具有活性的子宫内膜组织在子宫内膜以外部位生长被称为子宫内膜异位症。尽管子宫内膜异位症被认为是一种良性妇科疾病, 但其具有侵袭、种植及复发率高等恶性生物学行为[1, 2]。子宫内膜异位症的发病机制并未明确, 最广为接受的经血逆流学说认为, 随经血逆流的子宫内膜组织发生侵袭转移是重要的步骤[3, 4]。
中医学认为子宫内膜异位症病机主要为瘀血阻滞胞脉、子宫[5, 6], 治法当以活血化瘀为主[7]。加味佛手散源自妇科经典名方佛手散, 是本课题组研制的一种新的中药单体复方, 由川芎嗪、阿魏酸加延胡索乙素组成。前期研究发现, 加味佛手散对子宫内膜异位症具有较好的疗效, 能抑制异位组织增生, 调节雌激素、孕激素水平, 抗炎和抗血管生成[8], 但对异位内膜侵袭转移的作用机制未见报道。
网络药理学基于生物分子网络构建“药物-靶点-通路”多层次网络分析药效活性成分和可能的分子网络机制, 旨在建立中药及其复方多成分、多途径、多靶点间的协同作用联系[9, 10]。本研究运用网络药理学的思路和方法, 预测加味佛手散抑制子宫内膜侵袭转移作用的靶点, 并利用大鼠模型进行体内靶点验证, 探讨加味佛手散抑制子宫内膜侵袭转移机制。
材料与方法药品与试剂 加味佛手散由西南大学中药研究所研制提供, 阿魏酸(批号: ZL2016030815, 纯度98%)、川芎嗪(批号: ZL2016061382, 纯度98%)和延胡索乙素(批号: ZL2016051235, 纯度98.1%)均购自于南京泽朗公司。BCA试剂盒、qPCR引物购自北京鼎国生物技术公司; PrimeScriptTM RT试剂盒购自Takara公司, SYBRTM Green Master Mix购自美国Thermo Fisher公司, MMP2和MMP9抗体购自武汉博士德公司, TIMP1和β-actin抗体购自武汉三鹰生物技术公司, 荧光素标记的山羊抗兔二抗购自北京中杉金桥生物技术公司。
动物 SPF级雌性SD大鼠(180~200 g), 购自重庆市中药研究院, 生产许可证号: SCXK (渝) 2012- 0006。本研究严格遵循西南大学动物伦理委员会要求(伦理批件编号0002183)。动物自然昼夜节律光照, 适应1周后进行实验。
仪器 荧光分光光度计(美国Thermo Fisher公司); 电泳仪(上海伯乐公司); Tanon5200全自动化学发光图像分析系统(上海天能科技公司); CFX 96 Real-Time PCR仪(美国BIO-RAD公司)。
靶点收集 利用中药综合数据库TCMID[11] (http://www.megabionet.Org/tcmid/search/)、中药成分系统药理学数据库TCMSP[12] (http://lsp.nwu.edu.cn)和相似性集合方法数据库SEA[13] (http://sea.bkslab.org/)收集加味佛手散中3种成分的作用靶点构建加味佛手散成分-成分靶点网络。利用人类在线孟德尔遗传OMIM[14] (https://omim.org/)、基因疾病数据库协会DisGeNET[15] (http://www.disgenet.org/)和高通量基因表达数据库GEO[16] (https://www.ncbi.nlm.nih.gov/geo/)构建加味佛手散成分-子宫内膜异位症靶点网络。使用STRING[17]数据库(https://string-db.org/)进行蛋白相互作用查询, 选择打分值高于0.4的置信度数据靶点。上述所有靶点均通过UniProt[18]数据库中的UniProtKB搜索功能(http://www.uniprot.org/)获取其官方的UniProt ID信息, 并用于后续网络构建。
网络构建与通路分析 基于以上靶点预测结果, 采用Cytoscape 3.5.0软件构建加味佛手散成分-成分靶点和加味佛手散成分-子宫内膜异位症靶点网络, 并将关键靶点的信息上传至DAVID[19]数据库(https://david.ncifcrf.gov/)进行GO (Gene Ontology)生物学过程富集分析和KEGG (KEGG pathway analysis)通路富集分析, 选取具有统计学意义(P < 0.05)的所有通路。
动物造模与分组 60只发情期SD雌鼠进行子宫内膜自体移植手术。造模28天后再次开腹观察, 模型成功标准[20]: ①移植物体积增大, 表面覆盖有结缔组织或大网膜, 呈隆起透亮小囊状, 表面有血管, 内有积液, 且积液高度≥2 mm; ②游标卡尺测量移植物的体积大小, 异位组织体积= 0.52×长×宽×高, 移植物体积≥8 mm3; ③切取移植物送检, 证实移植物中有子宫内膜上皮细胞、腺体及间质的生长。将造模成功的50只大鼠随机分为5个组:模型组, 加味佛手散低、中、高浓度组和孕三烯酮组, 各组给药前异位内膜体积无显著性差异, 另设正常雌性大鼠10只为空白组。空白组和模型组灌胃0.5%羧甲基纤维素钠混悬液, 加味佛手散低、中、高浓度组分别给予45、90、180 mg·kg-1加味佛手散, 孕三烯酮组给予50 mg·kg-1孕三烯酮, 每天灌胃给药1次, 连续给药4周。以体积变化率= (给药前移植内膜体积-给药后移植内膜体积) /给药前移植内膜体积× 100%作为指标[21]。
qPCR和Western blot法测MMP2、MMP9、TIMP1 空白组取正常子宫内膜组织, 模型组和药物组取异位内膜组织, Trizol法提取总RNA, 按照Takara试剂盒说明书进行逆转录反应合成cDNA, 按照SYBR试剂盒说明书检测MMP-2、MMP-9和TIMP-1的mRNA水平, MMP-2引物序列为:上游5'-GGCCCTGTCACTCCTGAGAT-3', 下游5'-GGCA TCCAGGTTATCGGGGA-3'; MMP-9引物序列为:上游5'-TGGACGATGCCTGCAACGTG-3', 下游5'-GTC GTGCGTGTCCAAAGGCA-3'; TIMP-1引物序列为:上游5'-CAATTCCGACCTCGTCATCAG-3', 下游5'- CTTGGAACCCTTTATACATCTTGG-3'; GAPDH引物序列为:上游5'-AATGGGCAGCCGTTAGGAAA- 3', 下游5'-GCCCAATACGACCAAATCAGAG-3'。用内源对照GAPDH标准化的2-△△Ct方法计算目的基因相对表达量。
取内膜组织, 提取总蛋白上样, 经过10% SDS- PAGE电泳使蛋白转移至PVDF膜上封闭后, 孵育一抗(MMP-2 1:300, MMP-9 1:300, TIMP-1 1:500) 4 ℃过夜, 洗膜后室温孵育二抗(1:2 000) 1 h。使用全自动化学发光图像分析系统进行图像显影定影。β-actin设为内参蛋白。
统计分析 计数资料以(x±s)表示。组间数据采用单因素方差分析进行比较, 采用SPSS21统计软件包进行统计学分析。P < 0.05有显著性差异, P < 0.01有极显著差异。
结果 1 加味佛手散成分-成分靶点网络构建与分析3种加味佛手散成分共获得275个潜在靶点, 其中涉及川芎嗪10个, 阿魏酸86个, 延胡索乙素179个。通过分析每一种成分的靶点构建网络发现, ADRB2、CA2、F3、LTA4H、PTGS1、PTGS2、SLC6A2和SLC6A3靶点是川芎嗪、阿魏酸和延胡索乙素3种成分共有靶点(图 1), 提示这些共同靶点可能是多成分、多靶点中药协同作用的基础。
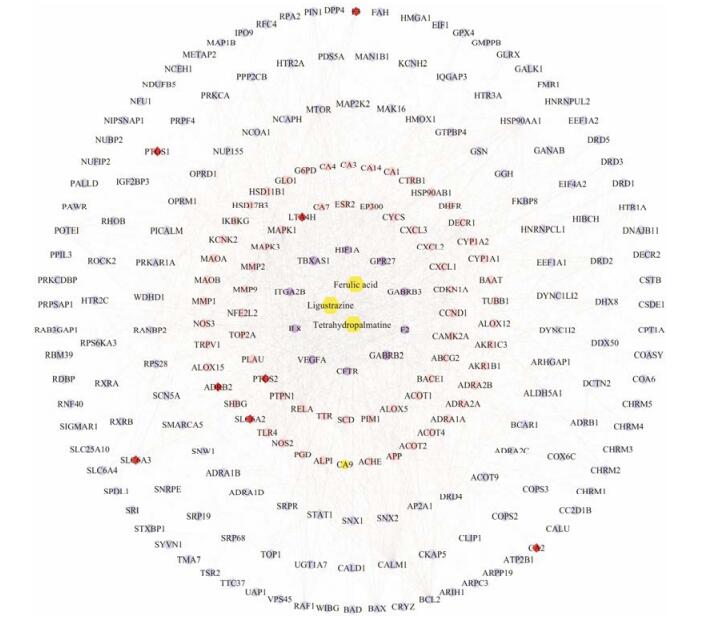
|
Figure 1 The JFS compound-target network. Purple circles, pink circles, blue circles and red diamonds represented the targets of ligustrazine, ferulic acid, tetrahydropalmatine and the common targets for all three components respectively. Yellow hexagons represented the three compounds of JFS |
整合数据获得与疾病相关靶点401个, 将3个成分的靶点映射到疾病靶点获得22个共同靶点(表 1)。然后提取22个共同靶点及其相邻的靶点构建核心蛋白质-蛋白质相互作用网络, 再对网络节点的拓扑属性分析进行靶点筛选, 节点的“度”和“介数”的平均值分别为29.860 3和0.003 9, 选择“度”≥29.860 3且“介数”≥0.003 9的66个节点作为关键靶点。其中, MMP2、MMP-9、TIMP-1、ICAM1和VEGFA被认为是与活血化瘀功效相关的靶点。
| Table 1 Common targets of Jiawei Foshou San (JFS)-treatment and endometriosis-development |
通过DAVID数据库对66个关键靶点进行GO生物学过程、细胞组分和分子功能分析和通路富集分析。图 2列出了GO分析生物学过程、细胞组分和分子功能各类别功能中显著富集排名前6的项目, 可以看出这些靶点主要涉及凋亡过程的负调节、DNA模板化、细胞增殖的正调节等生物学过程, 涉及核、细胞质、核质等细胞组分, 涉及蛋白质结合、酶结合、DNA结合等分子功能。在KEGG数据库中获得与关键靶点相关的通路115条, 结果表明加味佛手散可能主要通过调节雌激素、转录因子HIF-1、肿瘤坏死因子和促性腺释放激素等10条信号通路过程抑制内膜侵袭转移, 其中MMP/TIMP在通路中起关键作用(图 3)。
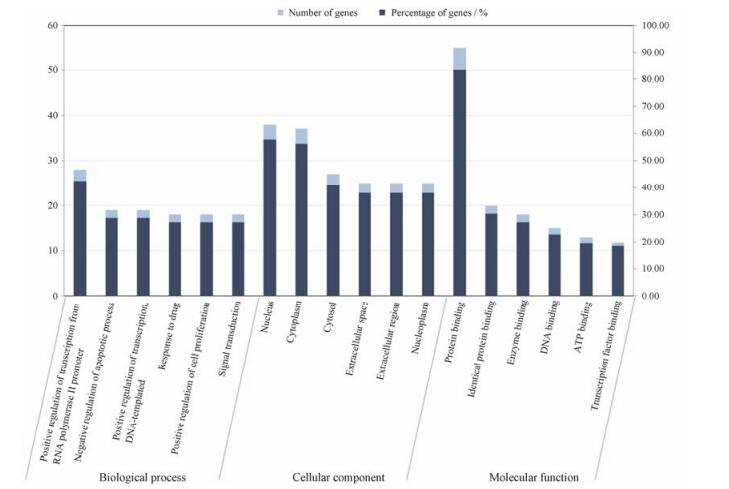
|
Figure 2 Gene ontology (GO) analysis of candidate targets. GO enrichment analysis in DAVID database showed the 6 remarkably enriched items in the biological processes, cell component, and molecular function |
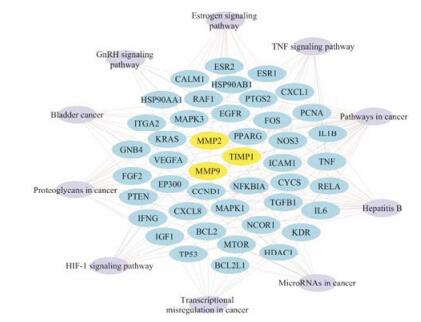
|
Figure 3 Pathway including MMP2, MMP9 and TIMP1 |
造模28天后, 模型组大鼠异位内膜生长良好, 体积增大, 黏连严重, 透亮小囊表面的血管清晰可见且丰富, 表面覆盖有结缔组织或大网膜, 液体积聚高度≥2 mm, 体积≥8 mm3, 送检结果显示为子宫内膜, 说明造模成功。分组给药28天后, 加味佛手散组体积明显缩小, 异位内膜黏连减少, 表面血管减少, 提示加味佛手散对异位内膜生长有较明显的抑制作用。其中加味佛手散90和180 mg·kg-1异位内膜体积变化率分别为0.58 ± 0.10、0.82 ± 0.04, 与模型组相比显著减小(P < 0.05)。与模型组相比, 孕三烯酮组异位内膜体积变化率为0.81 ± 0.25 (P < 0.05), 且加味佛手散的作用效果呈现剂量依赖性(图 4)。
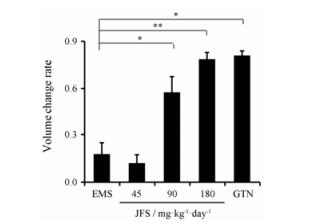
|
EMS: Endometriosis; GTN: Gestrinone. n = 6, x±s. *P < 0.05, **P < 0.01 Figure 4 Effect of JFS on the volume change rat of endometrium. |
结果表明, 与空白组相比, 模型组MMP-2、MMP-9 mRNA水平显著上调(P < 0.05), TIMP-1 mRNA水平显著下调(P < 0.05)。与模型组相比, 加味佛手散组显著下调MMP-2 (P < 0.05)和MMP-9 (P < 0.01) mRNA水平, 加味佛手散90和180 mg·kg-1组显著上调(P < 0.05) TIMP-1 mRNA水平(图 5)。
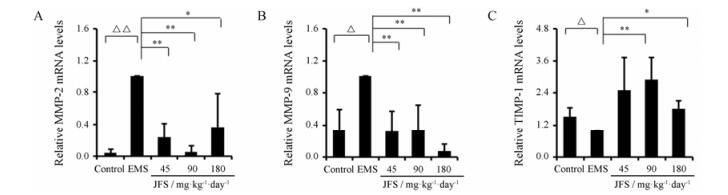
|
n = 3, x±s. △P < 0.05, △△P < 0.01; *P < 0.05, **P < 0.01 Figure 5 Effect of JFS on invasion and metastasis. The mRNA levels of MMP-2 (A), MMP-9 (B) and TIMP-1 (C) were detected by qPCR in different groups. |
Western blot结果表明, 与空白组相比, 模型组MMP-2、MMP-9蛋白表达水平显著上调(P < 0.05), TIMP-1蛋白表达水平显著下调(P < 0.05)。与模型组相比, 加味佛手散组MMP-2和MMP-9蛋白表达水平呈剂量依赖性显著下调(P < 0.05), TIMP-1蛋白表达水平均显著上调(P < 0.05) (图 6)。综上, 加味佛手散对侵袭转移的作用机制与调节MMP-2、MMP-9和TIMP-1基因和蛋白的表达有关。

|
n = 3, x±s. △P < 0.05; *P < 0.05, **P < 0.01 Figure 6 Effect of JFS on invasion and metastasis. The protein levels of MMP-2, MMP-9 and TIMP-1 were detected by Western blot (A), and the ratio of MMP-2 (B), MMP-9 (C) and TIMP-1 (D) with β-actin were shown. |
经血逆流学说认为, 异位内膜的侵袭转移是发生子宫内膜异位症的重要步骤。MMP-2、MMP-9和TIMP-1是重要的侵袭转移蛋白[22], MMP-2和MMP-9会促进细胞外基质降解, 增加异位内膜侵袭力, TIMP-1能与MMP-9形成复合体, 进而抑制细胞外基质的降解, 它们之间的平衡可调节细胞外基质的降解。通过查阅文献发现, 子宫内膜异位内膜的MMP-2、MMP-9表达明显增高, 而TIMP-1表达降低[23-26]。通过抑制MMP-2、MMP-9, 促进TIMP-1表达已被报道为抗子宫内膜异位症的有效疗法[24, 27, 28]。本研究发现, 在自体移植的子宫内膜异位症SD大鼠模型中, 异位内膜中MMP-2和MMP-9基因和蛋白的表达明显增强, 而TIMP-1基因和蛋白的表达则明显减弱, 这与已有研究结果相吻合。同时加味佛手散能显著抑制MMP-2和MMP-9的表达, 显著上调TIMP-1的表达, 从而抑制异位内膜的侵袭转移。因此, 其他如MMP-1、MMP-7和TIMP-2等在异位内膜侵袭转移中的作用, 以及加味佛手散对其的影响都值得进一步探讨研究。
加味佛手散来源于著名活血化瘀名方佛手散, 通过分析加味佛手散成分-子宫内膜异位症靶点网络中的66个核心靶点, 其中MMP-2、MMP-9、TIMP-1、ICAM1、ACE和VEGFA被认为是与活血化瘀功效相关的靶点。除了子宫内膜异位症, MMP-2、MMP-9和TIMP-1被发现是多种血瘀证的靶点, 包括肝癌、颈动脉血栓、毒热血瘀证、缺血再灌注损伤[29-32]。ICAM1、ACE和VEGFA也被认为是痛风、脑缺血、冠心病和子宫内膜异位症的靶点之一[33-36]。通过网络药理学, 对CPPI网络中的核心靶点进行通路富集分析, 发现GnRH、TNF和雌激素等信号通路与MMP-TIMP相关, 且GnRH、TNF和雌激素都是维持子宫内膜稳态的关键介质[37-40]。此外, 有研究表明MMP和TIMP蛋白表达受这3种途径调节[41-43]。课题组前期研究还发现, 加味佛手散可以抑制GnRH、雌激素和TNF-α的表达[8], 其作用机制可能与调节MMP/TIMP平衡有关。因此, 预测所得加味佛手散抑制子宫内膜侵袭转移机制可能与作用于MMP-TIMP靶点密切相关, 表明基于网络药理学的技术手段探讨中药复方药效具有一定的准确性与实用性, 为加味佛手散的评估提供合理支持, 也为进一步深入探讨药效及发病机制奠定了良好基础。
| [1] | Ma X, Hui Y, Lin L, et al. Possible relevance of tumor-related genes mutation to malignant transformation of endometriosis[J]. Eur J Gynaecol Oncol, 2016, 37: 89–94. |
| [2] | Mihailovici A, Rottenstreich M, Kovel S, et al. Endometriosis- associated malignant transformation in abdominal surgical scar: a PRISMA-compliant systematic review[J]. Medicine, 2017, 96: e9136. DOI:10.1097/MD.0000000000009136 |
| [3] | Borrelli GM, Abrao MS, Taube ET, et al. Immunohisto- chemical investigation of metastasis-related chemokines in deep- infiltrating endometriosis and compromised pelvic sentinel lymph nodes[J]. Reprod Sci, 2015, 22: 1632–1642. DOI:10.1177/1933719115592711 |
| [4] | Chui MH, Wang TL, Shih IM. Endometriosis: benign, malignant, or something in between?[J]. Oncotarget, 2017, 8: 78263–78264. |
| [5] | Shan J, Cheng W, Zhai DX, et al. Meta-analysis of Chinese traditional medicine Bushen Huoxue prescription for endometriosis treatment[J]. Evid Based Complement Alternat Med, 2017, 2017: 5416423. |
| [6] | Wen Y, Wang Y, Feng TT, et al. Differential proteomics analysis of endometriosis in blood stasis syndrome[J]. Chin J Integr Med, 2017. DOI:10.1007/s11655-017-2401-4 |
| [7] | Zhao RH. Strategies for activating blood circulation-regulating Gan (liver)-tonifying Shen (kidney) sequential therapy of endometriosis-associated infertility[J]. Chin J Integr Med, 2018. DOI:10.1007/s11655-018-2931-9 |
| [8] | Tang Q, Shang F, Wang X, et al. Combination use of ferulic acid, ligustrazine and tetrahydropalmatine inhibits the growth of ectopic endometrial tissue: a multi-target therapy for endometriosis rats[J]. J Ethnopharmacol, 2014, 151: 1218–1225. DOI:10.1016/j.jep.2013.12.047 |
| [9] | Ning K, Zhao X, Poetsch A, et al. Computational molecular networks and network pharmacology[J]. Biomed Res Int, 2017. DOI:10.1155/2017/7573904 |
| [10] | Wang Y, Lin W, Li C, et al. Multipronged therapeutic effects of Chinese herbal medicine Qishenyiqi in the treatment of acute myocardial infarction[J]. Front Pharmacol, 2017, 8: 98–110. |
| [11] | Ru JL. Construction and Utilization of Traditional Chinese Medicine System Pharmacology Database and Analysis Platform (中药系统药理学数据库和分析平台的构建和应用)[D]. Xianyang: North West Agriculture and Forestry University, 2015. |
| [12] | Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines[J]. J Cheminform, 2014, 6: 13–19. DOI:10.1186/1758-2946-6-13 |
| [13] | Keiser MJ, Roth BL, Armbruster BN, et al. Relating protein pharmacology by ligand chemistry[J]. Nat Biotechnol, 2007, 25: 197–206. DOI:10.1038/nbt1284 |
| [14] | Li X, Wu LH, Fan XH, et al. Network pharmacology study on major active compounds of Fufang Danshen formula[J]. China J Chin Mater Med (中国中药杂志), 2011, 36: 2911–2915. |
| [15] | Bravo A, Queralt-Rosinach N, Pinero J, et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants[J]. Nucleic Acids Res, 2017, 45: D833–D839. DOI:10.1093/nar/gkw943 |
| [16] | Li G, Xu B, Liang XZ, et al. Study on the molecular mechanism of osteoporosis treated by epimedium based on network pharmacology[J]. Chin Pharm Bull (中国药理学通报), 2018, 34: 267–273. |
| [17] | Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible[J]. Nucleic Acids Res, 2017, 45: D362–D368. DOI:10.1093/nar/gkw937 |
| [18] | Chai X, Meng YK, Bai ZF, et al. Mechanism of anti-hepatitis B virus activity of tonkinensis based on biological targets network[J]. Acta Pham Sin (药学学报), 2018, 53: 396–402. |
| [19] | Jiao X, Sherman BT, Huang DW, et al. DAVID-WS: a stateful web service to facilitate gene/protein list analysis[J]. Bioinformatics, 2012, 28: 1805–1806. DOI:10.1093/bioinformatics/bts251 |
| [20] | Liu W, Wang LX, Guo CX. The effects of lauromacrogol injection into rat endometrial cysts: a preliminary experimental study[J]. Arch Gynecol Obstet, 2016, 294: 556–559. |
| [21] | Duan LJ, Wang JY, Sun XT, et al. The role and significance of endomorphin-1 and µ-opioid receptor in rats with endometriosis[J]. Gynecol Endocrinol, 2016, 32: 912–915. DOI:10.1080/09513590.2016.1190816 |
| [22] | Li Z, Takino T, Endo Y, et al. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis[J]. Cancer Sci, 2017, 108: 347–353. DOI:10.1111/cas.13134 |
| [23] | Jana S, Chatterjee K, Ray AK, et al. Regulation of matrix metalloproteinase-2 activity by COX-2-PGE2-pAKT axis promotes angiogenesis in endometriosis[J]. PLoS One, 2016, 11: e0163540. DOI:10.1371/journal.pone.0163540 |
| [24] | Huang YH, Shen L, Cai A, et al. Effect of warming Yang and removing blood stasis method on matrix metalloproteinases / tissue inhibitor metalloproteinases levels secreted by cultured endometrial cells from patients with endometriosis[J]. J Tradit Chin Med (中医杂志), 2015, 35: 571–576. DOI:10.1016/S0254-6272(15)30141-2 |
| [25] | Yi KW, Kim SH, Ihm HJ, et al. Increased expression of p21-activated kinase 4 in adenomyosis and its regulationof matrix metalloproteinase-2 and -9 in endometrial cells[J]. Fertil Steril, 2015, 103: 1089–1097. DOI:10.1016/j.fertnstert.2014.12.124 |
| [26] | Szymanowski K, Mikolajczyk M, Wirstlein P, et al. Matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of matrix metalloproteinases (TIMP-1) and transforming growth factor-β2 (TGF-β2) expression in eutopic endometrium of women with peritoneal endometriosis[J]. Ann Agric Environ Med, 2016, 23: 649–653. DOI:10.5604/12321966.1226861 |
| [27] | Li Z, Liu H, He Z, et al. Effects of cisplatin and letrozole on surgically induced endometriosis and comparison of the two medications in a rat model[J]. Eur J Pharm Sci, 2016, 93: 132–140. DOI:10.1016/j.ejps.2016.07.018 |
| [28] | Kim JH, Woo JH, Kim HM, et al. Anti-endometriotic effects of pueraria flower extract in human endometriotic cells and mice[J]. Nutrients, 2017, 9: 212–226. DOI:10.3390/nu9030212 |
| [29] | Gao L, Wang KX, Zhou YZ, et al. Uncovering the anticancer mechanism of compound kushen injection against HCC by integrating quantitative analysis, network analysis and experimental validation[J]. Sci Rep, 2018, 8: 624–639. DOI:10.1038/s41598-017-18325-7 |
| [30] | Xue M, Zhang L, Yang L, et al. Effect of Chinese herbal medicine for activating blood circulation and detoxifying on expression of inflammatory reaction and tissue damage related factors in experimental carotid artery thrombosis rats[J]. Chin J Integr Med, 2010, 16: 247–251. DOI:10.1007/s11655-010-0247-4 |
| [31] | Sun J, Zhu Y, Zhang L, et al. Effects of xuelian injection on cerebral TNF-α, IL-1β and MMP-9 in rats experienced focal cerebral ischemia/reperfusion[J]. Int J Clin Exp Med, 2014, 7: 2632–2638. |
| [32] | Salameh A, Dhein S. Strategies for pharmacological organoprotection during extracorporeal circulation targeting ischemia- reperfusion injury[J]. Front Pharmacol, 2015, 6: 296–308. |
| [33] | Zhao FL, Li GC, Yang YH, et al. A network pharmacology approach to determine active ingredients and rationality of herb combinations of modified-Simiaowan for treatment of gout[J]. J Ethnopharmacol, 2015, 168: 1–16. DOI:10.1016/j.jep.2015.03.035 |
| [34] | Zhou W, Wang Y. A network-based analysis of the types of coronary artery disease from traditional Chinese medicine perspective: potential for therapeutics and drug discovery[J]. J Ethnopharmacol, 2014, 151: 66–77. DOI:10.1016/j.jep.2013.11.007 |
| [35] | Fei X, Zhang X, Wang Q, et al. Xijiao Dihuang decoction alleviates ischemic brain injury in MCAO rats by regulating inflammation, neurogenesis, and angiogenesis[J]. Evid Based Complement Alternat Med, 2018. DOI:10.1155/2018/5945128 |
| [36] | Chen ZZ, Gong X. Effect of Hua Yu Xiao Zheng decoction on the expression levels of vascular endothelial growth factor and angiopoietin-2 in rats with endometriosis[J]. Exp Ther Med, 2017, 14: 5743–5750. |
| [37] | Granese R, Perino A, Calagna G, et al. Gonadotrophin- releasing hormone analogue or dienogest plus estradiol valerate toprevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial[J]. Acta Obstet Gynecol Scand, 2015, 94: 637–645. DOI:10.1111/aogs.2015.94.issue-6 |
| [38] | Leavy O. Reproductive immunology: evading immunosurveillance in endometriosis[J]. Nat Rev Immunol, 2015, 15: 729. |
| [39] | Simmen RC, Kelley AS. Reversal of fortune: estrogen receptor-beta in endometriosis[J]. J Mol Endocrinol, 2016, 57: F23–F27. DOI:10.1530/JME-16-0080 |
| [40] | Tosti C, Biscione A, Morgante G, et al. Hormonal therapy for endometriosis: from molecular research to bedside[J]. Eur J Obstet Gynecol Reprod Biol, 2017, 209: 61–66. DOI:10.1016/j.ejogrb.2016.05.032 |
| [41] | Voloshenyuk TG, Gardner JD. Estrogen improves TIMP- MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females[J]. Am J Physiol Regul Integr Comp Physiol, 2010, 299: R683–R693. DOI:10.1152/ajpregu.00162.2010 |
| [42] | Raga F, Casan EM, Wen Y, et al. Independent regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-3 in human endometrial stromal cells by gonadotropin-releasing hormone: implications in early human implantation[J]. J Clin Endocrinol Metab, 1999, 84: 636–642. |
| [43] | Yang YN, Wang F, Zhou W, et al. TNF-alpha stimulates MMP-2 and MMP-9 activities in human corneal epithelial cells via the activation of FAK/ERK signaling[J]. Ophthalmic Res, 2012, 48: 165–170. DOI:10.1159/000338819 |
 2018, Vol. 53
2018, Vol. 53


