2. 中国医学科学院药物研究所, 神经科学中心, 天然药物活性物质与功能国家重点实验室, 北京 100050
2. State Key Laboratory of Bioactive Substances and Functions of Natural Medicine, Neuroscience Center, Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing 100050, China
近年来, 抑郁症已成为严重困扰人们生活的精神疾病[1-3]。其发病机制假说主要包括:单胺递质假说、受体假说、神经营养因子假说及下丘脑-垂体-肾上腺轴假说(hypothalamic-pituitary-adrenal, HPA)等[4], 但都未完全阐明抑郁发病原因。因此, 发病机制的深入研究和探讨对更好地治疗抑郁症极为重要。
星形胶质细胞是脑组织中分布最广泛的胶质细胞, 不仅可以给神经元提供代谢和营养支持, 还能够调节突触功能及帮助神经再生和修复, 具有重要的生理功能[5, 6]。对抑郁症患者的研究发现脑组织前额皮层(prefrontal cortex, PFC)区星形胶质细胞数目减少, 星形胶质细胞出现异常已成为抑郁症重要的病理生理学之一[7, 8]。有研究显示, Cx43和Cx30是星形胶质细胞内主要的缝隙连接蛋白, 是构成缝隙连接通道的主要蛋白[9, 10]。目前较多证据显示, Cx43蛋白构成的缝隙连接通道允许多种内源性生物活性分子通过, 但不能通过生物大分子[10, 11]。多个病理模型中也证实了Cx43的表达和缝隙连接功能相关。例如, 癫痫[12, 13]及阿尔茨海默病[14, 15]模型中同时观察到Cx43表达减少和缝隙连接功能降低。
本实验室前期研究表明星形胶质细胞缝隙连接功能与抑郁症密切相关, 研究通过慢性不可预知性应激(chronic unpredictability stress, CUS)诱导大鼠出现抑郁样行为, 并证明抑郁大鼠PFC脑区星形胶质细胞缝隙连接结构异常, 功能损伤且Cx43表达降低。但缝隙连接功能损伤机制尚未阐明[16, 17]。
HPA轴假说认为糖皮质激素水平升高导致的HPA轴过度兴奋与抑郁症发生密切相关[18-20]。抑郁症治疗的相关研究显示, 糖皮质激素受体(glucocorticoid receptor, GR)抑制剂米非司酮表现出快速有效的治疗作用[21, 22]。本课题组前期研究证实, 米非司酮可以逆转CUS诱导的抑郁症模型大鼠PFC脑区星形胶质细胞缝隙连接出现的损伤[16]。该结果提示PFC脑区星形胶质细胞缝隙连接损伤可能与糖皮质激素受体激活相关。
因此, 本研究旨在探讨糖皮质激素升高对大鼠前额皮层星形胶质缝隙连接功能的影响, 为抑郁症的HPA轴假说做出进一步阐释, 为抗抑郁药物研究提供进一步的实验依据。
材料与方法 药品与试剂米非司酮(北京紫竹药业有限公司); 皮质酮(corticosterone, CORT)、荧光黄(Sigma公司); 封闭用正常羊血清(北京中杉金桥生物技术有限公司); Cx43 rabbit pAb (Cell Signaling Technology公司); HRP-conjugated Goat Anti-Rabbit IgG (KPL公司); 其他试剂购自北京化工厂。
实验动物及处理雄性Sprague-Dawley大鼠36只, 每组12只, SPF级, 体重250~300 g, 由北京维通利华实验动物技术有限公司提供(合格证号: SCXK北京2012-0001), 饲养于清洁级动物房。保持平均室温在(22 ± 2) ℃, 相对湿度(55 ± 5) %。自由进食饮水, 动物房明暗交替(8:00~20:00白天, 20:00~8:00黑夜), 动物适应环境7天后开始进行实验。大鼠颈部皮下注射CORT溶液, 剂量40 mg·kg-1; 注射时间:每天09:00~11:30, 持续21天。CORT注射所选剂量和时间参考相关文献方法[23]。建模18天起灌胃给予米非司酮, 第21天进行糖水消耗实验, 第22天处死动物, 分两种处理方法取材用于后续实验: ①染料示踪实验, 在大鼠PFC区定位注射荧光黄30 min后灌流固定; ②免疫荧光和透射电子显微镜分析, 灌流固定。
糖水消耗实验糖水消耗实验(sucrose preference test, SPT)用于评价动物的快感缺失程度。通过测试每组大鼠在1 h内消耗1%蔗糖溶液百分比[1%蔗糖溶液消耗比= 1%蔗糖溶液/ (1%蔗糖溶液+自来水)]进行评价。正式实验前进行糖水预适应48 h, 第21天禁食禁水4 h, 之后每只大鼠给予两只相同的水瓶, 分别装有1%蔗糖溶液和饮用水, 统计1 h内大鼠消耗1%蔗糖溶液和饮用水量, 计算出1%蔗糖溶液的消耗比[16]。
荧光黄染料示踪实验用10%水合氯醛(400 mg·kg-1)腹腔注射(ip)麻醉SD大鼠。麻醉后, 固定于大鼠立体定位仪, 剪开皮肤2 cm, 3% H2O2推开皮下组织及骨膜, 充分暴露颅骨, 找出前囟点并读取坐标。根据前囟点计算在anteroposterior+3.2 mm, dorsolateral -0.5 mm (AP: 3.2, L: 0.5~1)的位置, 记号笔进行标记, 用钻头在颅骨标记位置钻直径为1 mm的小孔, 清除孔内骨屑。根据定位坐标将5 μL微量注射器插入, 深度4 mm, 即定位坐标是AP 3.2 mm, L 0.5 mm, H 4.0 mm (AP: 3.2, L: 0.5~1, H: 4.0)。用微量注射泵将2 μL荧光黄(lucifer yellow, LY)溶液(5 μg·μL-1)以0.25 μL·min-1恒速注入, 之后将针头缓慢退出, 并开始计时30 min, 然后迅速用4%多聚甲醛灌流固定取脑, 并固定过夜, 依次置于梯度蔗糖溶液中脱水, 冰冻切片, 厚度为60 μm。激光扫描共聚焦显微镜激光激发下观察荧光传递情况, 并应用MetaMorph 7.5.0.0软件测量各组大鼠针头痕迹同侧荧光传递的距离和荧光阳性的细胞数, 具体细节参考相关文献方法[16]。
缝隙连接超微结构的观察将大鼠迅速灌流固定取脑, 切取AP 3.2 mm附近(约1 mm厚) PFC区的组织, 并切成1 mm3小块, 放入装有新鲜配制的冷2.5%戊二醛固定液中, 4 ℃环境下固定2 h后, 用吸管吸出固定液, 加入0.1 mol·L-1磷酸盐缓冲液(PBS)漂洗3次, 每次10 min。2.5%戊二醛固定液及后续样品制备过程由北京神经外科研究所超微病理研究室提供。透射电子显微镜观察两个相邻的星形胶质细胞间连接部位结构, 拍下电子图片。用MetaMorph 7.5.0.0软件对图像中缝隙连接的缝隙宽度进行定量分析。
免疫荧光实验将大鼠迅速灌流固定取脑, 固定过夜后依次置于梯度蔗糖溶液中脱水, 切片厚度30 μm, 漂片进行下步实验。PBS漂洗脑片, PBS/0.3% Triton-X 100室温孵育30 min, 5%正常羊血清室温封闭1 h, 吸出封闭液, 加入400 μL特异性一抗(Cx43, 1:50), 4 ℃过夜。PBS漂洗3次, 加入FITC标记的羊抗兔抗体, 1:400, 室温避光孵育2 h, 此后的操作均需在避光条件下进行。封片, LSCM下进行观察。
统计学分析实验结果均以平均值±标准误(mean ± SEM)形式表示, 采用SPSS 13.0软件进行单因素方差分析(analysis of variance, ANOVA, SPSS 13.0), 通过Newman-Keuls post-hoc分析对两两进行比较。P < 0.05视为具有显著性差异。
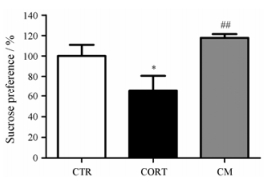
结果 1 米非司酮可逆转糖皮质激素水平升高诱导的大鼠快感缺失对大鼠皮下慢性注射皮质酮, 外源性提升动物体内糖皮质激素水平, 并通过SPT评价大鼠的行为学改变, 观察大鼠是否出现抑郁样行为。结果显示:与空白对照(CTR)组相比, CORT组大鼠糖水摄入百分比明显降低(P < 0.05), 而造模的最后4天给予米非司酮(50 mg·kg-1·day-1)有明显的改善作用(P < 0.01)。见图 1。
|
Figure 1 Mifepristone reversed behavioral deficits induced by chronic corticosterone administration. Animals of the control (CTR) group were injected with vehicle (olive oil), chronic corticosterone administration (CORT) group received corticosterone injections for 21 days (40 mg·kg-1). Animals received mifepristone (50 mg·kg-1) for the 4 last days of the experiment. Error bars represent SEM (n = 12). *P < 0.05 vs CTR group; ##P < 0.01 vs CORT group. CM: CORT + mifepristone group |
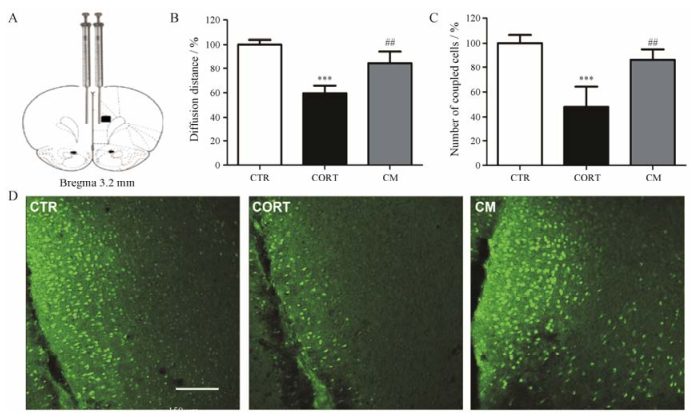
LY是一种不能透过细胞膜, 但可通过缝隙连接从标记的细胞传输到临近细胞的小分子化合物。通过分析LY在PFC区的扩散情况来评价PFC缝隙连接的功能。LY在PFC区扩散30 min后, 可以观察到明显的细胞间偶联, 与之前的研究结果一致。在每只动物的LY注射位置选取3张切片并对针头痕迹同侧荧光分子传递的距离和偶联的细胞数进行统计(图 2)。结果显示, 与CTR组相比, CORT组大鼠PFC区LY扩散的距离明显缩短(P < 0.001), 偶联的细胞数明显减少(P < 0.001), 而给予米非司酮后可明显改善CORT对细胞间偶联的损伤作用(P < 0.01)。
|
Figure 2 Mifepristone reversed intercellular diffusion decrease of lucifer yellow in the prefrontal cortex (PFC) induced by CORT (n = 6). A: The infusion illustration is shown and the distance of diffusion and the number of coupled cells in the black square are quantified; B: The diffusion distance was significantly reduced by CORT and this effect was reversed by mifepristone treatment; C: CORT significantly decreased the number of coupled cells and mifepristone significantly reversed this effect. Error bars represent SEM. ***P < 0.001 vs CTR group; ##P < 0.01 vs CORT group. D: Representative dye coupling images from animals of all groups are illustrated. Scale bar = 150 μm |
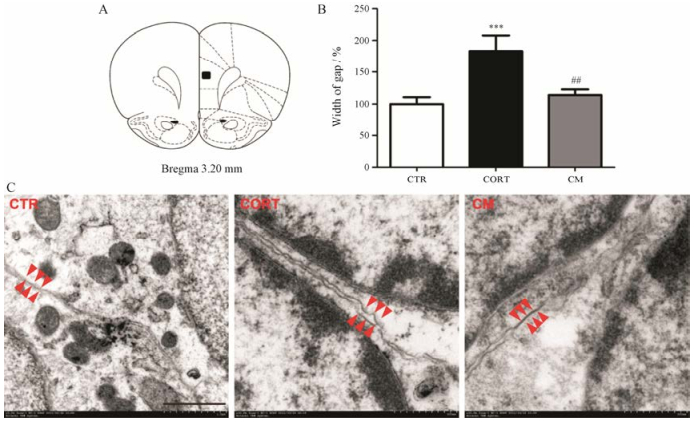
在透射电子显微镜下, 根据细胞核的特点找到两个相邻的星形胶质细胞, 对星形胶质细胞缝隙连接部位的超微结构进行研究(图 3)。结果显示, 给予慢性CORT组的星形胶质细胞出现胞体和突起明显肿胀, 连接部位结构不完整, 而CTR组和CORT应激后给予米非司酮治疗组星形胶质细胞胞体和突起以及连接部位的结构均显示正常。选取每只大鼠的4对相邻星形胶质细胞连接部位结构作为统计对象, 连接部位的缝隙宽度作为定量指标, 随机测量每对细胞的6个位置。结果显示, 与CTR组相比, CORT组星形胶质细胞缝隙连接处的缝隙明显变宽(P < 0.001), 给予米非司酮后可明显改善CORT对缝隙连接结构的损伤作用(P < 0.01)。
|
Figure 3 Mifepristone reversed ultrastructural alterations of astrocyte gap junction in the prefrontal cortex (PFC) induced by CORT (n = 6). A: The sections shown in the black square were cut from the brains of all groups; B: The width of gap was significantly enlarged by CORT and this effect was reversed by mifepristone treatment. Error bars represent SEM. ***P < 0.001 vs CTR group; ##P < 0.01 vs CORT group; C: Electron micrographs showing astrocytic gap junction in the PFC of each group (magnification: ×100 000). Astrocytic gap junctions are indicated by red arrowheads. Scale bar = 500 nm |
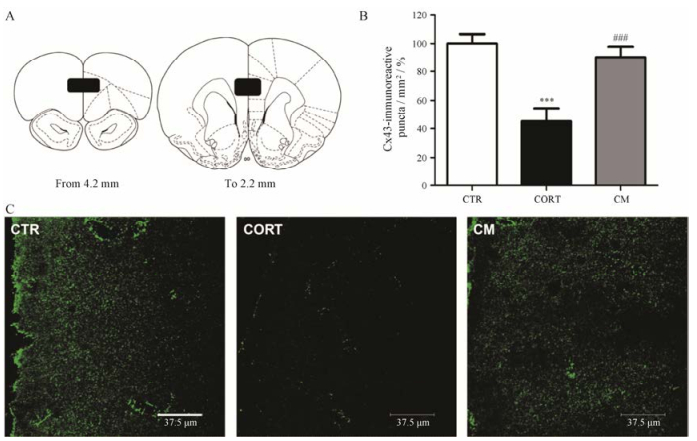
通过免疫荧光组化方法对Cx43在各组动物PFC区的表达分布进行研究(图 4)。每只动物选取6张PFC区前后范围(即AP 4.2 mm~2.2 mm)切片定量分析(图 4A)。Cx43的免疫阳性反应在PFC区呈点状分布(图 4C)。结果显示, 与CTR组相比, CORT组PFC区Cx43免疫反应阳性点数明显减少(P < 0.001), 而给予米非司酮后有明显的改善作用(P < 0.001)。上述所有的改变在PFC区的前后范围内都是一致的。
|
Figure 4 Mifepristone reversed decrease of the number of connexin 43 (Cx43)-immunoreactive puncta in PFC induced by CORT (n = 6). A: Numbers of Cx43-immunoreactive puncta were quantified throughout the PFC as shown in black squares; B: The number of Cx43-immunoreactive puncta/mm2 was significantly decreased by CORT and this effect was reversed by mifepristone treatment. Error bars represent SEM. ***P < 0.001 vs CTR group; ###P < 0.001 vs CORT group; C: Representative Cx43-stained sections from animals of all groups are illustrated at approximately 3.2 mm from bregma. Scale bar = 37.5 μm |
星形胶质细胞结构和功能的损伤已成为抑郁症发病机制的重要关注点[24]。无论是对抑郁症患者还是对抑郁症模型动物的研究都发现PFC区星形胶质细胞数目明显减少[25-27], 然而关于其功能研究少有报道。细胞缝隙连接通信功能是星形胶质细胞重要的功能之一, 其发生病理改变后会参与多种中枢神经系统疾病的发生, 如脑缺血、癫痫、阿尔茨海默病及抑郁症等。本课题组前期对PFC区缝隙连接功能障碍与动物抑郁样行为的关系进行了初步研究, 研究表明CUS激活HPA轴后引起糖皮质激素分泌增加, 而CUS同时破坏了星形胶质细胞功能, 这提示CUS引起的PFC区星形胶质细胞功能障碍可能与糖皮质激素受体的激活相关。以上研究中使用了米非司酮拮抗糖皮质激素受体, 并且证明单独使用米非司酮时对PFC区缝隙链接结构和功能无影响[16]。另外, 研究显示米非司酮对CUS导致的大鼠抑郁症状和学习记忆功能障碍有改善作用[28], 充分证明了米非司酮对抑郁样大鼠的治疗作用。
本研究选择外源性地增加机体的糖皮质激素水平, 模拟CUS诱导的生物学变化, 进一步研究糖皮质激素水平升高对大鼠PFC区缝隙连接结构和功能的影响, 探索抑郁症的发病机制。研究结果充分表明长期给予皮质酮构建抑郁症模型成功。注射皮质酮的模型大鼠中星形胶质细胞缝隙连接通道功能受损, 缝隙连接结构出现异常, 两相邻细胞缝隙连接处的缝隙变宽, 胞体和突起肿大, PFC区Cx43免疫反应阳性点数明显降低。同时, 米非司酮改善了以上长期给予皮质酮造成的一系列变化, 进一步证实星形胶质细胞缝隙连接通讯功能的损伤作用与GR的激活有关。
皮质酮是由肾上腺皮质产生的糖皮质激素[29]。当HPA轴激活后, 皮质酮的释放增加, 进而诱导抑郁症状的出现[30, 31]。本课题组前期研究指出皮质酮会降低体外培养的原代PFC区星形胶质细胞活性, 破坏其缝隙连接功能, 抑制Cx43蛋白合成以及促进Cx43蛋白的降解[32]。此项研究进一步提供了理论依据, 充分证明了皮质酮对星形胶质细胞的直接毒性作用, 以及其对Cx43蛋白水平的间接调控作用。因此, 无论是前期CUS导致大鼠体内皮质酮水平增加, 还是本研究中直接外源增加大鼠体内皮质酮水平, 均造成了大鼠抑郁样症状出现, 破坏了其PFC区星形胶质细胞缝隙连接功能。当用米非司酮阻断GR时, 均改善了上述现象。
综上所述, 米非司酮对大鼠PFC区星形胶质细胞缝隙连接通讯功能的损伤有改善作用, 星形胶质细胞异常及星形胶质细胞缝隙连接通讯功能的损伤作用与GR的激活有关。
| [1] | Cui R. Editorial:a systematic review of depression[J]. Curr Neuropharmacol, 2015, 13: 480. |
| [2] | Mårtensson B, Andersson G, Wålinder J, et al. Depression treatment-then, now and in the future[J]. Lakartidningen, 2013, 110: 493–495. |
| [3] | Sarris J, O'Neil A, Coulson CE, et al. Lifestyle medicine for depression[J]. BMC Psychiatry, 2014, 14: 107. |
| [4] | Eby GA 3rd, Eby KL. Magnesium for treatment-resistant depression:a review and hypothesis[J]. Med Hypotheses, 2010, 74: 649–660. DOI:10.1016/j.mehy.2009.10.051 |
| [5] | Pérez-Alvarez A, Araque A. Astrocyte-neuron interaction at tripartite synapses[J]. Curr Drug Targets, 2013, 14: 1220–1224. DOI:10.2174/13894501113149990203 |
| [6] | Torrente D, Cabezas R, Avila MF, et al. Cortical spreading depression in traumatic brain injuries:is there a role for astrocytes?[J]. Neurosci Lett, 2014, 565: 2–6. |
| [7] | Miguel-Hidalgo JJ, Waltzer R, Whittom AA, et al. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity[J]. J Affect Disord, 2010, 127: 230–240. |
| [8] | Krügel U, Koles L, Illés P. Integration of neuronal and glial signalling by pyramidal cells of the rat prefrontal cortex; control of cognitive functions and addictive behaviour by purinergic mechanisms[J]. Neuropsychopharmacol Hung, 2013, 15: 206–213. |
| [9] | Rash JE, Yasumura T, Davidson KG, et al. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord[J]. Cell Commun Adhes, 2001, 8: 315–320. DOI:10.3109/15419060109080745 |
| [10] | Liebmann M, Stahr A, Guenther M, et al. Astrocytic Cx43 and Cx30 differentially modulate adult neurogenesis in mice[J]. Neurosci Lett, 2013, 545: 40–45. DOI:10.1016/j.neulet.2013.04.013 |
| [11] | Chen MJ, Kress B, Han X, et al. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury[J]. Glia, 2012, 60: 1660–1670. DOI:10.1002/glia.v60.11 |
| [12] | Aronica E, Gorter JA, Jansen GH, et al. Expression of connexin 43 and connexin 32 gap-junction proteins in epilepsy-associated brain tumors and in the perilesional epileptic cortex[J]. Acta Neuropathol, 2001, 101: 449–459. |
| [13] | Carlen PL. Curious and contradictory roles of glial connexins and pannexins in epilepsy[J]. Brain Res, 2012, 1487: 54–60. DOI:10.1016/j.brainres.2012.06.059 |
| [14] | Braganza O, Bedner P, Huttmann K, et al. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling[J]. Epilepsia, 2012, 53: 1898–1906. DOI:10.1111/epi.2012.53.issue-11 |
| [15] | Sayyah M, Kaviani B, Khoshkholgh-Sima B, et al. Effect of chronic intracerebroventricluar administration of lipopolysaccharide on connexin43 protein expression in rat hippocampus[J]. Iran Biomed J, 2012, 16: 25–32. |
| [16] | Sun JD, Liu Y, Yuan YH, et al. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats[J]. Neuropsychopharmacology, 2012, 37: 1305–1320. DOI:10.1038/npp.2011.319 |
| [17] | Jin C, Wang ZZ, Zhou H, et al. Ginsenoside Rg1-induced antidepressant effects involve the protection of astrocyte gap junctions within the prefrontal cortex[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2017, 75: 183–191. |
| [18] | Lopez-Duran NL, McGinnis E, Kuhlman K, et al. HPA-axis stress reactivity in youth depression:evidence of impaired regulatory processes in depressed boys[J]. Stress, 2015, 18: 545–553. DOI:10.3109/10253890.2015.1053455 |
| [19] | Maric NP, Adzic M. Pharmacological modulation of HPA axis in depression-new avenues for potential therapeutic benefits[J]. Psychiatr Danub, 2013, 25: 299–305. |
| [20] | Palazidou E. The neurobiology of depression[J]. Br Med Bull, 2012, 101: 127–145. DOI:10.1093/bmb/lds004 |
| [21] | Howland RH. Mifepristone as a therapeutic agent in psychiatry[J]. J Psychosoc Nurs Ment Health Serv, 2013, 51: 11–14. |
| [22] | Nihalani ND, Schwartz TL. Mifepristone, a glucocorticoid antagonist for the potential treatment of psychotic major depression[J]. Curr Opin Investig Drugs, 2007, 8: 563–569. |
| [23] | Iyo AH, Feyissa AM, Chandran A, et al. Chronic corticosterone administration down-regulates metabotropic glutamate receptor 5 protein expression in the rat hippocampus[J]. Neuroscience, 2010, 169: 1567–1574. |
| [24] | Li J, Sun JD, Liu Y, et al. Mifepristone repairs alteration of learning and memory abilities in rat model of depression[J]. Acta Pharm Sin (药学学报), 2013, 48: 1221–1226. |
| [25] | Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors[J]. Biol Psychiatry, 2008, 64: 863–870. DOI:10.1016/j.biopsych.2008.06.008 |
| [26] | Di Benedetto B, Malik VA, Begum S, et al. Fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity of astrocyte processes[J]. Front Cell Neurosci, 2016, 10: 8. |
| [27] | Öngür D, Pohlman J, Dow AL, et al. Electroconvulsive seizures stimulate glial proliferation and reduce expression of Sprouty 2 within the prefrontal cortex of rats[J]. Biol Psychiatry, 2007, 62: 505–512. DOI:10.1016/j.biopsych.2006.11.014 |
| [28] | Ren Q, Xia CY, Wang ZZ, et al. Protective effects of ginsenoside Rg1 against corticosterone-induced primary astrocytes injury[J]. Acta Pharm Sin (药学学报), 2017, 52: 1410–1415. |
| [29] | Wolff C, Krinner K, Schroeder JA, et al. Inadequate corticosterone levels relative to arthritic inflammation are accompanied by altered mitochondria/cholesterol breakdown in adrenal cortex:a steroid-inhibiting role of IL-1β in rats[J]. Ann Rheum Dis, 2015, 74: 1890–1897. DOI:10.1136/annrheumdis-2013-203885 |
| [30] | Silvestre JS, Robert V, Heymes C, et al. Myocardial production of aldosterone and corticosterone in the rat[J]. J Biol Chem, 1998, 273: 4883–4891. |
| [31] | Niraula A, Wang Y, Godbout JP, et al. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression[J]. J Neurosci, 2018, 38: 2328–2340. DOI:10.1523/JNEUROSCI.2568-17.2018 |
| [32] | Xia CY, Wang ZZ, Zhang Z, et al. Corticosterone impairs gap junctions in the prefrontal cortical and hippocampal astrocytes via different mechanisms[J]. Neuropharmacology, 2018, 131: 20–30. DOI:10.1016/j.neuropharm.2017.12.003 |
 2018, Vol. 53
2018, Vol. 53


