阿尔茨海默病是以记忆、认知功能障碍和行为改变为主要特征的神经退行性疾病, 发病通常具有年龄相关性。根据世界阿尔茨海默病报告报道, 2016年全球有近5 000万AD患者, 预计到2050年, 这一数字将超过1.31亿[1]。到目前为止, 尚无有效的AD治疗策略, 相关药物研发也未取得突破性进展。随着人口老龄化加剧, 日益增长的AD患者人群将为其家庭和社会带来沉重的负担。因此, 深入探究AD的发病机制, 发掘潜在的药物治疗靶点具有重要意义。AD主要病理特征包括细胞外的β淀粉样蛋白(β-amyloid protein, Aβ)沉积形成老年斑, 细胞内高度磷酸化的tau蛋白积聚形成的神经纤维缠结等[2]。其中, 线粒体功能紊乱参与了AD早期的发病过程。
电压依赖性阴离子通道1 (voltage-dependent anion channel 1, VDAC1)主要位于线粒体外膜, 细胞膜也有一定的分布[3], 可转运多种离子、活性物质和代谢物进出线粒体, 在线粒体介导的细胞凋亡过程中发挥了重要作用。VDAC1还可与来自胞浆、内质网和线粒体的多种具有不同功能的蛋白质产生相互作用, 共同参与细胞代谢、信号转导和凋亡等过程, 被认为是一种具有多种功能的线粒体中枢蛋白[4]。研究表明, VDAC1在AD进程中发生了表达的改变, 与多种疾病相关蛋白存在相互作用, 被认为可能参与了AD的发病过程。
1 VDACs特征、分类和功能VDACs是线粒体外膜的通道蛋白, 分子质量约30 kDa。同时, 与线粒体内膜中的腺嘌呤核苷酸转运体(adenine nucleotide translocator, ANT)和基质中的亲环素D (cyclophilin D, CypD)共同组成线粒体渗透性转换孔(mitochondrial permeability transition pore, mPTP)。不同的状态下, VDACs的离子选择性不同:开放状态下, 倾向于允许阴离子通过(每通过2个Cl-通过1个K+), 电导约为4 nS (1 mol·L-1 NaCl或KCl); 关闭状态时, 则优先降低阴离子渗透性(Cl-的渗透性降低70%~90%, 而K+渗透性降低25%~50%, 数值与VDACs来源、测试条件等因素相关)[5, 6]。高等生物VDACs包括VDAC1、VDAC2和VDAC3三种亚型, 三者的翻译后修饰有所不同, 导致分子质量和电泳迁移速率等存在差异。三种亚型在大多数组织中均有表达, 其中VDAC1的表达具有明显优势, 而VDAC2和VDAC3的表达水平普遍较低[7, 8]。但在哺乳动物睾丸和精子中, 以VDAC2和VDAC3表达为主, 而VDAC1主要存在于支撑细胞中[9, 10]。
VDAC1功能包括:转运离子、ATP和代谢物等进出线粒体; 其发生表达、活性变化, 与凋亡相关蛋白相互作用等, 影响细胞的生存和死亡; 通过与能量代谢、信号转导、抗氧化等不同功能的蛋白质或分子相互作用, 参与能量供给, 维持Ca2+动态平衡, 氧化应激保护以及多种疾病发病和药物调控过程, 在维持线粒体功能和细胞活性方面发挥重要作用[7]。
此外, 电生理研究发现细胞膜也存在VDAC (plasmalemmal VDAC, pl-VDAC), 在不同细胞显示出VDAC样电压敏感性、单通道电导和离子选择性[11]。在海马神经元研究表明, 通常情况下, pl-VDAC呈关闭状态, 在处于凋亡状态时通道开放, 并起到促凋亡作用[12]。现阶段, pl-VDAC的具体生理功能仍不明确, 认为其可能参与了氯离子通道的组成, 与细胞ATP释放, 细胞体积调节和NADH-铁氰化物氧化还原等过程相关[3]。
2 VDAC1的结构分析VDAC1为19个跨膜β折叠组成的桶状结构, 通道孔径约为3~3.8 nm[13], N末端位于桶状结构内侧, 为α-螺旋结构(N-terminal α-helix, N-α), 该结构域可根据通道状态进行移位, 并可将通道孔径缩窄至约1.4 nm[14]。N-α结构缺失导致桶状结构扭曲, 并改变阴、阳离子的选择性[15]。小鼠VDAC1的结构分析结果表明, N-α结构具有阴离子选择性, 其中, 12位赖氨酸(K12)、15位精氨酸(R15)和20位赖氨酸(K20)组成的KRK片段被认为是ATP结合位点, ATP与K20发生相互作用[16]。VDAC1中N端结构域与β折叠连接处的富甘氨酸残基(21GYGFG25序列)[7], 也是重要的结构原件, 参与通道门控、二聚体形成, 且可与胆固醇、凋亡相关调节蛋白如Bcl-2家族、己糖激酶(hexokinase, HK)发生相互作用[17, 18]。此外, 73位谷氨酸(E73)侧链指向VDAC1结构中膜的中间平面, 在小鼠VDAC1中, 该位点突变可抑制HK介导的抗凋亡保护作用[19, 20], 在人VDAC1中, 认为该位点与Ca2+结合、HK调节及VDAC1寡聚反应有关[21-24]。VDAC1的电压依赖构象动力学表明, VDAC1通道调控和关闭机制涉及桶状结构扭曲、N-α氨基酸的置换以及E73与膜和离子的相互作用[19]。
生理状态下, 线粒体膜上VDAC1单体与二聚体处于动态平衡; 当处于凋亡过程时, VDAC1二聚体发生构象变化并组成寡聚体, 从而形成更大的孔道以介导细胞色素C (cytochrome C, Cyt C)等大分子物质释放[7, 25]。研究表明, 线粒体膜上的特殊脂质组成和细胞凋亡诱导剂均可促进VDAC1由单体向寡聚体形式转变[26, 27]。
3 VDAC1与细胞凋亡 3.1 线粒体与细胞凋亡细胞凋亡激活途径包括外源性和内源性两种途径。外源性途径由肿瘤坏死因子等配体与死亡受体结合激活含半胱氨酸的天冬氨酸蛋白水解酶(caspase), 主要为caspase-8, 继而caspase-3前体蛋白分解产生活性的caspase-3, 诱导细胞发生凋亡[4];内源性途径又称线粒体介导的细胞凋亡途径, 由氧化应激、Ca2+超载、DNA损伤等因素引发线粒体结构改变, 导致线粒体内的Cyt C释放到细胞质中, 并与凋亡蛋白酶激活因子-1结合, 形成多聚体, 激活caspase-9, 进而激活caspase-3等其他caspase, 导致凋亡发生[28]。研究表明, 以上两种途径均可通过促凋亡蛋白Bid裂解产生tBid (truncated Bid), 后者转位到线粒体, 引发Cyt C释放。因此, 实际内、外源两种细胞凋亡途径均有线粒体的参与[29]。
3.2 VDAC1参与线粒体介导的细胞凋亡过程由于线粒体凋亡蛋白均位于线粒体膜间隙, 因此只有提高线粒体外模渗透性才能使其释放。虽然促凋亡因子从线粒体释放到胞质的具体过程尚不明确, 但很多证据表明VDAC1在细胞凋亡过程中发挥了重要作用:如VDAC1抗体可减少Cyt C释放, 从而抑制细胞的凋亡过程[30];下调VDAC1的表达可对抗顺铂诱导的细胞凋亡[31];一些对VDAC1具有抑制作用的化合物, 如钌红(ruthenium red, RuR)、4, 4'-二异硫氰基二苯乙烯-2, 2'-二磺酸(4, 4'-diisothiocyanatostilbene- 2, 2'-disulfonic acid, DIDS)等, 同样可以对抗不同诱导因素导致的细胞凋亡[32, 33]。而过表达VDAC1则可诱导细胞产生凋亡[32, 34]。
3.3 VDAC1自身调节对凋亡相关蛋白的影响现阶段认为VDAC1参与细胞凋亡可能的模式有:由于VDAC1长期处于关闭状态而导致线粒体肿胀、线粒体外膜破裂和Cyt C释放[4]; VDAC1为mPTP的组成成分之一, mPTP为高传导性非特异性孔, 可由活性氧自由基(reactive oxygen species, ROS)和Ca2+等多种因子激活, 导致细胞内Cyt C释放[35]; VDAC1可与促凋亡蛋白(Bax/Bak)发生相互作用, 构成Cyt C释放通道[36]; VDAC1发生构象变化和寡聚化, 形成Cyt C释放通道, 引发细胞凋亡[37, 38]。此外, VDAC1与抗凋亡蛋白(如Bcl-2、Bcl-xL和HK)发生相互作用, 可对抗细胞凋亡过程[37] (图 1)。

|
Figure 1 Different models proposed for voltage-dependent anion channel 1 (VDAC1) function in apoptosis and inhibition of apoptosis. These models include: outer mitochondrial membrane rupture caused by VDAC1 closure (A); mitochondrial permeability transition pore (mPTP), composed of VDAC1, adenine nucleotide translocase (ANT) and cyclophilin D, allows apoptogenic protein release (B); VDAC1 oligomer as a channel for the apoptotic proteins release (C); oligomeric pro-apoptotic proteins (Bax/Bak) and VDAC1 as a channel for the apoptotic proteins release (D); anti-apoptotic proteins, hexokinase or Bcl-2 interact with VDAC1 to prevent cytochrome C release (E) |
肿瘤细胞代谢旺盛, 由于VDAC1具有调节线粒体能量代谢的能力, 可与糖酵解的关键酶HK及多种代谢相关蛋白形成复合体, 在肿瘤细胞中表达上调[39]。同时, 肿瘤细胞中线粒体结合HK的水平升高[40], 可抑制经VDAC1作用的线粒体介导的细胞凋亡过程[32], 结合VDAC1在细胞凋亡中的重要作用, 因此, 其有望成为抗肿瘤药物研发的潜在靶点。
4 VDAC1与AD等神经退行性疾病 4.1 线粒体功能障碍与AD神经退行性疾病包括AD、帕金森病(Parkinson’s disease, PD)和亨廷顿舞蹈病(Huntington's disease, HD)等, 普遍存在线粒体功能受损, 线粒体介导的细胞凋亡过程[41, 42]。线粒体功能障碍是AD发病机制中的早期事件, 表现为代谢降低、Ca2+稳态被破坏、自由基产生增加和脂质过氧化反应等[43]。研究发现, AD患者和AD转基因动物模型中存在线粒体转录、翻译、相关蛋白表达障碍, 电子传递和线粒体动力学等被损坏。线粒体出现明显的能量代谢障碍, 如ATP水平、呼吸链氧化酶系活性降低。此外, ROS生成增加, 线粒体DNA损伤, 线粒体膜流动性降低[44-47]。Aβ可定位于线粒体并发生聚集, 与线粒体蛋白发生相互作用, 可能通过线粒体结合内质网膜(mitochondria-associated endoplasmic reticulum membranes, MAM)线粒体外膜移位酶(translocase of the outer mitochondrial membrane, TOM)复合体进入线粒体[44], 影响线粒体的结构和功能, 诱导mPTP形成, 降低线粒体膜电位[48], 激活凋亡诱导因子、Cyt C和caspase前体蛋白释放到胞浆, 启动细胞凋亡通道[49, 50]。而在线粒体功能耗竭的细胞中, Aβ不会引起毒性[51]。此外, 磷酸化的tau也可导致如能量代谢降低、氧化损伤等线粒体功能障碍[52]。
4.2 VDAC1与AD的相关性已证实VDAC1在AD的发生、发展过程中发生了表达的变化, 在AD患者和转基因小鼠脑组织均可检测出VDAC1表达随病情发展程度的加重而逐渐升高。VDAC1与APP、Aβ和磷酸化tau蛋白均存在相互作用, 可能堵塞线粒体孔道, 导致线粒体功能发生紊乱[53]。VDAC1参与了Aβ诱导的毒性反应过程, 采用VDAC抗体与不同神经细胞预孵育, 可对抗Aβ引发的细胞凋亡[54]。采用小干扰RNA (small interfering RNA, siRNA)下调SH-SY5Y细胞中VDAC1表达, 抑制了Aβ的细胞渗透和毒性, 表明VDAC1是Aβ进入细胞介导细胞凋亡所必需的[55]。在APP稳转的HEK293细胞上的研究发现, 使用siRNA下调VDAC1表达可减少Aβ的生成, 而过表达VDAC1并未导致Aβ的水平升高[56]。
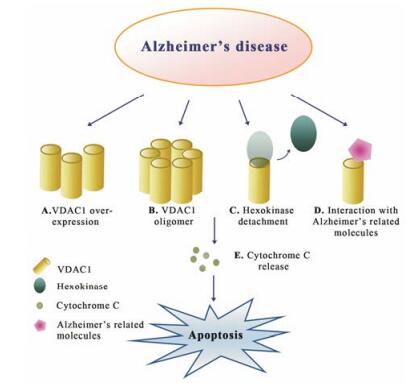
对于Aβ与VDAC1的相互作用机制, 有研究证明二者为直接相互作用, Aβ通过与VDAC1的N末端富甘氨酸结构序列结合, 导致线粒体结合的HK分离, VDAC1发生寡聚化, Cyt C释放, 从而引发细胞凋亡[55] (图 2)。在AD患者皮层组织制备的脂笩模型中, 研究者认为, 一方面, VDAC1与Aβ相互作用, 在脂筏中形成毒性多聚物, 并与γ-分泌酶存在相关性, 影响APP剪切过程; 另一方面, Aβ通过诱导VDAC1酪氨酸残基去磷酸化引发细胞凋亡, 采用酪氨酸蛋白激酶抑制剂降低VDAC1酪氨酸去磷酸化水平可减弱Aβ的毒性作用[56, 57]。
|
Figure 2 Different changes proposed for VDAC1 function in Alzheimer's disease. These changes include increasing in VDAC1 expression (A); oligomeric VDAC1 as a channel for the apoptotic proteins release (B); Alzheimer's related molecules induce hexokinase detachment and interact with VDAC1 (C and D); these changes of VDAC1 lead to cytochrome C release and cell death (E) |
在AD进程中, VDAC1还存在翻译后修饰过程, 如糖原合成酶激酶3β (glycogen syntheses kinase 3β, GSK3β)可诱导VDAC1磷酸化, 引发线粒体结合的HK分离[58]。在AD模型动物Tg2576小鼠海马组织中检测到GSK3β活性增强, 使得磷酸化VDAC1水平升高, 线粒体的HK水平下降[59]。AD患者海马组织中VDAC1硝化水平增加, 可能参与了mPTP的功能改变和线粒体的氧化损伤[60]。此外, VDAC1在AD患者和相关转基因动物营养不良神经突的Aβ斑块处大量沉积, 可能对细胞能量和代谢稳态产生影响[61]。质膜中的VDAC也参与了维持氧化还原稳态和调节细胞凋亡的过程, VDAC可在质膜小窝样结构域中富集, 与雌激素受体α发生相互作用, 可能参与了雌激素激活MAPK信号通路发挥对抗Aβ毒性的神经保护作用的过程[54]。本实验室也在近期研究中证明, APP/PS1小鼠脑中VDAC1表达显著增加, 并进一步发现AD患者血清中VDAC1表达明显升高, 且与患者的认知能力(mini-mental state examination, MMSE评分)呈负相关[62]。以上结果均表明, VDAC1可能成为AD诊断的生物标志物及潜在的治疗靶标。
4.3 VDAC1与其他神经退行性疾病的相关性在PD中, α-synuclein (α-syn)的错误折叠和线粒体功能紊乱被认为是两大主要发病因素。过表达α-syn的大鼠中, 可检测到VDAC1和α-syn存在相互作用。在PD患者含有α-syn阳性包涵体的黑质神经元中, 检测到VDAC1的表达下调, 且VDAC1在神经元中的表达与α-syn的表达改变和聚集有关[63]。不同神经细胞在线粒体复合物1 (complex 1)抑制剂或其他细胞损伤刺激剂作用后, VDAC1的mRNA和蛋白表达水平上调[64, 65]。6-羟基多巴胺给药的大鼠纹状体提取物中可检测到VDAC1表达增加[66]。在HD中, PC12细胞模型表明, 亨廷顿蛋白可介导VDAC1功能改变, 从而影响线粒体功能和细胞存活率[67]。
5 VDAC1表达或功能改变对学习记忆的影响 5.1 基因敲除动物研究VDAC基因敲除动物研究表明, VDAC1敲除纯合子小鼠(VDAC1-/-)产生部分胚胎致死现象, 导致动物出生数量减少。存活的VDAC1-/-小鼠具有繁殖能力, 但存在轻度的生长迟缓, 学习能力及突触可塑性遭到破坏[68]。VDAC2-/-小鼠则无法存活, VDAC3-/-雄性小鼠无繁殖能力[68, 69]。VDAC1和VDAC3杂合子小鼠(VDAC1+/-和VDAC3+/-)具有繁殖能力, 且寿命正常[68]。
VDAC基因敲除动物一般感觉和行为活动均无异常。VDAC3-/-小鼠、VDAC1-/-/VDAC3-/-小鼠的背景恐惧条件反射表现异常, VDAC1、VDAC3单敲除或双敲除小鼠的线索恐惧条件反射减弱, 且在Morris水迷宫实验中表现出空间学习记忆障碍。此外, VDAC1敲除对小鼠脑片海马双脉冲易化(paired-pulse facilitation, PPF)、强直后增强(post-tetanic potentiation, PTP)不产生明显影响, 但产生明显的长时程增强(long term potentiation, LTP)衰减[68]。
VDAC1+/-小鼠的mRNA表达检测结果表明, AD相关基因如APP、tau、早老素2、GSK3β的表达降低; 线粒体相关基因如线粒体融合蛋白、HK1和HK2表达增加, 线粒体动力学基因腺嘌呤核苷酸转运体和亲环素D的表达降低; 突触相关基因如突触蛋白、神经颗粒素等表达上调。此外, VDAC1+/-小鼠的自由基水平和脂质过氧化水平降低, 细胞色素氧化酶活性升高, 表明其线粒体功能增强。以上结果提示, 减少VDAC1表达, 有利于改善突触活动和突触功能[70]。但结合VDAC1-/-小鼠表现出一定的学习记忆损伤, 推测在一定范围内下调VDAC1的表达有利于突触活动和功能的改善, 但VDAC1在学习记忆生理功能中仍发挥了一定的作用, 表达水平过低时将产生学习记忆功能损伤。以上假设需要进一步的实验数据支持。
5.2 调节VDAC1表达相关研究在鼠神经母细胞瘤(Neuro-2a)细胞的研究表明, 过表达鼠源VDAC1, 可诱导Cyt C释放并导致细胞凋亡增加[34]。在人神经母细胞瘤(SH-SY5Y)细胞采用siRNA, 分别下调APP、tau和VDAC1表达, 对比研究发现, 三者变化规律一致, 均可导致AD相关基因(如APP、β分泌酶1、早老素等)的mRNA水平下降, 线粒体动力学基因表达下降, 突触相关基因表达上调, H2O2、脂质过氧化、GTP酶活性降低, 细胞色素氧化酶活性和ATP产生增加。表明下调SH-SY5Y细胞中APP、tau和VDAC1水平均可增强突触活性, 改善线粒体功能[71]。另有研究报道, 12周龄雄性Swiss albino小鼠连续7天以3 mg·kg-1剂量腹腔注射东莨菪碱, 海马VDAC1表达水平降低。正常小鼠双侧海马注射VDAC1 siRNA后表现出新物体识别记忆受损[72]。
microRNA (miR)为小非编码RNA, 可对基因转录后表达过程进行调节, 已发现miR-7、miR-29和miR-320a等可调节VDAC1表达[73-75]。其中, miR-29在多种神经退行性疾病中表达降低, 在小鼠海马、小脑和皮层下调miR-29表达可导致VDAC1表达上调并引发神经元凋亡[74]; HIV-1感染伴有轻度认知障碍的患者中可检测到VDAC1和miR-320a表达异常, 人星形胶质细胞使用miR-320a模拟物可下调VDAC1表达并导致ATP释放减少[75]。由于目前报道的调节VDAC1表达在神经保护、突触活性及线粒体相关的能量代谢中作用并不完全一致, 因此仍需进一步的研究确证。
6 VDAC抑制剂目前, VDAC1特异性抑制剂仍处于不断寻找和优化的阶段, 报道较多的对其产生抑制作用的分子可能是通过不同的生物化学途径影响细胞的功能。
环孢菌素A (cyclosporine A, CsA)为常用的mPTP抑制剂[76], 通过抑制钙调磷酸酶对突触可塑性产生影响[77]。正常小鼠海马切片与CsA共孵育后产生双脉冲易化和长时程增强衰减现象[68]。
已知的阴离子转运抑制剂如DIDS及其衍生物SITS、H2DIDS、DNDS和DPC都可与VDAC1发生相互作用, 降低其通道电导。相同浓度下, DIDS和H2DIDS的作用强度相同, 与SITS、DNDS和DPC相比可更大程度地降低VDAC1的通道电导。该类化合物可抑制由ROS、Ca2+超载、线粒体膜电位损耗、VDAC1表达和寡聚化增加而导致的细胞凋亡[33, 78]。其中, DIDS对Aβ诱导的细胞大鼠皮质神经元细胞凋亡具有明显的保护作用[79]。此外, DIDS还可对抗胞外K+浓度降低、生长因子去除和星形孢菌素诱发的小脑颗粒神经元凋亡[80]。在以上研究中, DIDS单独作用对神经元均无明显的毒性作用。也有研究指出, DIDS可导致培养的小鼠海马神经元凋亡, 但没有表现出如线粒体裂变和质膜完整性的丧失等凋亡标志[81]。上述作用主要是由于影响VDAC1功能还是其他因素的作用仍不明确, 因此, 关于DIDS神经保护作用需要进一步的实验进行确证。
VDAC1具有Ca2+结合位点, 其通道活性可由二价金属离子进行调节。有机金属六价染料RuR和钌胺双核配合物(ruthenium amine binuclear complex, Ru360)可阻断瞬时感受器电位(transient receptor potential, TRP)通道, 特异性地与Ca2+结合蛋白发生相互作用, 降低VDAC1的通道电导[35]。其中, RuR可对抗Aβ引发的大鼠海马脑片细胞损伤[82], 对VDAC1过表达诱导的细胞凋亡具有保护作用[32]。也有研究表明, RuR是通过与VDAC1直接相互作用从而介导细胞保护作用[20]。
VBIT-4是Shoshan-Barmatz实验室[83]通过化合物筛选得到的VDAC1特异性抑制剂, 可通过与VDAC1发生直接相互作用而降低其通道电导, 抑制多种细胞系的VDAC1寡聚化及Cyt C的释放, 对线粒体功能紊乱而导致的细胞凋亡具有保护作用, 且在体实验也显示出较好的安全性, 提出了抑制VDAC1寡聚化可作为未来抑制剂开发的方向。此外, 通过VDAC1的N末端和Aβ序列分析从而制备的非穿膜VDAC1 N末端肽(VDAC1-N-Ter peptide), 已证明可与Aβ结合, 抑制其进入细胞引发毒性作用[55]。在肿瘤细胞的研究中表明, 一些基于VDAC1的细胞穿透肽可干扰细胞能量代谢稳态, 诱导细胞凋亡[84]。以上研究显示, VDAC1相关肽在线粒体功能调节、神经退行性疾病和癌症治疗中的前景。
7 结语根据以往研究, VDAC1的表达、功能调节在神经退行性疾病, 特别是AD中发挥了重要作用。但是, 其能否作为AD治疗的新靶点有待更为深入的探讨, 如VDAC1选择性抑制剂的筛选和发现, 抑制剂的血脑屏障通过率, 即增加其脑组织选择性。此外, 开展对VDAC1自身寡聚化, VDAC1与其他生物分子的相互作用研究, 如抑制VDAC1表达、功能或从阻断VDAC1与Aβ等AD相关生物分子间的相互作用入手进行化合物的设计和筛选, 并在多种动物模型上进行验证。
| [1] | Alzheimer's Disease International. World Alzheimer report 2016[R]. London, ADI, 2016. |
| [2] | Hardy JA, Higgins GA. Alzheimer's disease:the amyloid cascade hypothesis[J]. Science, 1992, 256: 184–185. DOI:10.1126/science.1566067 |
| [3] | De Pinto V, Messina A, Lane DJ, et al. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane[J]. FEBS Lett, 2010, 584: 1793–1799. DOI:10.1016/j.febslet.2010.02.049 |
| [4] | Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A, et al. Voltage-dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics[J]. Front Oncol, 2017, 7: 154. DOI:10.3389/fonc.2017.00154 |
| [5] | Colombini M. Voltage gating in the mitochondrial channel, VDAC[J]. J Membr Biol, 1989, 111: 103–111. DOI:10.1007/BF01871775 |
| [6] | Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane[J]. Ann N Y Acad Sci, 1980, 341: 552–563. DOI:10.1111/nyas.1980.341.issue-1 |
| [7] | Shoshan-Barmatz V, De Pinto V, Zweckstetter M, et al. VDAC, a multi-functional mitochondrial protein regulating cell life and death[J]. Mol Aspects Med, 2010, 31: 227–285. DOI:10.1016/j.mam.2010.03.002 |
| [8] | Yamamoto T, Yamada A, Watanabe M, et al. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues[J]. J Proteome Res, 2006, 5: 3336–3344. DOI:10.1021/pr060291w |
| [9] | Hinsch KD, Asmarinah, Hinsch E, et al. VDAC2(porin-2) expression pattern and localization in the bovine testis[J]. Biochim Biophys Acta, 2001, 1518: 329–333. DOI:10.1016/S0167-4781(01)00199-3 |
| [10] | Hinsch KD, De Pinto V, Aires VA, et al. Voltage-dependent anion-selective channels VDAC2 and VDAC3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum[J]. J Biol Chem, 2004, 279: 15281–15288. DOI:10.1074/jbc.M313433200 |
| [11] | Buettner R, Papoutsoglou G, Scemes E, et al. Evidence for secretory pathway localization of a voltage-dependent anion channel isoform[J]. Proc Natl Acad Sci U S A, 2000, 97: 3201–3206. DOI:10.1073/pnas.97.7.3201 |
| [12] | Akanda N, Tofighi R, Brask J, et al. Voltage-dependent anion channels (VDAC) in the plasma membrane play a critical role in apoptosis in differentiated hippocampal neurons but not in neural stem cells[J]. Cell Cycle, 2008, 7: 3225–3234. DOI:10.4161/cc.7.20.6831 |
| [13] | Bayrhuber M, Meins T, Habeck M, et al. Structure of the human voltage-dependent anion channel[J]. Proc Natl Acad Sci U S A, 2008, 105: 15370–15375. DOI:10.1073/pnas.0808115105 |
| [14] | Ujwal R, Cascio D, Colletier JP, et al. The crystal structure of mouse VDAC1 at 2.3 resolution reveals mechanistic insights into metabolite gating[J]. Proc Natl Acad Sci U S A, 2008, 105: 17742–17747. DOI:10.1073/pnas.0809634105 |
| [15] | Zachariae U, Schneider R, Briones R, et al. β-Barrel mobility underlies closure of the voltage-dependent anion channel[J]. Structure, 2012, 20: 1540–1549. DOI:10.1016/j.str.2012.06.015 |
| [16] | Choudhary OP, Paz A, Adelman JL, et al. Structure-guided simulations illuminate the mechanism of ATP transport through VDAC1[J]. Nat Struct Mol Biol, 2014, 21: 626–632. DOI:10.1038/nsmb.2841 |
| [17] | Tinnes FP, Burckhardt G. On a fully closed state of native human type-1 VDAC enriched in Nonidet P40[J]. Mol Genet Metab, 2012, 107: 632–633. DOI:10.1016/j.ymgme.2012.08.015 |
| [18] | Geula S, Ben-Hail D, Shoshan-Barmatz V, et al. Structure- based analysis of VDAC1:N-terminus location, translocation, channel gating and association with anti-apoptotic proteins[J]. Biochem J, 2012, 444: 475–485. DOI:10.1042/BJ20112079 |
| [19] | Briones R, Weichbrodt C, Paltrinieri L, et al. Voltage dependence of conformational dynamics and subconducting states of VDAC-1[J]. Biophys J, 2016, 111: 1223–1234. DOI:10.1016/j.bpj.2016.08.007 |
| [20] | Israelson A, Zaid H, Abu-Hamad S, et al. Mapping the ruthenium red-binding site of the voltage-dependent anion channel-1[J]. Cell Calcium, 2008, 43: 196–204. DOI:10.1016/j.ceca.2007.05.006 |
| [21] | Gincel D, Zaid H, Shoshan-Barmatz V, et al. Calcium binding and translocation by the voltage-dependent anion channel:a possible regulatory mechanism in mitochondrial function[J]. Biochem J, 2001, 358: 147–155. DOI:10.1042/bj3580147 |
| [22] | Ge L, Villinger S, Mari SA, et al. Molecular plasticity of the human voltage-dependent anion channel embedded into a membrane[J]. Structure, 2016, 24: 585–594. DOI:10.1016/j.str.2016.02.012 |
| [23] | Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC[J]. J Bioenerg Biomembr, 2008, 40: 171–182. DOI:10.1007/s10863-008-9148-8 |
| [24] | Keinan N, Pahima H, Ben-Hail D, et al. The role of calcium in VDAC1 oligomerization and mitochondria-mediated apop tosis[J]. Biochim Biophys Acta, 2013, 1833: 1745–1754. DOI:10.1016/j.bbamcr.2013.03.017 |
| [25] | Geula S, Naveed H, Liang J, et al. Structure-based analysis of VDAC1 protein:defining oligomer contact sites[J]. J Biol Chem, 2012, 287: 2179–2190. DOI:10.1074/jbc.M111.268920 |
| [26] | Keinan N, Tyomkin D, Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis[J]. Mol Cell Biol, 2010, 30: 5698–5709. DOI:10.1128/MCB.00165-10 |
| [27] | Betaneli V, Petrov EP, Schwille P. The role of lipids in VDAC oligomerization[J]. Biophys J, 2012, 102: 523–531. DOI:10.1016/j.bpj.2011.12.049 |
| [28] | Liu AY, Hou XS, Ding Y. Advances of antineoplastic agents based on mitochondrial targeting mechanism[J]. Acta Pharm Sin (药学学报), 2017, 52: 879–887. |
| [29] | Kantari C, Walczak H. Caspase-8 and Bid:caught in the act between death receptors and mitochondria[J]. Biochim Biophys Acta, 2011, 1813: 558–563. DOI:10.1016/j.bbamcr.2011.01.026 |
| [30] | Shimizu S, Matsuoka Y, Shinohara Y, et al. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells[J]. J Cell Biol, 2001, 152: 237–250. DOI:10.1083/jcb.152.2.237 |
| [31] | Tajeddine N, Galluzzi L, Kepp O, et al. Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death[J]. Oncogene, 2008, 27: 4221–4232. DOI:10.1038/onc.2008.63 |
| [32] | Zaid H, Abu-Hamad S, Israelson A, et al. The voltage- dependent anion channel-1 modulates apoptotic cell death[J]. Cell Death Differ, 2005, 12: 751–760. DOI:10.1038/sj.cdd.4401599 |
| [33] | Ben-Hail D, Shoshan-Barmatz V. VDAC1-interacting anion transport inhibitors inhibit VDAC1 oligomerization and apop tosis[J]. Biochim Biophys Acta, 2016, 1863: 1612–1623. DOI:10.1016/j.bbamcr.2016.04.002 |
| [34] | Ghosh T, Pandey N, Maitra A, et al. A role for voltage- dependent anion channel Vdac1 in polyglutamine-mediated neuronal cell death[J]. PLoS One, 2007, 2: e1170. DOI:10.1371/journal.pone.0001170 |
| [35] | Shoshan-Barmatz V, Gincel D. The voltage-dependent anion channel:characterization, modulation, and role in mitochondrial function in cell life and death[J]. Cell Biochem Biophys, 2003, 39: 279–292. DOI:10.1385/CBB:39:3:279 |
| [36] | Shimizu S, Tsujimoto Y. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage- dependent anion channel activity[J]. Proc Natl Acad Sci U S A, 2000, 97: 577–582. DOI:10.1073/pnas.97.2.577 |
| [37] | Shoshan-Barmatz V, Israelson A, Brdiczka D, et al. The voltage-dependent anion channel (VDAC):function in intracellular signalling, cell life and cell death[J]. Curr Pharm Des, 2006, 12: 2249–2270. DOI:10.2174/138161206777585111 |
| [38] | Abu-Hamad S, Arbel N, Calo D, et al. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins[J]. J Cell Sci, 2009, 122: 1906–1916. DOI:10.1242/jcs.040188 |
| [39] | Ko JH, Gu W, Lim I, et al. Expression profiling of mito chondrial voltage-dependent anion channel-1 associated genes predicts recurrence-free survival in human carcinomas[J]. PLoS One, 2014, 9: e110094. DOI:10.1371/journal.pone.0110094 |
| [40] | Pedersen PL, Mathupala S, Rempel A, et al. Mitochondrial bound type Ⅱ hexokinase:a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention[J]. Biochim Biophys Acta, 2002, 1555: 14–20. DOI:10.1016/S0005-2728(02)00248-7 |
| [41] | Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders[J]. Neuron, 2008, 60: 748–766. DOI:10.1016/j.neuron.2008.10.010 |
| [42] | Friedlander RM. Apoptosis and caspases in neurodegenera tive diseases[J]. N Engl J Med, 2003, 348: 1365–1375. DOI:10.1056/NEJMra022366 |
| [43] | Shoshan-Barmatz V, Nahon-Crystal E, Shteinfer-Kuzmine A, et al. VDAC1, mitochondrial dysfunction, and Alzheimer's disease[J]. Pharmacol Res, 2018, 131: 87–101. DOI:10.1016/j.phrs.2018.03.010 |
| [44] | Pinho CM, Teixeira PF, Glaser E. Mitochondrial import and degradation of amyloid-β peptide[J]. Biochim Biophys Acta, 2014, 1837: 1069–1074. DOI:10.1016/j.bbabio.2014.02.007 |
| [45] | Casley CS, Canevari L, Land JM, et al. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities[J]. J Neurochem, 2002, 80: 91–100. |
| [46] | Calkins MJ, Manczak M, Mao P, et al. Impaired mitochon drial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease[J]. Hum Mol Genet, 2011, 20: 4515–4529. DOI:10.1093/hmg/ddr381 |
| [47] | Parker WD Jr, Mahr NJ, Filley CM, et al. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease[J]. Neurology, 1994, 44: 1086–1090. DOI:10.1212/WNL.44.6.1086 |
| [48] | Rao VK, Carlson EA, Yan SS. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration[J]. Biochim Biophys Acta, 2014, 1842: 1267–1272. DOI:10.1016/j.bbadis.2013.09.003 |
| [49] | Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer's disease[J]. J Neurosci, 2001, 21: 3017–3023. DOI:10.1523/JNEUROSCI.21-09-03017.2001 |
| [50] | Aleardi AM, Benard G, Augereau O, et al. Gradual alteration of mitochondrial structure and function by beta-amyloids:importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release[J]. J Bioenerg Biomembr, 2005, 37: 207–225. DOI:10.1007/s10863-005-6631-3 |
| [51] | Morais Cardoso S, Swerdlow RH, Oliveira CR. Induction of cytochrome c-mediated apoptosis by amyloid beta 25-35 requires functional mitochondria[J]. Brain Res, 2002, 931: 117–125. DOI:10.1016/S0006-8993(02)02256-4 |
| [52] | Ebneth A, Godemann R, Stamer K, et al. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum:implications for Alzheimer's disease[J]. J Cell Biol, 1998, 143: 777–794. DOI:10.1083/jcb.143.3.777 |
| [53] | Manczak M, Reddy PH. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease[J]. Hum Mol Genet, 2012, 21: 5131–5146. DOI:10.1093/hmg/dds360 |
| [54] | Marin R, Ramírez CM, González M, et al. Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor alpha in septal and hippocampal neurons[J]. Mol Membr Biol, 2007, 24: 148–160. DOI:10.1080/09687860601055559 |
| [55] | Smilansky A, Dangoor L, Nakdimon I, et al. The voltage- dependent anion channel 1 mediates amyloid beta toxicity and represents a potential target for Alzheimer disease therapy[J]. J Biol Chem, 2015, 290: 30670–30683. DOI:10.1074/jbc.M115.691493 |
| [56] | Hur JY, Teranishi Y, Kihara T, et al. Identification of novel γ-secretase-associated proteins in detergent-resistant mem branes from brain[J]. J Biol Chem, 2012, 87: 11991–12005. |
| [57] | Fernandez-Echevarria C, Díaz M, Ferrer I, et al. Aβ promotes VDAC1 channel dephosphorylation in neuronal lipid rafts. Relevance to the mechanisms of neurotoxicity in Alzheimer's disease[J]. Neuroscience, 2014, 278: 354–366. DOI:10.1016/j.neuroscience.2014.07.079 |
| [58] | Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3 beta disrupts the binding of hexokinase Ⅱ to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity[J]. Cancer Res, 2005, 65: 10545–10554. DOI:10.1158/0008-5472.CAN-05-1925 |
| [59] | Cuadrado-Tejedor M, Vilariño M, Cabodevilla F, et al. Enhanced expression of the voltage-dependent anion channel 1(VDAC1) in Alzheimer's disease transgenic mice:an insight into the pathogenic effects of amyloid-β[J]. J Alzheimers Dis, 2011, 23: 195–206. DOI:10.3233/JAD-2010-100966 |
| [60] | Sultana R, Poon HF, Cai J, et al. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach[J]. Neurobiol Dis, 2006, 22: 76–87. DOI:10.1016/j.nbd.2005.10.004 |
| [61] | Ferrer I. Altered mitochondria, energy metabolism, voltage- dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer's disease[J]. J Bioenerg Biomembr, 2009, 41: 425–431. DOI:10.1007/s10863-009-9243-5 |
| [62] | Sun Y, Rong X, Lu W, et al. Translational study of Alzheimer's disease (AD) biomarkers from brain tissues in AβPP/PS1 mice and serum of AD patients[J]. J Alzheimers Dis, 2015, 45: 269–282. DOI:10.3233/JAD-142805 |
| [63] | Chu Y, Goldman JG, Kelly L, et al. Abnormal alpha- synuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental Parkinson's disease[J]. Neurobiol Dis, 2014, 69: 1–14. DOI:10.1016/j.nbd.2014.05.003 |
| [64] | Burté F, De Girolamo LA, Hargreaves AJ, et al. Alterations in the mitochondrial proteome of neuroblastoma cells in response to complex 1 inhibition[J]. J Proteome Res, 2011, 10: 1974–1986. DOI:10.1021/pr101211k |
| [65] | Xiong Y, Ding H, Xu M, et al. Protective effects of asiatic acid on rotenone- or H2O2-induced injury in SH-SY5Y cells[J]. Neurochem Res, 2009, 34: 746–754. DOI:10.1007/s11064-008-9844-0 |
| [66] | Lessner G, Schmitt O, Haas SJ, et al. Differential proteome of the striatum from hemiparkinsonian rats displays vivid structural remodeling processes[J]. J Proteome Res, 2010, 9: 4671–4687. DOI:10.1021/pr100389u |
| [67] | Karachitos A, Grobys D, Kulczyńska K, et al. The association of VDAC with cell viability of PC12 model of Huntington's disease[J]. Front Oncol, 2016, 6: 238. |
| [68] | Weeber EJ, Levy M, Sampson MJ, et al. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity[J]. J Biol Chem, 2002, 277: 18891–18897. DOI:10.1074/jbc.M201649200 |
| [69] | Reddy PH. Is the mitochondrial outermembrane protein VDAC1 therapeutic target for Alzheimer's disease?[J]. Biochim Biophys Acta, 2013, 1832: 67–75. DOI:10.1016/j.bbadis.2012.09.003 |
| [70] | Manczak M, Sheiko T, Craigen WJ, et al. Reduced VDAC1 protects against Alzheimer's disease, mitochondria, and synaptic deficiencies[J]. J Alzheimers Dis, 2013, 37: 679–690. DOI:10.3233/JAD-130761 |
| [71] | Manczak M, Reddy PH. RNA silencing of genes involved in Alzheimer's disease enhances mitochondrial function and synaptic activity[J]. Biochim Biophys Acta, 2013, 1832: 2368–2378. DOI:10.1016/j.bbadis.2013.09.008 |
| [72] | Baghel MS, Thakur MK. Differential proteome profiling in the hippocampus of amnesic mice[J]. Hippocampus, 2017, 27: 845–859. DOI:10.1002/hipo.v27.8 |
| [73] | Chaudhuri AD, Choi DC, Kabaria S, et al. MicroRNA-7 regulates the function of mitochondrial permeability transition pore by targeting VDAC1 expression[J]. J Biol Chem, 2016, 291: 6483–6493. DOI:10.1074/jbc.M115.691352 |
| [74] | Roshan R, Shridhar S, Sarangdhar MA, et al. Brain-specific knockdown of miR-29 results in neuronal cell death and at axia in mice[J]. RNA, 2014, 20: 1287–1297. DOI:10.1261/rna.044008.113 |
| [75] | Fatima M, Prajapati B, Saleem K, et al. Novel insights into role of miR-320a-VDAC1 axis in astrocyte-mediated neuronal damage in neuroAIDS[J]. Glia, 2017, 65: 250–263. DOI:10.1002/glia.v65.2 |
| [76] | Brustovetsky N, Dubinsky JM. Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria[J]. J Neurosci, 2000, 20: 8229–8237. DOI:10.1523/JNEUROSCI.20-22-08229.2000 |
| [77] | Mulkey RM, Endo S, Shenolikar S, et al. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression[J]. Nature, 1994, 369: 486–488. DOI:10.1038/369486a0 |
| [78] | Huang L, Han J, Ben-Hail D, et al. Diterpene induces VDAC1-dependent apoptosis in Bax/Bak-defcient cells[J]. J Biol Chem, 2015, 290: 23563–23578. DOI:10.1074/jbc.M115.648774 |
| [79] | Xia Z, Tauskela J, Small DL. Disulfonic stilbenes prevent beta-amyloid (25-35) neuronal toxicity in rat cortical cultures[J]. Neurosci Lett, 2003, 340: 53–56. DOI:10.1016/S0304-3940(03)00075-2 |
| [80] | Himi T, Ishizaki Y, Murota SI. 4, 4'-Diisothiocyano-2, 2'- stilbenedisulfonate protects cultured cerebellar granule neurons from death[J]. Life Sci, 2002, 70: 1235–1249. DOI:10.1016/S0024-3205(01)01503-X |
| [81] | Pamenter ME, Perkins GA, Gu XQ, et al. DIDS (4, 4- diisothiocyanatostilbenedisulphonic acid) induces apoptotic cell death in a hippocampal neuronal cell line and is not neuroprotective against ischemic stress[J]. PLoS One, 2013, 8: e60804. DOI:10.1371/journal.pone.0060804 |
| [82] | Bai JZ, Lipski J. Involvement of TRPV4 channels in Aβ40- induced hippocampal cell death and astrocytic Ca2+ signalling[J]. Neurotoxicology, 2014, 41: 64–72. DOI:10.1016/j.neuro.2014.01.001 |
| [83] | Ben-Hail D, Begas-Shvartz R, Shalev M, et al. Novel compounds targeting the mitochondrial protein VDAC1 inhibit apoptosis and protect against mitochondrial dysfunction[J]. J Biol Chem, 2016, 291: 24986–25003. DOI:10.1074/jbc.M116.744284 |
| [84] | Shteinfer-Kuzmine A, Arif T, Krelin Y, et al. Mitochondrial VDAC1-based peptides:attacking oncogenic properties in glioblastoma[J]. Oncotarget, 2017, 8: 31329–31346. |
 2018, Vol. 53
2018, Vol. 53


