炎症是人类疾病中最常见且复杂的一种病理过程。早在1863年, Virchow[1]就提出炎症和肿瘤之间存在某种联系。随后研究表明人类大约20%的肿瘤源于微生物感染引起的炎症反应[2]。炎症是促进肿瘤发生的重要原因, 慢性炎症可显著增加组织癌变风险。炎症不仅参与肿瘤发生过程, 也是肿瘤进展过程中的加速剂。肿瘤微环境中通常有大量炎症细胞浸润, 并分泌生长因子、促炎细胞因子和趋化因子等, 维持抑制性免疫微环境, 促进肿瘤恶性进展。临床肿瘤样本也显示出了炎症相关信号通路的显著活化, 例如NF-κB和STAT3信号[3]。
巨噬细胞迁移抑制因子(macrophage migration inhibitory factor, MIF)是一种多功能蛋白, 既可以作为细胞因子发挥促炎作用, 又具有类似趋化因子的功能, 调节其他促炎因子产生。MIF的一个重要特性是具有D-多巴色素互变异构酶、苯丙酮酸盐互变异构酶以及硫醇蛋白氧化还原酶活性, 可以催化多种生化反应。MIF是炎症反应过程中重要的调节因子, 也是先天性免疫应答过程中主要的上游介导物。但近年来的研究发现MIF在乳腺癌[4]、前列腺癌[5]、胰腺癌[6]等实体肿瘤组织中的表达水平显著高于正常组织, 且表达量与肿瘤的转移侵袭能力明显相关。MIF一方面直接参与肿瘤细胞的调节; 另一方面通过炎症反应、免疫反应以及肿瘤微环境等间接参与肿瘤相关疾病的发展进程。
随着对MIF生物学功能的深入研究, MIF逐渐被认为是治疗炎症、免疫相关疾病, 乃至抗肿瘤药物研发的重要靶点。全面了解MIF的结构特性、信号调节, 阐明MIF在炎症、肿瘤发生发展过程中的调节作用和机制, 对开发以MIF为靶点的抗炎和抗肿瘤药物具有重要意义。本文主要对MIF的特性和分布特点; MIF相关信号传导通路; MIF在炎症、肿瘤发生发展过程中的调节以及靶向MIF药物研发进展进行综述。
1 MIF的特性和分布特点 1.1 MIF命名的由来及特性1966年, Bloom等[7]首次发现活化的T细胞能产生一种细胞因子, 该因子不仅能促进炎症反应, 而且能抑制单核/巨噬细胞随意迁移、黏附, 因此将其命名为MIF。人MIF基因由3个外显子和2个内含子组成, 定位于21q22.3, 相对分子质量约为12.5 kDa。X射线晶体衍射图像显示人重组蛋白MIF为α/β结构, 以同源三聚体形式存在, 单体由两个反向平行的α螺旋和4个β链形成的一个β片层组成[8]。
与其他细胞因子不同, MIF是一种多功能蛋白[9-11]。MIF不仅是多效性的细胞因子, 也是一种内分泌因子, 由下丘脑、垂体和肾上腺等内分泌器官组织表达分泌。值得一提的是, MIF至少具有3个不同的酶催化活性: D-多巴色素互变异构酶、羟苯丙酮酸互变异构酶和氧化还原酶。研究发现, MIF氨基末端脯氨酸(Pro)残基对互变异构酶的催化活性具有重要作用, 其他重要的残基有Lys-32、Ile-64、Tyr-95和Asn-97, 这些氨基酸在进化上大多较为保守[9]。MIF的氧化还原酶活性依赖于CALC (Cys57-Ala-Leu-Cys60)氨基酸序列, 而且Cys57和Cys60之间可以形成分子内二硫键[12]。MIF氧化还原酶活性对细胞内氧化还原的平衡、抵抗氧化应激、抑制细胞凋亡和巨噬细胞活化等有重要的调节作用。此外, MIF还是唯一一种拮抗糖皮质激素的免疫因子。
1.2 MIF分布特点T淋巴细胞、单核细胞、巨噬细胞、肺、肝、肾以及泌尿生殖系统等多种组织细胞均能表达MIF, 但MIF主要由垂体前叶细胞、外周单核/巨噬细胞和活化的T细胞产生[13]。MIF属于组成型表达因子, 提前合成并储存在胞浆内, 受到刺激时可以直接分泌, 不需要重新合成。虽然多种免疫细胞和非免疫细胞均能有效地合成MIF, 但MIF本身不具有转运至内质网的N端先导信号肽序列, 因此其分泌过程相对特殊, 是一种非经典的分泌途径。Merk等[14]研究发现MIF的分泌需要与胞内高尔基体复合物相关蛋白p115相互作用; Flieger等[15]发现格列苯脲和丙磺舒能减少MIF分泌到胞外。进一步研究结果表明ABC转运家族成员ABCA1转运子参与MIF分泌过程; 此外MIF还能形成自分泌环进行调节, Lue等[16]研究发现JAB1参与MIF的自分泌过程。但有关MIF分泌的确切机制尚待阐明。
2 MIF的调控功能及信号传导通路 2.1 MIF的调控功能MIF作为一种多功能的细胞因子, 主要与膜受体CXC家族(如CXCR2、CXCR4、CXCR7、CXCR12)、CD74以及CD44相互作用, 活化下游信号通路, 发挥生物学功能。Pantouris等[17]研究发现膜蛋白CD74是MIF的高亲和力受体, MIF可以调节CD74的活性, 引起机体稳态失调如炎症、肿瘤和自身免疫病等。MIF通过CD74, 不仅激活MAPK信号通路, 而且还持续活化ERK信号。Rajasekaran等[18]发现, MIF与趋化因子CXCR2、CXCR4结合, 招募白细胞, 加速动脉粥样硬化进程。进一步研究发现这些膜受体通常以复合物(如CXCR4/CD74、CXCR2/CD74、CD74/ CD44)的形式与MIF结合, 调节细胞功能, 如MIF与CD74/CD44复合物结合, 活化Syk、Akt、NF-κB等信号, 调节免疫反应[19]。
MIF不仅在胞外发挥功能, 胞浆内的MIF同样具有生物学功能, 胞内MIF通过蛋白-蛋白相互作用, 调控细胞功能。如MIF与丝氨酸蛋白酶HTRA1相互作用, 抑制HTRA1蛋白的水解活性, 影响细胞生长和分化能力[20]; Kim等[21]研究发现MIF与硫氧还原蛋白TXNIP结合, 一方面上调NF-κB, 另一方面抑制TXNIP的抑肿瘤功能, 从而发挥促肿瘤作用; Shen等[22]发现MIF与细胞凋亡相关蛋白BNIPL相互作用, 参与细胞凋亡过程。
2.2 MIF信号转导通路MIF相关信号分子主要有JAB1/JNK、ERK、PI3K/Akt、p53等。如图 1所示, MIF能直接或间接调节这些信号分子, 调控相关信号通路, 影响细胞功能。MIF与JAB1间的调节是相互的, MIF抑制JAB1对JNK、AP-1的磷酸化, 改变细胞周期, 调控细胞增殖、生长和凋亡; 而JAB1也能反馈性抑制MIF的合成[16]。MIF通过受体CD74/CD44复合物间接上调ERK1/2信号分子, 促进PLA2、COX-2、p53、MSK1和RSK1等一系列下游分子表达。其中, COX-2的上调, 在促炎反应中发挥重要作用; 同时, COX-2能够抑制p53在细胞内的聚集, 抑制细胞凋亡, 在自身免疫病与癌症的发生中有重要作用[12, 23]。此外, MIF通过ERK信号参与基质金属蛋白酶的调节, 如Yu等[24]研究发现MIF活化ERK/MAPK信号通路诱导MMP9表达。
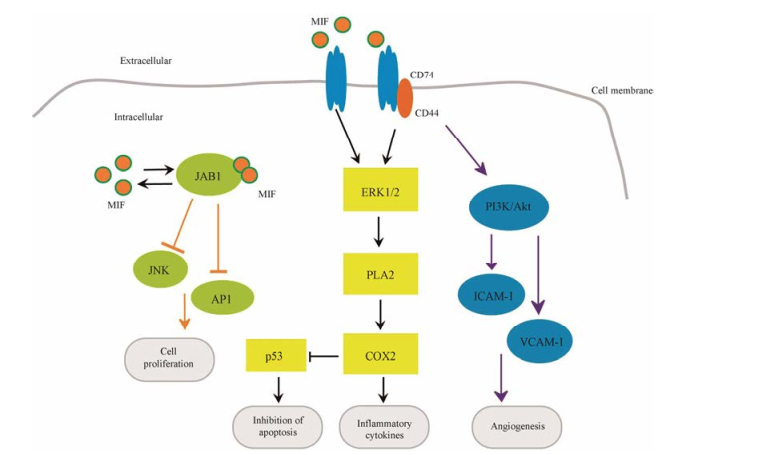
|
Figure 1 Macrophage migration inhibitor factor (MIF) signaling pathways. MIF regulates cell function through receptor-mediated and intracellular interactions. MIF interacts with the JAB1 and promotes cell proliferation; MIF forms a complex with CD74 or CD74/ CD44, leading to ERK1/2 activation, which triggers the release of pro-inflammatory cytokines. In the process, the tumor suppressor p53 is down-regulated along with activation of phospholipase A2 (PLA2) and cyclooxygenase 2 (COX2) leads to the inhibition of apoptosis. The PI3K/Akt pathway is also activated, leading to the release of angiogenesis factor such as ICAM-1, VCAM-1, which further contributes to angiogenesis |
MIF抗凋亡、促进血管新生的作用与PI3K/Akt信号分子的活化密切相关, Akt可使BAD发生磷酸化, 促进Bcl-2和Bcl-xL的抗凋亡作用[25]; 在胶质瘤细胞中MIF/Akt信号通路还参与血管生成拟态的调节[26]。MIF通过PI3K信号分子促进VCAM-1、ICAM-1等因子的释放, 参与血管调节[27]。MIF不仅间接调控p53, 而且MIF第81位半胱氨酸与p53相互作用直接介导p53降解[28]。由以上研究可以看出, MIF与这些信号通路间的关系并不是孤立的, 而是以调节网络的形式存在。而且MIF对这些信号通路的调控也不能一概而论, 因此有关MIF信号分子和信号通路的研究有待进一步探索。
3 MIF参与炎症、肿瘤调节 3.1 MIF的促炎功能早在1966年, David等[29]研究发现MIF参与迟发型超敏反应, 而且过敏反应的程度与MIF表达量呈正相关, 表明MIF是一种促炎因子。随后大量研究发现, MIF在感染性休克、肾小球肾炎、慢性肠炎和类风湿关节炎等炎症相关疾病的发展进程中具有重要的促炎作用。在强直性脊柱炎中, MIF诱导TNF上调进而增加成骨细胞活性, 促进疾病进程[30]; 在慢性肾炎患者血清中, MIF表达水平显著升高, 并通过活化ERK/MAPK、JAB1/AP-1和NF-κB等信号通路, 引起一系列促炎级联反应, 加速慢性肾炎的发展进程[31]; 然而Stoppe等[32]发现心脏手术后血清中MIF含量增加, 减少了并发症急性肾损伤的发生, 但具体机制还不清楚, 表明MIF可能也具有保护急性肾损伤的作用。MIF除了特异性中和糖皮质激素诱导的抗炎反应外, 还具有类似趋化因子的功能, 参与其他促炎因子的产生, 如TNF-α、IFN-γ、IL-1β、IL-6、IL-8和IL-12等, 调节自身免疫病、感染等炎症反应[33]。此外MIF还通过影响肠道微生物群的组成参与肠道相关炎症的发生发展[34]。
3.2 MIF促肿瘤作用MIF作为经典的促炎因子, 在炎症和免疫系统中发挥重要功能。近年来的研究发现在常见的实体肿瘤包括肝癌、前列腺癌、结肠癌、膀胱癌和肺腺癌中, MIF表达水平显著高于正常组织[33]。MIF参与肿瘤细胞无限增殖、血管新生、抗凋亡、免疫逃逸等特性维持。MIF是一种独特的促肿瘤蛋白, 不仅直接参与肿瘤调节, 而且通过诱导促肿瘤微环境间接加速肿瘤相关疾病的发展进程。
3.2.1 MIF促进肿瘤细胞增殖MIF作为一种促肿瘤因子, 参与肿瘤细胞多种生物学特性的调节。Takahashi等[35]早期研究发现抑制内源性MIF表达后, 肿瘤细胞生长减慢; 敲低PLC和HepG2等肝癌细胞中的MIF可抑制肿瘤细胞增殖[36]。肿瘤抑制子能启动细胞凋亡, 是维持细胞衰老和增殖间动态平衡的关键。研究表明50%人类肿瘤中p53存在功能异常[37], Bucala等[38]发现MIF通过抑制p53磷酸化, 降低p21、Mdm2和cyclin G1的转录活性, 抑制细胞的衰老凋亡。在宫颈癌细胞中敲低MIF后, Bax、caspase-3、cleaved-PARP等促凋亡蛋白表达上调, 而Bcl-2、pAkt等抑凋亡蛋白则表达下调, 表明MIF抑制了宫颈癌细胞的凋亡[39]。与MIF抑制凋亡作用相应, 研究发现miR-451通过下调MIF加速了骨肉瘤细胞的凋亡[40]; 而miR-146a通过抑制MIF的表达来诱导肺癌细胞凋亡[41]。以上研究表明MIF能够抑制肿瘤细胞凋亡, 促进肿瘤细胞增殖。
3.2.2 MIF促进肿瘤血管新生肿瘤生长到一定大小时会形成自己的血管系统, 以便提供丰富的营养和氧气, 如果没有新生血管对肿瘤细胞的营养供给, 肿瘤组织的直径一般不超过3 mm[42], 由此可见新生血管对肿瘤生长、扩散至关重要。肺动脉患者体内MIF的表达水平与Ang1、VEGF、PDGF-BB、FGF-basic、PLGF等血管生长因子呈正相关, 促进新生血管的形成[43]; Choudhary等[44]发现在N-丁基-N-(4-羟丁基)亚硝基胺诱导的膀胱癌模型中, 敲低MIF能显著降低VEGF和PECAM的表达, 抑制新生血管生成; Jia等[45]研究发现在胃癌中miR-1228通过靶向抑制MIF, 抑制肿瘤血管生成; 这些证据都表明MIF参与肿瘤血管新生, 促进肿瘤发展进程。此外, MIF本身也是一种有效的促血管生长因子, 能直接促进内皮细胞的生长和血管系统的形成[46]。
3.2.3 MIF诱导并维持肿瘤微环境的特性肿瘤微环境是由肿瘤细胞、多种基质细胞、组织液及浸润的炎症细胞等共同组成的内环境, 主要特征是低氧、低pH值、富含大量生长因子、趋化因子、炎症因子和蛋白水解酶等[47], 这些特性对肿瘤细胞的增殖、侵袭转移、新血管形成至关重要。炎症细胞尤其肿瘤相关巨噬细胞(tumor associated macrophages, TAM)是肿瘤微环境重要的组成成分, 在炎症-肿瘤轴中扮演重要角色。MIF可以调节TAM的极性, 在肿瘤中巨噬细胞通常分化形成促肿瘤的M2型TAM, 加速肿瘤细胞的增殖、转移并抑制抗肿瘤免疫反应[48]。MIF是肿瘤微环境中的免疫调节者, 诱导肿瘤抑制性免疫微环境的形成。在晚期黑色素瘤中, MIF通过诱导抑制性免疫细胞分化, 促进肿瘤生长和转移[49]; Drews-Elger等[50]研究发现肿瘤细胞向微环境中分泌MIF因子, 进而募集大量S100A8阳性骨髓细胞, 形成抑制性免疫微环境, 有利于肿瘤细胞免疫逃逸。这些证据表明MIF通过影响免疫细胞功能, 降低机体的免疫防御, 促进肿瘤发生发展。
低氧是肿瘤微环境的主要特征之一, 低氧的环境可以诱导肿瘤细胞低氧诱导因子(hypoxia inducible factor, HIF)表达, HIF与低氧反应元件HREs结合, 启动LOX、CTG和VEGF等靶基因的表达[51], 赋予肿瘤细胞快速增殖生长的优势。Hahne等[52]发现MIF是HIF-1α和HIF-2α的靶基因, HIF通过上调MIF参与血管新生, 促进炎症和肿瘤发展进程。事实上, MIF与HIF-1α之间的调节是相互的, Oda等[53]研究发现乳腺癌细胞系MCF-7和MDA-MB-231高表达MIF, 高表达的MIF参与维持HIF-1α的稳定性及活性; 活化的HIF-1α促进MIF表达, 由此MIF和HIF-1α之间形成正反馈调节, 提高肿瘤细胞对低氧环境的适应能力, 加速肿瘤进展。
大量研究表明, 肿瘤微环境中存在的MIF因子通过上调促炎因子、金属基质蛋白酶、胞外基质降解酶等, 增强了肿瘤细胞浸润转移能力, 有利于肿瘤细胞发生转移。如Oliveira等[54]研究发现转移性黑色素瘤中MIF的表达水平显著高于非转移性黑色素瘤; Hu等[55]研究发现肝内皮细胞向周围环境中分泌MIF因子, 进而促进直肠癌细胞系的生长转移。Zeng等[56]研究发现敲低口腔鳞癌中的MIF后, 上皮间质转变标志物Twist1和基质金属蛋白酶MMP9的表达也随之下降, 表明肿瘤细胞中高表达的MIF, 有利于肿瘤细胞侵袭转移。
4 靶向MIF治疗策略MIF具有多效性的生理功能和催化活性, 参与多种炎症、免疫、肿瘤等疾病的发生发展过程, 这些特性使其逐渐成为抗炎和抗肿瘤药物研发的重要靶点。图 2展现了当前靶向MIF治疗的主要策略: ①降低MIF酶活性; ②减少MIF蛋白量; ③抗MIF抗体。
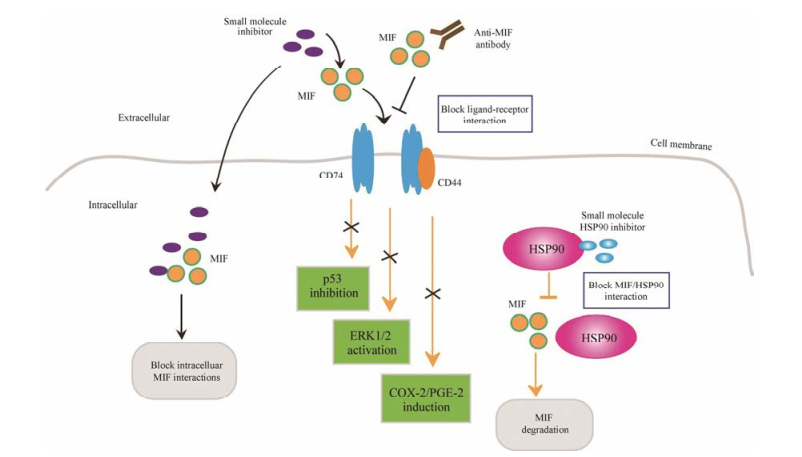
|
Figure 2 Strategies for targeted MIF treatment. MIF’s functional activity can be suppressed in many ways. Small molecule inhibitors block the binding of MIF to receptors CD74 or CD74/CD44, and the downstream signal pathway is suppressed, such as p53-dependent apoptosis, ERK1/2 activation and COX-2/PGE-2 production. Some MIF inhibitors can also enter the cell to prevent the protein interaction of MIF. Anti-MIF antibodies can generate similar functionality. HSP90 can stabilize intracellular MIF, so the HSP90 inhibitors can promote MIF degradation |
目前针对MIF的药物主要是靶向其酶活性的小分子抑制剂, 这些抑制剂多数是通过计算机辅助药物设计(computer aided drug design, CADD)和虚拟筛选技术(virtual screening, VS)发现[12]。当前已经发现的小分子抑制剂至少有11类, 它们的作用机制主要是: ①结合到酶活性位点, 使其失去酶活性; ②抑制互变异构反应; ③对酶活性位点进行共价修饰; ④抑制三聚体(具有互变异构酶活性)形成; ⑤诱导三聚体解离[57]。
Lubetsky等[58]早期研究发现小分子抑制剂Iso-1能够抑制MIF酶活性, 减少A549、DU145、LN229和HS683等一系列肿瘤细胞系的增殖、侵袭和转移; 在体内肿瘤模型中, Iso-1也具有良好的抑肿瘤效果。尽管Iso-1已经广泛应用于临床前研究, 但Iso-1在酶动力学和腹腔给药方面存在一定的缺陷, 随后该团队在Iso-1的基础上优化得到了Iso-66, Iso-66相比Iso-1更稳定、细胞毒性更小[59]。Rajasekaran等[60]发现4-吲哚-6-苯基哌啶(4-IPP)是针对MIF/D-DT双靶点的一种抑制剂。目前报道的MIF变构抑制剂主要有依布硒啉、异丁司特(AV411、AV1013)和p425等[12], 它们能显著降低MIF活性, p425还能阻断MIF与CD74的结合, 抑制MIF下游信号的活化, 这些抑制剂的抗肿瘤活性还有待于进一步研究。最近发现一种异香豆素化合物(SCD-19)[61]、维生素E[62]等能够靶向MIF抑制其生物学功能。一些制药公司也开展了靶向MIF的药物研发, 如Cytokine Pharma Sciences公司对MIF抑制剂CPSI-2705和CPSI-1306展开了研究[44]; Avanir Pharmaceuticals制药公司也发现了MIF的一种抑制剂AVP-13546[63]。
4.2 减少MIF蛋白量Schulz等[64]研究发现肿瘤细胞中热休克蛋白90 (heat shock protein 90, HSP90)能维持MIF稳定性, HSP90的抑制剂17AAG则促进MIF降解, 抑制肿瘤细胞增殖; 并且该团队发现抑制HER2导致HSP90失活也可以破坏MIF稳定性[65], 表明HER2抑制剂也能加速MIF降解, 减少胞内MIF蛋白量。由此可见通过破坏MIF稳定性促进其降解, 或抑制MIF转录等减少细胞内MIF表达量, 也是靶向MIF药物研究的一种策略。如Kim等[66]研究发现NADPH氧化酶4能够上调MIF mRNA和蛋白水平, 因此靶向NADPH氧化酶4的研究也许可以找到针对MIF表达量的新抑制剂, 对靶向MIF的药物研究提供新思路。
4.3 抗MIF抗体由于抗体存在半衰期短、成本高、潜在的免疫原性等问题, 其开发应用受到很大的限制。但随着越来越多单克隆抗体的成功应用(如利妥昔单抗、曲妥珠单抗、阿伦珠单抗), 抗体药物逐渐受到人们的青睐。靶向MIF的单克隆抗体也相继出现: BaxG03、BaxB01和BaxM159, 它们通过靶向MIF抑制MAPK/ ERK1/2活性, 降低前列腺癌细胞PC3的增殖、侵袭能力, 并显著抑制肿瘤的生长。随后MIF单克隆抗体的Ⅰ期临床也已启动[67]; 同样在CT26结肠癌模型中, 抗MIF的中和抗体也具有良好的抑肿瘤效果[68]。
虽然靶向MIF的药物研究已取得一定进展, 但这些抑制剂仍存在一些不足, 如活性低、效力低、特异性不高、不良反应多等。尽管目前已有多种MIF抑制剂被报道, 并且在体外显示出良好的抗肿瘤作用, 但至今并没有靶向MIF的药物上市, 因此寻找靶向MIF药物研发的新策略具有重要意义。靶向MIF的多肽药物是目前新兴策略之一, 多肽具有分子质量小、结构简单、免疫原性低或无免疫原性等特点。基于此筛选与MIF特异性结合的多肽药物, 诱导MIF失活或促进其降解是有效的MIF靶向策略。靶向MIF的siRNA药物是另一新兴策略。siRNA可以特异性靶向某个基因, 具有高效、持久、特异性强等优点。如Zeng等[56]研究发现瞬时转染MIF siRNA降低了口腔鳞癌的增殖、转移能力; 此外寻找靶向MIF的抗体偶联药物(antibody-drug conjugate, ADC), 即将抗体和具有生物活性的小分子药物偶联起来, 药物直接靶向肿瘤, 从而降低化疗药物的不良反应。
5 结语与展望大量研究表明, MIF不仅直接影响肿瘤细胞分裂和癌基因诱导的恶性转化, 而且间接通过调节免疫反应、炎症反应等参与肿瘤发展进程, 包括肿瘤细胞的生长、侵润转移、血管新生以及诱导肿瘤抑制性免疫微环境的形成等, 因此MIF是治疗肿瘤相关疾病的重要靶点之一。总而言之, MIF是一种重要的多功能蛋白, 既具有细胞因子作用, 又具有多种酶功能, 是连接炎症-肿瘤轴的关键蛋白。随着对MIF蛋白的深入研究, 对其功能的不断阐明, 将扩大研究者对MIF相关疾病的了解, 也有利于靶向MIF的药物研发。目前针对MIF功能研究已有部分临床应用, 如作为一些疾病的诊断标志物。但有关MIF病理生理学功能、酶活性、信号传导机制及相关的生物学功能还未完全阐明, 有待进一步的探索。
| [1] | Virchow R. Cellular Pathology as Based upon Physiological and Pathological Histology[M]. London: John Churchill, 1863. |
| [2] | Kanda Y, Osaki M, Okada F. Chemopreventive strategies for inflammation-related carcinogenesis: current status and future direction[J]. Int J Mol Sci, 2017, 18: 867–903. DOI:10.3390/ijms18040867 |
| [3] | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3[J]. Nat Rev Cancer, 2009, 9: 798–809. DOI:10.1038/nrc2734 |
| [4] | Bando H, Matsumoto G, Bando M, et al. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread[J]. Jpn J Cancer Res, 2002, 93: 389–396. DOI:10.1111/cas.2002.93.issue-4 |
| [5] | Meyer-Siegler KL, Iczkowski KA, Vera PL. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer[J]. BMC Cancer, 2005, 5: 73. DOI:10.1186/1471-2407-5-73 |
| [6] | Funamizu N, Hu C, Lacy C, et al. Macrophage migration inhibitory factor Induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma[J]. Int J Cancer, 2013, 132: 785–794. DOI:10.1002/ijc.27736 |
| [7] | Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity[J]. Science, 1966, 153: 80–82. DOI:10.1126/science.153.3731.80 |
| [8] | Lolis E, Bucala R. Crystal structure of macrophage migration inhibitory factor (MIF), a glucocorticoid-induced regulator of cytokine production, reveals a unique architecture[J]. Proc Assoc Am Physicians, 1996, 108: 415–419. |
| [9] | Bloom J, Sun S, Al-Abed Y. MIF, a controversial cytokine: a review of structural features, challenges, and opportunities for drug development[J]. Expert Opin Ther Targets, 2016, 20: 1463–1475. DOI:10.1080/14728222.2016.1251582 |
| [10] | Mokshagundam SPL. Macrophage migration inhibitory factor-A potential target for diabetes prevention[J]. Am J Med Sci, 2018, 18: 519–520. |
| [11] | Lerch JK, Puga DA, Bloom O, et al. Glucocorticoids and macrophage migration inhibitory factor (MIF) are neuroendocrine modulators of inflammation and neuropathic pain after spinal cord injury[J]. Semin Immunol, 2014, 26: 409–414. DOI:10.1016/j.smim.2014.03.004 |
| [12] | Xu L, Li Y, Sun H, et al. Current developments of macrophage migration inhibitory factor (MIF) inhibitors[J]. Drug Discov Today, 2013, 18: 592–600. DOI:10.1016/j.drudis.2012.12.013 |
| [13] | Kyoung WK, Hae RK. Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis[J]. Korean J Intern Med, 2016, 31: 634–642. DOI:10.3904/kjim.2016.098 |
| [14] | Merk M, Baugh J, Zierow S, et al. The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor (MIF)[J]. J Immunol, 2009, 182: 6896–6906. DOI:10.4049/jimmunol.0803710 |
| [15] | Flieger O, Engling A, Bucala R, et al. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter[J]. FEBS Lett, 2003, 551: 78–86. DOI:10.1016/S0014-5793(03)00900-1 |
| [16] | Lue H, Thiele M, Franz J, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity[J]. Oncogene, 2007, 26: 5046–5059. DOI:10.1038/sj.onc.1210318 |
| [17] | Pantouris G, Ho J, Shah D, et al. Nanosecond dynamics regulate the MIF-induced activity of CD74[J]. Angew Chem Int Ed, 2018, 57: 7116–7119. DOI:10.1002/anie.v57.24 |
| [18] | Rajasekaran D, Gröning S, Schmitz C, et al. Macrophage migration inhibitory factor-CXCR4 receptor interactions: evidence for partial allosteric agonism in comparison with CXCL12 chemokine[J]. J Biol Chem, 2016, 291: 15881–15895. DOI:10.1074/jbc.M116.717751 |
| [19] | Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex[J]. J Biol Chem, 2008, 5: 2784–2792. |
| [20] | FexSvenningsen Å, Löring S, Sørensen AL, et al. Macrophage migration inhibitory factor (MIF) modulates trophic signaling through interaction with serine protease HTRA1[J]. Cell Mol Life Sci, 2017, 74: 4561–4572. DOI:10.1007/s00018-017-2592-z |
| [21] | Kim MJ, Kim WS, Kim DO, et al. Macrophage migration inhibitory factor interacts with thioredoxin-interacting protein and induces NF-κB activity[J]. Cell Signal, 2017, 34: 110–120. DOI:10.1016/j.cellsig.2017.03.007 |
| [22] | Shen L, Hu J, Lu H, et al. The apoptosis-associated protein BNIPL interacts with two cell proliferation-related proteins, MIF and GFER[J]. FEBS Lett, 2003, 540: 86–90. DOI:10.1016/S0014-5793(03)00229-1 |
| [23] | Gesser B, Rasmussen M, Raaby L, et al. Dimethylfumarate inhibits MIF-induced proliferation of keratinocytes by inhibiting MSK1 and RSK1 activation and by inducing nuclear p-c-Jun (S63) and p-p53 (S15) expression[J]. Inflamm Res, 2011, 60: 643–653. DOI:10.1007/s00011-011-0316-7 |
| [24] | Yu X, Lin SG, Huang XR, et al. Macrophage migration inhibitory factor induces MMP-9 expression in macrophages via the MEK-ERK MAP kinase pathway[J]. J Interferon Cytokine Res, 2007, 27: 103–109. DOI:10.1089/jir.2006.0054 |
| [25] | Chatterjee M, Borst O, Walker B, et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling[J]. Circ Res, 2014, 11: 939–949. |
| [26] | Guo X, Xu S, Gao X, et al. Macrophage migration inhibitory factor promotes vasculogenic mimicry formation induced by hypoxia via CXCR4/AKT/EMT pathway in human glioblastoma cells[J]. Cancer Lett, 2018, 412: 289–296. DOI:10.1016/j.canlet.2017.10.018 |
| [27] | Yeh TM, Liu SH, Lin KC, et al. Dengue virus enhances thrombomodulin and ICAM-1 expression through the macrophage migration inhibitory factor induction of the MAPK and PI3K signaling pathways[J]. PLoS One, 2013, 8: e55018. DOI:10.1371/journal.pone.0055018 |
| [28] | Jung H, Seong HA, Ha H. Critical role of cysteine residue 81 of macrophage migration inhibitory factor (MIF) in MIF-induced inhibition of p53 activity[J]. J Biol Chem, 2008, 283: 20383–20396. DOI:10.1074/jbc.M800050200 |
| [29] | David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction[J]. Proc Natl Acad Sci U S A, 1966, 56: 72–77. DOI:10.1073/pnas.56.1.72 |
| [30] | Ranganathan V, Ciccia F, Zeng F, et al. Macrophage migration inhibitory factor induces inflammation and predicts spinal progression in ankylosing spondylitis[J]. Arthritis Rheumatol, 2017, 69: 1796–1806. DOI:10.1002/art.40175 |
| [31] | Liu Y, Zhang X, Liu G, et al. Expressions of macrophage migration inhibitory factor in patients with chronic kidney disease[J]. Niger J Clin Pract, 2016, 19: 778–783. DOI:10.4103/1119-3077.183239 |
| [32] | Stoppe C, Averdunk L, Goetzenich A, et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery[J]. Sci Transl Med, 2018. DOI:10.1126/scitranslmed.aan4886 |
| [33] | Nobre CC, de Araújo JM, Fernandes TA, et al. Macrophage migration inhibitory factor (MIF): biological activities and relation with cancer[J]. Pathol Oncol Res, 2017, 23: 235–244. DOI:10.1007/s12253-016-0138-6 |
| [34] | Vujicic M, Saksida T, Despotovic S, et al. The role of macrophage migration inhibitory factor in the function of intestinal barrier[J]. Sci Rep, 2018, 8: 6337–6349. DOI:10.1038/s41598-018-24706-3 |
| [35] | Takahashi N, Nishihira J, Sato Y, et al. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth[J]. Mol Med, 1998, 4: 707–714. DOI:10.1007/BF03401765 |
| [36] | Huang XH, Jian WH, Wu ZF, et al. Small interfering RNA (siRNA)-mediated knockdown of macrophage migration inhibitory factor (MIF) suppressed cyclin D1 expression and hepatocellular carcinoma cell proliferation[J]. Oncotarget, 2014, 5: 5570–5580. |
| [37] | Brock SE, Rendon BE, Xin D, et al. MIF family members cooperatively inhibit p53 expression and activity[J]. PLoS One, 2014, 9: e99795. DOI:10.1371/journal.pone.0099795 |
| [38] | Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer[J]. Immunity, 2007, 26: 281–285. DOI:10.1016/j.immuni.2007.03.005 |
| [39] | Guo P, Wang J, Liu J, et al. Macrophage immigration inhibitory factor promotes cell proliferation and inhibits apoptosis of cervical adenocarcinoma[J]. Tumour Biol, 2015, 36: 5095–5102. DOI:10.1007/s13277-015-3161-4 |
| [40] | Liu W, Liu SY, He YB, et al. MiR-451 suppresses proliferation, migration and promotes apoptosis of the human osteosarcoma by targeting macrophage migration inhibitory factor[J]. Biomed Pharmacother, 2017, 87: 621–627. DOI:10.1016/j.biopha.2016.12.121 |
| [41] | Wang WM, Liu JC. Effect and molecular mechanism of miR-146a on proliferation of lung cancer cells by targeting and regulating MIF gene[J]. Asian Pac J Trop Med, 2016, 9: 806–811. DOI:10.1016/j.apjtm.2016.06.001 |
| [42] | White ES, Strom SR, Wys NL, et al. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity[J]. J Immunol, 2001, 166: 7549–7555. DOI:10.4049/jimmunol.166.12.7549 |
| [43] | Marshall JD, Sauler M, Tonelli A, et al. Complexity of macrophage migration inhibitory factor (MIF) and other angiogenic biomarkers profiling in pulmonary arterial hypertension[J]. Pulm Circ, 2017, 7: 730–733. DOI:10.1177/2045893217724141 |
| [44] | Choudhary S, Hegde P, Pruitt JR, et al. Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis[J]. Carcinogenesis, 2013, 34: 2891–2899. DOI:10.1093/carcin/bgt239 |
| [45] | Jia L, Chen J, Xie C, et al. MicroRNA-1228* impairs the pro-angiogenic activity of gastric cancer cells by targeting macrophage migration inhibitory factor[J]. Life Sci, 2017, 180: 9–16. DOI:10.1016/j.lfs.2017.04.023 |
| [46] | Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis[J]. Thromb Haemost, 2013, 109: 391–398. DOI:10.1160/TH12-11-0831 |
| [47] | LeBleu VS. Imaging the tumor microenvironment[J]. Cancer J, 2015, 21: 174–178. DOI:10.1097/PPO.0000000000000118 |
| [48] | Figueiredo CR, Azevedo RA, Mousdell S, et al. Blockade of MIF-CD74 signalling on macrophages and dendritic cells restores the antitumour immune response against metastatic melanoma[J]. Front Immunol, 2018, 9: 1132. DOI:10.3389/fimmu.2018.01132 |
| [49] | Yaddanapudi K, Rendon BE, Lamont G, et al. MIF is necessary for late-stage melanoma patient MDSC immune suppression and differentiation[J]. Cancer Immunol Res, 2016, 4: 101–112. DOI:10.1158/2326-6066.CIR-15-0070-T |
| [50] | Drews-Elger K, Iorns E, Dias A, et al. Infiltrating S100A8+ myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcome[J]. Breast Cancer Res Treat, 2014, 148: 41–59. DOI:10.1007/s10549-014-3122-4 |
| [51] | Conroy H, Mawhinney L, Donnelly SC, et al. Inflammation and cancer: macrophage migration inhibitory factor (MIF) - the potential missing link[J]. QJM, 2010, 103: 831–836. DOI:10.1093/qjmed/hcq148 |
| [52] | Hahne M, Schumann P, Mursell M, et al. Unraveling the role of hypoxia-inducible factor (HIF)-1α and HIF-2α in the adaption process of human microvascular endothelial cells (HMEC-1) to hypoxia: redundant HIF-dependent regulation of macrophage migration inhibitory factor[J]. Microvasc Res, 2017, 116: 34–44. |
| [53] | Oda S, Oda T, Nishi K, et al. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner[J]. PLoS One, 2008, 3: e2215. DOI:10.1371/journal.pone.0002215 |
| [54] | Oliveira CS, de Bock CE, Molloy TJ, et al. Macrophage migration inhibitory factor engages PI3K/Akt signaling and is a prognostic factor in metastatic melanoma[J]. BMC Cancer, 2014, 14: 630–642. DOI:10.1186/1471-2407-14-630 |
| [55] | Hu CT, Guo LL, Feng N, et al. MIF, secreted by human hepatic sinusoidal endothelial cells, promotes chemotaxis and outgrowth of colorectal cancer in liver prometastasis[J]. Oncotarget, 2015, 6: 22410–22423. |
| [56] | Zeng J, Quan J, Xia X. Transient transfection of macrophage migration inhibitory factor small interfering RNA disrupts the biological behavior of oral squamous carcinoma cells[J]. Mol Med Rep, 2016, 13: 174–180. DOI:10.3892/mmr.2015.4525 |
| [57] | O'Reilly C, Doroudian M, Mawhinney L, et al. Targeting MIF in cancer: therapeutic strategies, current developments, and future opportunities[J]. Med Res Rev, 2016, 36: 440–460. DOI:10.1002/med.2016.36.issue-3 |
| [58] | Lubetsky JB, Dios A, Han J, et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents[J]. J Biol Chem, 2002, 277: 24976–24982. DOI:10.1074/jbc.M203220200 |
| [59] | Ioannou K, Cheng KF, Crichlow GV, et al. ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models[J]. Int J Oncol, 2014, 45: 1457–1468. DOI:10.3892/ijo.2014.2551 |
| [60] | Rajasekaran D, Zierow S, Syed M, et al. Targeting distinct tautomerase sites of D-DT and MIF with a single molecule for inhibition of neutrophil lung recruitment[J]. FASEB J, 2014, 28: 4961–4971. DOI:10.1096/fj.14-256636 |
| [61] | Tynan A, Mawhinney L, Armstrong ME. Macrophage migration inhibitory factor enhances pseudomonas aeruginosa biofilm formation, potentially contributing to cystic fibrosis pathogenesis[J]. FASEB J, 2017, 31: 5102–5110. DOI:10.1096/fj.201700463R |
| [62] | Hegde B, Vadnal P, Sanghavi J. Vitamin E is a MIF inhibitor[J]. Biochem Biophys Res Commun, 2012, 418: 384–389. DOI:10.1016/j.bbrc.2012.01.031 |
| [63] | Garai J, Lóránd T. Macrophage migration inhibitory factor (MIF) tautomerase inhibitors as potential novel anti-inflammatory agents: current developments[J]. Curr Med Chem, 2009, 16: 1091–1114. DOI:10.2174/092986709787581842 |
| [64] | Schulz R, Moll UM. Targeting the heat shock protein 90: a rational way to inhibit macrophage migration inhibitory factor function in cancer[J]. Curr Opin Oncol, 2014, 26: 108–113. DOI:10.1097/CCO.0000000000000036 |
| [65] | Schulz R, Streller F, Scheel AH, et al. HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer[J]. Cell Death Dis, 2014, 5: e980. DOI:10.1038/cddis.2013.508 |
| [66] | Kim JH, Lee J, Bae SJ, et al. NADPH oxidase 4 is required for the generation of macrophage migration inhibitory factor and host defense against Toxoplasma gondii infection[J]. Sci Rep, 2017, 7: 6361–6374. DOI:10.1038/s41598-017-06610-4 |
| [67] | Hussain F, Freissmuth M, Volkel D, et al. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo[J]. Mol Cancer Ther, 2013, 12: 1223–1234. DOI:10.1158/1535-7163.MCT-12-0988 |
| [68] | He XX, Chen K, Yang J, et al. Macrophage migration inhibitory factor promotes colorectal cancer[J]. Mol Med, 2009, 15: 1–10. |
 2018, Vol. 53
2018, Vol. 53


