近年来, 随着肿瘤免疫逃逸机制相关研究的逐渐深入, 肿瘤免疫治疗进入了人们的视野。嵌合抗原受体T细胞免疫疗法在急性白血病和非霍奇金淋巴瘤的治疗中表现出显著疗效, 而免疫检查点相关药物则通过恢复免疫细胞对肿瘤的监视和杀伤作用而起到治疗效果, 使得众多肿瘤患者从中获益。例如靶向程序性死亡受体(programmed cell death protein 1, PD-1)的单克隆抗体pembrolizumab目前已经用于治疗黑色素瘤、头颈部肿瘤和霍奇金淋巴瘤等, 是程序性死亡配体1 (programmed death-ligand 1, PD-L1)阳性的非突变型非小细胞肺癌一线用药, 但其响应率不佳、无明确生物标志物的特点大大限制了该药物的应用。近年来, 同为免疫检查点的吲哚胺2, 3-双加氧酶1 (indoleamine 2, 3-dioxygenase 1, IDO1)受到广泛关注, 成为各大医药公司争相研究的药物靶点。目前在临床研究阶段的IDO1抑制剂大多为小分子化合物, 在临床试验中与多种类型药物联合用药, 能够提高肿瘤对免疫疗法的敏感性且具有安全性高、成本较低的特点。
IDO1是胞浆内由403个氨基酸组成的含亚铁血红素的酶, 由位于第8号染色体上的INDO基因编码[1]。正常生理状态下, IDO1在人体多种组织中广泛表达, 如小肠、附睾、肺和胎盘等[2]。研究表明, IDO1是催化色氨酸分子中吲哚环氧化裂解, 使其沿犬尿氨酸途径分解代谢的限速酶。肿瘤微环境中, 树突细胞(dendritic cells, DCs)和巨噬细胞高表达IDO1, 减少了底物色氨酸并增加代谢产物的生成, 影响了免疫细胞的状态, 是免疫检查点中至关重要的一环。
早在1967年IDO1就已从兔小肠中被分离出来[3], 而其在肿瘤中的发现甚至可以追溯到1956年, Boyland等[4]发现患有膀胱癌的患者尿液中色氨酸的代谢产物明显增多。但IDO1在肿瘤中的生物学作用一直都不明确。直到1998年, Munn等[5, 6]才将IDO1与免疫结合起来, 较为完整地阐述了IDO1表达引起的免疫抑制效应。
1 IDO1与色氨酸代谢色氨酸是细胞重要生命活动所必需的氨基酸, 而IDO1主要的功能是将色氨酸代谢成犬尿氨酸等代谢产物, 是该代谢过程中至关重要的限速酶。一方面, IDO1的过表达会导致色氨酸耗竭, 使得不带电荷的色氨酸-转运核糖核酸蓄积, 继而激活一般性调控阻遏蛋白激酶2 (general control nonderepressible 2 kinase, GCN2)从而抑制T细胞的增殖和功能[7]; 另一方面, IDO1催化产生的代谢产物能够结合并激活芳烃受体(arylhydrocarbon receptor, AhR), 导致一系列生物学效应包括诱导调节性T细胞(regulatory T cell, Treg)的生成、促进DCs和巨噬细胞向免疫抑制表型的转化等[8]。另外, 研究表明代谢产物如犬尿氨酸、犬尿喹啉酸和3-羟基犬尿氨酸等也能够直接抑制T细胞的功能[9]。
2 IDO1的调控多种因素影响IDO1的表达和活性, 包括转录、转录后和降解等过程(图 1)。Ⅰ型(αβ)和Ⅱ型(γ)干扰素(interferon, IFN)通过激活Janus激酶/信号转导与转录激活子(the Janus kinase/signal transducers and activators of transcription, JAK/STAT)信号通路调节一系列干扰素刺激基因(IFN-stimulated genes, ISGs)的表达。INDO作为一种ISG, 其启动子区域存在干扰素刺激反应元件(IFN-stimulated response elements, ISRE)以及干扰素γ激活序列(IFN-gamma activation sequences, GAS), 二者是IFN响应的必要元件。根据文献报道, 转录因子DNAX激活蛋白12 (DNAX-activating protein 12, DAP12)调节IFNs诱导的IDO1转录[10]。除此之外, 其他细胞因子例如肿瘤坏死因子α (tumor necrosis factor α, TNF-α)和白细胞介素1 (interleukin-1, IL-1)等也能够调节IDO1的表达[11]。肿瘤细胞中IDO1的表达受到基因Bin1的调控, 这一基因的缺失会导致信号转导及转录激活蛋白1/核因子活化B细胞κ轻链增强子(signal transducers and activators of transcription 1/nuclear factor kappa-light-chain-enhancer of activated B cells, STAT1/NF-κB)依赖的IDO1表达上调[12]。
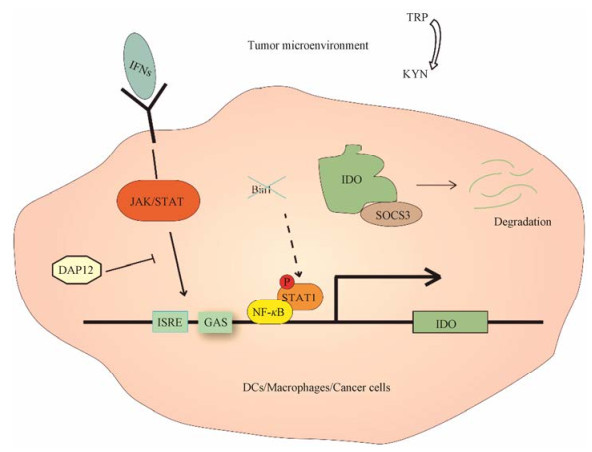
|
Figure 1 Regulation of indoleamine 2, 3-dioxygenase 1 (IDO1). In tumor microenvironment, IDO1 expresses in DCs, macrophages, and tumor cells. IFNs promote IDO1 transcription through ISRE and GAS elements located in IDO1 promoter, while DAP12 inhibits this process. Deletion of Bin1 gene in tumor cells leads to IDO1 overexpression, which is mediated by STAT1/NF-κB pathway. Moreover, IDO1 could also be recognized by SOCS3, resulting in IDO1 degradation by ubiquitin protease system. KYN: Kynurenine; DCs: Dendritic cells; IFNs: Interferon; ISRE: IFN-stimulated response elements; GAS: IFN-gamma activation sequences; DAP12: DNAX-activating protein 12; SOCS3: Suppressor of cytokine signaling 3; JAK/STAT: The Janus kinase/signal transducers and activators of transcription; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells |
IDO1的调控不仅仅局限于转录水平, 研究显示其酶学功能还受到转录后水平的一系列因素影响, 比如血红素、酶辅助因子、铁的氧化还原状态和一氧化氮等[13, 14]。据报道, 抗氧化剂可通过影响血红素与IDO1的结合而抑制其酶学活性[14]。
研究表明, 免疫调节机制中重要的分子细胞信号转导抑制因子3 (suppressor of cytokine signaling 3, SOCS3)作为泛素蛋白酶系统中E3连接酶的一部分, 能够在白细胞介素6 (interleukin-6, IL-6)的作用下与IDO1的酪氨酸磷酸化多肽区域结合, 促进IDO1被泛素-蛋白酶系统降解[15]。另外, 激活的AhR可负反馈调节IDO1, 促进其泛素化降解以维持细胞内稳态[16]。
3 肿瘤中的IDO1 3.1 IDO1在肿瘤中的异常表达报道显示乳腺癌[17, 18]、宫颈癌[19]和胶质瘤[20]多种原位和转移的肿瘤组织中存在IDO1的异常表达, 并与生存期的缩短、肿瘤浸润淋巴细胞的减少和Treg的增多等不良预后密切相关[21, 22]。在肿瘤微环境中, 不仅肿瘤细胞自身, 抗原递呈细胞尤其是DCs和巨噬细胞也呈现IDO1过表达的状态。
3.2 IDO1的反向调节作用正常生理学状态下, IDO1能够在感染时为了保护宿主而抑制机体的固有免疫[23], 以维持免疫系统的平衡。有研究表明, 肿瘤微环境中浸润性CD8+ T (T cells expressing cluster of differentiation 8)细胞的存在可诱导肿瘤中IDO1的表达[24]。也就是说, 肿瘤局部炎症或者T细胞激活信号刺激了IDO1的表达, 而其表达能够抑制免疫反应, 这就是IDO1的反向调节作用。但这种机制只有在机体对抗过激的炎症引起的免疫效应时有利, 对肿瘤治疗来说十分不利[25]。
3.3 IDO1与免疫逃逸肿瘤微环境中, IDO1的表达导致色氨酸的耗竭而激活GCN2, 引起肿瘤浸润免疫细胞的变化。IDO1诱导的GCN2激活会导致CD8+ T细胞周期阻滞以及失去功能[7]。而在CD4+ T (T cells expressing cluster of differentiation 4)细胞中则表现为抑制辅助型T细胞的分化[26]。
肿瘤浸润的Treg与预后明显相关[27], 其激活是IDO1影响T细胞免疫反应的重要方式, 也是肿瘤免疫耐受的主要机制[28]。体内IDO1引起的Treg激活是由于效应T细胞被激活的生物学信号引起的[29]。据报道, IDO1有促进Treg分化以及增强成熟Treg的免疫抑制功能[30]。IDO1的表达能够减少局部色氨酸的浓度, 激活Treg中的GCN2从而抑制哺乳动物雷帕霉素靶蛋白复合物2 (mammalian target of rapamycin complex 2, mTORC2), 减少蛋白激酶B (protein kinase B, PKB/AKT) S473位点的磷酸化[31]。与效应T细胞中AKT的作用不同, Treg中AKT的抑制会增强其免疫抑制活性[32]。在Treg中, IDO1还可以通过抑制AKT/mTOR通路上调叉形头转录因子3, 进而促进其表面PD-1的表达[31]。
DCs中IFNs能够激活IDO1及AhR, IDO1的酶活性和AhR的激活又能够继续促进IDO1的转录, 这一机制参与维持肿瘤中长期IDO1诱导的肿瘤免疫逃逸[33]。此外, IDO1的功能不仅仅局限在催化活性, 研究表明IDO1还能够通过非催化的方式诱导DCs释放转化生长因子β (transforming growth factor β, TGF-β), 继而通过非经典途径激活NF-κB维持DCs的免疫抑制表型, 参与肿瘤免疫耐受过程[34]。
4 IDO1抑制剂的研究进展越来越多的证据表明, IDO1的表达及反向调节机制对肿瘤免疫治疗不利。2006年公布的4-苯基咪唑与IDO1蛋白共结晶结构掀起了特异性靶向IDO1的热潮, 此后一系列IDO1抑制剂如navoximod等应运而生。目前大多数IDO1抑制剂在临床上推进时都是与免疫治疗剂或者化疗药物联合应用(表 1)。
| Table 1 IDO1 inhibitors in clinical trials (all informations come from www.clinicaltrials.gov). N/A stands for not applicable |
Epaca dostat是2009年通过高通量筛选得到的IDO1特异性抑制剂, 给药的动物血清中表现出犬尿氨酸浓度的降低, 并能够在动物黑色素瘤模型中达到超过50%抑瘤率[35]。进一步研究显示, epacadostat在细胞内抑制人源IDO1的半数有效浓度(EC50)约为10 nmol·L-1, 对其同工酶吲哚胺-2, 3双加氧酶2 (indoleamine 2, 3-dioxygenase 2, IDO2)和色氨酸-2, 3双加氧酶(tryp tophan 2, 3-dioxygenase, TDO)的抑制作用则很弱[36]。DCs与T细胞或自然杀伤细胞(natural killer cell, NK)共培养实验结果提示, epacadostat通过抑制IDO1来促进T细胞和NK细胞的增殖, 增加IFNγ的分泌并减少Treg[36]。Ⅰ期临床(NCT01195311)结果表明, 52例肿瘤进展期的患者中, 所有患者血浆中犬尿氨酸/色氨酸比值均随用药剂量的增加呈剂量依赖地降低, 且每天两次给予100 mg epacadostat即可对IDO1有80%~90%抑制作用, 用药后试验组中7例患者的病情稳定期都超过了16周[37]。
Epacadostat与PD-L1单抗pembrolizumab或nivolumab合用能够提高响应率, 控制病情, 同时又不引起严重的不良反应[38, 39]。根据2017年Ⅱ期临床试验(NCT02178722)结果的报道, pembrolizumab联用epacadostat应答率为56%[40], 相较单独使用pembrolizumab的响应率33%要高很多[41]。令人意外的是, 二者联合使用治疗黑色素瘤的Ⅲ期临床试验(NCT02752074)结果不尽人意, 可能与未能确定对IDO1抑制剂敏感的入组标准有关。日前Incyte宣布停止该试验受试者的招募。
4.2 Indoximod (D-1-MT, NewLink Genetics)1-甲基色氨酸(1-methyltryptophan, 1-MT)最早因是色氨酸类似物而被开发成为IDO1抑制剂, 并显示出抑制肿瘤的生物学活性[42]。然而后来的研究证实, 此前使用的外消旋体1-MT中的L-1-MT虽然与IDO1有一定的结合, 但并无抑制肿瘤的功能。反而是酶学水平无活性的indoximod对动物肿瘤模型显示出抑制作用[43]。有研究表明, indoximod能够在体内逆转由色氨酸耗竭引起的哺乳动物雷帕霉素靶蛋白复合物1 (mammalian target of rapamycin complex 1, mTORC1)的抑制, 其EC50仅为70 nmol·L-1, 因此indoximod可能通过激活mTORC1抑制细胞自噬, 恢复T细胞的功能, 从而达到抗肿瘤效果[44]。
Indoximod Ⅰ期的剂量爬坡试验结果表明, 与多西他赛共同使用时患者对每天两次1 200 mg indoximod耐受, 证实了其安全性[45]。目前正在开展indoximod与细胞毒性T淋巴细胞相关抗原4 (cytotoxic T- lymphocyte antigen 4, CTLA-4)单抗ipilimumab或PD-1单抗pembrolizumab/nivolumab联用的Ⅱ期临床试验(NCT02073123)[46]。最新报道提示, 该项试验中药物联用后响应率能达到52%[47]。Indoximod也已进入Ⅲ期临床试验阶段, 计划考察其与pembrolizumab或nivolumab联用治疗不可切除或转移性黑色素瘤的疗效。
4.3 Navoximod (GDC-0919, NLG919, NewLink Genetics)Navoximod是NewLink Genetics公司对4-苯基咪唑进行结构改造得到的(原为NLG919, 后在临床试验中更改名称为GDC-0919)。我国科研人员曾结合IDO1抑制剂可使肿瘤细胞对化疗药增敏的特点[12], 将navoximod与化疗药物多柔比星共同构建到新型反应性免疫刺激聚合药物载体中, 能够在体内实验中显著抑制小鼠结直肠癌[48]。临床前实验结果表明, navoximod在细胞水平抑制IDO1活性的EC50为75 nmol·L-1, 且对IDO1酶活性抑制作用约为TDO的10~20倍, 因此也被定义为IDO/TDO双靶点抑制剂。Ia临床试验结果证明, 复发或处于进展期的肿瘤患者对navoximod的耐受性较好, 在每天两次用药量800 mg的情况下能够使血中犬尿氨酸浓度降低30%[49]。最新报道的Ib临床试验(NCT02471846)中, navoximod与PD-L1单抗atezolizumab联合用药的安全性和部分药效学已经得到了验证[50]。但2017年Newlink Genetics披露出的消息证实, navoximod在联合紫杉烷治疗转移性乳腺癌的Ⅱ期临床试验中疗效不佳。尽管未达到理想试验终点可能是患者入组的选择等众多原因引起的, 但这一结果也让IDO/TDO双靶点抑制剂的发展受到限制。
4.4 其他IDO1抑制剂近年来, 新型IDO1抑制剂不断涌现。除了以上走在前列且有代表性的化合物之外, 也有其他公司开发的IDO1抑制剂进入临床研究。百时美施贵宝公司研发的BMS-986205是与IDO1不可逆结合的抑制剂, 其药效学活性要优于epacadostat和navoximod, 且对IDO1具有很强的选择性, EC50为1.1 nmol·L-1[50], Ⅰ期临床数据显示患者对BMS-986205有较好的耐受性[51]。目前BMS-986205正在进行Ⅰ/Ⅱ期临床试验(NCT02658890), 与PD-1单抗nivolumab或同时再应用CTLA-4单抗ipilimumab治疗进展期或已转移的肿瘤患者。与此同时, BMS-986205联用PD-1单抗nivolumab的Ⅲ期临床试验也正在开展(NCT03329846), 用于治疗进展期的黑色素瘤。
辉瑞公司开发的PF-06840003突出特点是能够透过血脑屏障, 因此可能具有抑制肿瘤脑转移的作用。PF-06840003能够逆转IDO1引起的T细胞失活, 且在动物模型中使血中犬尿氨酸浓度下降80%, 与免疫检查点抑制剂联用能够抑制肿瘤生长[52]。目前PF-06840003已经在Ⅰ期临床试验(NCT02764151)中, 以恶性胶质瘤的患者为对象进行安全性和药代动力学评价。
5 结语及展望作为肿瘤免疫检查点中的“刹车”之一, IDO1在肿瘤微环境中通过抑制T细胞的增殖和激活、促进Treg的功能等方式帮助肿瘤细胞逃脱免疫监视。在认识到了IDO1的重要作用之后, 近几年IDO1抑制剂开发和临床试验如火如荼。各项Ⅰ期临床试验结果已经证明了大多IDO1抑制剂单独或联合使用的安全性。许多Ⅱ期临床试验结果提示, 其与免疫检查点相关的药物联合使用能显著增加患者应答率, 那么Ⅲ期临床试验中会不会看到比单药更加优异的肿瘤治疗效果也是很值得期待的。
将IDO1抑制剂与其他作用于免疫检查点的药物联用原因如下: IDO1在多种肿瘤组织中异常表达, 其反向调节机制限制了现有的免疫治疗剂的作用。Holmgaard等[53]的研究显示, 在动物黑色素瘤模型中, IDO1的表达是限制CTLA-4及PD-1单抗疗效的主要因素, 抑制IDO1能够有效逆转这一现象。不仅如此, CTLA-4、PD-L1和IDO1在肿瘤微环境中关系密切, 呈现互补效果。这也可能是IDO1抑制剂单药使用药效不佳的原因。值得注意的是, 有文献报道相比较ipilimumab, IDO1抑制剂与pembrolizumab的联用既能起到相似作用, 又不会造成不良反应的叠加, 所以具有很大的优势[54]。因此, 目前临床上多作为重要的辅剂来增强免疫治疗效果。
肿瘤免疫治疗通过重新启动肿瘤中的免疫细胞, 恢复机体正常的抗肿瘤免疫反应来抑制肿瘤发展, 是近年来肿瘤领域革命性的突破。但以免疫检查点抑制剂为代表的免疫治疗药物一直存在着响应率低的问题, 很大程度上限制了这一疗法的应用。IDO1抑制剂的发展使得免疫治疗应答率有了大幅度的改善, 助力研究人员在攻克癌症的道路上加速前行。相信随着基础研究的继续深入和临床试验的同步结合, IDO1相关研究成果会为更多的肿瘤患者带来希望。
| [1] | Najfeld V, Menninger J, Muhleman D, et al. Localization of indoleamine 2, 3-dioxygenase gene (INDO) to chromosome 8p12—— > p11 by fluorescent in situ hybridization[J]. Cytogenet Cell Genet, 1993, 64: 231–232. DOI:10.1159/000133584 |
| [2] | Theate I, van Baren N, Pilotte L, et al. Extensive profiling of the expression of the indoleamine 2, 3-dioxygenase 1 protein in normal and tumoral human tissues[J]. Cancer Immunol Res, 2015, 3: 161–172. DOI:10.1158/2326-6066.CIR-14-0137 |
| [3] | Yamamoto S, Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes[J]. J Biol Chem, 1967, 242: 5260–5266. |
| [4] | Boyland E, Williams DC. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder[J]. Biochem J, 1956, 64: 578–582. DOI:10.1042/bj0640578 |
| [5] | Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism[J]. Science, 1998, 281: 1191–1193. DOI:10.1126/science.281.5380.1191 |
| [6] | Munn DH, Shafizadeh E, Attwood JT, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism[J]. J Exp Med, 1999, 189: 1363–1372. DOI:10.1084/jem.189.9.1363 |
| [7] | Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2, 3-dioxygenase[J]. Immunity, 2005, 22: 633–642. DOI:10.1016/j.immuni.2005.03.013 |
| [8] | Mezrich JD, Fechner JH, Zhang XJ, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells[J]. J Immunol, 2010, 185: 3190–3198. DOI:10.4049/jimmunol.0903670 |
| [9] | Berthon C, Fontenay M, Corm S, et al. Metabolites of tryp tophan catabolism are elevated in sera of patients with myelo dysplastic syndromes and inhibit hematopoietic progenitor amplification[J]. Leuk Res, 2013, 37: 573–579. DOI:10.1016/j.leukres.2013.02.001 |
| [10] | Orabona C, Puccetti P, Vacca C, et al. Toward the identifica tion of a tolerogenic signature in IDO-competent dendritic cells[J]. Blood, 2006, 107: 2846–2854. DOI:10.1182/blood-2005-10-4077 |
| [11] | Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells[J]. Cytokine, 2000, 12: 588–594. DOI:10.1006/cyto.1999.0661 |
| [12] | Muller AJ, DuHadaway JB, Donover PS, et al. Inhibition of indoleamine 2, 3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemo therapy[J]. Nat Med, 2005, 11: 312–319. DOI:10.1038/nm1196 |
| [13] | Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, et al. Differential regulation of indoleamine 2, 3-dioxygenase expres sion by nitric oxide and inflammatory mediators in IFN- gamma-activated murine macrophages and microglial cells[J]. J Immunol, 1997, 159: 419–426. |
| [14] | Thomas SR, Salahifar H, Mashima R, et al. Antioxidants inhibit indoleamine 2, 3-dioxygenase in IFN-gamma-activated human macrophages:posttranslational regulation by pyrrolidine dithiocarbamate[J]. J Immunol, 2001, 166: 6332–6340. DOI:10.4049/jimmunol.166.10.6332 |
| [15] | Orabona C, Pallotta MT, Volpi C, et al. SOCS3 drives proteasomal degradation of indoleamine 2, 3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis[J]. Proc Natl Acad Sci U S A, 2008, 105: 20828–20833. DOI:10.1073/pnas.0810278105 |
| [16] | Pallotta MT, Fallarino F, Matino D, et al. AhR-mediated, non-genomic modulation of IDO1 function[J]. Front Immunol, 2014, 5: 497. |
| [17] | Chen JY, Li CF, Kuo CC, et al. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2, 3-dioxygenase promotes breast cancer progression[J]. Breast Cancer Research, 2014, 16: 410. DOI:10.1186/s13058-014-0410-1 |
| [18] | Soliman H, Rawal B, Fulp J, et al. Analysis of indoleamine 2-3 dioxygenase (IDO1) expression in breast cancer tissue by immunohistochemistry[J]. Cancer Immunol Immunother, 2013, 62: 829–837. DOI:10.1007/s00262-013-1393-y |
| [19] | Inaba T, Ino K, Kajiyamaa H, et al. Indoleamine 2, 3-dioxy genase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy[J]. Gynecol Oncol, 2010, 117: 423–428. DOI:10.1016/j.ygyno.2010.02.028 |
| [20] | Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival[J]. Clin Cancer Res, 2012, 18: 6110–6121. DOI:10.1158/1078-0432.CCR-12-2130 |
| [21] | Speeckaert R, Vermaelen K, van Geel N, et al. Indoleamine 2, 3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients[J]. Eur J Cancer, 2012, 48: 2004–2011. DOI:10.1016/j.ejca.2011.09.007 |
| [22] | Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer:effect on tumor-infiltrating T cells[J]. Clin Cancer Res, 2006, 12: 1144–1151. DOI:10.1158/1078-0432.CCR-05-1966 |
| [23] | Munn DH, Mellor AL. Indoleamine 2, 3 dioxygenase and metabolic control of immune responses[J]. Trends Immunol, 2013, 34: 137–143. DOI:10.1016/j.it.2012.10.001 |
| [24] | Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T-regs in the melanoma tumor microenvi ronment is driven by CD8+ T cells[J]. Sci Transl Med, 2013, 5: 200ra116. |
| [25] | Munn DH, Mellor AL. IDO in the tumor microenvironment:inflammation, counter-regulation, and tolerance[J]. Trends Immunol, 2016, 37: 193–207. DOI:10.1016/j.it.2016.01.002 |
| [26] | Sundrud MS, Koralov SB, Feuerer M, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response[J]. Science, 2009, 324: 1334–1338. DOI:10.1126/science.1172638 |
| [27] | Curiel TJ, Coukos G, Zou LH, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival[J]. Nat Med, 2004, 10: 942–949. DOI:10.1038/nm1093 |
| [28] | Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy[J]. Curr Opin Immunol, 2014, 27: 1–7. DOI:10.1016/j.coi.2013.12.005 |
| [29] | Liu Z, Gerner MY, Van Panhuys N, et al. Immune homeostasis enforced by co-localized effector and regulatory T cells[J]. Nature, 2015, 528: 225–230. DOI:10.1038/nature16169 |
| [30] | Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2, 3-dioxygenase[J]. J Clin Invest, 2007, 117: 2570–2582. DOI:10.1172/JCI31911 |
| [31] | Sharma MD, Shinde R, McGaha TL, et al. The PTEN pathway in T-regs is a critical driver of the suppressive tumor microenvironment[J]. Sci Adv, 2015, 1: e1500845. DOI:10.1126/sciadv.1500845 |
| [32] | Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+ CD25+ T regulatory cells[J]. Blood, 2007, 109: 2014–2022. DOI:10.1182/blood-2006-07-035279 |
| [33] | Li Q, Harden JL, Anderson CD, et al. Tolerogenic phenotype of IFN-gamma-Induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop[J]. J Immunol, 2016, 197: 962–970. DOI:10.4049/jimmunol.1502615 |
| [34] | Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2, 3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells[J]. Nat Immunol, 2011, 12: 870–878. DOI:10.1038/ni.2077 |
| [35] | Yue EW, Douty B, Wayland B, et al. Discovery of potent competitive inhibitors of indoleamine 2, 3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model[J]. J Med Chem, 2009, 52: 7364–7367. DOI:10.1021/jm900518f |
| [36] | Liu XD, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity[J]. Blood, 2010, 115: 3520–3530. DOI:10.1182/blood-2009-09-246124 |
| [37] | Beatty GL, O'Dwyer PJ, Clark J, et al. First-in-human phase Ⅰ study of the oral inhibitor of indoleamine 2, 3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies[J]. Clin Cancer Res, 2017, 23: 3269–3276. DOI:10.1158/1078-0432.CCR-16-2272 |
| [38] | Gangadhar TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors:updated phase 1 results from ECHO-202/KEYNOTE-037[J]. Ann Oncol, 2016, 27: 379–400. DOI:10.1093/annonc/mdv617 |
| [39] | Perez RP, Riese MJ, Lewis KD, et al. Epacadostat plus nivolumab in patients with advanced solid tumors:preliminary phase Ⅰ/Ⅱ results of ECHO-204[J]. J Clin Oncol, 2017, 35: 3003. |
| [40] | Hamid O, Gajewski TF, Frankel AE, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma:phase 1 and 2 efficacy and safety results from ECHO-202/KEYNOTE- 037[J]. Ann Oncol, 2017, 28. |
| [41] | Ribas A, Hamid O, Daud A, et al. Association of pembroli zumab with tumor response and survival among patients with advanced melanoma[J]. JAMA, 2016, 315: 1600–1609. DOI:10.1001/jama.2016.4059 |
| [42] | Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase[J]. Nat Med, 2003, 9: 1269–1274. DOI:10.1038/nm934 |
| [43] | Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2, 3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses[J]. Cancer Res, 2007, 67: 792–801. DOI:10.1158/0008-5472.CAN-06-2925 |
| [44] | Metz R, Rust S, DuHadaway JB, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR a novel IDO effector pathway targeted by D-1-methyl-tryptophan[J]. Oncoimmunology, 2012, 1: 1460–1468. DOI:10.4161/onci.21716 |
| [45] | Soliman HH, Antonia S, Sullivan D, et al. Overcoming tumor antigen anergy in human malignancies using the novel indeolamine 2, 3-dioxygenase (IDO) enzyme inhibitor, 1-methyl- D-tryptophan (1MT)[J]. J Clin Oncol, 2009, 15: 3004. |
| [46] | Zakharia Y, Drabick J, Khleif S, et al. Phase Ⅱ trial of theilndoleamine 2, 3-dioxygenase pathway (IDO) inhibitor indoximod plus immune checkpoint inhibitors for the treatment of unresectable stage 3 or 4 melanoma[C]//AACR Annual Meeting. New Orleans: American Association for Cancer Research, 2016, 76: CT087. |
| [47] | Indoximod combo triggers responses in melanoma[J]. Cancer Discov, 2017, 7: 542-543. |
| [48] | Sun JJ, Chen YC, Huang YX, et al. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemothera peutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier[J]. Acta Pharmacol Sin, 2017, 38: 823–834. DOI:10.1038/aps.2017.44 |
| [49] | Nayak A, Hao Z, Sadek R, et al. Phase 1a study of the safety, pharmacokinetics, and pharmacodynamics of GDC-0919 in patients with recurrent/advanced solid tumors[J]. Eur J Cancer, 2015, 51: S69. |
| [50] | Burris HA, Gordon MS, Hellmann MD, et al. A phase Ib dose escalation study of combined inhibition of IDO1(GDC-0919) and PD-L1(atezolizumab) in patients (pts) with locally advanced or metastatic solid tumors[J]. J Clin Oncol, 2017, 35: 105. |
| [51] | Siu LL, Gelmon K, Chu Q, et al. BMS-986205, an optimized indoleamine 2, 3-dioxygenase 1(IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial[C]//AACR Annual Meeting. Washington: American Association for Cancer Research, 2017, 77: CT116. |
| [52] | Tumang J, Gomes B, Wythes M, et al. PF-06840003: a highly selective IDO-1 inhibitor that shows good in vivo efficacy in combination with immune checkpoint inhibitors[C]//AACR Annual Meeting. New Orleans: American Association for Cancer Research, 2016, 76: 4863. |
| [53] | Holmgaard RB, Zamarin D, Munn DH, et al. Indoleamine 2, 3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4[J]. J Exp Med, 2013, 210: 1389–1402. DOI:10.1084/jem.20130066 |
| [54] | Zakharia Y, McWilliams R, Shaheen M, et al. Interim analysis of the phase 2 clinical trial of the IDO pathway inhibitor indoximod in combination with pembrolizumab for patients with advanced melanoma[C]//AACR Annual Meeting. Washington: American Association for Cancer Research, 2017, 77: CT117. |
 2018, Vol. 53
2018, Vol. 53


