肥胖和糖尿病等代谢综合征可以引起轻、中度认知功能障碍和学习记忆能力下降[1]。血液中高游离脂肪酸状态是肥胖和糖尿病患者的共同病理特征之一, 肥胖患者血浆脂肪酸含量约是正常人的1.2倍[2], 糖尿病患者夜间和餐后血浆脂肪酸含量分别是正常人的1.5和3倍左右[3], 且代谢综合征患者大脑对脂肪酸的摄取率也增加约50%[4]。临床研究表明, 在60岁以上的老年群体中血浆非饱和脂肪酸水平与认知功能呈负相关[5]。动物实验也表明, 高脂和肥胖可引起认知能力下降[6]。由此可见脂毒性可能是引起代谢综合征患者大脑功能损伤的一个重要因素。人脑约由1×1012个细胞组成, 其中10%为神经元, 其余主要为神经胶质细胞, 而星形胶质细胞是中枢神经系统数量最多的神经胶质细胞类型。星形胶质细胞具有许多重要的生理功能, 如星形胶质细胞包绕神经元并填充神经元间的间隙起结构支撑和隔离绝缘的作用并维持突触传递, 参与血脑屏障的构成和脑血流量的调节, 维持大脑内环境K+、H+离子浓度稳态, 代谢谷氨酸、γ-氨基丁酸等神经递质, 分泌神经营养因子促进神经元生长和修复, 协助神经元抗氧化损伤等[7, 8]。星形胶质细胞正常生理功能的异常或丧失是亨廷顿病、阿尔兹海默病、帕金森病和肌萎缩性脊髓侧索硬化症等神经退行性疾病的重要病理机制[9]。综上所述, 脂肪酸对星形胶质细胞的损伤可能是引起代谢综合征相关脑病的重要机制。棕榈酸(palmitic acid, PA)是具有16个碳原子的饱和脂肪酸, 也是人血浆和脑脊液中含量最高的一类脂肪酸, 约占总游离脂肪酸的25%[10]。因而本文重点研究PA进入星形胶质细胞的转运和对星形胶质细胞的损伤机制。
已有文献报道, PA引起的巨噬细胞[11]和肾脏足细胞[12]凋亡是通过分化抗原簇36 (cluster of differentiation, CD36)脂肪酸转运功能介导的。CD36又名脂肪酸转位酶, 是一种广泛存在于巨噬细胞、血小板、内皮细胞、心肌细胞、周皮细胞和胰岛细胞等细胞的膜糖蛋白, 其主要功能之一是参与细胞对脂肪酸的摄取[13]。然而尚未有文献报道CD36在星形胶质细胞中的功能。因此本研究采用PA损伤模型, 研究CD36在PA诱导的星形胶质细损伤中的功能及CD36抑制剂可能的保护作用。
材料与方法动物 新生12 h内的Wistar乳鼠(SPF级), 购于北京维通利华实验动物技术有限公司, 动物证号SCXK (京) 2015-0001。全部实验和操作过程均符合中国医学科学院、北京协和医学院动物保护委员会规定的相关条例。
药品和试剂 DMEM/F12培养基、胎牛血清购自美国Gibco公司; 棕榈酸钠、棕榈油酸、BSA、MTT购自于美国Sigma-Aldrich公司; CD36抑制剂sulfo- N-succinimidyloleate (SSO)购自于美国Cayman Chemical公司; 4, 4-difluoro-5, 7-dimethyl-4-bora-3a, 4a- diaza-s-indacene-3-hexadecanoic acid (BODIPY FL C16)购自于美国Invitrogen公司; CD36 (SMΦ)小鼠单克隆抗体购自于美国Santa Cruz公司; 山羊抗小鼠IgG H & L (Alexa Fluor® 594)、IP3介导的钙释放抑制剂2-aminoethyl diphenylborinate (APB)购自于美国Abcam公司; 一步法TUNEL细胞凋亡检测试剂盒(绿色荧光)购自于中国Beyotime公司; SERCA抑制剂thapsigargin (TG)购自于中国JK chemical公司; 5-(and 6)-carboxy-2', 7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA)购自于中国ABP Biotech公司; FLIPR Calcium 6 Assay Kit购自于美国Molecular Devices公司。
星形胶质细胞的原代培养 新生12 h内的Wistar乳鼠取脑, 剥除脑膜, 分离皮层组织并剪碎, 37 ℃下经0.125%胰酶消化20 min, 终止消化后使用玻璃滴管轻柔吹打, 用40 μm筛网过滤。离心后将沉淀重悬于含10%胎牛血清的DMEM/F12培养基, 接种于包被多聚赖氨酸的培养瓶中, 置于37 ℃的CO2培养箱中进行培养。接种24 h后, 换全液。之后每3天进行换液。约12天后星形胶质细胞铺满培养瓶底部, 将培养瓶放入恒温振荡器中, 37 ℃下250 r·min-1振荡培养5 h后消化传代, 继续培养24 h后进行实验。
MTT细胞活性检测 星形胶质细胞以每毫升3×105个细胞数接种于96孔板中, 经相应处理后, 每孔加入MTT (5 mg·mL-1) 10 μL, 将细胞板置于37 ℃的CO2培养箱内继续孵育4 h。弃去培养基, 每孔加入DMSO 150 μL, 振荡15 min使结晶充分溶解, 在酶标仪上以波长570 nm检测每孔的OD值。
TUNEL细胞凋亡检测 按照一步法TUNEL细胞凋亡检测试剂盒(绿色荧光)说明书进行操作。星形胶质细胞以每毫升3×105个细胞数接种于96孔板中, 经相应处理后, 弃去培养基, HBSS洗涤1次。含0.3% Triton X-100的PBS室温孵育破膜5 min, 每孔加入TUNEL检测液50 μL, 37 ℃避光孵育1 h, HBSS洗涤3次后用抗荧光淬灭封片液封片, 激发波长/发射波长为488/530 nm在荧光显微镜下观察, 绿色荧光细胞为凋亡细胞。每孔随机选取3个视野进行统计。
细胞内钙释放检测 星形胶质细胞以每毫升5×105个细胞数接种于Costar 384孔黑色透明底酶标板内, 生长至完全汇合时开始实验。按照检测试剂盒说明书用不含钙镁HBSS配制适量的FLIPR Calcium 6 Assay染液, 并加入适量的丙磺舒阻止细胞将荧光探针被动运输或主动分泌到胞外。每孔加入染液20 μL, CD36抑制剂组同时加入0.2 mmol·L-1 SSO, 室温避光孵育2 h后进行FLIPR实时荧光检测, 每1 s读取1次荧光值, 1 min时加入PA 5 μL使其终浓度为0.2 mmol·L-1, 继续读取荧光值9 min。
脂肪酸摄取检测 星形胶质细胞以每毫升1×105个细胞数接种于6孔板内。实验开始前, 对SSO+ BODIPY组加入0.2 mmol·L-1 SSO孵育30 min, 其他组不进行处理。配制10 μmol·L-1 BODIPY工作液。弃去6孔板内的培养基, 每孔加入预热的BODIPY工作液1 mL, CO2培养箱内37 ℃孵育20 min后分别加入预冷的HBSS 2 mL, 弃去6孔板内液体, HBSS洗1次, 加入0.25%胰酶500 μL放置于CO2培养箱内37 ℃消化1 min, 加入FACS缓冲液(含10% FBS, 10 mmol·L-1 EDTA的HBSS, 冰浴预冷) 500 μL终止消化, 收集细胞悬液, 4 ℃, 600 ×g离心5 min, 弃上清, 加入FACS缓冲液500 μL重悬细胞, 流式上机检测2×104个细胞, 检测参数为激发波长/发射波长为488/510 nm, 统计各组平均荧光强度。
免疫荧光染色 星形胶质细胞以每毫升1×105个细胞数接种于96孔板内培养24 h, 弃去培养基, 用0.01 mol·L-1 PBS洗涤2遍, 4% PFA室温固定1 h, PBS洗涤3次, 5%山羊血清室温封闭30 min。弃去封闭液, CD36抗体(SMΦ)小鼠单克隆抗体(1:200稀释), 4 ℃孵育过夜。PBS洗涤3次, 加入山羊抗小鼠IgG H & L (Alexa Fluor® 594) (1:500稀释)室温孵育2 h, PBS洗涤3次, DAPI染核, 荧光显微镜下观察并进行图像采集, DAPI的激发/发射波长为360/460 nm呈蓝色荧光, Alexa Fluor® 594的激发/发射波长为590/617 nm呈红色荧光。
ROS检测 星形胶质细胞以每毫升1×105个细胞数接种于Costar 96孔黑色透明底板内培养24 h, HBSS洗涤2次, 加入CM-H2DCFDA 50 μL (20 μmol·L-1)于CO2培养箱内37 ℃避光孵育30 min。HBSS洗涤3次, 每孔加入HBSS 100 μL, 荧光酶标仪进行读数, 激发波长/发射波长为495/525 nm。
结果统计 所有结果以mean ± SEM表示。采用单因素方差分析(one-way ANOVA)结合Dunnett's post hoc test检验比较各组间差异, P < 0.05认为存在显著性差异。
结果 1 PA对星形胶质细胞形态、活性和凋亡的影响体外培养条件下, Wistar大鼠皮层原代星形胶质细胞呈不规则的扁平状, 单层铺路石样排列。PA损伤后使星形胶质细胞收缩变圆, 胞体变小, 间隙增大, 少数细胞悬浮脱落(图 1A)。PA可以浓度依赖性地引起星形胶质细胞活性下降, 0.2 mmol·L-1 PA损伤24 h可以使星形胶质细胞活性下降(36.1 ± 1.2) % (P < 0.001, 图 1B)。TUNEL染色结果(图 1C)表明, 0.2 mmol·L-1 PA损伤24 h后星形胶质细胞凋亡率为(16.6 ± 1.5) %, 与对照组相比凋亡率显著增加(P < 0.01), 同等浓度的棕榈油酸(palmitoleic acid, PO)对星形胶质细胞凋亡无影响(图 1D)。
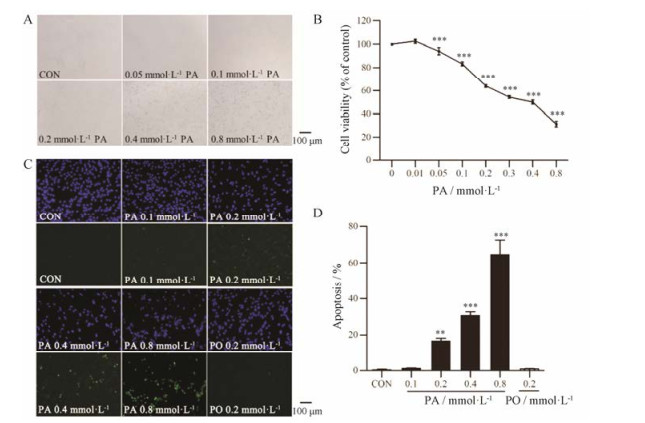
|
Figure 1 Effects of palmitic acid (PA) on astrocyte cell morphology (A), viability (B, n = 8) and apoptosis (C and D, n = 5). Astrocytes were treated with different doses of palmitate for 24 h, and then cell viability was detected by MTT assay, cell apoptosis was detected by TUNEL assay. Nuclei were stained by DAPI (blue), and apoptotic cells were stained by TUNEL (green). x± s. **P < 0.01, ***P < 0.001 vs CON group |
细胞免疫荧光染色表明, CD36在星形胶质细胞表达(图 2A)。BODIPY FL C16为氟化硼二吡咯荧光标记的PA类似物, 在激发波长/发射波长为488/ 515 nm时可在荧光显微镜下观察到绿色荧光。10 μmol·L-1 BODIPY孵育20 min后可观察到星形胶质细胞内有较强的绿色荧光, 提前使用0.2 mmol·L-1 CD36抑制剂SSO孵育或0.2 mmol·L-1 PA孵育30 min, 再用10 μmol·L-1 BODIPY FL C16孵育20 min后可观察到星形胶质细胞内绿色荧光较弱, 表明SSO可以抑制星形胶质细胞对BODIPY FL C16的摄取(图 2B)。流式细胞仪定量分析表明, 0.2 mmol·L-1 SSO可使星形胶质细胞对BODIPY FL C16的摄取率降低(69.9 ± 1.9) % (P < 0.001), PA通过竞争CD36抑制星形胶质细胞对BODIPY FL C16的摄取(图 2C和D)。
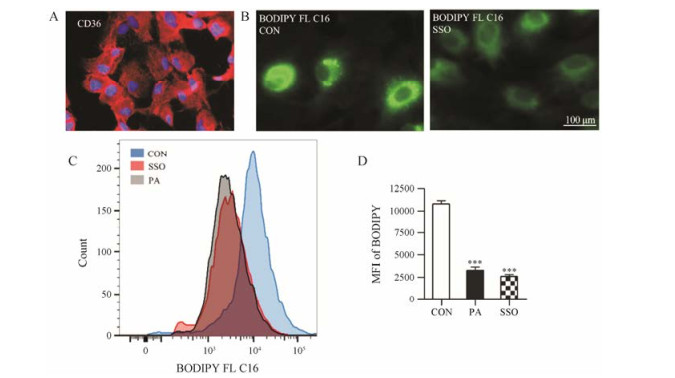
|
Figure 2 Cluster of differentiation (CD36) meditates the uptake of PA by astrocytes. A: Expression of CD36 in astrocytes confirmed by cellular immunofluorescence staining. Nuclei were stained by DAPI (blue), and CD36 were stained by Alexa Fluor® 594 (red); B: Observation of BODIPY FL C16 uptake and effect of CD36 inhibitor sulfo-N-succinimidyloleate (SSO) by fluorescence microscope; C: Quantitative analysis of BODIPY FL C16 uptake and effects of CD36 inhibitor and PA by flow cytometry (n = 3, mean ± SEM). MFI: Mean fluorescence intensity. ***P < 0.001 vs CON group |
SSO是CD36抑制剂, 广泛作为工具药用于CD36相关功能的研究[14-17]。0.2 mmol·L-1 PA损伤星形胶质细胞24 h后细胞凋亡率为(16.6 ± 1.5) %, 与对照组相比具有显著差异(P < 0.001), 而PA+SSO组星形胶质细胞凋亡率为(2.8 ± 0.6) %, 与模型组相比具有显著差异(P < 0.001, 图 3A和B)。0.2 mmol·L-1 PA损伤24 h可以使星形胶质细胞活性下降(51.0 ± 0.7) %, 与对照组相比具有显著差异(P < 0.001), 而PA+SSO组星形胶质细胞活性下降(15.7 ± 1.9) %, 与模型组相比具有显著差异(P < 0.001, 图 3C)。以上结果表明, SSO通过抑制CD36减少星形胶质细胞对PA的摄取, 从而对星形胶质细胞起保护作用。
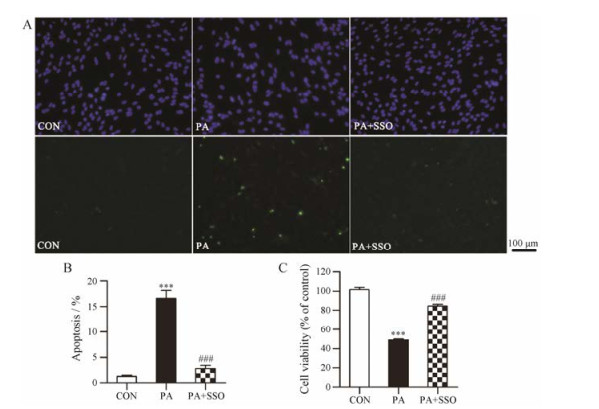
|
Figure 3 Effects of SSO on PA-induced apoptosis (A and B, n = 5) and cell viability decrease (C, n = 8) in astrocytes. Astrocytes were treated with 0.2 mmol·L-1 PA or 0.2 mmol·L-1 PA + 0.2 mmol·L-1 SSO for 24 h, and then cell viability was detected by MTT assay, cell apoptosis was detected by TUNEL assay. Nuclei were stained by DAPI (blue), and apoptotic cells were stained by TUNEL (green). Mean ± SEM. ***P < 0.001 vs CON group; ###P < 0.001 vs PA group |
利用钙离子荧光染料Calcium 6 Assay染液和FLIPR实时荧光记录系统观察PA对星形胶质细胞内钙离子水平的影响。0.2 mmol·L-1 PA可使星形胶质细胞内钙离子水平显著增加, 同等浓度的PO无明显作用(图 4A和B)。正常生理情况下细胞内钙离子水平升高可能由胞外钙离子内流或内质网钙离子释放引起, 因本实验使用不含钙、镁的HBSS作为外液, 因此PA引起的星形胶质细胞内钙离子升高来自于内质网钙离子释放。同时加入0.2 mmol·L-1 CD36抑制剂SSO或10 μmol·L-1 IP3R抑制剂APB可以显著抑制PA诱导的星形胶质细胞内质网钙离子释放(P < 0.001, 图 4A和B)。毒胡萝卜素(thapsigagin, TG)是内质网Ca2+-ATP酶抑制剂, 能够阻止细胞内钙离子向内质网的转运。1 μmol·L-1 TG即可引起内质网钙离子衰竭, TG引起的细胞内钙离子升高的水平可间接反映内质网钙储存量[18]。PA、PA+SSO或PA+APB分别与星形胶质细胞孵育4 h, 然后加入TG并观察细胞内钙离子升高水平。0.2 mmol·L-1 PA孵育后星形胶质细胞钙离子释放明显减少, 说明PA损伤使星形胶质细胞内质网钙离子储存能力显著下降(P < 0.001), 同时加入0.2 mmol·L-1 CD36抑制剂SSO或10 μmol·L-1 IP3R抑制剂APB对此具有一定的保护作用(P < 0.05, 图 4C和D)。
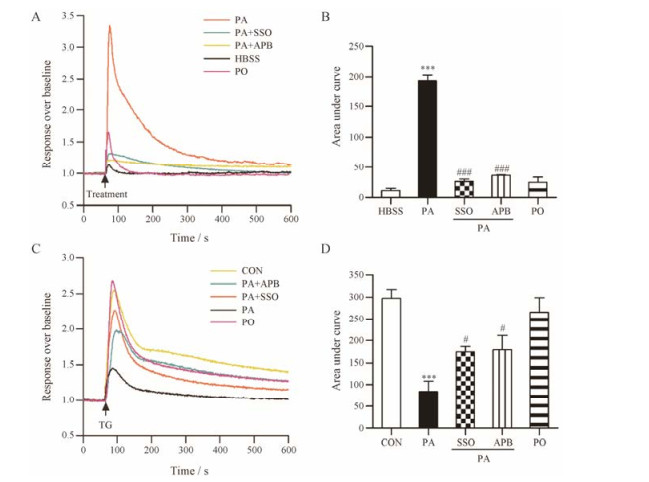
|
Figure 4 PA causes a significant [Ca2+]i elevation (A and B) and [Ca2+]ER depletion (C and D, n = 4) in astrocytes by activating IP3R, which can be suppressed by SSO. A, B: For HBSS, PA or PO group, astrocytes were incubated with Calcium 6 Assay working solution for 2 h, then calcium-induced fluorescence was captured by FLIPR real-time fluorescence recording system, at the time point of 60 s, HBSS, 0.2 mmol·L-1 PA or 0.2 mmol·L-1 PO was added. For PA+SSO or PA+APB group, astrocytes were incubated with Calcium 6 Assay working solution containing 0.2 mmol·L-1 SSO or 10 μmol·L-1 APB for 2 h, then calcium-induced fluorescence was captured by FLIPR real-time fluorescence recording system, at the time point of 60 s, 0.2 mmol·L-1 PA was added. n = 4, mean ± SEM. ***P < 0.001 vs HBSS group; ###P < 0.001 vs PA group. C, D: Astrocytes were incubated with 0.2 mmol·L-1 PA, 0.2 mmol·L-1 PO, 0.2 mmol·L-1 PA + 0.2 mmol·L-1 SSO or 0.2 mmol·L-1 PA + 10 μmol·L-1 APB for 4 h, then incubated with Calcium 6 Assay working solution for 2 h, calcium-induced fluorescence was captured by FLIPR real-time fluorescence recording system, at the time point of 60 s, 1 μmol·L-1 TG was added. n = 4, mean ± SEM. ***P < 0.001 vs CON group; #P < 0.05 vs PA group |
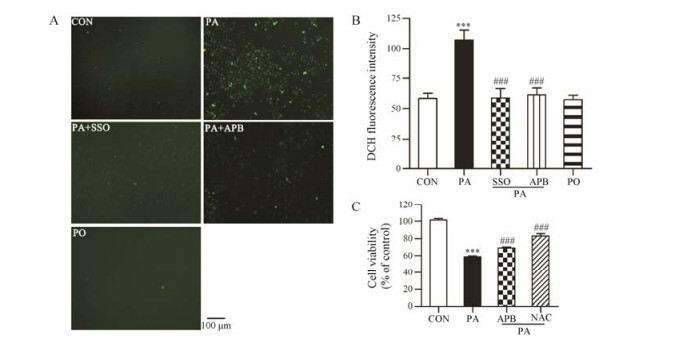
利用CM-H2DCFDA活性氧荧光探针检测星形胶质细胞内ROS水平, 与对照组相比, 棕榈损伤星形胶质细胞4 h后使细胞内ROS探针的荧光强度增加至1.9倍(P < 0.001), 同等浓度的PO对星形胶质细胞内ROS水平无明显影响。0.2 mmol·L-1 CD36抑制剂SSO或10 μmol·L-1 IP3R抑制剂APB可以阻断PA引起的星形胶质细胞内ROS的升高(P < 0.001) (图 5)。提示PA导致星形胶质细胞内氧化应激可能与其引起内质网钙离子释放导致细胞内钙超载有关。0.2 mmol·L-1 PA损伤24 h可以使星形胶质细胞活性下降(42.4 ± 0.6) %, 与对照组相比具有显著差异(P < 0.001), 而PA+APB组星形胶质细胞活性下降(32.5 ± 1.2) %, 与模型组相比具有显著差异(P < 0.05), PA+NAC组星形胶质细胞活性下降(18.7 ± 2.4) %, 与模型组相比具有显著差异(P < 0.001, 图 5C)。表明抑制细胞内钙离子释放和抗氧化均可抑制PA引起的星形胶质细胞损伤。
|
Figure 5 Effects of SSO on PA-induced ROS increase in astrocytes. A, B: Astrocytes were treated with 0.2 mmol·L-1 PA, 0.2 mmol·L-1 PA + 0.2 mmol·L-1 SSO or 0.2 mmol·L-1 PA + 10 μmol·L-1 APB for 4 h, then astrocytes were incubated with 25 μmol·L2-1 CM-HDCFDA for 30 min, fluorescence was measured by fluorescence microscope (A) or multi-mode microplate reader (B). C: Astrocytes were treated with 0.2 mmol·L-1 PA, 0.2 mmol·L-1 PA + 10 μmol·L-1 APB or 0.2 mmol·L-1 PA + 0.5 mmol·L-1 NAC for 24 h, and then cell viability was detected by MTT assay. n = 8, mean ± SEM. ***P < 0.001 vs CON group; ###P < 0.001 vs PA group |
本研究采用PA损伤模型, 研究CD36在PA诱导的星形胶质细胞凋亡中的作用, 及CD36抑制剂SSO的保护作用。实验结果表明: ① PA可引起原代星形胶质细胞活性显著下降, 细胞凋亡显著增加; ② CD36在星形胶质细胞中表达并介导PA的摄取; ③ PA可引起IP3R依赖的细胞内钙释放和内质网钙衰竭, 此作用可被CD36抑制剂所抑制; ④ PA引起星形胶质细胞ROS水平显著增加, 此作用可被CD36抑制剂及IP3R抑制剂所抑制; ⑤ CD36抑制剂、IP3R抑制剂和抗氧化剂均可显著抑制PA引起的星形胶质细胞损伤。
虽然PA损伤星形胶质细胞的机制研究已有报道, 但主要集中于氧化应激损伤[19, 20]、内质网应激[21]、线粒体损伤[19, 22]、神经酰胺毒性[23]和炎症反应[24, 25]等。PA的转运机制仍不清楚。本研究通过细胞免疫荧光方法证明, CD36在星形胶质细胞中表达(图 2A), 进一步通过荧光标记脂肪酸摄取实验证明, CD36在星形胶质细胞中也具有脂肪酸摄取功能(图 2B和C), 并且CD36抑制剂SSO可显著抑制PA引起的星形胶质细胞凋亡(图 3)。
内质网是细胞内重要的钙离子储存库, 正常生理状态下细胞内钙离子浓度需要维持在约100 nmol·L-1, 而内质网钙离子浓度维持在约1 mmol·L-1左右[26]。内质网上主要存在3种钙离子通道或转运体, 包括激活后引起内质网钙释放的IP3受体、雷诺丁受体(ryanodine receptor, RYR)及消耗ATP将细胞内钙离子逆浓度梯度转运到内质网的Ca2+-ATP酶(Ca2+- ATPase, SERCA)。在肾脏足细胞[12]、肝细胞[27]和心肌细胞[28]中, PA可以激活PLC, 增加IP3的产生, 引起内质网钙离子释放。本研究发现, PA也可引起星形胶质细胞内质网钙离子释放, 使细胞内钙离子水平显著增加(图 4A和B)。PA损伤使星形胶质细胞内质网钙离子含量显著下降, 失去钙储存库的功能(图 4C和D), 提示PA引起星形胶质细胞钙水平升高是持续性的。与本研究结果一致, Wang等[19]在BALB/c小鼠星形胶质细胞内也观察到了PA引起的细胞内钙离子水平增加, 然而Wong等[20]在SD大鼠星形胶质细胞内并未观察到此现象, 实验结果不一致的机制仍不清楚。但本研究的意义在于, PA引起的细胞内钙离子水平增加和内质网钙衰竭需要在CD36介导PA转运的前提下实现。此外, 非饱和脂肪酸PO (PA的类似物)不能引起内质网钙离子释放。
胞浆内钙浓度升高可通过多种途径对细胞产生损伤作用。一方面线粒体钙离子单向转运体(mito chondria Ca2+ uniporter, MCU)摄取钙增加引起线粒体内钙超载及其功能障碍[29]。另一方面胞浆内钙浓度升高同时会激活多种钙依赖性降解酶, 破坏磷脂、核酸和蛋白质等重要的生命大分子。此外, 胞浆内钙浓度升高通过激活黄嘌呤氧化酶(xanthine oxidase, XO)和磷脂酶A2 (phospholipase A2, PLA2)等增加细胞内ROS的产生[29]。与其他文献[19, 20, 22]报道一致, 本研究也发现PA可引起星形胶质细胞内ROS水平显著增加, IP3R抑制剂可以抑制PA诱导的ROS升高(图 5)。提示PA引起星形胶质细胞ROS水平升高是由于内质网钙释放和线粒体钙超载所致, 可能的损伤机制如图 6所示。

|
Figure 6 CD36 mediates the apoptosis of astrocytes induced by PA via calcium overload and oxidative stress |
综上所述, CD36是星形胶质细胞摄取脂肪酸的关键转运体, 脂肪酸进入细胞后激活IP3R促进内质网钙离子释放, 进而引起钙超载和氧化损伤。本研究提示抑制CD36功能, 可以从源头阻断PA的毒性, 可能成为潜在的保护星形胶质细胞免受脂肪酸损伤的靶点。
| [1] | Elias MF, Elias PK, Sullivan LM, et al. Obesity, diabetes and cognitive deficit:the Framingham Heart Study[J]. Neurobiol Aging, 2005, 26 Suppl 1: 11–16. |
| [2] | Arner P, Ryden M. Fatty acids, obesity and insulin resis-tance[J]. Obes Facts, 2015, 8: 147–155. DOI:10.1159/000381224 |
| [3] | Miles JM, Wooldridge D, Grellner WJ, et al. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects:effects of insulin sensitization therapy[J]. Diabetes, 2003, 52: 675–681. DOI:10.2337/diabetes.52.3.675 |
| [4] | Karmi A, Iozzo P, Viljanen A, et al. Increased brain fatty acid uptake in metabolic syndrome[J]. Diabetes, 2010, 59: 2171–2177. DOI:10.2337/db09-0138 |
| [5] | Marília B, Vencato PH, Luiza O, et al. Fatty acid status and its relationship to cognitive decline and homocysteine levels in the elderly[J]. Nutrients, 2014, 6: 3624–3640. DOI:10.3390/nu6093624 |
| [6] | Farr SA, Yamada KA, Butterfield DA, et al. Obesity and hypertriglyceridemia produce cognitive impairment[J]. Endocrinology, 2008, 149: 2628–2636. DOI:10.1210/en.2007-1722 |
| [7] | Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets[J]. Neurotherapeutics, 2010, 7: 338–353. DOI:10.1016/j.nurt.2010.07.006 |
| [8] | Liu DM, Zhang M, Jiang XM, et al. Protective effects and the mechanisms of donepezil and galantamine on neuronal injury induced by glucose deprivation/reoxygenation[J]. Acta Pharm Sin (药学学报), 2017, 52: 928–935. |
| [9] | Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease[J]. Cold Spring Harb Perspect Biol, 2015, 7: a020628. DOI:10.1101/cshperspect.a020628 |
| [10] | Pilitsis JG, Diaz FG, Wellwood JM, et al. Quantification of free fatty acids in human cerebrospinal fluid[J]. Neurochem Res, 2001, 26: 1265–1270. DOI:10.1023/A:1014227231130 |
| [11] | Kim DH, Cho YM, Lee KH, et al. Oleate protects macro-phages from palmitate-induced apoptosis through the downregulation of CD36 expression[J]. Biochem Biophys Res Commun, 2017, 488: 477–482. DOI:10.1016/j.bbrc.2017.05.066 |
| [12] | Xu S, Nam SM, Kim JH, et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress[J]. Cell Death Dis, 2015, 6: e1976. DOI:10.1038/cddis.2015.331 |
| [13] | Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells:CD36, FATP and FABPm[J]. Biochim Biophys Acta, 1999, 1441: 4–13. DOI:10.1016/S1388-1981(99)00137-7 |
| [14] | Harmon CM, Abumrad NA. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins:isolation and ammo-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids[J]. J Membr Biol, 1993, 133: 43–49. |
| [15] | Han WQ, Zhu Q, Hu J, et al. Hypoxia-inducible factor prolyl- hydroxylase-2 mediates transforming growth factor beta 1- induced epithelial-mesenchymal transition in renal tubular cells[J]. Biochim Biophys Acta, 2013, 1833: 1454–1462. DOI:10.1016/j.bbamcr.2013.02.029 |
| [16] | Moon JS, Karunakaran U, Elumalai S, et al. Metformin prevents glucotoxicity by alleviating oxidative and ER stress-induced CD36 expression in pancreatic beta cells[J]. J Diabetes Complications, 2016, 31: 21–30. |
| [17] | Kuda O, Pietka TA, Demianova Z, et al. Sulfo-N-suc-cinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164:SSO also inhibits oxidized low density lipoprotein uptake by macrophages[J]. J Biol Chem, 2013, 288: 15547–15555. DOI:10.1074/jbc.M113.473298 |
| [18] | Thastrup O, Dawson AP, Scharff O, et al. Thapsigargin, a novel molecular probe for studying intracellular calcium re-lease and storage[J]. Agents Actions, 1989, 27: 17–23. DOI:10.1007/BF02222186 |
| [19] | Wang Z, Liu D, Wang J, et al. Cytoprotective effects of melatonin on astroglial cells subjected to palmitic acid treat-ment in vitro[J]. J Pineal Res, 2012, 52: 253–264. DOI:10.1111/jpi.2012.52.issue-2 |
| [20] | Wong KL, Wu YR, Cheng KS, et al. Palmitic acid-induced lipotoxicity and protection by (+)-catechin in rat cortical astrocytes[J]. Pharmacol Rep, 2014, 66: 1106–1113. |
| [21] | Frago LM, Canelles S, Freire-Regatillo A, et al. Estradiol uses different mechanisms in astrocytes from the hippocampus of male and female rats to protect against damage induced by palmitic acid[J]. Front Mol Neurosci, 2017, 10: 330. DOI:10.3389/fnmol.2017.00330 |
| [22] | González-Giraldo Y, Garcia-Segura LM, Echeverria V, et al. Tibolone preserves mitochondrial functionality and cell morphology in astrocytic cells treated with palmitic acid[J]. Mol Neurobiol, 2017. DOI:10.1007/s12035-017-0667-3 |
| [23] | Gómez dPT, Velasco G, Sánchez C, et al. De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis[J]. Biochem J, 2002, 363: 183–188. DOI:10.1042/bj3630183 |
| [24] | Liu L, Chan C. IPAF inflammasome is involved in inter-leukin- 1β production from astrocytes, induced by palmitate; implications for Alzheimer's disease[J]. Neurobiol Aging, 2014, 35: 309–321. DOI:10.1016/j.neurobiolaging.2013.08.016 |
| [25] | Gupta S, Knight AG, Gupta S, et al. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes[J]. J Neurochem, 2012, 120: 1060–1071. |
| [26] | Clapham DE. Calcium signaling[J]. Cell, 2007, 131: 1047–1058. DOI:10.1016/j.cell.2007.11.028 |
| [27] | Egnatchik RA, Leamy AK, Jacobson DA, et al. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload[J]. Mol Metab, 2014, 3: 544–553. DOI:10.1016/j.molmet.2014.05.004 |
| [28] | Rabkin SW, Huber M, Krystal G. Modulation of palmitate- induced cardiomyocyte cell death by interventions that alter intracellular calcium[J]. Prostaglandins Leukot Essent Fatty Acids, 1999, 61: 195–201. DOI:10.1054/plef.1999.0090 |
| [29] | Liao Y, Hao Y, Chen H, et al. Mitochondrial calcium uni-porter protein MCU is involved in oxidative stress-induced cell death[J]. Protein Cell, 2015, 6: 434–442. DOI:10.1007/s13238-015-0144-6 |
 2018, Vol. 53
2018, Vol. 53


