时至今日, 癌症仍是威胁人类生命和健康的重大疾病之一。世界卫生组织发布的《2017世界卫生统计》报告中指出, 2015年全球死亡人数达5 600万人, 其中880万死于恶性肿瘤, 占总死亡人数的15.7%。2015年中国新增癌症患者429.2万例, 死亡281.4万例, 其中, 胰腺癌死亡人数约7.94万[1]。2017年国家癌症中心公布的最新数据显示, 胰腺癌死亡人数占所有患恶性肿瘤死亡人数的2.82%。胰腺癌是一种恶性程度高、诊断和治疗都极为困难的消化道肿瘤, 是预后最差的恶性肿瘤之一, 被誉为“癌中之王”, 5年相对生存率仅为8%[2]。
胰腺癌一般被分为外分泌型(分泌消化酶)和内分泌型(分泌激素)两类。胰腺导管腺癌属于外分泌型, 具有腺体结构, 是最常见和最致命的胰腺癌, 占胰腺癌的90%以上[3, 4]。未经治疗的胰腺导管腺癌患者平均生存期为3个月, 而术后的平均生存期为10~20个月, 5年总体生存率不到5%[5]。胰腺内分泌肿瘤, 又称神经内分泌型肿瘤, 起源于胰岛细胞, 发病率较低, 年发病率为0.32例/10万人[6], 约占胰腺癌的1.3%[7]。此外, 胰腺囊性肿瘤也是一种较为罕见的胰腺癌, 可在胰头、胰身、胰尾形成聚集液体, 主要特征是产生黏蛋白[8]。由于缺乏特异性症状和生物标志物以及有效的筛查方法, 早期胰腺癌很难诊断。只有15%~20%患者在早期被诊断出胰腺癌并可进行手术治疗, 而80%~85%患者在确诊后已是晚期并且高度转移, 状况已无法进行手术切除[9, 10]。对于这些胰腺癌患者来说, 化疗和放疗的效果尤为关键。而胰腺癌患者在术后常发生侵袭性转移, 转移肿瘤对常规化疗和放疗具有高度抵抗性。胰腺癌患者预后极差, 局部晚期癌症患者中位生存期为6~10个月, 转移性癌症患者中位生存期为3~6个月[11, 12]。
吉西他滨是目前临床胰腺癌治疗的一线用药。单用吉西他滨化疗的胰腺癌患者的中位生存期为5.91个月, 而吉西他滨和厄洛替尼联合治疗可延长中位生存期至6.24个月[13], 但总体生存率仍然比较低, 因此探索更加有效的治疗方法十分迫切。近年来, 基于纳米技术的纳米递药系统的研究和发展为胰腺癌的治疗带来了一丝曙光[14, 15]。纳米载体可以改善化疗药物的成药性, 提高药物的水溶性, 并且纳米载体对药物的包裹作用可降低血液中酶对药物的降解和破坏。更重要的是负载化疗药物的纳米载体(纳米药物)能够有效改善药物的体内分布, 增加药物在肿瘤组织的富集, 降低在正常脏器的分布, 发挥增效减毒作用。2015年, 美国食品药品监督管理局批准了伊立替康脂质体注射液(Onivyde)与氟尿嘧啶和亚叶酸联用治疗晚期(转移性)胰腺癌患者, 将平均生存期从单用氟尿嘧啶和亚叶酸的4.2个月延长到6.1个月。但是, 单用伊立替康脂质体治疗的患者与氟尿嘧啶和亚叶酸联用的患者相比, 生存期没有改善, 其主要原因是胰腺癌的肿瘤微环境阻碍了纳米药物的有效递送, 从而影响其疗效[16]。
胰腺肿瘤组织的90%以上都是致密的肿瘤基质。这种细胞外基质(extracellular matrix, ECM)由胰腺癌细胞、胰腺星状细胞和成纤维细胞等分泌的胶原蛋白、弹性蛋白、纤粘蛋白和透明质酸等组成, 大大阻碍了纳米药物在肿瘤组织中的深层递送[17]。胰腺肿瘤中坍塌的血管导致间质液压(interstitial fluid pressure, IFP)增大, 超过集合血管的静水压力和血管壁渗透压, 使大粒径的纳米药物渗透难度增加。并且, 除胰腺癌细胞和胰腺星状细胞外, 胰腺肿瘤组织中还存在肿瘤相关巨噬细胞(tumor-associated macrophage, TAM)等免疫细胞, 其在肿瘤的发生和发展中扮演着重要的角色, 促进肿瘤的生长、浸润和转移。此外, 肿瘤细胞群体中一类具有干细胞特性的细胞亚群, 具有自我更新、强致瘤性、多重耐药性和放疗耐受性, 被称为肿瘤干细胞, 是肿瘤复发和转移的根源。胰腺癌组织中高表达的蛋白酶在肿瘤侵袭转移中发挥着重要作用, 如基质金属蛋白酶(matrix metalloproteinase, MMP), 可降解ECM中多种蛋白成分, 破坏组织学屏障, 促进肿瘤细胞转移。因此, 基于胰腺癌病理学特征和纳米药物递送的关键问题, 需要更高效的药物递送系统来提高胰腺癌治疗效果。为提高药物在胰腺癌组织中的浓度以达到治疗效果, 可采用靶向胰腺癌的特殊分子标记物策略, 同时利用纳米技术, 研发具有靶向能力和渗透能力的纳米制剂。本文综述了近年来针对胰腺癌的靶向纳米药物递送系统, 从靶向胰腺癌细胞、靶向胰腺癌基质、靶向胰腺癌相关细胞和靶向胰腺癌干细胞4个方面进行介绍, 以期为胰腺癌的临床治疗提供新技术和新方法。
1 靶向胰腺癌细胞基于胰腺癌细胞表面高表达的受体, 通过配体修饰技术构建靶向胰腺癌细胞的纳米递送系统。靶向递送系统可增加药物在胰腺癌组织的富集量, 通过受体介导的胞吞作用将药物有效递送至胰腺癌细胞内, 提高胞内药物浓度, 杀死癌细胞, 增强疗效。转铁蛋白受体(transferrin receptor, TfR)、表皮生长因子受体(epidermal growth factor receptor, EGFR)、整合素受体和尿激酶纤溶酶原激活受体(urokinase plasminogen activator receptor, uPAR)等已被证实在胰腺癌细胞表面过表达。
1.1 转铁蛋白受体铁作为维持细胞快速增殖的必需元素, 在肿瘤的生长过程中发挥着重要作用。转铁蛋白(transferrin, Tf)是分子质量约为80 kDa的铁结合血浆糖蛋白[18]。Tf可结合细胞表面的TfR, 形成复合物, 继而通过内吞进入细胞[19]。TfR在许多恶性肿瘤组织中表达上调[17]。超过90%的恶性胰腺癌细胞高表达TfR[20]。
Frasco等[21]制备了Tf修饰的聚乳酸-羟基乙酸共聚物(Tf-PLGA)纳米粒, 递送蛋白酶体抑制剂硼替佐米, 用于胰腺癌治疗。研究表明, 50%人源胰腺癌S2-013细胞摄取了荧光标记的Tf-PLGA纳米粒, 而仅在10%人正常胰腺hTERT-HPNE细胞中检测到荧光信号, 证明了Tf-PLGA纳米粒对胰腺癌细胞的靶向性。并且Tf修饰的纳米粒显著增强了硼替佐米对胰腺癌细胞的杀伤能力。Camp等[22]报道了Tf修饰的负载p53基因的脂质体在荷胰腺肿瘤小鼠中的肿瘤靶向效率相比于未修饰脂质体提高了3倍。与Tf修饰的负载p53脂质体和吉西他滨单独用药组相比, 两者联合用药组小鼠的生存期延长了10天。
1.2 表皮生长因子受体EGFR是一种酪氨酸激酶, 也是人表皮生长因子(EGF)家族的受体成员之一。EGFR在多种肿瘤细胞中表达上调[23], 在90%以上的胰腺癌中过表达[24], 特别是在人源胰腺癌BxPc-3、PANC-1和HPAC细胞中表达较高[25]。EGF与EGFR结合, 发生二聚化和自身磷酸化, 从而激活RAS/ MAPK和PI3K-AKT信号通路, 促进胰腺癌细胞的增殖[26]。EGFR上调会促进肿瘤生长、增殖、血管生成、侵袭和转移。
Mondal等[27]报道了GE11肽(氨基酸序列: YHWYGYTPQNVI)修饰的聚合物胶束递送吉西他滨, 通过靶向EGFR将药物递送至肿瘤组织(图 1)。全长的EGFR配体由于具有较高的促有丝分裂和促血管生成能力限制了其在临床上的应用。经噬菌体展示技术筛选得到的GE11肽能够特异性靶向EGFR, 并且促有丝分裂能力低[28]。静脉给药4 h后, GE11肽修饰的胶束在肿瘤组织中的富集量高于在血液和正常脏器中的分布量。GE11肽修饰的载药胶束治疗组小鼠的肿瘤体积是游离吉西他滨治疗组的50%。在此基础上, Xu等[29]研发了EGFR靶向肽修饰的还原响应型硫醇化明胶纳米粒共递送p53质粒和吉西他滨用于胰腺癌联合治疗。该靶向肽段(氨基酸序列:YHWYGYTPQNVIGGGGC)是修饰了4个甘氨酸间隔区和1个末端半胱氨酸的GE11肽。靶向纳米共递药系统显著抑制了胰腺肿瘤的生长。单载吉西他滨和p53质粒的纳米粒抑瘤率分别为61.7%和50.1%, 而共载吉西他滨和p53质粒的纳米粒的抑瘤率可达77.3%。Mondal等[30]还通过西妥昔单抗修饰的聚合物胶束共递送吉西他滨和miR-205靶向胰腺癌细胞表面的EGFR, 通过化疗与基因治疗联用治疗胰腺癌。单抗修饰的胶束的肿瘤靶向效率是未修饰胶束的2倍, 且抗体修饰的共载吉西他滨和miR-205的胶束较单载吉西他滨或miR-205胶束治疗效果显著提高, 抑瘤率达到80%。
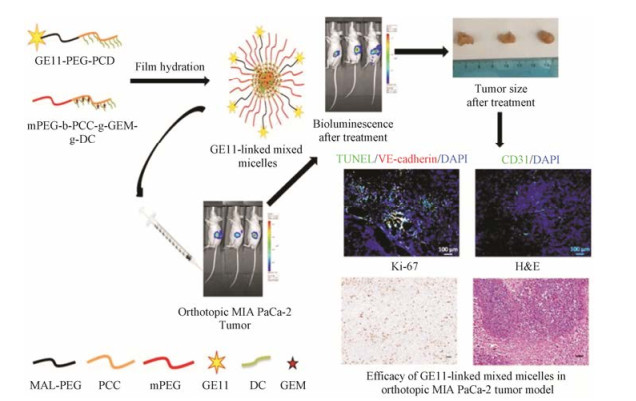
|
Figure 1 Tumor targetability and in vivo antitumor activity of epidermal growth factor receptor (EGFR)-targeted polymeric mixed micelles carrying gemcitabine[27]. PCD: Poly(2-methyl-2-carboxyl-propylene carbonate-graft-dodecanol); MAL-PEG: Maleimide poly (ethylene glycol); PCC: Poly(2-methyl-2-carboxylpropylene carbonate); mPEG: Methoxy poly(ethylene glycol); DC: Dodecanol; GEM: Gemcitabine; TUNEL: TdT-mediated dUTP nick-end labeling |
整合素来自异源二聚体细胞表面受体家族, 可直接结合细胞外基质成分, 作为双向信号转导子, 调控细胞的分化、迁移、增殖及死亡[31, 32]。所有的整合素都具有结合精氨酸-甘氨酸-天门冬氨酸(RGD)三肽的能力。因此, RGD常用作整合素受体的靶向配体。整合素αvβ3受体是许多病毒的内化受体[33, 34], 已被证实在恶性肿瘤等病变组织中高表达[35, 36]。
Ji等[37]研发了RGD修饰的白蛋白纳米粒靶向递送吉西他滨, 通过整合素受体介导的内吞作用进入胰腺癌细胞。RGD修饰的纳米粒在人源胰腺癌BXPC3细胞的摄取量显著高于无RGD修饰的普通纳米粒。在荷人源胰腺癌BXPC3细胞肿瘤小鼠模型中, 与无RGD修饰的制剂组相比, RGD修饰的纳米粒组的抑瘤效果增强近2倍。Murphy等[38]制备了荷载多柔比星(Dox)的RGD修饰的脂质体(RGD-Dox-NPs)用于治疗转移性胰腺癌。在胰腺癌原位模型中, RGD- Dox-NPs有效抑制了23%原位胰腺肿瘤, 并显著减少了82%肝门淋巴结转移。因此, 这种RGD修饰的纳米粒有潜力成为治疗胰腺癌转移的载体。
血胰屏障(blood-pancreas barrier, BPB)具有类似血脑屏障的结构, 存在于胰腺组织和血液之间, 由腺泡周围毛细血管内皮细胞层、基底膜层、腺泡周围间隙、腺泡细胞层、泡心细胞层及闰管等构成。BPB对不同结构和分子大小的药物具有选择通透性和滤过作用, 影响药物在胰腺内的分布及药效发挥[39]。Li和Luo等[40, 41]设计穿过BPB的载体, 可增强急性胰腺炎的治疗效果。BPB同样限制了药物在胰腺癌治疗中的作用。Shen等[42]制备了递送紫杉醇的靶向整合素受体的温敏性纳米胶(mPEG-PLGA-PLL-cRGD)。在荷人源胰腺癌Aspc-1细胞的小鼠模型中, 荧光染料溶液在注射3天后, 肿瘤部位的荧光消失, 而温敏性纳米胶静脉给药50天后在肿瘤部位仍有荧光。结果证明, mPEG-PLGA-PLL-cRGD通过克服血胰屏障提高了肿瘤分布, 增强抗肿瘤作用(图 2)。
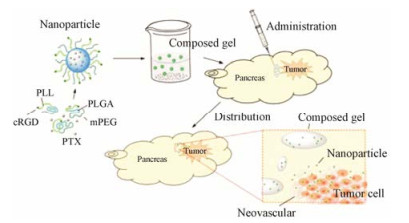
|
Figure 2 Thermosensitive nanogel targeting integrin receptors for pancreatic cancer treatment[42]. PLL: Poly(L-lysine); PLGA: Poly(D, L-lactide-co-glycolide); mPEG: Methoxy poly(ethylene glycol); PTX: Paclitaxel |
尿激酶纤溶酶原激活物受体(uPAR)与尿激酶纤溶酶原激活物(uPA)相互作用, 调控基质降解、细胞迁移和血管生成等多条通路[43]。uPAR在大多数正常组织和器官低表达[44], 而在大多数肿瘤组织中高表达, 包括乳腺癌、胰腺癌、肺癌和卵巢癌[44-46]。86%~90%胰腺癌组织高表达uPAR, 胰腺癌组织中uPAR的mRNA水平比邻近正常组织高9.6倍[47]。
Lee等[48]制备了uPA结构中与受体结合区域肽段(ATF)修饰的氧化铁纳米粒, 并以响应溶酶体蛋白酶可降解的四肽连结臂(氨基酸序列: GFLG)共价偶联吉西他滨于纳米粒上, 完成药物装载。纳米粒经受体介导的内吞进入胰腺癌细胞的溶酶体后, 连接臂被蛋白酶降解, 从而释放吉西他滨并发挥药效。荷载吉西他滨的靶向肽修饰的纳米粒在人源胰腺癌异位小鼠模型中的抑瘤率为50%, 相比之下, 无靶头制剂组仅为23% (图 3)。Hu等[49]制备了uPAR抗体修饰的金纳米壳, 并在动物模型中考察了介入光热疗法(interventional photothermal therapy, IPTT)的效果。与碘-125 (125I)间质近距离放射治疗方式相比, 接受IPTT治疗的小鼠的中位生存率提高25%, 并能够实现一次介入治疗就达到完全消除肿瘤的效果。Gao等[50]通过腹腔给药uPAR靶向磁性氧化铁纳米粒用于胰腺癌化疗。在鼠源胰腺癌PAN02原位模型中, 腹腔注射纳米粒在瘤内累积量可达到17%, 是静脉注射纳米粒累积量的3倍。当载体载入化疗药顺铂后, 对肿瘤的抑制率可达到40%, 游离顺铂的肿瘤抑制率仅为15%, 抑制率显著提高。
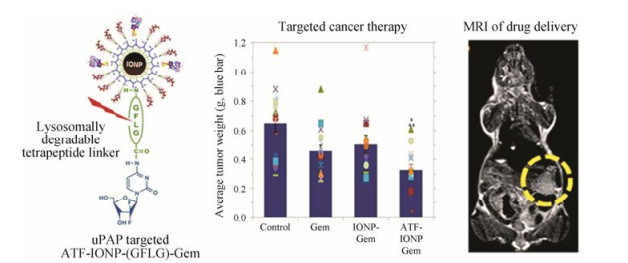
|
Figure 3 Antitumor efficacy and targetability of uPAR-decorated magnetic iron oxide nanoparticles (IONPs)[48]. ATF: Amino- terminal fragment; GFLG: Gly-Phe-Leu-Gly; MRI: Magnetic resonance imaging |
胰腺癌具有高度密集的肿瘤基质。基质微环境由ECM、活化的成纤维细胞、肌成纤维细胞、炎症细胞以及血液和淋巴管等组成[51]。该微环境严重阻碍了大分子甚至吉西他滨等小分子在肿瘤组织中的渗透性。近年来, 有很多研究聚焦于靶向胰腺肿瘤基质, 通过将酶或药物递送至肿瘤基质, 破坏肿瘤基质的致密结构, 从而增强胰腺癌治疗效果。
2.1 基质金属蛋白酶MMPs是锌离子依赖的结构和功能同源的内肽酶家族, 能降解ECM的多种蛋白成分, 如胶原蛋白、蛋白多糖、弹性蛋白和纤连蛋白[52]。由于MMPs能够改善一些生物活性化合物的活性, 如肿瘤坏死因子-α (TNF-α)、趋化因子(CXCL-8)和转化生长因子-β (TGF-β), 因此MMPs与许多炎症相关性疾病的发病密切有关[53]。MMPs水平通常在多种癌症的ECM中升高, 尤其在胰腺癌的组织重塑和肿瘤生长中起重要作用[54]。胰腺癌中过表达MMP-1 (胶原酶)、MMP-2 (明胶酶-A)、MMP-7 (基质溶解因子)、MMP-9 (明胶酶-B)、MMP-10和MMP-11 (基质溶素)[55, 56], 其中MMP-2和MMP-9与肿瘤的侵袭与转移关联最大。
Kulkarni等[57]构建了MMP-9响应型纳米囊泡递送吉西他滨。纳米囊泡表面的PEG修饰起到了长循环的作用, 增加肿瘤靶向性。在肿瘤部位, 二硫键连接的PEG层在高浓度还原型谷胱甘肽作用下脱落, 并将MMP-9响应的肽段暴露, 肿瘤基质中存在的高浓度MMP-9随之将纳米粒水解, 促进吉西他滨的快速释放。载药纳米粒在胰腺癌的动物模型中取得了良好的治疗效果, 有效抑制了肿瘤的生长。Ji等[58]报道了MMP-2响应型脂质体用于递送抗纤维化药物吡非尼酮, 抑制胰腺星状细胞中Ⅰ型胶原蛋白和TGF-β的表达, 下调纤维化, 减少基质屏障(图 4)。同时, 递送化疗药物吉西他滨靶向胰腺癌细胞。在胰腺癌细胞与胰腺星状细胞共接种的小鼠模型中, MMP-2响应型脂质体组的肿瘤渗透性更佳, 并且用药后, 肿瘤组织的纤维化得到改善, 基质屏障减少, 显著抑制肿瘤生长。
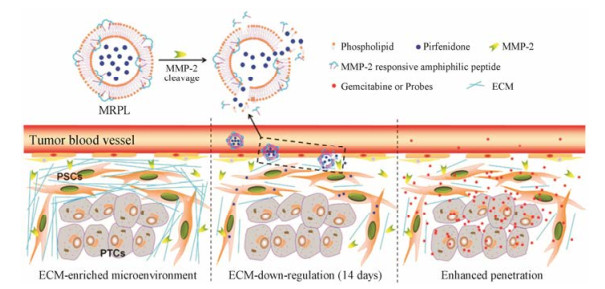
|
Figure 4 Down-regulation of multiple extracellular matrix (ECM) components including collagen Ⅰ by a MMP-2 responsive peptide- hybrid liposome (MRPL)[58] |
透明质酸(hyaluronic acid, HA)是存在于细胞外基质中的非硫酸化糖胺聚糖, 由长度可变的N-乙酰葡糖胺/葡糖醛酸二糖重复结构单元构成。HA可以通过与CD44结合发出信号以调节受体酪氨酸激酶和小GTP酶的活性, 参与血管生成、上皮细胞间质转化和化学抗性等过程[59]。此外, HA的阴离子重复结构也能隔离流动的阳离子和溶剂化水, 导致渗透性肿胀, 在富含HA的正常和恶性组织中提供结构支持[59]。HA在肿瘤细胞外基质能够形成较高的间质压, 因此造成血管的压缩和低灌注, 构成了一道物理屏障[16, 60-62]。多种胰腺癌细胞都会分泌HA, 主要聚集在基质和瘤周结缔组织[63]。
Provenzano等[64]报道了在注射聚乙二醇化的透明质酸酶(PEGPH20)消除肿瘤基质的HA后, 间质压正常化, 微血管重新扩大。在联合化疗药物吉西他滨共同治疗后, 可以永久改变肿瘤微环境, 提高肿瘤对药物的响应, 生存期延长1倍。在Ⅰ/Ⅰb期临床试验中, 28例Ⅳ期转移性胰腺癌患者中, 当PEGPH20与吉西他滨联合用药时, 具有良好的耐受性, 有助于晚期胰腺癌患者的治疗[65]。目前, PEGPH20与吉西他滨和蛋白结合型紫杉醇(NCT01839487)以及与FOLFIRINOX方案(氟尿嘧啶、亚叶酸、伊立替康和奥沙利铂)联用(NCT01959139)正处于Ⅱ期临床试验阶段。Buckway等[66]制备了靶向的N-(2-羟丙基)甲基丙烯酰胺(HPMA)共聚物。HPMA共聚物上修饰短肽配体cRGDfK和KCCYSL, 从而靶向到整合素αvβ3和HER2受体。在用透明质酸酶处理后, 去除了致密的基质障碍, 该HPMA共聚物能够更高效地靶向荷人源胰腺癌CAPAN-1肿瘤小鼠的肿瘤部位。
3 靶向肿瘤相关细胞在胰腺癌中, 胰腺星状细胞是肿瘤组织中主要的基质细胞类型, 并且是ECM产生的主要来源[67]。ECM的成分与胰腺癌细胞联系紧密, 创造了一个促进局部肿瘤生长和远端转移的微环境[68]。免疫细胞是胰腺癌进展中最重要的支持细胞之一, 并且使胰腺癌在治疗过程躲过免疫系统的攻击[69], 其中, 肿瘤相关巨噬细胞是胰腺癌微环境中重要的组成部分。因此, 靶向胰腺星状细胞和肿瘤相关巨噬细胞是增加药物对胰腺癌治疗效果的新策略。
3.1 胰腺星状细胞胰腺星状细胞是造成胰腺癌中高度纤维化的主要细胞[70, 71]。在正常胰腺中, 胰腺星状细胞处于静息状态, 但当胰腺受损伤或炎症时, 细胞会经历形态和功能上的变化而被激活, 表达α-平滑肌肌动蛋白(α-SMA)并分泌生长因子及多种促炎细胞因子, 如巨噬细胞趋化蛋白-1 (MCP-1)和白细胞介素-8 (IL-8), 以及过量的ECM蛋白如Ⅰ型胶原蛋白[70-72]。如果损伤或炎症持续和重复, 胰腺星状细胞将保持永久活化, 从而发展为胰腺纤维化和胰腺肿瘤[71]。
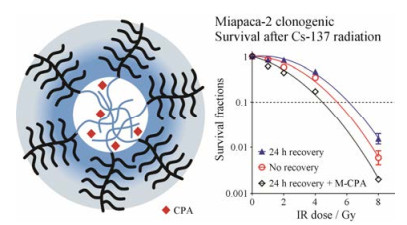
Gonzalez-Villasana等[73]报道了共载含氮双膦酸盐(NBPs)和紫杉醇的白蛋白纳米粒用于胰腺癌联合治疗。共载两药的白蛋白纳米粒给药后, 荷瘤小鼠肿瘤组织中的α-SMA的表达降低了55%;纤维化程度得到明显改善, 降低了47%;有效抑制了胰腺肿瘤的生长, 抑瘤率可达70%, 腹膜扩散率降低72%。通过胰腺癌位模型验证联合给药能够抑制肿瘤生长。Yalcin等[74]应用树状聚合物包被的磁性纳米粒(DcMNPs)共递送吉西他滨和全反式维甲酸。DcMNPs可同时靶向胰腺癌细胞和胰腺星状细胞, 有效杀灭两种细胞, 增强疗效。经DcMNPs处理后, 胰腺星状细胞的存活率为30%, 胰腺癌PANC1细胞的存活率为20%。Zhao等[75]构建了载有环巴胺(cyclopamine, CPA)的交联聚合物胶束(M-CPA) (图 5)。CPA是一种维持肿瘤干性Hedgehog信号通路的抑制剂, 可以扰乱肿瘤相关基质, 从而增强胰腺癌治疗效果。M-CPA消除了Gli-1的表达(hedgehog通路的关键组分), 并增强放射治疗效果。M-CPA与放疗联用对胰腺癌细胞系和胰腺星状细胞均有较强的毒性, 两种细胞的存活率低于40%。Ji等[76]制备了荷载吡非尼酮(PFD)的MMP-2响应型脂质体(MRPL)。MRPL能够特异性地在富含MMP-2的胰腺肿瘤部位释放PFD, 下调胰腺星状细胞分泌的多种组分, 包括Ⅰ型胶原蛋白、纤连蛋白、多功能蛋白聚糖和腱生蛋白C, 促进纳米粒在肿瘤组织中的渗透率。MRPL的渗透深度能达到972.2 μm, 是普通脂质体渗透深度的3倍, 并增强了吉西他滨对胰腺癌的治疗效果。
|
Figure 5 Therapeutic efficacy of cyclopamine (CPA)-loaded core-crosslinked polymeric micelles in combination with radiotherapy[75] |
TAM来源于循环单核细胞, 是实体瘤中免疫细胞的主要群体[77-81]。TAM能促进癌细胞增殖, 刺激肿瘤血管生成和细胞外基质分解, 进而增强肿瘤的侵袭和转移[82]。TAM也可抑制免疫反应, 在肿瘤发展过程中及从化疗或免疫治疗恢复后, 阻止肿瘤细胞被自然杀伤细胞和T细胞攻击[83]。
在一项研究中, 用趋化因子(C-C基序)受体2或集落刺激因子-1受体拮抗剂抑制TAM, 改善了化疗反应并增加了抗肿瘤T细胞应答[84]。由于TAM可能表现出促癌和抑癌双重效应, 以巨噬细胞为导向的免疫治疗需要具有抗基质和抗癌活性。Wang-Gillam等[85]报道了PF-04136309, 一种新型趋化因子(C-C基序)受体2抑制剂, 能够消耗发挥免疫抑制作用的炎性TAM, 与FOLFIRINOX联合用于晚期胰腺癌的治疗。结果发现, 在23例有效患者中, 有效率为52%, 但长期疗效尚不明确。这些关于靶向肿瘤微环境TAM的初步数据具有可喜的结果, 但仍需要进一步探索。
4 胰腺癌干细胞肿瘤组织中有一类胚胎样细胞, 称为肿瘤干细胞(cancer stem cell, CSC)或肿瘤启动细胞(cancer initiation cell, CIC), 具有自我更新和分化的能力, 与肿瘤的起始和增殖密切相关[86]。胰腺癌干细胞同时表达CD44、CD24和ESA[87]。Wickham等[33]发现CD44阳性(CD44+)的胰腺癌细胞是造成吉西他滨耐药的主要原因之一。在人源胰腺癌HPAC和CFPAC-1细胞中, 高剂量吉西他滨能杀死大部分细胞后, CD44+细胞能够增殖并重构细胞群体。因此, 消除CD44+细胞可能是胰腺癌深度治愈的关键。Wei等[88]利用HA纳米胶递送药物靶向到CD44+的肿瘤干细胞。MIA PaCa-2细胞高表达CD44。荷载姜黄素的HA纳米胶对人源胰腺癌MIA PaCa-2细胞的IC50为9 μmol·L-1, 而游离姜黄素的IC50值为19 μmol·L-1, 并且空白HA纳米胶几乎没有毒性(IC50 > 5 mg·mL-1)。Kesharwani等[89]制备了靶向CD44受体的透明质酸-苯乙烯-马来酸纳米胶束(HA-SMA-CDF)用于靶向递送3, 4-二氟亚苄基姜黄素衍生物(CDF)。游离CDF、SMA-CDF和HA-SMA-CDF对CD44+的人源胰腺癌MIA PaCa-2细胞的IC50值分别为410、245和170 nmol·L-1, 表明靶向制剂HA-SMA-CDF显著提高了CDF对CD44+胰腺癌细胞的毒性。
5 展望随着对胰腺癌本质及其微环境的逐步阐明, 研究者设计并构建了基于不同靶向策略的胰腺癌靶向纳米递药系统, 包括靶向胰腺癌细胞、靶向胰腺癌组织间质、靶向胰腺癌相关细胞和靶向胰腺癌干细胞等, 以期增加药物在胰腺癌组织的富集量, 克服肿瘤组织中的生物学屏障, 提高胰腺癌治疗效果。相比于游离化疗药物, 靶向胰腺癌细胞的纳米递药系统利用受体-配体结合的主动靶向作用, 增加药物在胰腺癌组织和细胞内的浓度, 杀死癌细胞。但是, 胰腺癌组织中致密的肿瘤基质导致了纳米递药系统的肿瘤渗透性差。因此, 靶向胰腺癌细胞的纳米递药系统在胰腺癌组织纤维化较高的情况下抗癌作用较差。利用靶向胰腺癌基质和胰腺癌相关细胞, 可起到调节肿瘤微环境, 增强化疗药物对胰腺癌的疗效[90]。目前还没有针对肿瘤干细胞的药物上市, 但鉴于肿瘤干细胞在恶性肿瘤发生发展过程中的重要作用, 靶向胰腺癌干细胞的治疗策略为临床胰腺癌的治疗带来了新的希望[91]。这4种靶向策略在提高胰腺癌治疗方面各有优势和缺陷, 因此未来两种或多种策略联用可能是提高胰腺癌治愈率的重要方法, 是未来胰腺癌治疗的发展趋势。
此外, 基于细胞毒T淋巴细胞相关抗原4 (CTLA-4)、程序性死亡受体1 (PD-1)和程序性死亡受体-配体1 (PD-L1)等的免疫疗法在白血病和黑色素瘤的治疗中取得了良好的疗效, 但在胰腺癌的治疗中免疫疗法并没有实质性进展[92-94], 主要原因是受到胰腺癌致密的肿瘤基质和免疫抑制的肿瘤微环境的限制[95]。Lu等[96]构建了共载吲哚胺2, 3-二氧化酶(IDO)抑制剂indoximod和奥沙利铂的脂质-介孔硅杂化纳米粒(OX/IND-MSNP), 用于克服胰腺癌肿瘤组织的免疫抑制性, 从而增强奥沙利铂的抗癌作用。OX/IND-MSNP治疗组与单用奥沙利铂治疗组相比, 小鼠的中位生存期延长了14天。因此, 有机结合靶向纳米递药系统与免疫疗法也为胰腺癌的治疗提供了新策略。
靶向纳米制剂也存在不可忽视的问题, 虽然相对于游离药物, 纳米制剂可以提高其在肿瘤组织的浓度, 但是相比于总的给药剂量, 在肿瘤组织中的累积药量依然很低。大量纳米粒分布于肝、肾等脏器, 可能导致长期毒性, 这也是限制纳米制剂发展的重要因素。纳米制剂评价体系的建立对其发展也十分必要, 系统地评价纳米制剂的优劣将为新型纳米制剂的研发提供参考。
| [1] | Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66: 115–132. DOI:10.3322/caac.21338 |
| [2] | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017[J]. CA Cancer J Clin, 2017, 67: 7–30. DOI:10.3322/caac.21387 |
| [3] | Pezzilli R, Fabbri D, Imbrogno A. Pancreatic ductal adenocarcinoma screening:new perspectives[J]. World J Gastroenterol, 2012, 18: 4973–4977. DOI:10.3748/wjg.v18.i36.4973 |
| [4] | Feldmann G, Maitra A. Molecular genetics of pancreatic ductal adenocarcinomas and recent implications for translational efforts[J]. J Mol Diagn, 2008, 10: 111–122. DOI:10.2353/jmoldx.2008.070115 |
| [5] | Hidalgo M. Pancreatic cancer[J]. New Engl J Med, 2010, 362: 1605–1617. DOI:10.1056/NEJMra0901557 |
| [6] | Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid":epidemiology of and prognostic factors for neuroendocrine tumors in 35825 cases in the United States[J]. J Clin Oncol, 2008, 26: 3063–3072. DOI:10.1200/JCO.2007.15.4377 |
| [7] | Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma[J]. Ann Surg Oncol, 2007, 14: 3492–3500. DOI:10.1245/s10434-007-9566-6 |
| [8] | Salvia R, Festa L, Butturini G. Pancreatic cystic tumors[J]. Minerva Chir, 2004, 59: 185–207. |
| [9] | Li D, Xie K, Wolff R, et al. Pancreatic cancer[J]. Lancet, 2004, 363: 1049–1057. DOI:10.1016/S0140-6736(04)15841-8 |
| [10] | Khare V, Alam N, Saneja A, et al. Targeted drug delivery systems for pancreatic cancer[J]. J Biomed Nanotechnol, 2014, 10: 3462–3482. DOI:10.1166/jbn.2014.2036 |
| [11] | Hawes RH, Xiong Q, Waxman I, et al. A multispecialty approach to the diagnosis and management of pancreatic cancer[J]. Am J Gastroenterol, 2000, 95: 17–31. DOI:10.1111/j.1572-0241.2000.01699.x |
| [12] | Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world[J]. HPB (Oxford), 2008, 10: 58–62. DOI:10.1080/13651820701883148 |
| [13] | Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer:a phase Ⅲ trial of the National Cancer Institute of Canada Clinical Trials Group[J]. J Clin Oncol, 2007, 25: 1960–1966. DOI:10.1200/JCO.2006.07.9525 |
| [14] | Gao HL, Jiang XG. The development of novel tumor targeting delivery strategy[J]. Acta Pharm Sin (药学学报), 2016, 51: 272–280. |
| [15] | Qiao MX, Zhang XJ, Ba S, et al. Progress in the study of targeted drug delivery systems for cancer stem cells[J]. Acta Pharm Sin (药学学报), 2013, 48: 477–483. |
| [16] | Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma[J]. Mol Cancer Ther, 2007, 6: 1186–1197. DOI:10.1158/1535-7163.MCT-06-0686 |
| [17] | Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment[J]. Clin Cancer Res, 2012, 18: 4266–4276. DOI:10.1158/1078-0432.CCR-11-3114 |
| [18] | De Jong G, van Dijk JP, van Eijk HG. The biology of transferrin[J]. Clin Chim Acta, 1990, 190: 1–46. DOI:10.1016/0009-8981(90)90278-Z |
| [19] | Qian ZM, Li H, Sun H, et al. Targeted drug delivery via the transferrin receptor mediated endocytosis pathway[J]. Pharmacol Rev, 2002, 54: 561–587. DOI:10.1124/pr.54.4.561 |
| [20] | Ryschich E, Huszty G, Knaebel HP, et al. Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas[J]. Eur J Cancer, 2004, 40: 1418–1422. DOI:10.1016/j.ejca.2004.01.036 |
| [21] | Frasco MF, Almeida GM, Santos-Silva F, et al. Transferrin surface-modified PLGA nanoparticles-mediated delivery of a proteasome inhibitor to human pancreatic cancer cells[J]. J Biomed Mater Res A, 2015, 103: 1476–1484. DOI:10.1002/jbm.a.35286 |
| [22] | Camp ER, Wang C, Little EC, et al. Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy[J]. Cancer Gene Ther, 2013, 20: 222–228. DOI:10.1038/cgt.2013.9 |
| [23] | Sebastian S, Settleman J, Reshkin SJ, et al. The complexity of targeting EGFR signalling in cancer:from expression to turnover[J]. Biochim Biophys Acta, 2006, 1766: 120–139. |
| [24] | Lemoine NR, Hughes CM, Barton CM, et al. The epidermal growth factor receptor in human pancreatic cancer[J]. J Pathol, 1992, 166: 7–12. DOI:10.1002/(ISSN)1096-9896 |
| [25] | Zhang Y, Banerjee S, Wang Z, et al. Antitumor activity of epidermal growth factor receptor-related protein is mediated by inactivation of ErbB receptors and nuclear factor-kappaB in pancreatic cancer[J]. Cancer Res, 2006, 66: 1025–1032. DOI:10.1158/0008-5472.CAN-05-2968 |
| [26] | Furukawa T. Molecular targeting therapy for pancreatic cancer:current knowledge and perspectives from bench to bedside[J]. J Gastroenterol, 2008, 43: 905–911. DOI:10.1007/s00535-008-2226-1 |
| [27] | Mondal G, Kumar V, Shukla SK, et al. EGFR-targeted polymeric mixed micelles carrying gemcitabine for treating pancreatic cancer[J]. Biomacromolecules, 2016, 17: 301–313. DOI:10.1021/acs.biomac.5b01419 |
| [28] | Li Z, Zhao R, Wu X, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics[J]. FASEB J, 2005, 19: 1978–1985. DOI:10.1096/fj.05-4058com |
| [29] | Xu J, Singh A, Amiji MM. Redox-responsive targeted gelatin nanoparticles for delivery of combination wt-p53 expressing plasmid DNA and gemcitabine in the treatment of pancreatic cancer[J]. BMC Cancer, 2014, 14: 75. DOI:10.1186/1471-2407-14-75 |
| [30] | Mondal G, Almawash S, Chaudhary AK, et al. EGFR-targeted cationic polymeric mixed micelles for codelivery of gemcitabine and miR-205 for treating advanced pancreatic cancer[J]. Mol Pharm, 2017, 14: 3121–3133. DOI:10.1021/acs.molpharmaceut.7b00355 |
| [31] | Bandyopadhyay A, Raghavan S. Defining the role of integrin αvβ6 in cancer[J]. Curr Drug Targets, 2009, 10: 645–652. DOI:10.2174/138945009788680374 |
| [32] | Hynes RO. Integrins:bidirectional, allosteric signaling machines[J]. Cell, 2002, 110: 673–687. DOI:10.1016/S0092-8674(02)00971-6 |
| [33] | Wickham TJ, Mathias P, Cheresh DA, et al. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment[J]. Cell, 1993, 73: 309–319. DOI:10.1016/0092-8674(93)90231-E |
| [34] | Wang X, Huang DY, Huong SM, et al. Integrin αvβ3 is a coreceptor for human cytomegalovirus[J]. Nat Med, 2005, 11: 515–521. DOI:10.1038/nm1236 |
| [35] | Brooks PC, Strömblad S, Klemke R, et al. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin[J]. J Clin Invest, 1995, 96: 1815–1822. DOI:10.1172/JCI118227 |
| [36] | Brooks PC, Montgomery AM, Rosenfeld M, et al. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels[J]. Cell, 1994, 79: 1157–1164. DOI:10.1016/0092-8674(94)90007-8 |
| [37] | Ji S, Xu J, Zhang B, et al. RGD-conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy[J]. Cancer Biol Ther, 2012, 13: 206–215. DOI:10.4161/cbt.13.4.18692 |
| [38] | Murphy EA, Majeti BK, Barnes LA, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis[J]. Proc Natl Acad Sci U S A, 2008, 105: 9343–9348. DOI:10.1073/pnas.0803728105 |
| [39] | Wang G, Sun B, Jiang HC. Blood-pancreatic barrier and its clinical significance[J]. Chin J Prac Surg (中国实用外科杂志), 2008, 28: 389–391. |
| [40] | Li J, Zhang J, Fu Y, et al. Dual pancreas- and lung-targeting therapy for local and systemic complications of acute pancreatitis mediated by a phenolic propanediamine moiety[J]. J Control Release, 2015, 212: 19–29. DOI:10.1016/j.jconrel.2015.06.011 |
| [41] | Luo S, Li PW, Li S, et al. N, N-Dimethyl tertiary amino group mediated dual pancreas- and lung-targeting therapy against acute pancreatitis[J]. Mol Pharm, 2017, 14: 1771–1781. DOI:10.1021/acs.molpharmaceut.7b00028 |
| [42] | Shen M, Xu YY, Sun Y, et al. Preparation of a thermosensitive Gel composed of a mPEG-PLGA-PLL-cRGD nanodrug delivery system for pancreatic tumor therapy[J]. ACS Appl Mater Interfaces, 2015, 7: 20530–20537. DOI:10.1021/acsami.5b06043 |
| [43] | Blasi F, Carmeliet P. uPAR:a versatile signalling orchestrator[J]. Nat Rev Mol Cell Biol, 2002, 3: 932–943. |
| [44] | Solberg H, Ploug M, Høyer-Hansen G, et al. The murine receptor for urokinase-type plasminogen activator is primarily expressed in tissues actively undergoing remodeling[J]. J Histochem Cytochem, 2001, 49: 237–246. DOI:10.1177/002215540104900211 |
| [45] | Hemsen A, Riethdorf L, Brünner N, et al. Comparative evaluation of urokinase-type plasminogen activator receptor expression in primary breast carcinomas and on metastatic tumor cell[J]. Int J Cancer, 2003, 107: 903–909. DOI:10.1002/ijc.v107:6 |
| [46] | Salden M, Splinter TAW, Peters HA, et al. The urokinase-type plasminogen activator system in resected non-small-cell lung cancer[J]. Ann Oncol, 2000, 11: 327–332. DOI:10.1023/A:1008312801800 |
| [47] | Xue A, Scarlett CJ, Jackson CJ, et al. Prognostic significance of growth factors and the urokinase-type plasminogen activator system in pancreatic ductal adenocarcinoma[J]. Pancreas, 2008, 36: 160–167. DOI:10.1097/MPA.0b013e31815750f0 |
| [48] | Lee GY, Qian WP, Wang L, et al. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer[J]. ACS Nano, 2013, 7: 2078–2089. DOI:10.1021/nn3043463 |
| [49] | Hu Y, Chi C, Wang S, et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer[J]. Adv Mater, 2017, 29: 1700448. DOI:10.1002/adma.v29.33 |
| [50] | Gao N, Bozeman EN, Qian W, et al. Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery[J]. Theranostics, 2017, 7: 1689–1704. DOI:10.7150/thno.18125 |
| [51] | Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer[J]. Gut, 2011, 60: 861–868. DOI:10.1136/gut.2010.226092 |
| [52] | Coleman JD, Thompson JT, Smith RW 3rd, et al. Role of peroxisome proliferator-activated receptor β/δ and B-cell lymphoma-6 in regulation of genes involved in metastasis and migration in pancreatic cancer cells[J]. PPAR Res, 2013. DOI:10.1155/2013/121956 |
| [53] | Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity[J]. Nat Rev Immunol, 2004, 4: 617–629. DOI:10.1038/nri1418 |
| [54] | Jones LE, Humphreys MJ, Campbell F, et al. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer:increased expression of matrix metalloproteinase-7 predicts poor survival[J]. Clin Cancer Res, 2004, 10: 2832–2845. DOI:10.1158/1078-0432.CCR-1157-03 |
| [55] | Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition?[J]. Nat Rev Drug Discov, 2014, 13: 904–927. DOI:10.1038/nrd4390 |
| [56] | Shay G, Lynch CC, Fingleton B. Moving targets:emerging roles for MMPs in cancer progression and metastasis[J]. Matrix Biol, 2015, 44-46: 200–206. DOI:10.1016/j.matbio.2015.01.019 |
| [57] | Kulkarni PS, Haldar MK, Nahire RR, et al. MMP-9 responsive PEG cleavable nanovesicles for efficient delivery of chemotherapeutics to pancreatic cancer[J]. Mol Pharm, 2014, 11: 2390–2399. DOI:10.1021/mp500108p |
| [58] | Ji T, Li S, Zhang Y, et al. An MMP-2 responsive liposome integrating antifibrosis and chemotherapeutic drugs for enhanced drug perfusion and efficacy in pancreatic cancer[J]. ACS Appl Mater Interfaces, 2016, 8: 3438–3445. DOI:10.1021/acsami.5b11619 |
| [59] | Toole BP. Hyaluronan:from extracellular glue to pericellular cue[J]. Nat Rev Cancer, 2004, 4: 528–539. DOI:10.1038/nrc1391 |
| [60] | Nguyen NC, Taalab K, Osman MM. Decreased blood flow with increased metabolic activity:a novel sign of pancreatic tumor aggressiveness[J]. Clin Cancer Res, 2010, 16: 367. |
| [61] | Xie D, Xie K. Pancreatic cancer stromal biology and therapy[J]. Genes Dis, 2015, 2: 133–143. DOI:10.1016/j.gendis.2015.01.002 |
| [62] | Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer[J]. Br J Cancer, 2013, 108: 1–8. DOI:10.1038/bjc.2012.569 |
| [63] | Kohi S, Sato N, Cheng XB, et al. A novel epigenetic mechanism regulating hyaluronan production in pancreatic cancer cells[J]. Clin Exp Metastasis, 2016, 33: 225–230. DOI:10.1007/s10585-015-9771-9 |
| [64] | Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma[J]. Cancer Cell, 2012, 21: 418–429. DOI:10.1016/j.ccr.2012.01.007 |
| [65] | Hingorani SR, Harris WP, Beck JT, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer[J]. Clin Cancer Res, 2016, 22: 2848–2854. DOI:10.1158/1078-0432.CCR-15-2010 |
| [66] | Buckway B, Wang Y, Ray A, et al. Overcoming the stromal barrier for targeted delivery of HPMA copolymers to pancreatic tumors[J]. Int J Pharm, 2013, 456: 202–211. DOI:10.1016/j.ijpharm.2013.07.067 |
| [67] | Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment[J]. Gastroenterology, 2013, 144: 1210–1219. DOI:10.1053/j.gastro.2012.11.037 |
| [68] | Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance[J]. J Cell Sci, 2010, 123: 4195–4200. DOI:10.1242/jcs.023820 |
| [69] | Sideras K, Braat H, Kwekkeboom J, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies[J]. Cancer Treat Rev, 2014, 40: 513–522. DOI:10.1016/j.ctrv.2013.11.005 |
| [70] | Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells:partners in crime with pancreatic cancer cells[J]. Cancer Res, 2008, 68: 2085–2093. DOI:10.1158/0008-5472.CAN-07-2477 |
| [71] | Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells[J]. J Gastroenterol, 2009, 44: 249–260. DOI:10.1007/s00535-009-0013-2 |
| [72] | Apte MV, Pirola RC, Wilson JS. Battle-scarred pancreas:role of alcohol and pancreatic stellate cells in pancreatic fibrosis[J]. J Gastroenterol Hepatol, 2006, 21: S97–S101. DOI:10.1111/jgh.2006.21.issue-s3 |
| [73] | Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound paclitaxel in pancreatic ductal adenocarcinoma[J]. Mol Cancer Ther, 2014, 13: 2583–2594. DOI:10.1158/1535-7163.MCT-14-0028 |
| [74] | Yalcin S, Erkan M, Ünsoy G, et al. Effect of gemcitabine and retinoic acid loaded PAMAM dendrimer-coated magnetic nanoparticles on pancreatic cancer and stellate cell lines[J]. Biomed Pharmacother, 2014, 68: 737–743. DOI:10.1016/j.biopha.2014.07.003 |
| [75] | Zhao J, Wu C, Abbruzzese J, et al. Cyclopamine-loaded core-cross-linked polymeric micelles enhance radiation response in pancreatic cancer and pancreatic stellate cells[J]. Mol Pharm, 2015, 12: 2093–2100. DOI:10.1021/mp500875f |
| [76] | Ji T, Lang J, Wang J, et al. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy[J]. ACS Nano, 2017, 11: 8668–8678. DOI:10.1021/acsnano.7b01026 |
| [77] | Balkwill FR, Mantovani A. Cancer-related inflammation:common themes and therapeutic opportunities[J]. Semin Cancer Biol, 2012, 22: 33–40. DOI:10.1016/j.semcancer.2011.12.005 |
| [78] | Fan QM, Jing YY, Yu GF, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma[J]. Cancer Lett, 2014, 352: 160–168. DOI:10.1016/j.canlet.2014.05.008 |
| [79] | Kurahara H, Takao S, Kuwahata T, et al. Clinical significance of folate receptor β-expressing tumor-associated macrophages in pancreatic cancer[J]. Ann Surg Oncol, 2012, 19: 2264–2271. DOI:10.1245/s10434-012-2263-0 |
| [80] | Ségaliny AI, Mohamadi A, Dizier B, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment[J]. Int J Cancer, 2015, 137: 73–85. DOI:10.1002/ijc.29376 |
| [81] | Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs[J]. Cancer Cell, 2011, 20: 538–549. DOI:10.1016/j.ccr.2011.08.025 |
| [82] | Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis[J]. Cell, 2010, 141: 39–51. DOI:10.1016/j.cell.2010.03.014 |
| [83] | Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation:a magic bullet[J]. Science, 2013, 339: 286–291. DOI:10.1126/science.1232227 |
| [84] | Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses[J]. Cancer Res, 2013, 73: 1128–1141. DOI:10.1158/0008-5472.CAN-12-2731 |
| [85] | Wang-Gillam A, Nywening TM, Sanford DE, et al. Phase IB study of FOLFIRINOX plus PF-04136309 in patients with borderline resectable and locally advanced pancreatic adenocarcinoma (PC)[J]. J Clin Oncol, 2015, 33: 338. |
| [86] | Marhaba R, Klingbeil P, Nuebel T, et al. CD44 and EpCAM:cancer-initiating cell markers[J]. Curr Mol Med, 2008, 8: 784–804. DOI:10.2174/156652408786733667 |
| [87] | Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells[J]. Cancer Res, 2007, 67: 1030–1037. DOI:10.1158/0008-5472.CAN-06-2030 |
| [88] | Wei X, Senanayake T H, Warren G, et al. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors[J]. Bioconjug Chem, 2013, 24: 658–668. DOI:10.1021/bc300632w |
| [89] | Kesharwani P, Banerjee S, Padhye S, et al. Hyaluronic acid engineered nanomicelles loaded with 3, 4-difluorobenzylidene curcumin for targeted killing of CD44+ stem-like pancreatic cancer cells[J]. Biomacromolecules, 2015, 16: 3042–3053. DOI:10.1021/acs.biomac.5b00941 |
| [90] | Kota J, Hancock J, Kwon J, et al. Pancreatic cancer:stroma and its current and emerging targeted therapies[J]. Cancer Lett, 2017, 391: 38–49. DOI:10.1016/j.canlet.2016.12.035 |
| [91] | Zhao J, Li J, Schlöer HA, et al. Targeting cancer stem cells and their niche:current therapeutic implications and challenges in pancreatic cancer[J]. Stem Cells Int, 2017. DOI:10.1155/2017/6012810 |
| [92] | Loo K, Daud AI. Inhibitors of cytotoxic T lymphocyte antigen 4 and programmed death 1/programmed death 1 ligand for metastatic melanoma, dual versus monotherapy-summary of advances and future directions for studying these drugs[J]. Cancer J, 2017, 23: 3–9. DOI:10.1097/PPO.0000000000000238 |
| [93] | Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer[J]. Oncologist, 2017, 22: 81–88. DOI:10.1634/theoncologist.2016-0189 |
| [94] | Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer[J]. Cancer Lett, 2017, 407: 57–65. DOI:10.1016/j.canlet.2017.08.006 |
| [95] | Guo S, Contratto M, Miller G, et al. Immunotherapy in pancreatic cancer:unleash its potential through novel combinations[J]. World J Clin Oncol, 2017, 8: 230–240. DOI:10.5306/wjco.v8.i3.230 |
| [96] | Lu J, Liu X, Liao YP, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppress sion[J]. Nat Commun, 2017, 8: 1811. DOI:10.1038/s41467-017-01651-9 |
 2018, Vol. 53
2018, Vol. 53


