s, 3-OCOCH3), 1.10, 0.89, 0.87, 0.86, 0.85, 0.76, 0.67 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.57, 169.44, 165.77, 163.54, 147.35, 137.28, 135.21, 130.40, 121.25, 116.28, 115.02, 54.24, 47.46, 46.28, 46.53, 46.19, 42.18, 41.56, 39.53, 39.58, 39.23, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.65, 27.25, 26.36, 25.60, 23.68, 23.37, 19.23, 17.47, 16.68, 14.96。
Ⅱ5, 白色固体粉末状固体, 收率75%。mp 153.2~155.1 ℃; [α]D25 +42.5 (c 0.50, CHCl3); HR-MS m/z: 675.465 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.79 (2H, d, J = 8.8 Hz, ph-H-2', H-6'), 7.48 (1H, s, CONHCH2CH2NH), 6.91 (2H, d, J = 8.8 Hz, ph-H-3', H-5'), 6.48 (1H, d, J = 5.7 Hz, CONH), 5.40 (1H, t, J = 3.4 Hz, H-12), 4.47 (1H, dd, J = 10.4, 5.8 Hz, H-3), 3.82 (3H, s, ph-OCH3), 3.40~3.33 (4H, m, CONHCH2CH2), 2.67 (1H, dd, J = 10.2, 3.4 Hz, H-18), 2.05 (3H, s, 3-OCOCH3), 1.14, 0.89, 0.87, 0.86, 0.85, 0.84, 0.70 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.65, 169.37, 165.86, 162.42, 148.57, 135.26, 132.25, 127.44, 118.09, 115.65, 114.12, 56.28, 53.87, 47.46, 46.73, 46.59, 46.12, 42.38, 41.95, 39.55, 39.20, 38.13, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.60, 27.23, 26.35, 25.60, 23.18, 22.37, 19.13, 17.45, 16.65, 14.95。
Ⅱ6, 白色固体粉末g, 产率80%。mp 161.6~163.7 ℃; [α]D25 +29.6 (c 0.40, CHCl3); HR-MS m/z: 690.440 2 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.39 (1H, s, CONHCH2CH2NH), 8.28 (2H, d, J = 8.8 Hz, ph-H-2', H-6'), 8.03 (2H, d, J = 8.8 Hz, ph-H-3', H-5'), 6.51 (1H, t, J = 5.8 Hz, CONH), 5.41 (1H, t, J = 3.3 Hz, H-12), 4.47 (1H, dd, J = 10.8, 5.3 Hz, H-3), 3.57 (2H, dd, J = 10.0, 4.3 Hz, CONHCH2CH2), 3.44 (2H, dd, J = 10.4, 5.3 Hz, CONHCH2CH2), 2.59 (1H, dd, J = 10.2, 3.4 Hz, H-18), 2.05 (3H, s, 3-OCOCH3), 1.15, 0.91, 0.88, 0.84, 0.86, 0.69, 0.58 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.75, 169.45, 165.33, 148.45, 144.47, 144.41, 137.33, 130.91, 128.32, 124.29, 123.71, 123.43, 123.03, 55.21, 47.45, 46.69, 46.57, 43.15, 42.16, 42.09, 39.30, 39.20, 39.10, 36.65, 34.09, 34.00, 32.87, 32.66, 31.80, 30.69, 27.18, 26.35, 25.61, 24.13, 23.53, 23.16, 21.48, 19.45, 16.75, 14.97。
Ⅱ7, 白色粉末状固体, 收率70%。mp 155.2~157.1 ℃; [α]D25 +30.4 (c 0.45, CHCl3); HR-MS m/z: 690.4402 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 8.41 (1H, s, CONHCH2CH2NH), 8.33 (1H, m, ph-H-2'), 7.98~7.82 (3H, m, ph-H-3', 4', 5'), 6.65 (1H, t, J = 5.8 Hz, CONH), 5.39 (1H, t, J = 3.3 Hz, H-12), 4.49 (1H, dd, J = 10.8, 5.3 Hz, H-3), 3.52 (2H, dd, J = 10.0, 4.3 Hz, CONHCH2CH2), 3.45 (2H, dd, J = 10.4, 5.3 Hz, CONHCH2CH2), 2.52 (1H, dd, J = 10.2, 3.4 Hz, H-18), 2.10 (3H, s, 3-OCOCH3), 1.16, 1.10, 0.93, 0.89, 0.86, 0.72, 0.60 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.76, 169.25, 165.31, 147.45, 145.46, 143.42, 137.34, 130.95, 128.35, 124.25, 123.73, 123.45, 123.05, 55.23, 47.43, 46.68, 46.57, 43.14, 42.16, 41.95, 39.32, 39.20, 39.08, 36.64, 34.09, 34.02, 32.85, 32.65, 31.80, 30.65, 27.18, 26.34, 25.62, 24.82, 23.64, 23.13, 21.48, 19.16, 16.80, 14.86。
Ⅱ8, 白色固体粉末, 收率65%。mp 130.3~ 131.4 ℃; [α]D25 +48.6 (c 0.55, CHCl3); HR-MS m/z: 703.460 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.89~7.84 (2H, m, ph-H-2', H-6'), 7.16~7.14 (2H, m, ph-H-3', H-5'), 6.49 (1H, t, J = 5.6 Hz, CONH), 5.40 (1H, t, J = 3.4 Hz, H-12), 4.47 (1H, dd, J = 10.0, 6.1 Hz, H-3), 3.66~3.30 (4H, m, CONHCH2CH2), 2.58 (1H, dd, J = 10.2, 3.4 Hz, H-18), 2.36 (3H, s, ph-OAc), 2.05 (3H, s, 3-OCOCH3), 1.143 (3H, s, -CH3), 0.87 (6H, s, 2CH3), 0.90, 0.85, 0.84, 0.71 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 180.54, 179.62, 169.20, 165.92, 163.52, 153.19, 147.35, 131.75, 131.28, 128.60, 122.97, 121.68, 147.45, 133.63, 131.23, 129.40, 115.67, 115.58, 115.12, 55.24, 47.46, 46.73, 46.59, 46.42, 42.28, 41.90, 39.54, 39.20, 39.23, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.65, 27.25, 26.36, 25.60, 23.68, 23.37, 21.48, 19.39, 17.38, 16.68, 14.97。
Ⅱ9, 白色粉末状固体, 收率60%。mp 127.3~129.6 ℃; [α]D25 +52.1 (c 0.65, CHCl3); HR-MS m/z: 695.437 8 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 7.73 (2H, d, J = 8.2 Hz, ph-H-2', H-6'), 7.29 (2H, d, J = 8.1 Hz, ph-H-3', H-5'), 6.32 (1H, t, J = 5.5Hz, CONHCH2CH2NH), 5.41 (1H, d, J = 3.4 Hz, H-12), 4.49 (1H, dd, J = 10.2, 5.7 Hz, H-3), 3.46~3.05 (4H, CONHCH2CH2), 2.51 (1H, d, J = 9.8 Hz, H-18), 2.42 (3H, s, ph-CH3), 2.05 (3H, s, 3-OCOCH3), 1.15, 0.93, 0.91, 0.90, 0.87, 0.86, 0.68 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl) δ 180.65, 169.25, 166.59, 163.52, 142.45, 132.63, 131.23, 129.40, 115.67, 115.58, 115.12, 55.24, 47.46, 46.73, 46.59, 46.42, 42.28, 41.90, 39.54, 39.20, 39.23, 36.76, 33.09, 32.95, 32.67, 31.85, 30.60, 29.65, 27.25, 26.36, 25.60, 23.68, 23.37, 21.48, 19.39, 17.38, 16.68, 14.95。
Ⅱ10, 白色固体, 收率51%。mp 127.2~130.2 ℃; [α]D25 +48.3 (c 0.55, CHCl3); HR-MS m/z: 619.406 4 [M+H]+; 1H NMR (400 MHz, CDCl3) δ 6.35 (1H, d, J = 5.3 Hz, CONH), 5.45~5.37 (1H, d, J = 3.4 Hz, H-12), 4.49 (1H, dd, J = 10.2, 5.7 Hz, H-3), 3.57~3.10 (4H, m, CONHCH2CH2), 2.96 (3H, m, S-CH3), 2.54 (1H, m, H-18), 2.15 (3H, s, CH3), 1.83~1.66 (1H, m, H-2), 1.65~1.31 (1H, m, H-2), 1.19, 1.11, 0.96, 0.92, 0.88, 0.75 (each 3H, s, CH3); 13C NMR (101 MHz, CDCl3) δ 181.55, 169.25, 165.92, 163.52, 147.45, 129.40, 115.67, 55.24, 47.46, 46.73, 46.59, 46.42, 42.28, 41.90, 39.54, 39.20, 38.25, 36.76, 33.12, 32.75, 32.67, 31.25, 30.45, 29.65, 27.25, 26.36, 25.60, 24.12, 23.52, 21.58, 19.52, 18.02, 16.85, 14.97。
2 初步体外细胞毒活性测试取对数生长期的各株细胞以每孔5×103个接种于96孔板内, 每组3个复孔, CO2培养箱中培养24 h后备用。待细胞贴壁后, 分别加入待测化合物和舒尼替尼, 药品浓度为10 μmol·L-1, 空白组为阴性对照组, 舒尼替尼为阳性对照组。培养箱中继续培养72 h。加入MTT溶液(5 mg·mL-1, 20 μL), 继续培养4 h, 吸去每孔上清液, 加入二甲基亚砜200 μL, 震荡器上震荡5 min, 然后用酶标仪于570 nm处测OD值, 重复3次实验, 取平均值, 计算各组细胞的IC50, 抑制率= (对照组OD值-药物组OD值)/对照组OD值×100%。
| [1] | Sawyers C. Targeted cancer therapy[J]. Nature, 2004, 432: 294–297. DOI:10.1038/nature03095 |
| [2] | Newman DJ, Giddings IA. Natural products as leads to antitumor drugs[J]. Phytochem Rev, 2014, 13: 123–137. DOI:10.1007/s11101-013-9292-6 |
| [3] | Qian S, Li HJ, Chen Y, et al. Synthesis and biological evaluation of oleanolic acid derivatives as inhibitors of protein tyrosine phosphatase 1B[J]. J Nat Prod, 2010, 73: 1743–1750. DOI:10.1021/np100064m |
| [4] | Lin C, Huang C, Mong M, et al. Antiangiogenic potential of three triterpenic acids in human liver cancer cells[J]. J Agric Food Chem, 2011, 59: 7557–7562. DOI:10.1021/jf104237y |
| [5] | Ivy SP, Wick JY, Kaufman BM. An overview of small- molecule inhibitors of VEGFR signaling[J]. Clin Oncol, 2009, 6: 569–579. |
| [6] | Pang X, Yi Z, Zhang X, et al. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis[J]. Cancer Res, 2009, 69: 5893–5900. DOI:10.1158/0008-5472.CAN-09-0755 |
| [7] | Tang C, Chen Y, Bai S, et al. Advances in the study of structural modification and biological activities of oleanolic acid[J]. Chin J Org Chem (有机化学), 2013, 33: 46–65. DOI:10.6023/cjoc201207019 |
| [8] | Liu J, Wu N, Ma LN, et al. Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type M isoforms[J]. PLoS One, 2014, 9: e91606. DOI:10.1371/journal.pone.0091606 |
| [9] | Wei JT, Liu M, Liu HZ, et al. Oleanolic acid inhibits proliferation of HUVECs, and inhibits migration and tube formation via VEGF pathway[J]. Acta Pharm Sin (药学学报), 2012, 47: 1457–1462. |
| [10] | Small J, Washburn E, Millington K, et al. The addition of abemaciclib to sunitinib induces regression of renal cell carcinoma xenograft tumors[J]. Oncotarget, 2017, 56: 95116–95134. |
| [11] | Liu P, Zhou YF, Zhang Y, et al. Research progress in VEGFR-2 inhibitors[J]. Acta Pharm Sin (药学学报), 2017, 52: 531–540. |
| [12] | Mol CD, Fabbro D, Hosfield DJ. Structural insights into the conformational selectivity of STI-571 and related kinase inhibitors[J]. Curr Opin Drug Discov Devel, 2004, 7: 639–648. |
| [13] | Weng Y, Zhang LJ, Lu Q, et al. Molecular design and computer simulation of small molecule thrombin inhibitor[J]. Central South Pharm (中南药学), 2013, 11: 565–568. |
| [14] | Duan AX, Chen J, Liu HD, et al. Applications and developments of molecular docking method[J]. J Anal Sci (分析科学学报), 2009, 25: 473–477. |
| [15] | Liu L, Ma HY, Tang YP, et al. Discovery of estrogen receptor α modulators from natural compounds in Si-Wu-Tang series decoctions using estrogen-responsive MCF-7 breast cancer cells[J]. Bioorg Med Chem Lett, 2012, 22: 154–163. DOI:10.1016/j.bmcl.2011.11.041 |
| [16] | Kitchen DB, Decornez H, Furr JR, et al. Docking and scoring in virtual screening for drug discovery: methods and applications[J]. Nat Rev Drug Discov, 2004, 3: 935–949. DOI:10.1038/nrd1549 |
| [17] | Backes AC, Zech B, Felber B, et al. Small-molecule inhibitors binding to protein kinase. Part Ⅱ: the novel pharmacophore approach of type Ⅱ and type Ⅲ inhibition[J]. Expert Opin Drug Discov, 2008, 3: 1427–1449. DOI:10.1517/17460440802580106 |
| [18] | Meng YQ, Nie HH, Wang XC, et al. Synthesis and anti- tumor activity oleanolic acid derivatives[J]. Acta Pharm Sin (药学学报), 2011, 46: 1215–1220. |
| [19] | Ma M, Nakamura N, Miyashiro H, et al. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease[J]. Chem Pharm Bull, 1999, 47: 141–145. DOI:10.1248/cpb.47.141 |
 2018, Vol. 53
2018, Vol. 53


