癌症是全球第二大死亡原因, 每6人中就有1人死于癌症。2014年世界癌症日前夕, 世界卫生组织(WHO)发表的《2014年世界癌症报告》显示, 2012年全球新增癌症病例数约1 400万, 并有820万人死亡, 预计未来20年, 新病例数将上升57%左右[1]。随着分子靶向疗法的出现, 癌症的治疗已取得了巨大的进步, 如2004年, 美国FDA批准了首个用于非小细胞肺癌(non-small cell lung cancer, NSCLC)的EGFR抑制剂, 约1/4 NSCLC患者能够获得有效治疗[2]。但遗憾的是, 由于肿瘤恶性演进过程中通常会产生异质性, 多数靶向药物因而失效[2, 3]。
针对肿瘤发展的研究大多局限于新生血管生成、细胞间/细胞−细胞基质间黏附作用等方面[4], 关注肿瘤细胞本身恶性化进程潜能的研究较少。以肿瘤转移为例, 即使有部分研究关注到了肿瘤细胞本身的运动能力, 但是大多难以有针对性地开发干预策略。如文献[5]报道CD133、CD87等细胞干性标记物在肺癌中表达增加, 与肿瘤耐药及远端转移等恶性进程密切相关, 但目前缺乏精细靶向肿瘤细胞干性的有效手段。由此可见, 目前对相关分子机制的理解尚比较局限, 这使得探索调控肿瘤恶性演进、转移的新分子机制迫在眉睫。
肿瘤的发生、发展被认为是细胞内基因调控紊乱所致, 近年来, 包括DNA修饰、蛋白翻译后修饰在内的表观遗传学逐渐成为研究热点[6]。常见的蛋白翻译后修饰有磷酸化、乙酰化、甲基化和泛素化[7]。其中组蛋白及非组蛋白乙酰化状态失衡与肿瘤的发生、发展密切相关, 是肿瘤治疗的新靶点[7, 8]。因此, 深入研究组蛋白去乙酰化酶在肿瘤恶性演进特别是浸润、转移过程中的作用, 为新型抗肿瘤药物的研发以及临床恶性肿瘤的治疗提供理论依据, 对于肿瘤的控制与治疗具有重要意义。
1 Sirtuins家族蛋白蛋白质乙酰化是一种转录修饰, 它调控DNA识别、蛋白质相互作用、蛋白质催化活性和稳定性, 因而在细胞凋亡、线粒体生成、脂质代谢、细胞应激、细胞衰老及炎症等生理学功能中发挥重要作用[9, 10]。组蛋白乙酰转移酶(HATs)和组蛋白脱乙酰酶(HDACs)分别催化链中的赖氨酸残基(ε氨基)乙酰化和脱乙酰化[9]。Sir (silent information regulator)基因首先在酵母菌中被发现, 因其与寿命的延长相关而备受关注, 哺乳动物同源基因sirtuins也随之成为研究热点[11]。
1.1 Sirtuins生物化学性质Sirtuins家族蛋白是NAD+依赖酶, 属于Ⅲ类HDACs, 其核心区域由Rossmann折叠组成并高度保守[12]。哺乳动物共编码7种sirtuins, SIRT1~SIRT7, 其亚细胞定位和功能各不相同。SIRT1、SIRT6和SIRT7主要为核蛋白, SIRT3、SIRT4和SIRT5定位于线粒体而SIRT2通常存在于细胞质中[11]。SIRT1~SIRT3具有较强的去乙酰化酶活性, 然而SIRT4~SIRT7则被认为较弱甚至难以检测到去乙酰化酶活性; SIRT4主要有ADP-核糖基转移酶活性[11]。Sirtuins去乙酰化过程分为两步:首先, sirtuins水解NAD+产生NAM, 之后乙酰基从底物蛋白转移到ADP-核糖基产生O-acetyl-ADP和去乙酰化的产物。各家族蛋白的细胞内定位及酶活等如表 1[12]所示。
| Table 1 The location and substrates of sirtuins[12]. NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; HIF1α, hypoxia inducible factor-1; HIF2α, hypoxia inducible factor-2; CTIP2, chicken ovalbumin upstream promoter transcription factor interacting protein 2; Tat, transactivator; LXR, liver X receptor; FXR, farnesoid X receptor; eNOS, endothelial nitric oxide synthase; MEF2, myocyte enhancer factor-2; WRN, werner syndrome protein; NBS1, nijmegen breakage syndrome 1; LKB1, liver kinase B1; hMOF, human ortholog of the Drosophila males-absent-on-the-firs; AceCS, acetyl-CoA synthase; PARP1, poly (ADP-ribose) polymerase 1; PEPCK1, phosphoenolpyruvate carboxykinase; FOX, forkhead box protein; Par-3, protease activated receptor 3; CDK9, cyclin-dependent kinase 9; G6PD, glucose-6-phosphate dehydrogenase; PGAM, phosphoglycerate mutase; ALDH, aldehyde dehydrogenase; HMGCS, 3-hydroxy-3-methylglutaryl CoA synthase; LCAD, long-chain acyl coenzyme A dehydrogenase; SDH, succinate dehydrogenase; SOD, superoxide dismutase; GDH, glutamate dehydrogenase; PDH, pyruvate dehydrogenase; Skp2, S-phase kinase associated protein 2; OGG1, 8-oxoguanine-DNA glycosylase 1; Hsp10, heat shock protein 10; GOT2, glutamate oxaloacetate transaminase 2; MCD, malonyl CoA decarboxylase; PML, peroxiredoxin; VLCAD, very long-chain acyl coenzyme A dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; TNFα, tumor necrosis factor; GCN, general control non-repressed protein; KAP1, KRAB-associated protein 1; PAF53, polymerase-associated factor 53; DNA-PK, DNA-dependent protein kinase; GABPβ1, GA binding protein 1 |
细胞内基因组不稳定将导致致瘤性突变, 促进肿瘤的存活与增殖。进化过程中, 一个高效、紧密的DNA损伤应激系统(DNA damage response, DDR)得以建立起来[13]。Sirtuins家族蛋白因其在调节细胞周期及染色质结构方面的重要作用而在该系统中占据一席之地。
细胞核内, DNA双链断裂(double strand break, DSB)的修复过程中, SIRT1对γ-H2AX焦点的形成和DDR相关蛋白Rad51、NBS1和BRCA1 (breast cancer susceptibility gene 1)在损伤位点的积聚尤为重要[14, 15]。研究发现SIRT1缺失的小鼠胚胎成纤维细胞对这些蛋白的招募能力大大减弱, 导致大量染色体异常, 加速p53+/-小鼠肿瘤生长[16]。DSB修复通常有同源重组(homologous recombination HR)和非同源末端连接(non-homologous end joining, NHEJ)两种方式[17]。Cagnetta等[18]证明SIRT6分别通过对CtIP蛋白去乙酰化、稳定DNA-PK (DNA-dependent protein kinase)增强以上两种修复方式。除此之外, sirtuins家族蛋白也能够通过维尔纳氏综合征蛋白(Werner syndrome protein, WRN)发挥维持基因组稳定性的作用。WRN是一种既可以和DNA结合又可以和多种蛋白质结合的RecQ DNA解旋酶家族蛋白, 在DNA复制、重组、损伤修复、维持端粒稳定性等过程中发挥重要作用。SIRT1通过对其去乙酰化, 调节其从核仁到核质的转运, 来促进同源重组DNA修复[19]。SIRT6与端粒酶相互作用并对组蛋白H3K9去乙酰化, 增强了WRN蛋白对这些区域的作用, 维持染色体端粒结构。实验证明SIRT6敲除的小鼠对DNA损伤更为敏感[20]。另外, Zhang等[21]研究发现, 在G2/M过渡期, 核SIRT2对组蛋白H4K16脱乙酰化, 并直接去乙酰化α微管蛋白, 调控有丝分裂形成正常的单倍体细胞。SIRT7则能够直接去乙酰化并激活p53肿瘤抑制因子, 因此, SIRT7敲除的骨肉瘤对DNA毒性应激的能力明显减弱[22]。
1.2.2 调控能量代谢自“Warburg效应”提出以来, 越来越多的研究表明, 脂质代谢和糖代谢异常是恶性肿瘤的重要特征, 在肿瘤的发生、发展过程具有重要作用[23]。肿瘤组织主要以糖酵解方式进行糖代谢, 并且肿瘤的恶性程度越高、转移能力越强, 糖酵解能力越强[24]。由于sirtuins能够调节脂肪和葡萄糖的新陈代谢, 从而成为能量应激的关键调节因子, 与肿瘤的进程息息相关[23]。
脂质代谢包括脂质酸合成、脂质摄取、脂肪酸氧化、脂质分解及利用。在脂质合成过程中, 肝X受体(liver X receptor, LXR)作为转录因子, 通过调节下游固醇调节元件结合蛋白1c (sterol regulatory element- binding protein 1, SREBP-1c)信号通路, 在多种脂代谢关键酶的表达过程中发挥重要作用[25]。而SIRT1能够对LXR去乙酰化并增强其转录活性, 从而促进脂肪酸合成。SIRT1同样能够使SREBP-1c去乙酰化, 降低其稳定性, 抑制脂肪酸的合成。这两者失去平衡, LXR过度激活, 将促进乳腺癌的生长和转移[23, 25]。在脂质储存过程中, 过氧化物酶体增殖剂激活受体(peroxisome proliferators-activated receptors, PPAR) γ通过调节代谢相关基因的转录, 控制肝脏中的脂肪摄取, Tian等[26]研究证明, PPARγ表达活性异常, 将导致肝癌发生风险升高。在高度分化的脂肪细胞内, SIRT1通过促进该受体下游基因启动子抑制复合物(NCoR1和SMRT)的装配, 抑制热量限制(caloric restriction, CR)条件下脂肪的贮存, 促进脂质代谢[23]。另外, SIRT2也被证明可以通过脱乙酰化来激活FOXO1, 促进FOXO1与PPARγ结合, 抑制其转录活性, 从而促进脂肪的分解[27]。在脂肪酸氧化和脂质利用过程中, 长链脂肪酸首先需要从细胞质转移到线粒体中, 发生β-氧化产生乙酰辅酶A, 乙酰辅酶A再通过TCA循环和氧化磷酸化用以ATP合成。SIRT1通过激活PPARα和PGC1α促进β-氧化, 这一作用对于肝脏的脂肪酸氧化尤为重要, 实验证明, 敲除SIRT1的小鼠易发生肝变性[27]。除了SIRT1, 其他sirtuins家族蛋白也在长链脂肪酸氧化过程中发挥重要作用, 如SIRT3通过去乙酰化激活长链脂酰辅酶A脱氢酶(LCAD)促进氧化过程[28], 而SIRT4则产生负性调控作用[29]。值得一提的是, AMPK作为细胞内的能量传感器, 与SIRT1相互作用, 形成了一条独特的脂质代谢调节回路。在该回路中, AMPK上调NAD+的水平, 同时SIRT1能够激活AMPK上游蛋白[30]。
糖酵解是葡萄糖利用的主要途径, 不仅提供大量的能量, 其代谢产物还通过氨基酸及脂质合成等途径, 为肿瘤快速增殖提供所必需的蛋白质、核酸及脂肪。研究表明, SIRT1通过激活PGC1α参与糖酵解过程, PGC1α激活后减弱了糖酵解基因的转录[31]。除此之外, SIRT1、SIRT3和SIRT6均能够抑制转录因子HIF1α的活性, 从而通过柠檬酸循环抑制葡萄糖氧化[32-34]。其中, SIRT1直接作用于HIF1α使其去乙酰化[34]; 而SIRT3则通过激活超氧化物歧化酶2 (superoxide dismutase 2, SOD2)增加还原型谷胱甘肽, 间接抑制ROS诱导下HIF1α稳定性的增加[33]; SIRT6则以HIF1α辅阻遏物的方式抑制糖酵解[32]。众所周知, 胰岛素的分泌调控着血糖浓度, 因而也与肿瘤的发生、发展密不可分。SIRT1通过对解偶联蛋白2 (mitochondrial uncoupling proteins 2, UCP2)的转录抑制诱导葡萄糖氧化产生ATP, 反馈刺激了胰岛素的分泌[35]。SIRT3和SIRT4主要定位于线粒体, 可作用于谷氨酸脱氢酶, 通过TCA循环和氧化磷酸化产生ATP, 因此SIRT3和SIRT4会影响氨基酸诱导的胰岛素释放通路[27]。SIRT2在糖酵解过程中的作用尚待研究, 目前认为, SIRT2主要通过脱乙酰化来激活磷酸烯醇式丙酮酸羧化(phosphoenolpyruvate carboxykinase, PEPCK)和M2型丙酮酸激酶(pyruvate kinase subtype M2, PKM2), 在葡萄糖缺乏的时候增强糖异生[36, 37]。
1.2.3 调节肿瘤干细胞功能肿瘤干细胞是一群具有自我更新、多向分化、重建肿瘤组织表型能力的细胞。普遍认为, 肿瘤干细胞参与了肿瘤的转移、复发并增加对化疗和放疗耐受。目前对SIRT1在肿瘤干细胞中的作用研究最为广泛。SIRT1在包括神经胶质瘤、结肠癌、白血病、卵巢癌等癌症中高表达, 并被认为是细胞干性所不可或缺的[38-41]。敲除SIRT1后, 干细胞标志物如OCT4、NANOG、TERT均明显下降, 细胞球形成效率也随之降低, 对药物的敏感性则显著增加[39, 41]。研究表明, SIRT1在Nanog+的肝癌干细胞高表达, 但随着分化的进行, SIRT1水平下降, 并通过对SOX2启动子的调控, 维持肝癌干细胞自我更新[42]。除此之外, 研究发现, SIRT1也参与调控了Wnt信号通路。蓬乱蛋白(Dvl)能够将Wnt信号从卷曲蛋白受体(Frizzled)传递到下游组分, SIRT1降低了Wnt拮抗剂SFRP1、SFRP2和DKK1的活性, 从而正向调控这一传递作用[43]。Simmons等[43]发现在乳腺癌细胞中, 抑制SIRT1/2降低了Frizzled7蛋白水平, 同时抑制了β-链蛋白和c-Jun与FZD7启动子结合。除了调控特定的干细胞信号通路, sirtuins也被报道能够直接作用于肿瘤干细胞标志物, 如ALDH1A1是乙醛脱氢酶(ALDH)家族的重要成员, 在多种肿瘤细胞中均可检测到异常表达, 而SIRT2则能够对ALDH1A1进行转录后修饰。乳腺癌细胞内, Notch信号通路诱导SIRT2对ALDH1A1去乙酰化, 导致其乙醛脱氢酶活性增加, 使得肿瘤干细胞增殖[44]。
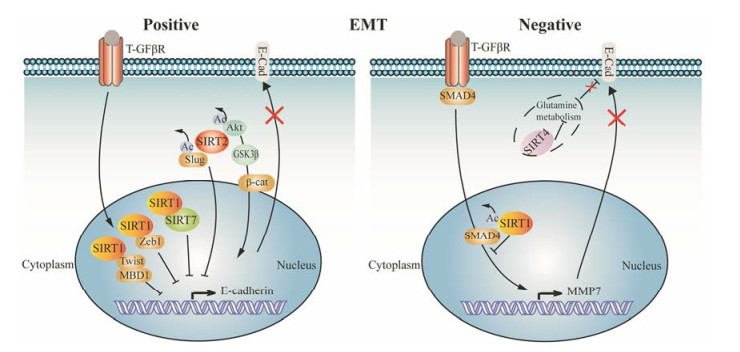
上皮-间质转化(epithelial-mesenchymal transitions, EMT)在胚胎发育以及肿瘤侵袭转移过程中均起重要作用, 且与上皮样细胞的干性获得密切相关[45]。据报道, sirtuins蛋白能够促进或抑制EMT进程[45-48], 如图 1所示。SIRT1被锌指转录因子ZEB1招募至钙黏蛋白启动子, 使组蛋白H3去乙酰化, 阻遏RNA聚合酶Ⅱ的结合, 导致E-钙黏蛋白发生转录抑制[45, 49]。Xu等[48]研究发现在胰腺癌中, SIRT1与Twist及甲基化CpG结合域蛋白1 (methyl-CpG binding domain protein 1, MBD1)相互作用, 从而沉默E-钙黏素。有趣的是, 尽管SIRT2-/-小鼠胚胎成纤维细胞E-钙黏蛋白表达减少, 但SIRT2仍然对EMT具有促进作用。除了对EMT的正向调控, sirtuins家族蛋白中的SIRT4通常被认为具有负性调控作用。由于SIRT4能够抑制谷氨酸脱氢酶的活性, 从而抑制谷氨酰胺代谢, 因此, SIRT4能够间接调控E-钙黏素[47]。另外, 也有研究指出SIRT1具有双重作用, 能够通过对Smad4去乙酰化, 抑制TGF-β信号通路和EMT[46]。除此之外, Geng等[50]研究发现, SIRT6依赖于其组蛋白H3K9去乙酰化活性促进Snail基因表达并抑制TET-1 (ten eleven translocation-1)的转录, 在结肠癌EMT和转移过程中发挥重要作用, 成为治疗结肠癌的潜在靶点。
|
Figure 1 Schematic representation of positive and negative regulation of epithelial-mesenchymal transitions (EMT) by sirtuins. E-cad, E-cadherin; GSK3β, glycogen synthase kinase-3β; MBD1, methyl-CpG binding domain protein-1; MMP7, metalloproteinase 7; TGF-βR, transforming growth factor-β receptor; β-cat, β-catenin |
如上所述, sirtuins家族蛋白在调控细胞蛋白质组中具有重要作用并与肿瘤的发生息息相关, 在生物体的进化过程中逐渐衍生出一系列相关调控网络以调控其表达与活性。
2.1 转录水平p53是目前研究最为充分的SIRT1底物蛋白, 有趣的是, 该蛋白同时也直接作用于SIRT1基因启动子区域, 扮演着转录抑制因子的角色[51]。肿瘤高甲基化-1 (hypermethylated in cancer-1, HIC-1)与CtBP形成复合物, 结合于SIRT启动子区域, 抑制其表达。肿瘤细胞内HIC-1的失活导致SIRT1上调, 从而去乙酰化p53, 使细胞得以逃脱DNA损伤所致的凋亡[51, 52]。Sun等[53]研究发现, 肿瘤微环境中的缺氧条件也能够促进HIC-1/CtBP结合于SIRT1启动子区域, 并阻碍转录因子Sp1的结合, 且该作用依赖于HIC-1的sumo化修饰。除此, 由于SIRT2启动子区域含有进化上高度保守的HIF-1α反应元件, 在缺氧和营养过剩条件下, HIF-1α高表达, 显著降低了SIRT2的蛋白水平, 是饮食诱导肥胖发生的主要原因[51, 54]。
腺苷酸活化蛋白激酶(AMPK)是AMP依赖的丝/苏氨酸蛋白激酶, 作用于包括PGC-1α在内的多种转录调控因子, 以维持细胞能量的供求平衡。研究表明, 依赖于PGC-1α, 在肝细胞中, AMPK持续激活显著增加了SIRT3的mRNA水平[51, 54, 55]。除此之外, AICAR[56]、美拉酮宁[57]、二氢杨梅素[58]均被报道可通过AMPK诱导SIRT3的表达。SIRT5也受到PGC1α和AMPK的调控, 不同的是, 在该调控通路中PGC-1α协同ERRα和PPARα促进SIRT5的表达, 而AMPK则抑制了SIRT5的mRNA水平[59], 目前尚缺乏更深层次的机制研究。
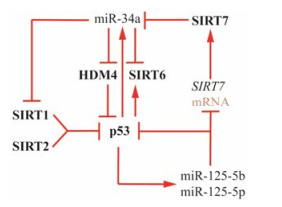
2.2 转录后修饰Sirtuins家族蛋白的转录后修饰主要依赖于microRNA (miRNA), 这是一类长度约为23个核苷酸的非编码单链RNA分子, 能够结合于目标mRNA的3' UTR区, 介导其降解或导致翻译阻滞[60]。随着研究的深入, 有报道指出p53可通过microRNA调控SIRT1, 在该通路中p53不仅能够直接作用于SIRT1基因启动子区域发挥转录抑制作用, 还能够促进miR-34a的表达, 在不增加SIRT1 mRNA降解的情况下, 发挥抑制作用[51, 61]。相似的, SIRT6也受到p53-miR-34a的调控, 在肿瘤细胞内, p53缺失降低了miR-34a的转录水平, 从而导致SIRT6蛋白水平显著增加[62]。除了miR-34a、miR-766和miR-122也相继被报道与SIRT6存在相互作用[51]。SIRT7在肝癌和膀胱癌中的促癌作用也受到miRNAs的调控。miR-34a通过抑制HDM4促进p53的表达, 从而促进了miR- 125a-5p和miR-125-5b的转录, 抑制SIRT7的翻译, 同时SIRT7能够对miR-34a启动子H3K18去乙酰化, 进而发挥表观遗传学调节作用[51, 62-64]。综上所述, 以p53-miR-34a为中心, 构成如图 2所示调控网络[51]。
|
Figure 2 Schematic representation of sirtuins, p53 and miR-34a |
Sirtuins家族蛋白的翻译后修饰对于其活性和稳定性也至关重要。目前, 磷酸化修饰研究最为广泛。细胞周期性依赖激酶cyclin B/Cdk1磷酸化SIRT1的T530和S540位点, 显著提高了蛋白活性, Sasaki等[65]研究指出在基因敲除的小鼠胚胎成纤维细胞中转染野生型SIRT1, 细胞能正常增殖, 而转染T530A和S540A双突变的SIRT1质粒, 则产生明显的增殖抑制。位点T530也能够被c-JNK磷酸化, 增强SIRT1的核转位。JNK1选择性地影响SIRT的功能:增强对组蛋白H3的去乙酰化作用而不影响其对p53的作用。JNK2则磷酸化S27并延长SIRT1的半衰期[51]。SIRT2的磷酸化修饰对其活性的调控较为复杂, 研究指出cyclin B/Cdk1复合物作用于SIRT2的S368位点, E-Cdk2、A-Cdk2作用于S331位点, 抑制SIRT2的酶活, 而细胞外调节蛋白激酶ERK1/2磷酸化SIRT2后则显著增加其去乙酰化活性[51]。
3 Sirtuins激动剂及抑制剂概况由于sirtuins在多种病理生理过程中扮演着重要的角色, 随着其去乙酰化机制和活性位点的揭示, 小分子激动剂和抑制剂的开发逐渐成为研究热点。SIRT1与SIRT2的激动剂和抑制剂最早被发现与合成, 在临床前和临床试验过程中均显示出良好的药效[66, 67]。受此鼓舞, 其他sirtuin家族蛋白相关小分子化合物也逐渐被报道, 以期提高人类的健康水平[68]。
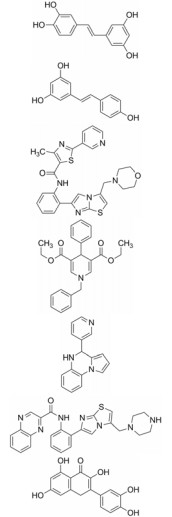
3.1 Sirtuins的激动剂植物提取物中的多酚类化合物如白藜芦醇、紫铆花素、白皮杉醇、异甘草素等被证明能激活SIRT1, 并延长了酿酒酵母的寿命, 其中白藜芦醇能够使SIRT1活性提高10倍以上而得到广泛的研究, 是肿瘤的化学预防剂[68]。随后更多的高效小分子化合物被报道并进入临床前或临床研究阶段, 其中, SRT2104已进入临床Ⅱ期, 有望用于治疗银屑病[69] (表 2)[66, 68, 70-77]。
| Table 2 The table of sirtuins activators |
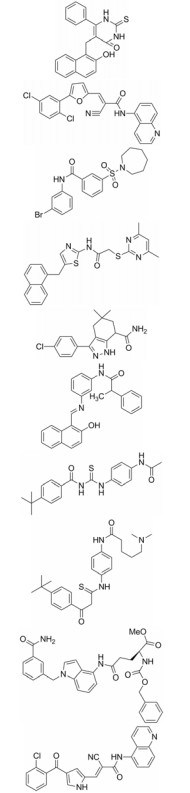
如上所述, 在一些病理学过程如乳腺癌、肝癌的发生发展过程中, sirtuins家族蛋白显著上调, 因此高效的抑制剂也亟待研究[42, 44]。大多数已报道的sirtuin抑制剂(表 3)[66, 68, 78-83]作用于多肽结合位点或NAD+结合口袋发挥竞争性抑制作用, 如cambinol、AK7、SirReal2等[66]。除此之外, selisistat是目前唯一进入临床研究的高效选择性SIRT1抑制剂, 有望用于治疗亨廷顿氏舞蹈病[66, 68]。
| Table 3 The table of sirtuins inhibitors |
SIRT2广泛存在于多种组织和器官中, 在大脑、肌肉、肝脏、胰腺、肾脏等新陈代谢相关器官中高表达。主要为胞浆蛋白, 调控细胞衰老、能量代谢和基因组的稳定性等, 由于其在肿瘤演进过程中具有双重作用而受到越来越多的关注[11, 27]。因此本文进一步对其做重点阐述。
4.1 SIRT2在肿瘤发生中的双重作用目前SIRT2公认的去乙酰化底物蛋白包括histone H4、α-tublin、β-catenin、p53、FOXO1、PEPCK1等, 通过调控这些底物蛋白的去乙酰化从而影响其生物学功能[37, 84]。如有研究指出, SIRT2能通过去乙酰化蛋白CDH1和CDC20, 增加它们与APC/C的结合, 促进有丝分裂过程中APC/C复合物的活性[85]; ANKLE2蛋白的K302位受SIRT2去乙酰化调控后会影响细胞核被膜正常组装, 干扰细胞周期[86]。
越来越多的实验证明, 在肿瘤发生过程中, SIRT2具有双重作用, 即在不同的细胞来源和肿瘤中, 分别扮演着肿瘤抑制因子或促进因子的角色[87]。在神经胶质瘤和黑色素瘤中, SIRT2的表达明显减少[38]。如上所述, SIRT2能够调节APC/C乙酰化水平, 通过此机制, SIRT2敲除的小鼠肿瘤灶点数多于野生型小鼠[38]。而乳腺癌中SIRT2的表达呈时间依赖性增加, 并与Slug相互结合并且使其去乙酰化, 从而能促进Slug发生蛋白酶体降解, 影响乳腺癌的侵袭[88]。
4.2 SIRT2与肿瘤增殖细胞恶性化的核心事件是正常的细胞周期进程被破坏, 使肿瘤细胞顺利通过限制点, 失去分化能力, 发生恶性增殖。有报道指出, SIRT2在皮肤癌中表达较正常组织少。体内实验发现, SIRT2敲除后, 角蛋白19和15上调从而使分化标志物Loricrin的表达下降, 同时干细胞标志物CD34增加, 促进肿瘤增殖[89]。Du等[90]通过免疫组化发现与卵巢上皮细胞相比, 在浆液性卵巢癌(serous ovarian carcinoma, SOC)中, SIRT2的表达明显降低, 导致细胞周期蛋白-依赖激酶4 (cyclin dependent kinase 4, CDK4)表达下降, 细胞增殖加快。Song等[91]通过质谱检测发现KRAS K147位点的乙酰化直接调控其的活性。SIRT2缺失的KrasG12D小鼠肿瘤细胞内KRAS乙酰化水平提高, 增殖加快, 体外克隆形成率增加。Li团队[92]研究发现, SIRT2作用于NF-κB-miR-21信号通路, 使p65 K310位点去乙酰化, 阻止p65结合于miR-21启动子区域, 抑制其转录, 从而抑制肿瘤增殖和克隆形成, 并通过上调caspase 3和Bax蛋白水平诱导细胞凋亡。
4.3 SIRT2与肿瘤耐药化疗是恶性肿瘤治疗的重要手段之一, 然而常常因为肿瘤细胞耐药性的产生而导致治疗失败[93]。实验证明, SIRT2可以通过抑制MAP激酶通路调控肿瘤增殖, SIRT2缺失导致细胞对作用于RTK-RAS/ RAF-MEK-ERK通路的药物耐受[94]。如Xu等[93]研究发现, SIRT2在急性髓细胞白血病(AML)患者中高表达, 而在HL60/A细胞中沉默SIRT2后, 多药耐药性相关蛋白(MRP1)水平下降, 能够促进多柔比星或蒽环类药物-阿糖胞苷(DNR/Ara-C)的积累, 使得细胞大量凋亡。这一作用的产生与磷酸化ERK1/2水平的降低密切相关, 这意味着SIRT2蛋白水平与AML耐药以及ERK1/2信号通路活性成正相关。除此之外, Bajpe等[94]研究发现, 尽管西妥昔单抗能够抑制表皮生长因子受体(EGFR)活性, 但是SIRT2缺失仍会导致下游MEK1乙酰化水平增加, 促使该激酶通路异常激活, 产生显著的耐药性。利用微管抑制剂(microtubule inhibitors, MTIs)如紫杉醇、长春新碱阻断恶性肿瘤细胞有丝分裂进程是肿瘤治疗的另一重要手段, 近年来, SIRT2在该治疗耐药机制中的作用也得到了广泛关注[95, 96]。研究指出, SIRT2下调, 使得细胞核周围乙酰化微管水平的升高, 导致CYLD移位至近核区域, CYLD与Bcl-3相互作用, 显著地延长了细胞周期, 防止细胞发生有丝分裂滑脱, 即防止细胞在没有修复损伤纺锤体的情况下, 突破纺锤体组装检查点进入G1期形成多倍体而凋亡[95]。另外, 在对微管抑制剂如诺考达唑耐受的结肠癌细胞中, SIRT2去乙酰化有丝分裂检查点蛋白BubR的K250位点, 影响BubR的降解, 延长慢性有丝分裂阻滞, 阻止细胞死亡[96]。
4.4 SIRT2与肿瘤转移恶性肿瘤的转移往往是治疗失败的主要原因, 因此, 大量的研究致力于探寻其转移机制, 并开发新的治疗手段[97]。近年来, SIRT2影响肿瘤转移及其机制逐渐成为研究的热点。与其生物学功能相一致, SIRT2主要通过影响细胞代谢和细胞黏附与运动性能调控肿瘤转移过程。有报道[98]指出, 与正常组织细胞相比, HCC细胞内SIRT2表达水平显著升高, 且该水平与患者存活率成负相关。HCC肿瘤发生过程中, 磷酸烯醇式丙酮酸羧化酶1 (PEPCK1)和谷氨酰胺酶(GLS)能够促进葡萄糖和谷氨酰胺的合成代谢, 在许多组织细胞和肿瘤细胞中表达。实验结果表明SIRT2所介导的去乙酰化翻译后修饰能够稳定PEPCK1和GLS的蛋白水平, 促进葡萄糖的利用并抑制钙黏蛋白信号通路, 在HCC细胞转移和入侵过程中具有重要作用。除此之外, 在胰腺癌中高表达的SIRT2还能去乙酰化乳酸脱氢酶LDH-A, 增加其酶活性, 促进乳酸积累[99]。其中, 乳酸已被报道是几种侵袭性肿瘤的主要营养物来源, 对肿瘤细胞生长极为重要[100]。肝癌中, SIRT2调节蛋白质激酶B (p-Akt)的脱乙酰化和活化, 从而影响Akt/GSK3β/β-catenin信号轴来促进肿瘤的迁移侵袭和EMT[101]。研究发现SIRT6与SIRT2具有协同作用, 两者高表达将促进膀胱癌细胞迁移[102]。SIRT1与SIRT2也高度相关, 共沉默SIRT1、SIRT2影响包括CRMP2、stathmin、transglutaminase 2在内的多种转移相关基因的表达[103]。另外, Saxena等[104]提出SIRT2去乙酰化并正向调控鸟嘌呤核苷酸转换因子(TIAM1)和Rac1-GTP的活性, 由于DVL-TIAM1-Rac轴对细胞内信号转导具有分子开关的作用, 激活后将促进T淋巴瘤细胞的运动、侵袭和转移等恶性化过程。
5 总结Sirtuins家族蛋白在正常及病理状态下均具有重要的生物学功能, 与衰老及肿瘤密切相关。随着分子生物学的发展, sirtuins家族逐渐成为疾病预测靶标以及肿瘤治疗靶点。虽然HDAC抑制剂已经上市, 但是同属去乙酰化酶的sirtuins抑制剂/激动剂则仍处于开发阶段, 因此对于每个sirtuins蛋白在某种特定刺激下的作用机制尚需要更全面的研究, 从而使得利用天然或人工合成的小分子干预疾病或肿瘤的进程成为可能。目前研究最为广泛的去乙酰化蛋白为SIRT1, 由于SIRT2与其高度同源, 并且SIRT2在不同肿瘤之间抑癌、促癌的双重性尤为突出, 近年来已成为研究的热点。根据SIRT2的靶蛋白, 深入研究肿瘤生物学功能和机制, 对于肿瘤的靶向治疗具有十分重要的意义。
| [1] | McGuire S. World Cancer Report 2014. Geneva, Switzerland:World Health Organization, International Agency for Research on Cancer, WHO Press, 2015[J]. Adv Nutr, 2016, 7: 418–419. DOI:10.3945/an.116.012211 |
| [2] | Kazandjian D, Blumenthal GM, Yuan WS, et al. FDA approval of gefitinib for the treatment of patients with metastatic EGFR mutation-positive non-small cell lung cancer[J]. Clin Cancer Res, 2016, 22: 1307–1312. DOI:10.1158/1078-0432.CCR-15-2266 |
| [3] | Remon J, Steuer CE, Ramalingam SS, et al. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients[J]. Ann Oncol, 2018, 29: 20–27. DOI:10.1093/annonc/mdx704 |
| [4] | Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis:a com prehensive review[J]. Crit Rev Oncol Hematol, 2013, 87: 1–11. DOI:10.1016/j.critrevonc.2012.12.007 |
| [5] | Kubo T, Takigawa N, Osawa M, et al. Subpopulation of small-cell lung cancer cells expressing CD133 and CD87 show resistance to chemotherapy[J]. Cancer Sci, 2013, 104: 78–84. DOI:10.1111/cas.2013.104.issue-1 |
| [6] | Kanwal R, Gupta K, Gupta S. Cancer epigenetics: an intro duction[M]//Verma M. Cancer Epigenetics. New York: Humana Press, Methods Mol Biol, 2015, 1238: 3-25. |
| [7] | Azevedo C, Saiardi A. Why always lysine? The ongoing tale of one of the most modified amino acids[J]. Adv Biol Regul, 2016, 60: 144–150. DOI:10.1016/j.jbior.2015.09.008 |
| [8] | Hsu CC, Shi JJ, Yuan C, et al. Recognition of histone acetylation by the GAS41 YEATS domain promotes H2A.Z deposition in non-small cell lung cancer[J]. Genes Dev, 2018, 32: 58–69. DOI:10.1101/gad.303784.117 |
| [9] | Schiedel M, Robaa D, Rumpf T, et al. The current state of NAD+-dependent histone deacetylases (sirtuins) as novel therapeutic targets[J]. Med Res Rev, 2018, 38: 147–200. DOI:10.1002/med.2018.38.issue-1 |
| [10] | Mendes KL, Lelis DF, Santos SHS. Nuclear sirtuins and inflammatory signaling pathways[J]. Cytokine Growth Factor Rev, 2017, 38: 98–105. DOI:10.1016/j.cytogfr.2017.11.001 |
| [11] | O'Callaghan C, Vassilopoulos A. Sirtuins at the crossroads of stemness, aging, and cancer[J]. Aging Cell, 2017, 16: 1208–1218. DOI:10.1111/acel.2017.16.issue-6 |
| [12] | Poulose N, Raju R. Sirtuin regulation in aging and injury[J]. Biochim Biophys Acta, 2015, 1852: 2442–2455. DOI:10.1016/j.bbadis.2015.08.017 |
| [13] | Zhang J, Dai Q, Park D, et al. Targeting DNA replication stress for cancer therapy[J]. Genes, 2016, 7: 51. DOI:10.3390/genes7080051 |
| [14] | Langsfeld ES, Bodily JM, Laimins LA. The deacetylase sirtuin 1 regulates human papillomavirus replication by modu lating histone acetylation and recruitment of DNA damage factors NBS1 and Rad51 to viral genomes[J]. PLoS Pathog, 2015, 11: e1005181. DOI:10.1371/journal.ppat.1005181 |
| [15] | Kala R, Shah HN, Martin SL, et al. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer[J]. BMC Cancer, 2015, 15: 672. DOI:10.1186/s12885-015-1693-z |
| [16] | Ong ALC, Ramasamy TS. Role of sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming[J]. Ageing Res Rev, 2018, 43: 64–80. DOI:10.1016/j.arr.2018.02.004 |
| [17] | Liu T, Huang J. DNA end resection:facts and mechanisms[J]. Genomics Proteomics Bioinformatics, 2016, 14: 126–130. DOI:10.1016/j.gpb.2016.05.002 |
| [18] | Cagnetta A, Soncini D, Orecchioni S, et al. Depletion of SIRT6 enzymatic activity increases acute myeloid leukemia cells' vulnerability to DNA-damaging agents[J]. Haema tologica, 2018, 103: 80–90. |
| [19] | Lee SY, Lee H, Kim ES, et al. WRN translocation from nucleolus to nucleoplasm is regulated by SIRT1 and required for DNA repair and the development of chemoresistance[J]. Mutat Res, 2015, 774: 40–48. DOI:10.1016/j.mrfmmm.2015.03.001 |
| [20] | Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin[J]. Nature, 2008, 452: 492–496. DOI:10.1038/nature06736 |
| [21] | Zhang L, Hou XJ, Ma RJ, et al. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis[J]. FASEB J, 2014, 28: 1435–1445. DOI:10.1096/fj.13-244111 |
| [22] | Kiran S, Oddi V, Ramakrishna G. Sirtuin 7 promotes cellular survival following genomic stress by attenuation of DNA damage, SAPK activation and p53 response[J]. Exp Cell Res, 2015, 331: 123–141. DOI:10.1016/j.yexcr.2014.11.001 |
| [23] | Sebastián C, Mostoslavsky R. The role of mammalian sirtuins in cancer metabolism[J]. Semin Cell Dev Biol, 2015, 43: 33–42. DOI:10.1016/j.semcdb.2015.07.008 |
| [24] | Kelly RS, Sinnott JA, Rider JR, et al. The role of tumor metabolism as a driver of prostate cancer progression and lethal disease:results from a nested case-control study[J]. Cancer Metab, 2016, 4: 22. DOI:10.1186/s40170-016-0161-9 |
| [25] | Zhao Y, Li H, Zhang YY, et al. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade[J]. Cancer Res, 2016, 76: 4696–4707. DOI:10.1158/0008-5472.CAN-15-1734 |
| [26] | Tian JW, Hu LP, Li X, et al. microRNA-130b promotes lung cancer progression via PPARγ/VEGF-A/BCL-2-mediated suppression of apoptosis[J]. J Exp Clin Cancer Res, 2016, 35: 105. DOI:10.1186/s13046-016-0382-3 |
| [27] | Mei Z, Zhang X, Yi JR, et al. Sirtuins in metabolism, DNA repair and cancer[J]. J Exp Clin Cancer Res, 2016, 35: 182. DOI:10.1186/s13046-016-0461-5 |
| [28] | Chen TS, Liu JN, Li N, et al. Mouse SIRT3 attenuates hyper trophy-related lipid accumulation in the heart through the deacetylation of LCAD[J]. PLoS One, 2015, 10: e0118909. DOI:10.1371/journal.pone.0118909 |
| [29] | Guo L, Zhou SR, Wei XB, et al. Acetylation of mitochondrial trifunctional protein α-subunit enhances its stability to promote fatty acid oxidation and is decreased in nonalcoholic fatty liver disease[J]. Mol Cell Biol, 2016, 36: 2553–2567. DOI:10.1128/MCB.00227-16 |
| [30] | Ma CH, Chiu YC, Wu CH, et al. Homocysteine causes dysfunction of chondrocytes and oxidative stress through repression of SIRT1/AMPK pathway:a possible link between hyperhomocysteinemia and osteoarthritis[J]. Redox Biol, 2018, 15: 504–512. DOI:10.1016/j.redox.2018.01.010 |
| [31] | Vellinga TT, Borovski T, de Boer VCJ, et al. SIRT1/PGC1α- dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer[J]. Clin Cancer Res, 2015, 21: 2870–2879. DOI:10.1158/1078-0432.CCR-14-2290 |
| [32] | Shun CT, Lin SK, Hong CY, et al. Sirtuin 6 modulates hypoxia-induced autophagy in nasal polyp fibroblasts via inhibition of glycolysis[J]. Am J Rhinol Allergy, 2016, 30: 179–185. DOI:10.2500/ajra.2016.30.4282 |
| [33] | Wei L, Zhou Y, Qiao C, et al. Oroxylin A inhibits glycolysis- dependent proliferation of human breast cancer via promoting SIRT3-mediated SOD2 transcription and HIF1α destabilization[J]. Cell Death Dis, 2015, 6: e1714. DOI:10.1038/cddis.2015.86 |
| [34] | Yu Q, Dong L, Li Y, et al. SIRT1 and HIF1α signaling in metabolism and immune responses[J]. Cancer Lett, 2018, 418: 20–26. DOI:10.1016/j.canlet.2017.12.035 |
| [35] | Bordone L, Motta MC, Picard F, et al. Correction:Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells[J]. PLoS Biol, 2015, 13: e1002346. DOI:10.1371/journal.pbio.1002346 |
| [36] | Park SH, Ozden O, Liu GX, et al. SIRT2-mediated deacetyla tion and tetramerization of pyruvate kinase directs glycolysis and tumor growth[J]. Cancer Res, 2016, 76: 3802–3812. |
| [37] | Jiang WQ, Wang SW, Xiao MT, et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase[J]. Mol Cell, 2011, 43: 33–44. DOI:10.1016/j.molcel.2011.04.028 |
| [38] | Sayd S, Thirant C, El-Habr EA, et al. Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol[J]. Stem Cell Rev, 2014, 10: 103–113. DOI:10.1007/s12015-013-9465-0 |
| [39] | Li L, Bhatia R. Role of SIRT1 in the growth and regulation of normal hematopoietic and leukemia stem cells[J]. Curr Opin Hematol, 2015, 22: 324–329. |
| [40] | Chen XJ, Sun K, Jiao SF, et al. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients[J]. Sci Rep, 2014, 4: 7481. |
| [41] | Qin J, Liu Y, Lu YK, et al. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression[J]. Sci Rep, 2017, 7: 10592. DOI:10.1038/s41598-017-09244-8 |
| [42] | Ou X, Chae HD, Wang RH, et al. SIRT1 deficiency compro mises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse[J]. Blood, 2011, 117: 440–450. DOI:10.1182/blood-2010-03-273011 |
| [43] | Simmons Jr GE, Pandey S, Nedeljkovic-Kurepa A, et al. Frizzled 7 expression is positively regulated by SIRT1 and β-catenin in breast cancer cells[J]. PLoS One, 2014, 9: e98861. DOI:10.1371/journal.pone.0098861 |
| [44] | Zhao D, Mo Y, Li MT, et al. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells[J]. J Clin Invest, 2014, 124: 5453–5465. DOI:10.1172/JCI76611 |
| [45] | Byles V, Zhu L, Lovaas JD, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis[J]. Oncogene, 2012, 31: 4619–4629. DOI:10.1038/onc.2011.612 |
| [46] | Chen IC, Chiang WF, Huang HH, et al. Role of SIRT1 in regulation of epithelial-to-mesenchymal transition in oral squamous cell carcinoma metastasis[J]. Mol Cancer, 2014, 13: 254. DOI:10.1186/1476-4598-13-254 |
| [47] | Haley JA, Haughney E, Ullman E, et al. Altered transcrip tional control networks with trans-differentiation of isogenic mutant-KRas NSCLC models[J]. Front Oncol, 2014, 4: 344. |
| [48] | Xu J, Zhu W, Xu W, et al. Up-regulation of MBD1 promotes pancreatic cancer cell epithelial-mesenchymal transition and invasion by epigenetic down-regulation of E-cadherin[J]. Curr Mol Med, 2013, 13: 387–400. |
| [49] | Ray U, Chowdhury SR, Roy SS. Lysophosphatidic acid promotes epithelial to mesenchymal transition in ovarian cancer cells by repressing SIRT1[J]. Cell Physiol Biochem, 2017, 41: 795–805. DOI:10.1159/000458744 |
| [50] | Geng CH, Zhang CL, Zhang JY, et al. Overexpression of Sirt6 is a novel biomarker of malignant human colon carcinoma[J]. J Cell Biochem, 2018, 119: 3957–3967. DOI:10.1002/jcb.v119.5 |
| [51] | Buler M, Andersson U, Hakkola J. Who watches the watchmen? Regulation of the expression and activity of sirtuins[J]. FASEB J, 2016, 30: 3942–3960. DOI:10.1096/fj.201600410RR |
| [52] | Kwon HS, Ott M. The ups and downs of SIRT1[J]. Trends Biochem Sci, 2008, 33: 517–525. DOI:10.1016/j.tibs.2008.08.001 |
| [53] | Sun L, Li H, Chen J, et al. A SUMOylation-dependent pathway regulates SIRT1 transcription and lung cancer metastasis[J]. J Natl Cancer Inst, 2013, 105: 887–898. DOI:10.1093/jnci/djt118 |
| [54] | Salminen A, Kaarniranta K, Kauppinen A. AMPK and HIF signaling pathways regulate both longevity and cancer growth:the good news and the bad news about survival mechanisms[J]. Biogerontology, 2016, 17: 655–680. DOI:10.1007/s10522-016-9655-7 |
| [55] | Zhang X, Ren X, Zhang Q, et al. PGC-1α/ERRα-Sirt3 pathway regulates DAergic neuronal death by directly deacetylating SOD2 and ATP synthase β[J]. Antioxid Redox Signal, 2016, 24: 312–328. DOI:10.1089/ars.2015.6403 |
| [56] | Brandauer J, Andersen MA, Kellezi H, et al. AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD[J]. Front Physiol, 2015, 6: 85. |
| [57] | Chen Y, Qing W, Sun M, et al. Melatonin protects hepato cytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway[J]. Free Radic Res, 2015, 49: 1275–1284. DOI:10.3109/10715762.2015.1067806 |
| [58] | Shi LY, Zhang T, Zhou Y, et al. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPK-PGC-1α-Sirt3 signaling pathway[J]. Endocrine, 2015, 50: 378–389. DOI:10.1007/s12020-015-0599-5 |
| [59] | Buler M, Aatsinki SM, Izzi V, et al. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism[J]. FASEB J, 2014, 28: 3225–3237. DOI:10.1096/fj.13-245241 |
| [60] | Xue M, Li Y, Hu F, et al. High glucose up-regulates microRNA-34a-5p to aggravate fibrosis by targeting SIRT1 in HK-2 cells[J]. Biochem Biophys Res Commun, 2018, 498: 38–44. DOI:10.1016/j.bbrc.2017.12.048 |
| [61] | Reynolds RH, Petersen MH, Willert CW, et al. Perturbations in the p53/miR-34a/SIRT1 pathway in the R6/2 Huntington's disease model[J]. Mol Cell Neurosci, 2018, 88: 118–129. DOI:10.1016/j.mcn.2017.12.009 |
| [62] | Lefort K, Brooks Y, Ostano P, et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network[J]. EMBO J, 2013, 32: 2248–2263. DOI:10.1038/emboj.2013.156 |
| [63] | Kim JK, Noh JH, Jung KH, et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b[J]. Hepatology, 2013, 57: 1055–1067. DOI:10.1002/hep.26101 |
| [64] | Zhang S, Chen P, Huang ZA, et al. Sirt7 promotes gastric cancer growth and inhibits apoptosis by epigenetically inhibiting miR-34a[J]. Sci Rep, 2015, 5: 9787. DOI:10.1038/srep09787 |
| [65] | Sasaki T, Maier B, Koclega KD, et al. Phosphorylation regulates SIRT1 function[J]. PLoS One, 2008, 3: e4020. DOI:10.1371/journal.pone.0004020 |
| [66] | Zhou Z, Ma T, Zhu Q, et al. Recent advances in inhibitors of sirtuin1/2:an update and perspective[J]. Future Med Chem, 2018, 10: 907–934. DOI:10.4155/fmc-2017-0207 |
| [67] | Zhou YM, Cui HQ, Yu XM, et al. Synthesis of benzimidazole and benzothiazole derivatives as a sirtuins 2 inhibitor[J]. Acta Pharm Sin (药学学报), 2017, 52: 773–778. |
| [68] | Dai H, Sinclair DA, Ellis JL, et al. Sirtuin activators and inhibitors:promises, achievements, and challenges[J]. Phar macol Ther, 2018. DOI:10.1016/j.pharmthera.2018.03.004 |
| [69] | Krueger JG, Suárez-Fari as M, Cueto I, et al. A randomized, placebo-controlled study of SRT2104, a SIRT1 activator, in patients with moderate to severe psoriasis[J]. PLoS One, 2015, 10: e0142081. DOI:10.1371/journal.pone.0142081 |
| [70] | Lewandowska H, Kalinowska M, Lewandowski W, et al. The role of natural polyphenols in cell signaling and cytoprotection against cancer development[J]. J Nutr Biochem, 2016, 32: 1–19. DOI:10.1016/j.jnutbio.2015.11.006 |
| [71] | Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol[J]. Biochim Biophys Acta, 2015, 1852: 1209–1218. DOI:10.1016/j.bbadis.2015.01.012 |
| [72] | Ajami M, Pazoki-Toroudi H, Amani H, et al. Therapeutic role of sirtuins in neurodegenerative disease and their modula tion by polyphenols[J]. Neurosci Biobehav Rev, 2017, 73: 39–47. DOI:10.1016/j.neubiorev.2016.11.022 |
| [73] | Naia L, Rego AC. Sirtuins:double players in Huntington's disease[J]. Biochim Biophys Acta, 2015, 1852: 2183–2194. DOI:10.1016/j.bbadis.2015.07.003 |
| [74] | Zamora M, Pardo R, Villena JA. Pharmacological induction of mitochondrial biogenesis as a therapeutic strategy for the treatment of type 2 diabetes[J]. Biochem Pharmacol, 2015, 98: 16–28. DOI:10.1016/j.bcp.2015.06.032 |
| [75] | El-Hattab AW, Zarante AM, Almannai M, et al. Therapies for mitochondrial diseases and current clinical trials[J]. Mol Genet Metab, 2017, 122: 1–9. |
| [76] | Datar PA, Auti PB. Design and synthesis of novel 4-substituted 1, 4-dihydropyridine derivatives as hypotensive agents[J]. J Sadui Chem Soc, 2016, 20: 510–516. DOI:10.1016/j.jscs.2012.08.003 |
| [77] | Klusa V. A typical 1, 4-dihydropyridine derivatives, an approach to neuroprotection and memory enhancement[J]. Pharmacol Res, 2016, 113: 754–759. DOI:10.1016/j.phrs.2016.05.017 |
| [78] | Lugrin J, Ciarlo E, Santos A, et al. The sirtuin inhibitor cambinol impairs MAPK signaling, inhibits inflammatory and innate immune responses and protects from septic shock[J]. Biochim Biophys Acta, 2013, 1833: 1498–1510. DOI:10.1016/j.bbamcr.2013.03.004 |
| [79] | Yar Saglam AS, Yilmaz A, Onen HI, et al. HDAC inhibitors, MS-275 and salermide, potentiates the anticancer effect of EF24 in human pancreatic cancer cells[J]. EXCLI J, 2016, 15: 246–255. |
| [80] | Chen L, Ahmad N, Liu XQ. Combining p53 stabilizers with metformin induces synergistic apoptosis through regulation of energy metabolism in castration-resistant prostate cancer[J]. Cell Cycle, 2016, 15: 840–849. DOI:10.1080/15384101.2016.1151582 |
| [81] | Jin YL, Cao Q, Chen C, et al. Tenovin-6-mediated inhibition of SIRT1/2 induces apoptosis in acute lymphoblastic leukemia (ALL) cells and eliminates ALL stem/progenitor cells[J]. BMC Cancer, 2015, 15: 226. DOI:10.1186/s12885-015-1282-1 |
| [82] | Alhazzazi TY, Kamarajan P, Xu YL, et al. A novel sirtuin-3 inhibitor, LC-0296, inhibits cell survival and proliferation, and promotes apoptosis of head and neck cancer cells[J]. Anti cancer Res, 2016, 36: 49–60. |
| [83] | Carafa V, Nebbioso A, Cuomo F, et al. RIP1-HAT1-SIRT complex identification and targeting in treatment and preven tion of cancer[J]. Clin Cancer Res, 2018, 24: 2886–2990. DOI:10.1158/1078-0432.CCR-17-3081 |
| [84] | Harting K, Kn ll B. SIRT2-mediated protein deacetylation:an emerging key regulator in brain physiology and pathology[J]. Eur J Cell Biol, 2010, 89: 262–269. DOI:10.1016/j.ejcb.2009.11.006 |
| [85] | Kim HS, Vassilopoulos A, Wang RH, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regu lating APC/C activity[J]. Cancer Cell, 2011, 20: 487–499. DOI:10.1016/j.ccr.2011.09.004 |
| [86] | Kaufmann T, Kukolj E, Brachner A, et al. SIRT2 regulates nuclear envelope reassembly through ANKLE2 deacetylation[J]. J Cell Sci, 2016, 129: 4607–4621. DOI:10.1242/jcs.192633 |
| [87] | Bosch-Presegué L, Vaquero A. The dual role of sirtuins in cancer[J]. Genes Cancer, 2011, 2: 648–662. DOI:10.1177/1947601911417862 |
| [88] | Zhou WH, Ni TK, Wronski A, et al. The SIRT2 deacetylase stabilizes slug to control malignancy of basal-like breast cancer[J]. Cell Rep, 2016, 17: 1302–1317. DOI:10.1016/j.celrep.2016.10.006 |
| [89] | Ming M, Qiang L, Zhao BZ, et al. Mammalian SIRT2 inhibits keratin 19 expression and is a tumor suppressor in skin[J]. Exp Dermatol, 2014, 23: 207–209. DOI:10.1111/exd.12323 |
| [90] | Du YH, Wu J, Zhang HY, et al. Reduced expression of SIRT2 in serous ovarian carcinoma promotes cell proliferation through disinhibition of CDK4 expression[J]. Mol Med Rep, 2017, 15: 1638–1646. DOI:10.3892/mmr.2017.6183 |
| [91] | Song HY, Biancucci M, Kang HJ, et al. SIRT2 deletion enhances KRAS-induced tumorigenesis in vivo by regulating K147 acetylation status[J]. Oncotarget, 2016, 7: 80336–80349. |
| [92] | Li YN, Dai DW, Lu Q, et al. Sirt2 suppresses glioma cell growth through targeting NF-κB-miR-21 axis[J]. Biochem Biophys Res Commun, 2013, 441: 661–667. DOI:10.1016/j.bbrc.2013.10.077 |
| [93] | Xu H, Li YY, Chen L, et al. SIRT2 mediates multidrug resistance in acute myelogenous leukemia cells via ERK1/2 signaling pathway[J]. Int J Oncol, 2016, 48: 613–623. DOI:10.3892/ijo.2015.3275 |
| [94] | Bajpe PK, Prahallad A, Horlings H, et al. A chromatin modifier genetic screen identifies SIRT2 as a modulator of response to targeted therapies through the regulation of MEK kinase activity[J]. Oncogene, 2015, 34: 531–536. DOI:10.1038/onc.2013.588 |
| [95] | Inoue T, Nakayama Y, Yamada H, et al. SIRT2 downregula tion confers resistance to microtubule inhibitors by prolonging chronic mitotic arrest[J]. Cell Cycle, 2009, 8: 1279–1291. DOI:10.4161/cc.8.8.8245 |
| [96] | Suematsu T, Li YZ, Kojima H, et al. Deacetylation of the mitotic checkpoint protein BubR1 at lysine 250 by SIRT2 and subsequent effects on BubR1 degradation during the prometaphase/anaphase transition[J]. Biochem Biophys Res Commun, 2014, 453: 588–594. DOI:10.1016/j.bbrc.2014.09.128 |
| [97] | Zenitani M, Nojiri T, Hosoda H, et al. Chemotherapy can promote liver metastasis by enhancing metastatic niche forma tion in mice[J]. J Surg Res, 2018, 224: 50–57. DOI:10.1016/j.jss.2017.11.050 |
| [98] | Huang S, Zhao ZG, Tang DH, et al. Downregulation of SIRT2 inhibits invasion of hepatocellular carcinoma by inhibiting energy metabolism[J]. Transl Oncol, 2017, 10: 917–927. DOI:10.1016/j.tranon.2017.09.006 |
| [99] | Zhao D, Zou SW, Liu Y, et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer[J]. Cancer Cell, 2013, 23: 464–476. DOI:10.1016/j.ccr.2013.02.005 |
| [100] | Kim SY. Cancer energy metabolism:shutting power off cancer factory[J]. Biomol Ther, 2018, 26: 39–44. DOI:10.4062/biomolther.2017.184 |
| [101] | Chen J, Chan AWH, To KF, et al. SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling[J]. Hepatology, 2013, 57: 2287–2298. DOI:10.1002/hep.26278 |
| [102] | Zuo QQ, Wu WJ, Li X, et al. HDAC6 and SIRT2 promote bladder cancer cell migration and invasion by targeting cortactin[J]. Oncol Rep, 2012, 27: 819–824. |
| [103] | Wilking-Busch MJ, Ndiaye MA, Liu XQ, et al. RNA interference-mediated knockdown of SIRT1 and/or SIRT2 in melanoma:identification of downstream targets by large-scale proteomics analysis[J]. J Proteomics, 2018, 170: 99–109. DOI:10.1016/j.jprot.2017.09.002 |
| [104] | Saxena M, Dykes SS, Malyarchuk S, et al. The sirtuins promote dishevelled-1 scaffolding of TIAM1, Rac activation and cell migration[J]. Oncogene, 2015, 34: 188–198. DOI:10.1038/onc.2013.549 |
 2018, Vol. 53
2018, Vol. 53